Abstract
The inheritance and causal loci for resistance to blackleg, a devastating disease of Brassicaceous crops, are yet to be known in cabbage (Brassica oleracea L.). Here, we report the pattern of inheritance and linked molecular marker for this trait. A segregating BC1 population consisting of 253 plants was raised from resistant and susceptible parents, L29 (♀) and L16 (♂), respectively. Cotyledon resistance bioassay of BC1 population, measured based on a scale of 0–9 at 12 days after inoculation with Leptosphaeria maculans isolate 03–02 s, revealed the segregation of resistance and ratio, indicative of dominant monogenic control of the trait. Investigation of potential polymorphism in the previously identified differentially expressed genes within the collinear region of ‘B. napus blackleg resistant loci Rlm1′ in B. oleracea identified two insertion/deletion (InDel) mutations in the intron and numerous single nucleotide polymorphisms (SNPs) throughout the LRR-RLK gene Bol040029, of which six SNPs in the first exon caused the loss of two LRR domains in the susceptible line. An InDel marker, BLR-C-InDel based on the InDel mutations, and a high resolution melting (HRM) marker, BLR-C-2808 based on the SNP C2808T in the second exon were developed, which predicated the resistance status of the BC1 population with 80.24%, and of 24 commercial inbred lines with 100% detection accuracy. This is the first report of inheritance and molecular markers linked with blackleg resistance in cabbage. This study will enhance our understanding of the trait, and will be helpful in marker assisted breeding aiming at developing resistant cabbage varieties.
Keywords:
bioassay; blackleg; cabbage; disease resistance; high resolution melting (HRM); InDel; LRR-RLK gene; marker; PCR 1. Introduction
Blackleg or phoma stem canker, a severely devastating disease of oilseed rape (Brassica napus), and can cause significant economic loss in the farming of cabbage (Brassica oleracea) as well [1,2,3,4,5]. The disease is caused by dothideomycete fungal pathogens, Leptosphaeria maculans (Desm.) Ces. & de Not. (anamorph = Plenodomus lingam) and Leptosphaeria biglobosa Shoemaker & Brun (anamorph = P. biglobosus) [4,6]. The pathogen causes numerous circular to oval lesions on cotyledons, leaves, petioles, and stems; and characteristic black stem canker at the stem base (caused by more aggressive L. maculans), or less damaging lesions higher up the stem base (caused by less aggressive L. biglobosa) [7,8,9]. The fungus can reproduce both sexually and asexually [2], can complete multiple disease cycles in a single growing season [10], and survives on infected crop stubbles as a saprophyte [10]. Under favorable conditions, especially at moderate temperatures and high humidity during vegetative growth, the disease can cause up to 50% yield loss in brassica crops [11,12]. All the resistant loci controlling the resistance to this disease have been identified in B. napus, B. rapa, B. nigra, B. carinata, and B. juncea [5,13]. Several of these R genes have been introgressed in the commercial cultivars of Europe, Australia, and Canada [13,14,15,16,17,18,19]. Despite being substantially affected by the disease, no such quantitative trait loci (QTL) or R-gene have been identified in cabbage so far.
The disease is of particular concern in Asian region where the largest share (38%) of global cabbage is produced (Food and Agriculture Organization (FAO) Statistics Database 2017). In Asia, the disease is currently caused by the less aggressive species, L. biglobosa [6,20,21,22,23]. But, there is concern that the more aggressive L. maculans may spread in this region [21,23,24,25], which may threaten the Asian cabbage and oilseed rape industries [1,2,26,27]. So, developing resistant varieties is a prioritized breeding target in this region, which will not only safeguard the regional cabbage industry against the L. biglobosa now, but also against the eminent L. maculans invasion in future [20]. Such breeding programs require sources of resistance and efficient molecular markers. But, studies on blackleg disease in cabbage is scarce. So far, only a few screening studies was conducted on cabbage which identified few moderately resistant accessions [28,29,30]. Very recently, we identified two inbred lines that showed resistance against two L. maculans isolates 00–100 s and 03–02 s at cotyledon stage [1]. Both the isolates carry AvrLm1, however, the corresponding R-gene is yet to be identified in cabbage.
We have identified putative disease resistance related domain containing genes within the collinear region of major ‘A genome R-loci’ of B. napus (such as Rlm1, Rlm2, LepR1′ and LepR2′) and B. rapa (such as LepR4) in C-genome of B. oleracea via differential expression analysis against avirulent strains of L. maculans (I-S Nou, publication awaiting). In this study, we report the inheritance of blackleg resistance in cabbage and the characterization of polymorphisms, and development of molecular markers in the putative genes (within the collinear region of ‘B. napus R-loci Rlm1’ in cabbage) linked with blackleg resistance in cabbage.
2. Results
2.1. Inheritance of Blackleg Resistance in Cabbage
The pattern of inheritance was determined based on the bioassay results of the parental lines L29 (♀) and L16 (♂), their F1 hybrids, and 253 individuals of BC1 generation. A segregating F2 population could not be generated due to the male sterile nature of the female parent and the F1 hybrids. The susceptible parent, L16, showed characteristic ashy-gray lesions in the cotyledons at 12 dai and blackened stems at 30 dai (Figure 1). The symptoms on the leaves of their F1 hybrids resembled those of the resistant parent, indicating the dominant nature of inheritance of the trait in cabbage. Among the 253 BC1 plants, 122 and 131 plants were resistant and susceptible, respectively (Table 1; Table S1; Figure S1). The disease scores showed a continuous and normal distribution (Figure S1B). A Chi-square (χ2) test revealed that the resistance and susceptibility segregated at a 1:1 ratio in the BC1 population, which is suggestive of a monogenic control of the trait in the studied population (Table 1).
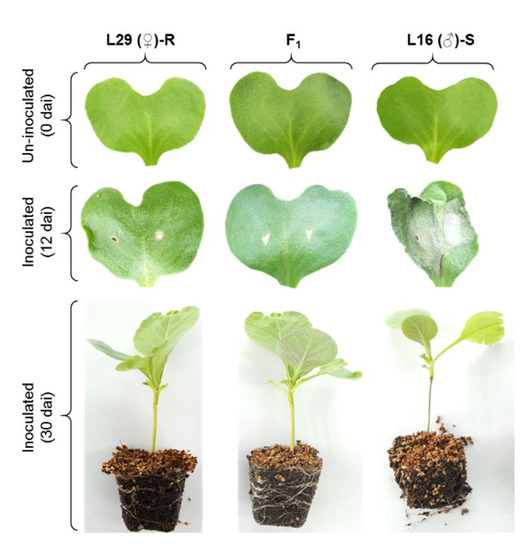
Figure 1.
Blackleg disease symptoms on the cotyledons and stems of the seedlings of parents and F1 hybrids at 12 and 30 days after inoculation with L. maculans isolate 03–02 s. Disease scores of all 253 BC1 lines are shown in Table S1. Color figure online.

Table 1.
