Nutritional Value, Mineral Composition, Secondary Metabolites, and Antioxidant Activity of Some Wild Geophyte Sedges and Grasses
Abstract
1. Introduction
2. Results and Discussion
2.1. Proximate Composition
2.2. Mineral Content
2.3. Secondary Metabolites
2.4. Antioxidant Activity
3. Materials and Methods
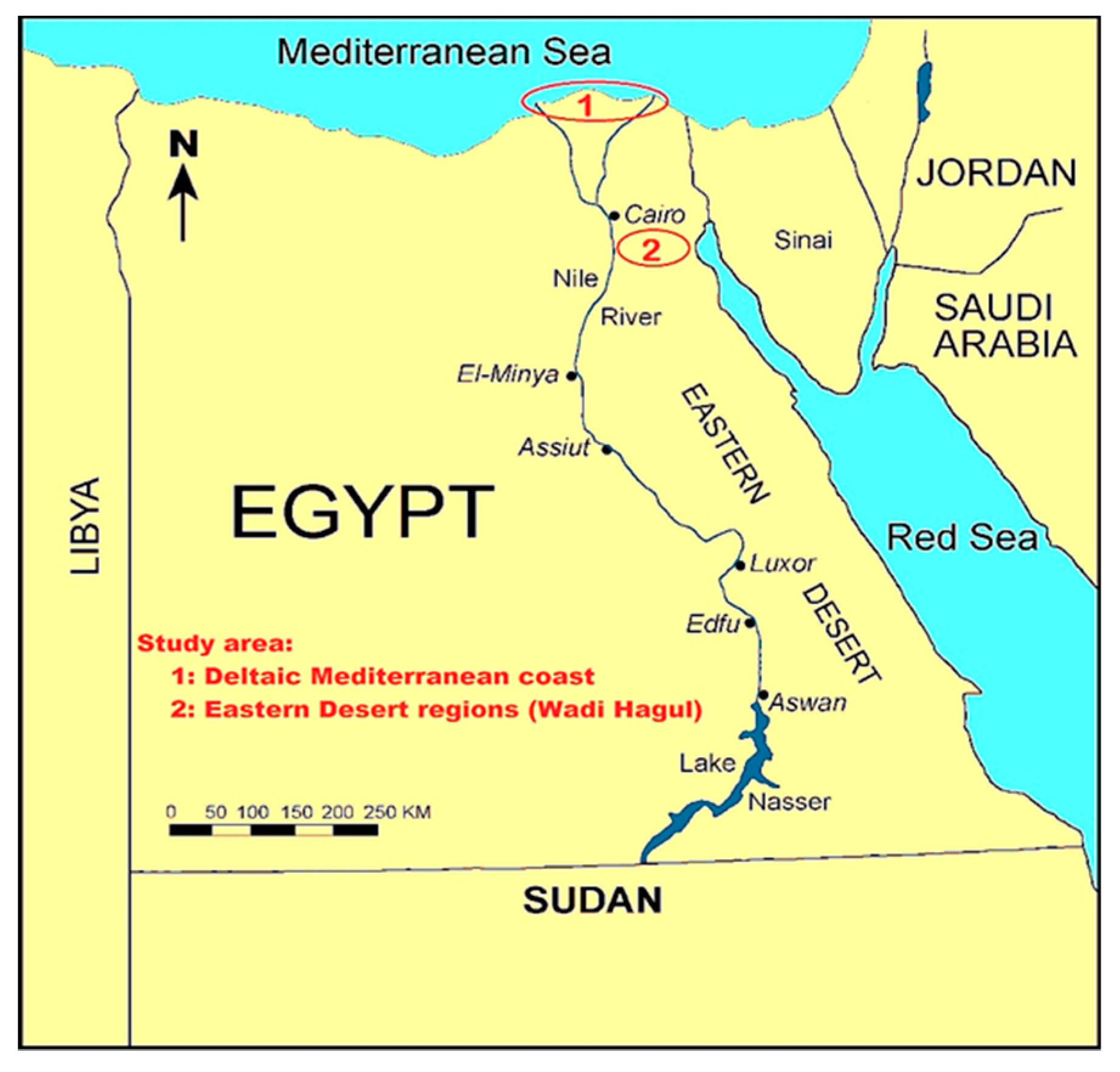
3.1. Collection and Preparation of Plant Materials
3.2. Proximate Composition
3.3. Minerals Content Analysis
3.4. Phytochemical Analysis
3.5. Determination of Antioxidant Activity
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zahran, M.A.; El-Amier, Y.A. Non-traditional fodders from the halophytic vegetation of the Deltaic Mediterranean Coastal Desert, Egypt. J. Biol. Sci. 2013, 13, 226–233. [Google Scholar]
- Rundel, P.W. Mediterranean-climate ecosystems: Defining their extent and community dominance. In Ecology, Conservation and Management of Mediterranean Climate Ecosystems; Arianoutsou, M., Panastasis, V.P., Eds.; Millpress: Rotterdam, The Netherlands, 2004; pp. 1–12. [Google Scholar]
- Berg, L. Introductory Botany: Plants, People, and the Environment; Thomson Higher Education: Belmont, CA, USA, 2007; p. 146. [Google Scholar]
- El-Amier, Y.A. Vegetation structure and soil characteristics of five common geophytes in desert of Egypt. Egypt. J. Basic Appl. Sci. 2016, 3, 172–186. [Google Scholar] [CrossRef][Green Version]
- Boval, M.; Dixon, R.M. The importance of grasslands for animal production and other functions: A review on management and methodological progress in the tropics. Animal 2012, 6, 748–762. [Google Scholar] [CrossRef] [PubMed]
- O’Mara, F.P. The role of grasslands in food security and climate change. Ann. Bot. 2012, 110, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Catling, P.M.; McElroy, A.R.; Spicer, K.W. Potential forage value of some eastern Canadian sedges (Cyperaceae: Carex). J. Range Manag. 1994, 47, 226–230. [Google Scholar] [CrossRef]
- Zahran, M.A.; El-Amier, Y.A. Ecology and establishment of fiber producing taxa naturally growing in the Egyptian deserts. Egypt. J. Basic Appl. Sci. 2014, 1, 144–150. [Google Scholar] [CrossRef][Green Version]
- Zaki, A.A.; Ross, S.A.; El-Amier, Y.A.; Khan, I.A. New flavans and stilbenes from Cyperus conglomeratus. Phytochem. Lett. 2018, 26, 159–163. [Google Scholar] [CrossRef]
- Mashaly, I.A.; El-Halawany, E.F.; Abd El-Gawad, A.M. Fodder potentiality and ecology of some non-conventional forage weeds in the Nile Delta region, Egypt. Egypt. J. Bot. 2007, 47, 119–142. [Google Scholar]
- Heneidy, S.Z.; Halmy, M.W. The nutritive value and role of Panicum turgidum Forssk. in the arid ecosystems of the Egyptian desert. Acta Bot. Croat. 2009, 68, 127–146. [Google Scholar]
- Sekeroglu, N.; Ozkutlu, F.; Deveci, M.; Dede, O.; Yilmaz, N. Evaluation of some wild plants aspect of their nutritional values used as vegetable in eastern Black Sea region of Turkey. Asian J. Plant Sci. 2006, 5, 185–189. [Google Scholar]