Inheritance of resistance to blackleg disease in cabbage against the Leptosphaeria maculans isolate 03–02 s.
2.2. Selection of Genes and Detection of Length Polymorphism
Among the 15 putative disease resistance related NB-ARC, LRR, TIR, CC, EREB, FBD, and RLK domain containing genes within the collinear region of ‘B. napus blackleg resistant loci Rlm1’ in B. oleracea chromosome C07 (Table 2), six genes that showed significant higher expressions in the resistant line L29 (♀) compared to that of the susceptible line L16 (♂) against the isolate 03–02 s (I-S Nou, unpublished data) were investigated for identifying the potential polymorphism between contrastingly resistant cabbage parental lines (Table 3; Figure 2). Gene specific primers (Table 3), designed covering the entire length of these genes (one set for shorter genes and multiple sets for longer genes), were used for PCR amplification. Among these six genes, conspicuous length polymorphism was only detected for the LRR-RLK gene Bol040029 by the primer pair 3F3 and 3R3 (Figure 3).

Table 2.
List of putative disease resistance related domain (NB-ARC, LRR, TIR, CC, EREB, FBD, RLK etc.) containing genes within the collinear region of ‘B. napus blackleg resistant locus Rlm1’ in B. oleracea.

Table 3.
List of primers designed on six differentially expressed genes for detecting size polymorphism between resistant (L29) and susceptible (L16) parental lines via PCR assay.
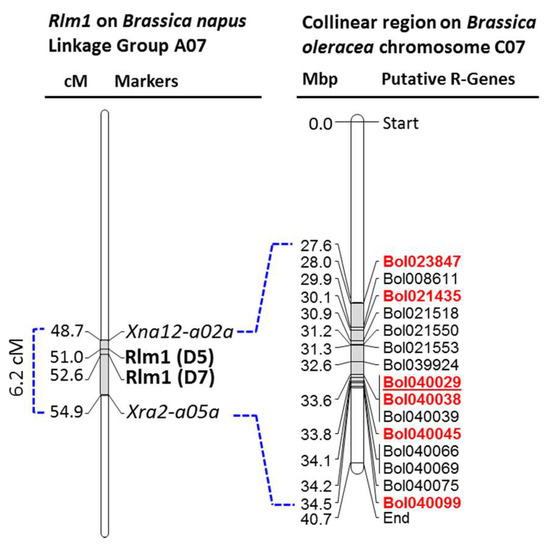
Figure 2.
Disease resistance related domain containing genes within the collinear region of ‘B. napus blackleg resistant locus Rlm1′ on B. oleracea chromosome C07. Details of the genes are shown in Table 2 and domain structures are shown in Figure S2. Rlm1 is collinear to a 2.6 Mb region on B. oleracea chromosome C07. Genes that showed significant higher expressions [1] in the Leptosphaeria maculans isolate 03–02s inoculated cotyledons of the resistant line L29 compared to that of susceptible line L16 within 12 days of inoculation are highlighted with bold and red text. Polymorphism between the resistant and susceptible cabbage lines are detected and markers linked with blackleg resistance is designed on the underlined gene. Color figure online.
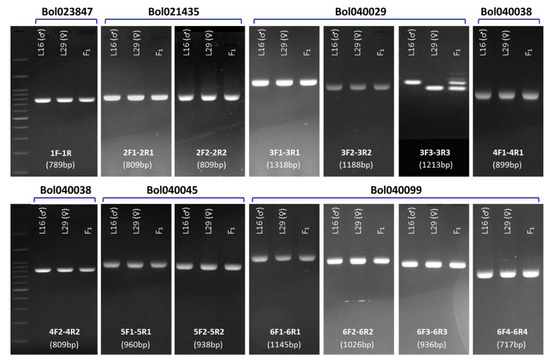
Figure 3.
Detection of length polymorphism between the resistant (L29) and susceptible (L16) cabbage lines in the six selected genes via PCR assay. Primer combinations and product size for each gel are shown in the bottom. Visible length polymorphism is observed in the gene Bol040029 by the primer pair’s 3F3–3R3. Details of the genes and corresponding primer specifications can be found in Table 2 and Table 3, respectively.
2.3. Cloning, Sequencing and Characterization of Polymorphism
To characterize the polymorphism, six fragments covering the entire length of Bol040029 were cloned and sequenced from both resistant and susceptible lines using six different primer sets (Table S2). Alignment of the gene identified InDel polymorphisms: deletion of 2508–2518 bp and 2597–2713 bp (total 128 bp deletion) in the only intron of the gene in the resistant line and numerous single nucleotide polymorphisms (SNPs): 54, 10, and 8 SNPs in the first exon, only intron, and second exon of the gene, respectively (Figure 4A; Figure S4). In-silico domain analysis of the translated protein sequences by the SMART domain analysis tool (http://smart.embl-heidelberg.de/) revealed that three non-synonymous SNPs (G268A, A271C, and G277C) between the susceptible to resistance parental lines caused the loss of an LRR domain and three other non-synonymous (T295A, C305A, and T308C) and one synonymous SNP (T324C) caused the loss of another LRR domain in the susceptible line L16 (Figure 4B; Figure S5).
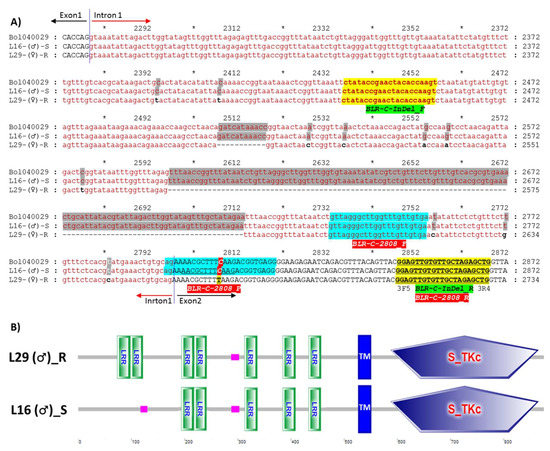
Figure 4.
Alignment of the segment of DNA sequences (A) and domain structures of translated protein sequences (B) of the gene Bol040029 from the susceptible (L16) and resistant (L29) lines showing the positions of the developed InDel and high resolution melting (HRM) Markers, BLR-C-InDel-F/R and BLR-C-2808, respectively. In figure A, Alignment of part of exon-1, intron-1 and part of exon-2 are shown only. The complete alignment is shown in Figure S4. The InDel (BLR-C-InDel-F/R) and the HRM (BLR-C-2808) marker developed for detecting blackleg resistant and susceptible genotypes are presented as yellow and green highlighted text, respectively. The 128 bp deleted region of the resistant line is highlighted gray and the C2808T SNP within the HRM probe BLR-C-2808-P is shown in green highlighted region. Black text = exon; red and lower case text = intron; shaded single nucleotides = SNPs. In figure B: LRR. Leucine rich repeat domain, TM. Transmembrane region, S_TKc. Serine/Threonine protein kinase domain, Pink box. Low complexity region. Color figure online.
2.4. Development of Markers Linked with Blackleg Resistance
One InDel marker, BLR-C-InDel, based on the 2508–2518 bp and 2597–2713 bp (total 128 bp) deletionS in the intronic region and one high resolution melting (HRM) marker, BLR-C-2808, based on the SNP C2808T in the second and last exon of the LRR-RLK gene Bol040029 were developed (Table 4; Figure 5A). The InDel marker BLR-C-InDel generated 433 bp and 305 bp amplicon from the susceptible and resistant parental lines, respectively, and a heterozygous amplicon for the F1 hybrid in PCR assay (Figure 5). The HRM marker BLR-C-2808 generated a melting peak at 55 °C and 63 °C for the resistant (T/T) and susceptible (C/C) alleles, respectively, and both peaks for the heterozygous (T/C) alleles (Figure S6).

Table 4.
Specifications of the developed InDel and high resolution melting (HRM) markers linked with blackleg resistance in cabbage.
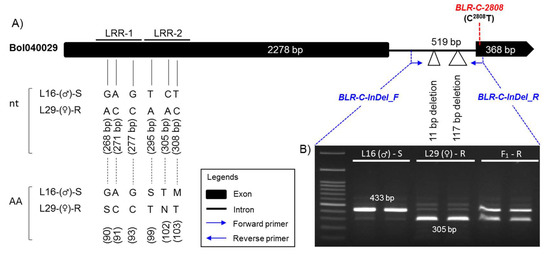
Figure 5.
(A) Gene structure of the LRR-RLP gene Bol040029 showing the positions of InDel marker BLR-C-InDel designed to characterize 128 bp InDel polymorphism and high resolution melting (HRM) marker BLR-C-2808 designed to detect the SNP C2808T between susceptible and resistant cabbage genotypes and (B) Polymorphic PCR amplicons of the resistant (R) and susceptible (S) parents, and their F1 plants by the primer pair BLR-C-InDel_F/R after 45 min of electrophoresis on 1.2% Agarose gel. The non-synonymous SNPs that cause loss of LRR-1 and LRR-2 domains in the susceptible lines and the SNP C2808T based on which the HRM marker BLR-C2808 is designed are shown here. All other SNPs throughout the length of the gene and the InDel segment is shown in Figure S5.
2.5. Validation of the Developed Markers
The efficacy of the developed markers was validated using 253 BC1 plants and 30 commercial inbred lines. The genotyping results of both InDel and HRM markers (BLR-C-InDel and BLR-C-2808, respectively) were same for all of the 30 commercial inbred lines and for 249 out of 253 BC1 plants (Figure 6; Table S1). In terms of accuracy in predicting the resistance status based on the bioassay phenotypes of 253 BC1 plants and 24 commercial inbred lines (no bioassay data is available for six BA lines, BA21-BA64), both the markers predicted 203 out of 253 BC1 individuals (80.24% detection accuracy) and all of the 24 commercial inbred lines correctly (Figure 6 and Figure 7). These indicate that the developed markers can be used for detecting the resistant and susceptible cabbage genotypes using a PCR based assay.
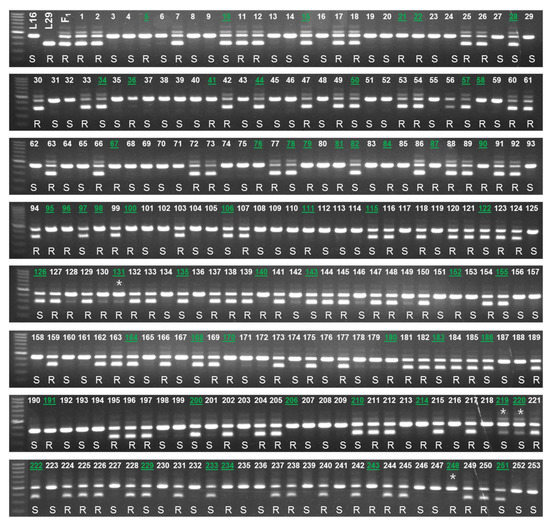
Figure 6.
Genotyping the BC1 population (1–253) raised from the resistant (L29) and susceptible (L16) parental lines using the developed InDel (BLR-C-F/R) and high resolution melting (HRM) (BLR-C-2808) markers. The bioassay phenotype is indicated as R (resistant) and S (susceptible). The green and underlined text indicate mismatch between the genotypic and phenotypic (bioassay) results. Mismatch between the InDel and HRM markers are indicated by an asterisk below the line number. Detailed disease scores (bioassay results) and HRM genotyping is shown in Table S1. The HRM plots of representative samples are shown in Figure S6.
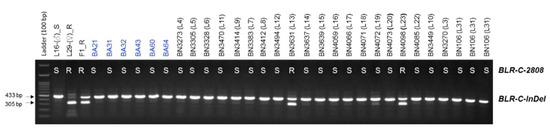
Figure 7.
Validation of the developed InDel marker BLR-C-F/R and high resolution melting (HRM) marker BLR-C-2808 using 30 commercial cabbage inbred lines. No bioassay data is available for BA lines BA21-BA64 (indicated by blue texts). Both markers perfectly predicted the resistance status of these inbred lines. R. resistant and S. susceptible as detected by the HRM marker BLR-C-2808.
3. Discussion
Despite being significantly damaging, the pattern of inheritance of resistance to blackleg disease, loci controlling the trait, and any marker linked to the trait in cabbage are yet to be identified. In this study, resistance to blackleg disease is determined to be controlled by a single dominant gene. In addition, one InDel and one HRM marker were developed that can distinguish resistant and susceptible cabbage genotypes via PCR assay.
Research on blackleg disease was mainly focused on A- and B-genome crops, with all the known R- loci (a total of 19 race-specific R-genes) being identified in the A genomes of B. rapa [31,32] and B. napus [33,34,35,36,37,38,39] and in the B genomes of B. nigra, B. carinata, and B. juncea [34,40,41,42]. A few major loci include the LepR1 on B. napus linkage group A02; Rlm1, Rlm3, Rlm4, Rlm7, and Rlm9 on A07; Rlm2, BlmR2, and LepR3 on A10 [18,41,43]; LepR2 on linkage group A02 [44]; rjlm2 in B. napus [45]; LMJR2 on LG J18 of B. juncea [40] and LepR4 on A06 of B. napus [18,46] etc.
Studies conducted on cabbage, on the other hand, were limited to screening the resistant cabbage lines only [28,29,30], including the identification of two resistant Korean cabbage inbred lines [1]. It would be better, if the loci could be mapped in cabbage, but this is time consuming and resource demanding [47]. Nonetheless, transferring those sources of resistance in elite cabbage lines may be enhanced via marker assisted breeding. We identified several disease resistance related domain containing genes within the collinear region of major R-loci of B. napus and B. rapa in cabbage such as Rlm1, Rlm2, LepR1, LepR2 and LepR4 via differential expression analysis against virulent L. maculans isolates (I-S Nou, unpublished data). Any polymorphism in the highly induced putative genes that is linked with inheritance of blackleg resistance can serve as molecular marker for this trait.