- Emelugo, B.N.; Umerie, S.C.; Okonkwo, I.F.; Achufusi, J.N. Evaluation of the tubers and oil of Cyperus rotundus Linn (Cyperaceae). Pak. J. Nutr. 2011, 10, 147–150. [Google Scholar] [CrossRef]
- Abdou Bouba, A.; Njintang Yanou, N.; Foyet, H.; Scher, J.; Montet, D.; Mbofung, C.M. Proximate composition, mineral and vitamin content of some wild plants used as spices in Cameroon. Food Nutr. Sci. 2012, 3, 423–432. [Google Scholar]
- El-Amier, Y.A.; Abdullah, T.J. Evaluation of nutritional value for four kinds of wild plants in Northern sector of Nile Delta, Egypt. Open J. Appl. Sci. 2015, 5, 393. [Google Scholar] [CrossRef]
- Imam, T.S.; Aliyu, F.G.; Umar, H.F. Preliminary phytochemical screening, elemental and proximate composition of two varieties of Cyperus esculentus (tiger nut). Niger. J. Basic Appl. Sci. 2013, 21, 247–251. [Google Scholar] [CrossRef]
- Turan, M.; Kordali, S.; Zengin, H.; Dursun, A.; Sezen, Y. Macro and micro mineral content of some wild edible leaves consumed in Eastern Anatolia. Acta Agric. Scand. Sec. B Soil Plant Sci. 2003, 53, 129–137. [Google Scholar] [CrossRef]
- Yildirim, E.; Dursun, A.; Turan, M. Determination of the nutrition contents of the wild plants used as vegetables in Upper Coruh Valey. Turk. J. Biol. 2001, 25, 367–371. [Google Scholar]
- Khan, A.D.; Ejaz, N.; Gilani, A.H. The use of berseem clover (Trifoliuma lexandrinum L.) pulp residue, after juice extraction in lamb finishing diets. Archivos de Zootecnia 2002, 51, 291–301. [Google Scholar]
- Musco, N.; Koura, I.B.; Tudisco, R.; Awadjihè, G.; Adjolohoun, S.; Cutrignelli, M.I.; Mollica, M.P.; Houinato, M.; Infascelli, F.; Calabrò, S. Nutritional characteristics of forage grown in south of Benin. Asian Australas. J. Anim. Sci. 2016, 29, 51–61. [Google Scholar] [CrossRef]
- Pamo, E.T.; Boukila, B.; Fonteh, F.A.; Tendonkeng, F.; Kana, J.R.; Nanda, A.S. Nutritive value of some grasses and leguminous tree leaves of the Central region of Africa. Anim. Feed Sci. Technol. 2007, 135, 273–282. [Google Scholar] [CrossRef]
- Borgnia, M.; Vilá, B.L.; Cassini, M.H. Foraging ecology of Vicuña, Vicugna vicugna, in dry Puna of Argentina. Small Rumin. Res. 2010, 88, 44–53. [Google Scholar] [CrossRef]
- El-Amier, Y.A.; Ejgholi, A.A. Fodder Potentialities of Three halophytes naturally growing in Egypt. J. Environ. Sci. 2014, 43, 647–662. [Google Scholar]
- Capstaff, N.M.; Miller, A.J. Improving the yield and nutritional quality of forage crops. Front. Plant Sci. 2018, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Razik, A.F.; Nassar, M.I.; El-Khrisy, E.-D.A.; Dawidar, A.-A.M.; Mabry, T.J. New prenylflavans from Cyperus conglomeratus. Fitoterapia 2005, 76, 762–764. [Google Scholar] [CrossRef] [PubMed]
- Gill, K.; Omokanye, A.; Pettyjohn, J.; Elsen, M. Evaluation of forage type barley varieties for forage yield and nutritive value in the Peace region of Alberta. J. Agric. Sci. 2013, 5, 24–36. [Google Scholar] [CrossRef]