Our bioassay assessment of the 253 BC1 individuals at the seedlings stage revealed the monogenic dominant nature of inheritance of the trait in the studied material. Investigating the segregation ratio in the F2 population would have further validated this finding but raising an F2 generation population was not possible since the resistant parent L29 (♀) and the F1 individuals were male sterile (causing selfing of F1 individuals impossible). At the seedling stage, such qualitative resistance conferred by a monogenic dominant gene in several B. napus cultivars such as Cresor, Dunkeld, Major, Maluka, and Skipton [48,49,50,51], and digenic-inheritance in other B. napus and B. juncea populations [38,52] have been reported. Contrastingly, in the adult plant stage, the quantitative polygenes explain a majority of the phenotypic variation for blackleg resistance [53,54,55,56].
Among the six highly differentially expressed genes within the collinear region of ‘B. napus blackleg resistant locus Rlm1’ in B. oleracea, the gene Bol040029 was polymorphic between the resistant and susceptible parental genotypes. This gene encodes a Leucine-rich repeat receptor-like protein kinase (LRR-RLK) and showed a seven-fold increase of expression in the cotyledons of the resistant parent within 24 h of inoculation with L. maculans isolates 03–02 s and 00–100 s (I-S Nou, unpublished data). A recent pangenome shows a total of 901 RLKs in B. oleracea [57]. A meta-analysis of the 314 cloned plant R-genes revealed that 60 out of these 314 R-genes are RLKs/RLPs [58]. These RLKs broadly play roles in both broad-spectrum elicitor-initiated defense responses (e.g., FLS2 against bacterial elicitor Flagellin in Arabidopsis) and pathogen specific dominant R-gene mediated defense responses (e.g., Stb6 gene conferring resistance against Zymoseptoria tritici [59,60,61,62,63]. In addition, among the cloned blackleg resistant R-genes, LepR3/Rlm2 is also found to encode LRR-RLP [64,65]. This indicates the putative role of the gene Bol040029 in conferring resistance to blackleg in this genotype against the tested isolate which, however, needs to be functionally verified.
Numerous SNPs between the R and S lines throughout the exonic and intronic regions, and two deletions totaling 128 bp in the intronic region of the R line were observed (Figure S4). Among these, six SNPs within the LRR1 and LRR2 regions caused the loss of these two LRR domains in the susceptible line. However, no HRM marker could be designed using these SNPs, since these SNPs were located very closely, hindering the development of a precise and effective HRM probe. The InDel marker was developed based on the 128 bp InDel mutation in the intron. Introns are reported to play important roles in mRNA export, transcription coupling [66], exon shuffling and alternative splicing [67,68,69], the synthesis of non-coding RNA [70], and regulation of gene expression [71,72,73]. Very recently, a marker based on intronic mutations in the gene BoFLC1.C9 was found to be associated with the inheritance of flowering time variation in winter cabbage [74]. Both the developed markers, the HRM marker BLR-C-2808 (designed on the synonymous SNP C2808T) and the InDel marker BLR-C-InDel, predicted the resistance status of 253 BC1 plants with 80.24% accuracy and of 24 commercial inbred lines with 100% accuracy. This is the first report of molecular markers linked with blackleg resistance in cabbage. Markers with perfect genotyping capability would have been ideal, however, since no such marker is available for blackleg resistance in cabbage, the developed markers will be useful in practical breeding programs, at least roughly, for detecting the resistant and susceptible cabbage genotypes using a PCR assay.
We have determined the pattern of inheritance of resistance to blackleg disease in the studied genotypes of cabbage and developed two co-dominant markers, one InDel and one HRM that can be used in marker assisted breeding programs, aiming to improve the trait in cabbage. The functional validation of the roles of the detected polymorphism in the gene Bol040029 remains to be performed. Work is underway to map the blackleg resistant loci in cabbage using partial genome sequence based approaches.
4. Materials and Methods
4.1. Plant Materials and Population Development
A segregating BC1 population consisting of 253 plants was developed from the resistant and susceptible cabbage lines, L29 (♀) and L16 (♂), respectively. In addition, 30 commercial cabbage lines were used for validation of the developed markers. All these plant materials were obtained from Asia Seeds Ltd., Seoul, Republic of Korea. Seeds were germinated on commercial nursery soil mix in a controlled plant growth chamber at 25 ± 2 °C, 16 h day length and 440 μmoles/m2/s light intensity at bench level.
4.2. L. maculans Isolate: Culture, Inoculation, and Disease Scoring
L. maculans isolate 03–02 s, collected from Agriculture and Agri-Foods (AAFC Saskatoon, Canada), was cultured on 20% V8 agar with a 0.1% streptomycin sulfate supplement at 22 °C and 16 h photoperiod under fluorescent light. Fungal spores were collected in 10 mL sterile distilled water, by scraping the spores off the culture plates with a plastic scraper followed by filtering the spore suspension with four layers of sterile Miracloth (EMD Millipore Corporation, Burlington, MA, USA). For the final inoculum, the spore concentration was adjusted to 2 × 107 spores/mL−1.
Four tiny puncture wounds were created in the center of four cotyledon lobes of 12 day-old seedlings of each plant using a sterile needle. Each wound was inoculated with a drop of (~10 μL) spore suspension. The trays of the inoculated seedlings were covered with a plastic cover to maintain high (90%) relative humidity. Plants were re-inoculated 24 h after the first inoculation to ensure no plants avoided inoculation and to eliminate false positives. Disease symptoms on the cotyledons were recorded based on a scale of 0–9 (Figure S1) at 12 days after inoculation (dai) and on the stems at 30 dai. Cotyledons with 0–5 and 6–9 scores were considered as resistant and susceptible, respectively, and the resulting ratio of the BC1 population was analyzed for goodness-of-fit using χ2 test.
4.3. Primer Design
The genomic sequences of the selected genes (Table 2) were retrieved from Bolbase (http://ocri-genomics.org/bolbase) database. All the primers for detecting length polymorphism (Table 3), for cloning the entire length of the polymorphic gene Bol040029 (Table S2) and the final InDel, as well as HRM markers on the gene Bol040029 (Table 4) were designed using the Primer3plus web tool and checked for any potential hairpin and self-annealing sites using the ‘Oligo Calc’ tool (http://biotools.nubic.northwestern.edu/OligoCalc.html).
4.4. DNA Extraction and PCR Based Detection of Length Polymorphism
Genomic DNA was isolated from the young leaves of four weeks old seedlings of parental lines, their F1 hybrids, 253 BC1 plants, and 30 commercial cabbage lines using DNeasy Plant Mini Kit (Qiagen, Hilden, Germany). The concentrations of genome DNA were determined spectrophotometrically using Nanodrop ND-1000 (Nano Drop, Wilmington, DE, USA) and diluted to a 100 ng µL−1 and store in a −20 °C refrigerator for further use.
A polymerase chain reaction (PCR) was carried out in a 20 μL reaction volume that consisted of 1 μL of 10 pmol forward and reverse primers each, 1 μL of genomic DNA (~100 ng), 9 μL of ultra-pure water, and 8 μL of 2× Prime Taq Premix containing 1 unit of Taq polymerase (GENETBIO Inc., Korea). PCR conditions were set at 95 °C for 5 min, 30 cycles of 95 °C for 30 s, (at primer specific annealing temperatures (Table 3) or at 60 °C, if not specifically mentioned) for 30 s, at 72 °C for 40 s, and 72 °C for 5 min. Electrophoresis was performed in 2% agarose gel stained with HiQ blue mango (BioD, Gwangmyeong, Korea) for 30 min and the banding patterns were visualized on an ENDURO™TM GDS gel documentation system under UV light for detecting any potential size polymorphism.
4.5. Cloning and Sequencing of the Polymorphic Gene
Six consecutive segments covering the entire length of the polymorphic gene Bol040029 were amplified by six pairs of primers (Table S2). The amplified bands were excised from the gel after electrophoresis and purified using the ‘Wizard SV gel and PCR cleanup system’ (Promega, Madison, WI, USA). The fragments were then cloned using TOPcloner™ Blunt Kit (Enzynomics, Daejeon, Korea) and three independent PCR-confirmed clones were sequenced (Macrogen Inc., Seoul, Korea) using the universal primers, M13FpUC and M13RpUC. The clone sequences of resistant and susceptible lines were then aligned using ‘Clustal Omega’ to identify the sequence variation.
4.6. High Resolution Melting (HRM) Analysis
High resolution melting (HRM) analysis of the C2808T SNP of the gene Bol040029 were analyzed in the BC1 population and in 30 commercial lines in a final reaction volume of 20 μL, containing 50 ng of genomic DNA, 10 μL of ‘HS Prime LP Premix’ (GeNet Bio, Deajeon, Republic of Korea), 0.6 μL of 2xSYTO9 green fluorescent nucleic acid stain (GeNet Bio, Deajeon, Republic of Korea), 0.2, 1.0 and 1.0 μL of forward, reverse and probe primers, respectively (Table 4), and ultra-pure water for the remainder of the volume. HRM was performed in a LightCycler96 software (Roche, Mannheim, Germany) using a 96-well plate in a 20μL/well final reaction mix based on the cycling condition of initial denaturation at 95 °C for 5 min followed by 40 cycles of 3-step amplifications at 95 °C for 10 sec, 60 °C for 15 sec and 72 °C for 15 sec. The HRM program included denaturation at 95 °C for 1 min, renaturation at 40 °C for 2 min, melting from 60–90 °C with a ramp of 0.3 °C per second, and 5 fluorescent acquisitions per degree centigrade. HRM data were analyzed using LightCycler® 96 software v1.1 (Roche, Mannheim, Germany).
4.7. Statistical Analysis
A Chi-square (χ2) test for goodness-of-fit was performed to determine deviations of observed data from the expected segregation ratios using Minitab®18 software package (Minitab Inc., State College, PA, USA).
Supplementary Materials
The following are available online at https://www.mdpi.com/2223-7747/8/12/583/s1, Figure S1: (A) Criteria for scoring the severity of blackleg disease symptoms in Leptosphaeria maculans isolate 03-02 s infected cotyledons at 12 days after inoculation (dai). Cotyledons with 0-5 and 6-9 scores were characterized as resistant and susceptible, respectively. (B) Frequency distributions of disease scores of the 253 BC1 population raised from the resistant (R) and susceptible (S) parental lines, L29 (♀) and L16 (♂), respectively. Figure S2: Domain structures of the putative disease resistance related domain (NB-ARC, LRR, TIR, CC EREB, FBD, RLK etc.) containing genes within the collinear region of B. napus blackleg resistant gene Rlm1 in B. oleracea. Figure S3: Exon-intron structures and primer positions on the selected six putative R-genes for detecting length polymorphism between blackleg resistant and susceptible cabbage lines. Figure S4: Alignment of nucleotide sequences of the gene Bol040029 from the susceptible (L16) and resistant lines (L29) using Clustal Omega showing the positions of the developed InDel and high resolution melting (HRM) Markers, BLR-C-InDel-F/R and BLR-C-2808, respectively. Figure S5: Alignment of translated protein sequences of the gene Bol040029 of the susceptible (L16) and resistant lines (L29). Figure S6: Normalized melting peaks (A), the difference plots (B) and normalized melting curves (C) of the high resolution melting analysis of 253 BC1 lines generated from the resistant (R) and susceptible (S) parental lines, L29 (♀) and L16 (♂), respectively using the developed HRM marker BLR-C-2808 (forward and reverse primers and C2808T SNP based probe). Table S1: Disease scores against the Leptosphaeria maculans isolate 03-02s and prediction of resistance by developed InDel and HRM markers in the 253 BC1 population raised from resistant and susceptible parental lines L29 (♀) and L16 (♂), respectively, Table S2: Specifications of primers designed for cloning the six consecutive fragments covering the entire length of the gene Bol040029.
Author Contributions
I.-S.N., J.-I.P. and H.-T.K. conceptualized, acquired the fund and supervised the work; A.H.K.R. selected the genes and generated F1 population. M.J.F., M.R.H. raised BC1 population, collected the DNA, designed the primers. D.M.I.J. conducted the in-silico analysis. M.J.F., H.-J.J. and M.R.H. conducted all wet lab works, analyzed the data, interpreted the results and wrote the manuscript. All authors read the article and approved the manuscript.