- Abbasi, M.K.; Tahir, M.M.; Shah, A.H.; Batool, F. Mineral nutrient composition of different ecotypes of white clover and their nutrient credit to soil at Rawalkot Azad Jammu and Kashmir. Pak. J. Bot. 2009, 41, 41–51. [Google Scholar]
- Vejnovic, J.; Djuric, B.; Lombnæs, P.; Singh, B.R. Concentration of trace and major elements in natural grasslands of Bosnia and Herzegovina in relation to soil properties and plant species. Acta Agric. Scand. Sec. B Soil Plant Sci. 2018, 68, 243–254. [Google Scholar] [CrossRef]
- ARC. Agricultural Research Council: The Nutrient Requirements of Ruminant Livestock; The Gresham Press: London, UK, 1980; p. 351. [Google Scholar]
- NRC. Nutrient Requirements of Small Ruminants, National Research Council (US), 1st ed.; National Academy Press: Washington, DC, USA, 2006; p. 362.
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Canty, M.J.; Fogarty, U.; Sheridan, M.K.; Ensley, S.M.; Schrunk, D.E.; More, S.J. Ergot alkaloid intoxication in perennial ryegrass (Lolium perenne): An emerging animal health concern in Ireland? Ir. Vet. J. 2014, 67, 1–7. [Google Scholar] [CrossRef]
- Kara, E.; Sürmen, M. The effects of secondary metabolites of rangeland and pasture plants on the animal health in Mediterranean ecological conditions. J. US China Med. Sci. 2019, 16, 63–72. [Google Scholar]
- Al-Hazmi, G.H.; Awaad, A.S.; Alothman, M.R.; Alqasoumi, S.I. Anticandidal activity of the extract and compounds isolated from Cyperus conglomertus Rottb. Saudi Pharm. J. 2018, 26, 891–895. [Google Scholar] [CrossRef]
- El Gendy, A.E.-N.G.; Abd El-Gawad, A.M.; Taher, R.F.; El-Khrisy, E.E.-D.A.; Omer, E.A.; Elshamy, A.I. Essential oils constituents of aerial parts of Cyperus capitatus L. and Cyperus difformis L. grown wild in Egypt. J. Essent. Oil Bear. Plants 2017, 20, 1659–1665. [Google Scholar] [CrossRef]
- Seabra, R.M.; Moreira, M.M.; Costa, M.C.; Paul, M.I. 6, 3′, 4′-trihydroxy-4-methoxy-5-methylaurone from Cyperus capitatus. Phytochemistry 1995, 40, 1579–1580. [Google Scholar] [CrossRef]
- Poutaraud, A.; Michelot-Antalik, A.; Plantureux, S.J.J.o.a. Grasslands: A source of secondary metabolites for livestock health. J. Agric. Food Chem. 2017, 65, 6535–6553. [Google Scholar] [CrossRef] [PubMed]
- Glencross, B. Understanding the nutritional and biological constraints of ingredients to optimize their application in aquaculture feeds. In Aquafeed Formulation; Nates, S.F., Ed.; Elsevier: Oxford, MS, USA, 2016; pp. 33–73. [Google Scholar]
- Matsuura, H.N.; Fett-Neto, A.G. Plant alkaloids: Main features, toxicity, and mechanisms of action. In Plant Toxins; Gopalakrishnakone, P., Carlini, C., Ligabue-Braun, R., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 243–261. [Google Scholar]
- Morimoto, M.; Fujii, Y.; Komai, K. Antifeedants in Cyperaceae: Coumaran and quinones from Cyperus spp. Phytochemistry 1999, 51, 605–608. [Google Scholar] [CrossRef]
- Abdel-Mogib, M.; Basaif, S.A.; Ezmirly, S.T. Two novel flavans from Cyperus conglomeratus. Pharmazie 2000, 55, 693–695. [Google Scholar]