Funding
This study was supported by the Golden Seed Project (Grant Number: 213007-05-4-CG100), Center for Horticultural Seed Development, Ministry of Agriculture, Food and Rural Affairs in the Republic of Korea (MAFRA). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Acknowledgments
We thank Nicholas J. Larkan and Hossein Borhan of Agriculture and Agri-Food Canada (AAFC), Saskatoon for providing the Leptosphaeria maculans isolates.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Robin, A.H.K.; Larkan, N.J.; Laila, R.; Park, J.I.; Ahmed, N.U.; Borhan, H.; Parkin, I.A.P.; Nou, I.S. Korean Brassica oleracea germplasm offers a novel source of qualitative resistance to blackleg disease. Eur. J. Plant Pathol. 2017, 149, 611–623. [Google Scholar] [CrossRef]
- West, J.S.; Kharbanda, P.D.; Barbetti, M.J.; Fitt, B.D.L. Epidemiology and management of Leptosphaeria maculans (phoma stem canker) on oilseed rape in Australia, Canada and Europe. Plant Pathol. 2001, 50, 10–27. [Google Scholar] [CrossRef]
- Howlett, B.J. Current knowledge of the interaction between Brassica napus and Leptosphaeria maculans. Can. J. Plant Pathol. 2004, 26, 245–252. [Google Scholar] [CrossRef]
- Fitt, B.D.L.; Brun, H.; Barbetti, M.J.; Rimmer, S.R. World-wide importance of phoma stem canker (Leptosphaeria maculans and L. biglobosa) on oilseed rape (Brassica napus). Eur. J. Plant Pathol. 2006, 114, 3–15. [Google Scholar] [CrossRef]
- Zhang, X.; Fernando, W.G.D. Insights into fighting against blackleg disease of Brassica napus in Canada. Crop Pasture Sci. 2018, 69, 40–47. [Google Scholar] [CrossRef]
- Mendes-Pereira, E.; Balesdent, M.-H.; Brun, H.; Rouxel, T. Molecular phylogeny of the Leptosphaeria maculans—L. biglobosa species complex. Mycol. Res. 2003, 107, 1287–1304. [Google Scholar] [CrossRef] [PubMed]
- Howlett, B.J.; Idnurm, A.; Pedras, M.S.C. Leptosphaeria maculans, the causal agent of blackleg disease of Brassicas. Fungal Genet. Biol. 2001, 33, 1–14. [Google Scholar] [CrossRef]
- Henderson, M.P. The black-leg disease of cabbage caused by Phoma lingam (Tode) Desmaz. Phytopathology 1918, 8, 379–431. [Google Scholar]
- Williams, P.H. Biology of Leptosphaeria maculans. Can. J. Plant Pathol. 1992, 14, 30–35. [Google Scholar] [CrossRef]
- Li, H.; Kuo, J.; Barbetti, M.J.; Sivasithamparam, K. Differences in the responses of stem tissues of spring-type Brassica napus cultivars with polygenic resistance and single dominant gene-based resistance to inoculation with Leptosphaeria maculans. Botany 2007, 85, 191–203. [Google Scholar] [CrossRef]
- Ghanbarnia, K.; Dilantha Fernando, W.G.; Crow, G. Developing rainfall-and temperature-based models to describe infection of canola under field conditions caused by pycnidiospores of Leptosphaeria maculans. Phytopathology 2009, 99, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fitt, B.D.L.; Welham, S.J.; Gladders, P.; Sansford, C.E.; West, J.S. Effects of severity and timing of stem canker (Leptosphaeria maculans) symptoms on yield of winter oilseed rape (Brassica napus) in the UK. Eur. J. Plant Pathol. 1999, 105, 715–728. [Google Scholar] [CrossRef]
- Raman, H.; Raman, R.; Larkan, N. Genetic Dissection of Blackleg Resistance Loci in Rapeseed (Brassica napus L.). In Plant Breeding from Laboratories to Fields; InTech: London, UK, 2013; pp. 85–120. [Google Scholar] [CrossRef]
- Sprague, S.J.; Balesdent, M.-H.; Brun, H.; Hayden, H.L.; Marcroft, S.J.; Pinochet, X.; Rouxel, T.; Howlett, B.J. Major gene resistance in Brassica napus (oilseed rape) is overcome by changes in virulence of populations of Leptosphaeria maculans in France and Australia. In Sustainable Strategies for Managing Brassica Napus (Oilseed Rape) Resistance to Leptosphaeria; Fitt, B.D.L., Evans, N., Howlett, B.J., Cooke, B.M., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 33–40. ISBN 978-1-4020-4525-7. [Google Scholar] [CrossRef]
- Brun, H.; Levivier, S.; Somda, I.; Ruer, D.; Renard, M.; Chèvre, A.M. A Field Method for Evaluating the Potential Durability of New Resistance Sources: Application to the Leptosphaeria maculans-Brassica napus Pathosystem. Phytopathology 2000, 90, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Barret, P.; Guérif, J.; Reynoird, J.P.; Delourme, R.; Eber, F.; Renard, M.; Chèvre, A.M. Selection of stable Brassica napus-Brassica juncea recombinant lines resistant to blackleg (Leptosphaeria maculans). A ‘to and fro’ strategy to localise and characterise interspecific introgressions on the B. napus genome. Theor. Appl. Genet. 1998, 96, 1097–1103. [Google Scholar] [CrossRef]
- Rouxel, T.; Penaud, A.; Pinochet, X.; Brun, H.; Gout, L.; Delourme, R.; Schmit, J.; Balesdent, M.-H. A 10-year Survey of Populations of Leptosphaeria maculans in France Indicates a Rapid Adaptation Towards the Rlm1 Resistance Gene of Oilseed Rape. Eur. J. Plant Pathol. 2003, 109, 871–881. [Google Scholar] [CrossRef]
- Yu, F.; Lydiate, D.J.; Rimmer, S.R. Identification and mapping of a third blackleg resistance locus in Brassica napus derived from B. rapa subsp. sylvestris. Genome 2008, 51, 64–72. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, M.; Wang, X.; Tong, C.; Huang, S.; Tehrim, S.; Liu, Y. Bolbase: A comprehensive genomics database for Brassica oleracea. BMC Genomics 2013, 14, 664. [Google Scholar] [CrossRef]
- Zhang, X.; White, R.P.; Demir, E.; Jedryczka, M.; Lange, R.M.; Islam, M.; Li, Z.Q.; Huang, Y.J.; Hall, A.M.; Zhou, G. Leptosphaeria spp., phoma stem canker and potential spread of L. maculans on oilseed rape crops in China. Plant Pathol. 2014, 63, 598–612. [Google Scholar] [CrossRef]
- Liu, Z.; Latunde-Dada, A.O.; Hall, A.M.; Fitt, B.D.L. Phoma stem canker disease on oilseed rape (Brassica napus) in China is caused by Leptosphaeria biglobosa ‘brassicae’. Eur. J. Plant Pathol. 2014, 140, 841–857. [Google Scholar] [CrossRef]
- Hong, S.-K.; Kim, W.-G.; Shin, D.-B.; Choi, H.-W.; Lee, Y.-K.; Lee, S.-Y. Occurrence of stem canker on rape caused by Leptosphaeria biglobosa in Korea. Plant Pathol. J. 2009, 25, 294–298. [Google Scholar] [CrossRef]
- Hao, L.; Song, P.; Huangfu, H.; Li, Z. Genetic diversity and differentiation of Leptosphaeria biglobosa on oilseed rape in China. Phytoparasitica 2015, 43, 253–263. [Google Scholar] [CrossRef]
- Cai, X.; Huang, Y.; Jiang, D. Evaluation of oilseed rape seed yield losses caused by Leptosphaeria biglobosa in central China. Eur. J. Plant Pathol. 2017, 150, 179–190. [Google Scholar] [CrossRef]
- Fitt, B.D.L.; Hu, B.C.; Li, Z.Q.; Liu, S.Y.; Lange, R.M.; Kharbanda, P.D.; Butterworth, M.H.; White, R.P. Strategies to prevent spread of Leptosphaeria maculans (phoma stem canker) onto oilseed rape crops in China; costs and benefits. Plant Pathol. 2008, 57, 652–664. [Google Scholar] [CrossRef]
- Liu, Z. Strategies for Managing Leptosphaeria Maculans and Leptosphaeria Biglobosa to Decrease Severity of Phoma Stem Canker Epidemics on Winter Oilseed Rape. Ph.D. Thesis, University of Hertfordshire, Hertfordshire, UK, 2008. [Google Scholar]
- Fitt, B.D.L.; Huang, Y.-J.; van den Bosch, F.; West, J.S. Coexistence of related pathogen species on arable crops in space and time. Annu. Rev. Phytopathol. 2006, 44, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Ananga, A.O.; Cebert, E.; Soliman, K.; Kantety, R.; Pacumbaba, R.P.; Konan, K. RAPD markers associated with resistance to blackleg disease in Brassica species. J. Biotechnol. 2006, 5, 2041–2048. [Google Scholar] [CrossRef]
- Badawy, H.M.A.; Hoppe, H.; Koch, E. Differential reactions between the genus Brassica and aggressive single spore isolates of Leptosphaeria maculans. J. Phytopathol. 1991, 131, 109–119. [Google Scholar] [CrossRef]
- Ferreira, M.E.; Dias, J.S.; Mengistu, A.; Williams, P.H. Screening of Portuguese cole landraces (Brassica oleracea L.) with Leptosphaeria maculans and Xanthomonas campestris pv. campestris. Euphytica 1992, 65, 219–227. [Google Scholar] [CrossRef]
- Leflon, M.; Brun, H.; Eber, F.; Delourme, R.; Lucas, M.O.; Vallée, P.; Ermel, M.; Balesdent, M.H.; Chèvre, A.M. Detection, introgression and localization of genes conferring specific resistance to Leptosphaeria maculans from Brassica rapa into B. napus. Theor. Appl. Genet. 2007, 115, 897–906. [Google Scholar] [CrossRef]
- Mithen, R.F.; Lewis, B.G.; Heaney, R.K.; Fenwick, G.R. Resistance of leaves of Brassica species to Leptosphaeria maculans. Trans. Br. Mycol. Soc. 1987, 88, 525–531. [Google Scholar] [CrossRef]
- Bohman, S.; Wang, M.; Dixelius, C. Arabidopsis thaliana-derived resistance against Leptosphaeria maculans in a Brassica napus genomic background. Theor. Appl. Genet. 2002, 105, 498–504. [Google Scholar] [CrossRef]
- Chèvre, A.M.; Barret, P.; Eber, F.; Dupuy, P.; Brun, H.; Tanguy, X.; Renard, M. Selection of stable Brassica napus-B. juncea recombinant lines resistant to blackleg (Leptosphaeria maculans). Identification of molecular markers, chromosomal and genomic origin of the introgression. Theor. Appl. Genet. 1997, 95, 1104–1111. [Google Scholar] [CrossRef]
- Delourme, R.; Chevre, A.M.; Brun, H.; Rouxel, T.; Balesdent, M.H.; Dias, J.S.; Salisbury, P.; Biop, U.M.R.; Cedex, L.R.; St-cyr, R. De Major gene and polygenic resistance to Leptosphaeria maculans in oilseed rape (Brassica napus). Eur. J. Plant Pathol. 2006, 114, 41–52. [Google Scholar] [CrossRef]
- Marcroft, S.J.; Wratten, N.; Purwantara, A.; Salisbury, P.A.; Potter, T.D.; Barbetti, M.J.; Khangura, R.; Howlett, B.J. Reaction of a range of Brassica species under Australian conditions to the fungus, Leptosphaeria maculans, the causal agent of blackleg. Aust. J. Exp. Agric. 2002, 42, 587–594. [Google Scholar] [CrossRef]
- Pang, E.C.K.; Halloran, G.M. The genetics of blackleg [Leptosphaeria maculans (Desm.) Ces. et De Not.] resistance in rapeseed (Brassica napus L.). Theor. Appl. Genet. 1996, 93, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Rimmer, S.R.; van den Berg, C.G.J. Resistance of oilseed Brassica spp. to blackleg caused by Leptosphaeria maculans. Can. J. Plant Pathol. 1992, 14, 56–66. [Google Scholar] [CrossRef]
- Snowdon, R.J.; Winter, H.; Diestel, A.; Sacristán, M.D. Development and characterisation of Brassica napus-Sinapis arvensis addition lines exhibiting resistance to Leptosphaeria maculans. Theor. Appl. Genet. 2000, 101, 1008–1014. [Google Scholar] [CrossRef]
- Christianson, J.A.; Rimmer, S.R.; Good, A.G.; Lydiate, D.J. Mapping genes for resistance to Leptosphaeria maculans in Brassica juncea. Genome 2006, 41, 30–41. [Google Scholar] [CrossRef]
- Balesdent, M.H.; Attard, A.; Kühn, M.L.; Rouxel, T. New Avirulence Genes in the Phytopathogenic Fungus Leptosphaeria maculans. Genet. Resist. 2002, 92, 1122–1133. [Google Scholar] [CrossRef]
- Plieske, J.; Struss, D.; Röbbelen, G. Inheritance of resistance derived from the B-genome of Brassica against Phoma lingam in rapeseed and the development of molecular markers. Theor. Appl. Genet. 1998, 97, 929–936. [Google Scholar] [CrossRef]
- Delourme, R.; Pilet-Nayel, M.L.; Archipiano, M.; Horvais, R.; Tanguy, X.; Rouxel, T.; Brun, H.; Renard, M.; Balesdent, M.H. A Cluster of Major Specific Resistance Genes to Leptosphaeria maculans in Brassica napus. Phytopathology 2004, 94, 578–583. [Google Scholar] [CrossRef]
- Yu, F.; Lydiate, D.J.; Rimmer, S.R. Identification of two novel genes for blackleg resistance in Brassica napus. Theor. Appl. Genet. 2005, 110, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Saal, B.; Brun, H.; Glais, I.; Struss, D. Identification of a Brassica juncea-derived recessive gene conferring resistance to Leptosphaeria maculans in oilseed rape. Plant Breed. 2004, 123, 505–511. [Google Scholar] [CrossRef]
- Yu, F.; Gugel, R.K.; Kutcher, H.R.; Peng, G.; Rimmer, S.R. Identification and mapping of a novel blackleg resistance locus LepR4 in the progenies from Brassica napus × B. rapa subsp. sylvestris. Theor. Appl. Genet. 2013, 126, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Delourme, R.; Laperche, A.; Bouchet, A.; Jubault, M.; Paillard, S.; Nesi, N. Genes and Quantitative Trait Loci Mapping for Major Agronomic Traits in Brassica napus L. In The Brassica Napus Genome; Springer International Publishing: New York, NY, USA, 2018; pp. 41–85. ISBN 9783319436944. [Google Scholar] [CrossRef]
- Raman, R.; Taylor, B.; Marcroft, S.; Stiller, J.; Eckermann, P. Molecular mapping of qualitative and quantitative loci for resistance to Leptosphaeria maculans causing blackleg disease in canola (Brassica napus L.). Theor. Appl. Genet. 2012, 125, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Dion, Y.; Gugel, R.K.; Rakow, G.F.; Seguin-Swartz, G.; Landry, B.S. RFLP mapping of resistance to the blackleg disease [causal agent, Leptosphaeria maculans (Desm.) Ces. et de Not.] in canola (Brassica napus L.). Theor. Appl. Genet. 1995, 91, 1190–1194. [Google Scholar] [CrossRef]
- Ferreira, M.E.; Rimmer, S.R.; Williams, P.H.; Osborn, T.C. Mapping loci controlling Brassica napus resistance to Leptosphaeria maculans under different screening conditions. Phytopathology 1995, 85, 213–217. [Google Scholar] [CrossRef]
- Mayerhofer, R.; Good, A.G.; Bansal, V.K.; Thiagarajah, M.R.; Stringam, G.R. Molecular mapping of resistance to Leptosphaeria maculans in Australian cultivars of Brassica napus. Genome 1997, 40, 294–301. [Google Scholar] [CrossRef]
- Saal, B.; Struss, D. RGA-and RAPD-derived SCAR markers for a Brassica B-genome introgression conferring resistance to blackleg in oilseed rape. Theor. Appl. Genet. 2005, 111, 281–290. [Google Scholar] [CrossRef]
- Huang, Y.J.; Pirie, E.J.; Evans, N.; Delourme, R.; King, G.J.; Fitt, B.D.L. Quantitative resistance to symptomless growth of Leptosphaeria maculans (phoma stem canker) in Brassica napus (oilseed rape). Plant Pathol. 2009, 58, 314–323. [Google Scholar] [CrossRef]
- Raman, H.; Raman, R.; Diffey, S.; Qiu, Y.; McVittie, B.; Barbulescu, D.M.; Salisbury, P.A.; Marcroft, S.; Delourme, R. Stable Quantitative Resistance Loci to Blackleg Disease in Canola (Brassica napus L.) Over Continents. Front. Plant Sci. 2018, 9, 1622. [Google Scholar] [CrossRef]
- Pilet, M.L.; Delourme, R.; Foisset, N.; Renard, M. Identification of loci contributing to quantitative field resistance to blackleg disease, causal agent Leptosphaeria maculans (Desm.) Ces. et de Not., in Winter rapeseed (Brassica napus L.). Theor. Appl. Genet. 1998, 96, 23–30. [Google Scholar] [CrossRef]
- Huang, Y.; Qi, A.; King, G.J.; Fitt, B.D.L. Assessing Quantitative Resistance against Leptosphaeria maculans (Phoma Stem Canker) in Brassica napus (Oilseed Rape) in Young Plants. PLoS ONE 2014, 9, e84924. [Google Scholar] [CrossRef] [PubMed]
- Bayer, P.E.; Golicz, A.A.; Tirnaz, S.; Chan, C.K.K.; Edwards, D.; Batley, J. Variation in abundance of predicted resistance genes in the Brassica oleracea pangenome. Plant Biotechnol. J. 2019, 17, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Kourelis, J.; van der Hoorn, R.A.L. Defended to the Nines: 25 Years of Resistance Gene Cloning Identifies Nine Mechanisms for R Protein Function. Plant Cell 2018, 30, 285–299. [Google Scholar] [CrossRef]
- Gómez-Gómez, L.; Boller, T. FLS2: An LRR receptor–like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol. Cell 2000, 5, 1003–1011. [Google Scholar] [CrossRef]
- Goff, K.E.; Ramonell, K.M. The Role and Regulation of Receptor-Like Kinases in Plant Defense. Gene Regul. Syst. Biol. 2007, 1, 167–175. [Google Scholar] [CrossRef]
- Liu, P.L.; Du, L.; Huang, Y.; Gao, S.M.; Yu, M. Origin and diversification of leucine-rich repeat receptor-like protein kinase (LRR-RLK) genes in plants. BMC Evol. Biol. 2017, 17, 47. [Google Scholar] [CrossRef]
- Sekhwal, M.K.; Li, P.; Lam, I.; Wang, X.; Cloutier, S.; You, F.M. Disease resistance gene analogs (RGAs) in plants. Int. J. Mol. Sci. 2015, 16, 19248–19290. [Google Scholar] [CrossRef]
- Saintenac, C.; Lee, W.-S.; Cambon, F.; Rudd, J.J.; King, R.C.; Marande, W.; Powers, S.J.; Bergès, H.; Phillips, A.L.; Uauy, C. Wheat receptor-kinase-like protein Stb6 controls gene-for-gene resistance to fungal pathogen Zymoseptoria tritici. Nat. Genet. 2018, 50, 368. [Google Scholar] [CrossRef]
- Larkan, N.J.; Ma, L.; Borhan, M.H. The I-like protein RLM2 is encoded by a second allele of the LepR3/Rlm2 blackleg resistance locus. Plant Biotechnol. J. 2015, 13, 983–992. [Google Scholar] [CrossRef]
- Larkan, N.J.; Lydiate, D.J.; Parkin, I.A.P.; Nelson, M.N.; Epp, D.J.; Cowling, W.A.; Rimmer, S.R.; Borhan, M.H. The Brassica napus blackleg resistance gene LepR3 encodes a receptor-like protein triggered by the Leptosphaeria maculans effector AVRLM1. New Phytol. 2013, 197, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Maniatis, T.; Reed, R. An extensive network of coupling among gene expression machines. Nature 2002, 416, 499. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-F.; Zhu, T.; Niu, D.-K. Association of intron loss with high mutation rate in Arabidopsis: Implications for genome size evolution. Genome Biol. Evol. 2013, 5, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Kalsotra, A.; Cooper, T.A. Functional consequences of developmentally regulated alternative splicing. Nat. Rev. Genet. 2011, 12, 715. [Google Scholar] [CrossRef] [PubMed]
- Gorlova, O.; Fedorov, A.; Logothetis, C.; Amos, C.; Gorlov, I. Genes with a large intronic burden show greater evolutionary conservation on the protein level. BMC Evol. Biol. 2014, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.-Y.; Lin, S.-L. Intronic microRNAs. Biochem. Biophys. Res. Commun. 2005, 326, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.B. The effect of intron location on intron-mediated enhancement of gene expression in Arabidopsis. Plant J. 2004, 40, 744–751. [Google Scholar] [CrossRef]
- Rose, A.B.; Emami, S.; Bradnam, K.; Korf, I. Evidence for a DNA-based mechanism of intron-mediated enhancement. Front. Plant Sci. 2011, 2, 98. [Google Scholar] [CrossRef]
- Chorev, M.; Carmel, L. The function of introns. Front Genet. 2012, 3, 55. [Google Scholar] [CrossRef]
- Abuyusuf, M.; Nath, U.K.; Kim, H.; Islam, R.; Park, J.; Nou, I. Molecular markers based on sequence variation in BoFLC1. C9 for characterizing early- and late-flowering cabbage genotypes. BMC Genet. 2019, 20, 42. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).