- Feizbakhsh, A.; Naeemy, A. Chemical composition of the essential oil of Cyperus conglomeratus Rottb. From Iran. Eur. J. Chem. 2011, 8, S293–S296. [Google Scholar]
- Hisham, A.; Rameshkumar, K.B.; Sherwani, N.; Al-Saidi, S.; Al-Kindy, S. The composition and antimicrobial activities of Cyperus conglomeratus, Desmos chinensis var. lawii and Cyathocalyx zeylanicus essential oils. Nat. Prod. Commun. 2012, 7, 663–666. [Google Scholar]
- El-Desoukey, R.M.A. Phytochemical and antimicrobial activity of Panicum turgidum (thummam) as a grazing herb against some animal pathogens. EC Microbiol. 2017, 5, 22–29. [Google Scholar]
- Farag, M.A.; El Fishawy, A.M.; El-Toumy, S.A.; Amer, K.F.; Mansour, A.M.; Taha, H.E. Antihepatotoxic effect and metabolite profiling of Panicum turgidum extract via -qTOF-MS. Pharmacogn. Mag. 2016, 12, S446–S453. [Google Scholar] [CrossRef]
- Lee, S.T.; Mitchell, R.B.; Wang, Z.; Heiss, C.; Gardner, D.R.; Azadi, P. Isolation, Characterization, and quantification of steroidal saponins in switchgrass (Panicum virgatum L.). J. Agric. Food Chem. 2009, 57, 2599–2604. [Google Scholar] [CrossRef]
- Zaki, A.A.; Ali, Z.; Wang, Y.H.; El-Amier, Y.A.; Khan, S.I.; Khan, I.A. Cytotoxic steroidal saponins from Panicum turgidum Forssk. Steroids 2017, 125, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Zakia, A.A.; Qiub, L.; Alib, Z.; Khanb, S.I.; Khanb, I.A. Anti-inflammatory steroidal saponins from Panicum turgidum. J. Agric. Basic Sci. 2016, 1, 1–6. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Pirie, N.W. Protein. In Modern Methods of Plant Analysis IV; Peack, K., Tracey, M.V., Eds.; Springer: Berlin, Germany, 1955; Volume 23. [Google Scholar]
- Feteris, A.W. A serum glucose method without protein precipitation. Am. J. Med. Technol. 1965, 31, 17–21. [Google Scholar] [PubMed]
- Handel, E.V. Direct micro determinations of sucrose. Anal. Biochem. 1968, 22, 280–283. [Google Scholar] [CrossRef]
- Joint FAO. Energy and Protein Requirements Report of a Joint Expert Consultation; Technical Report Series; No 724; World Health Organization: Geneva, Switzerland, 1985. [Google Scholar]
- Abu-El-Naga, M.A.; EL-Shazly, K. The Prediction of the Nutritive Value of Animal Feeds from Chemical Analyses. J. Agric. Sci. 1971, 77, 25–31. [Google Scholar] [CrossRef]
- Allen, S.E.; Grimshaw, H.M.; Parkinson, J.A.; Quarmby, C.; Roberts, J.D. Chemical Analysis of Ecological Materials; Blackwell Scientific Publications: Oxford, UK; London, UK, 1974. [Google Scholar]
- Sadasivam, S.; Manickam, A. Biochemical Methods, 3rd ed.; New Age International Publishers: New Delhi, India, 2008. [Google Scholar]
- Obadoni, B.O.; Ochuko, P.O. Phytochemical studies and comparative efficacy of the crude extracts of some haemostatic plants in Edo and Delta States of Nigeria. Glob. J. Pure Appl. Sci. 2002, 8, 203–208. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Harborne, J. Phytochemical Methods; Chapman and Hall. Ltd.: London, UK, 1973; Volume 4, pp. 49–188. [Google Scholar]
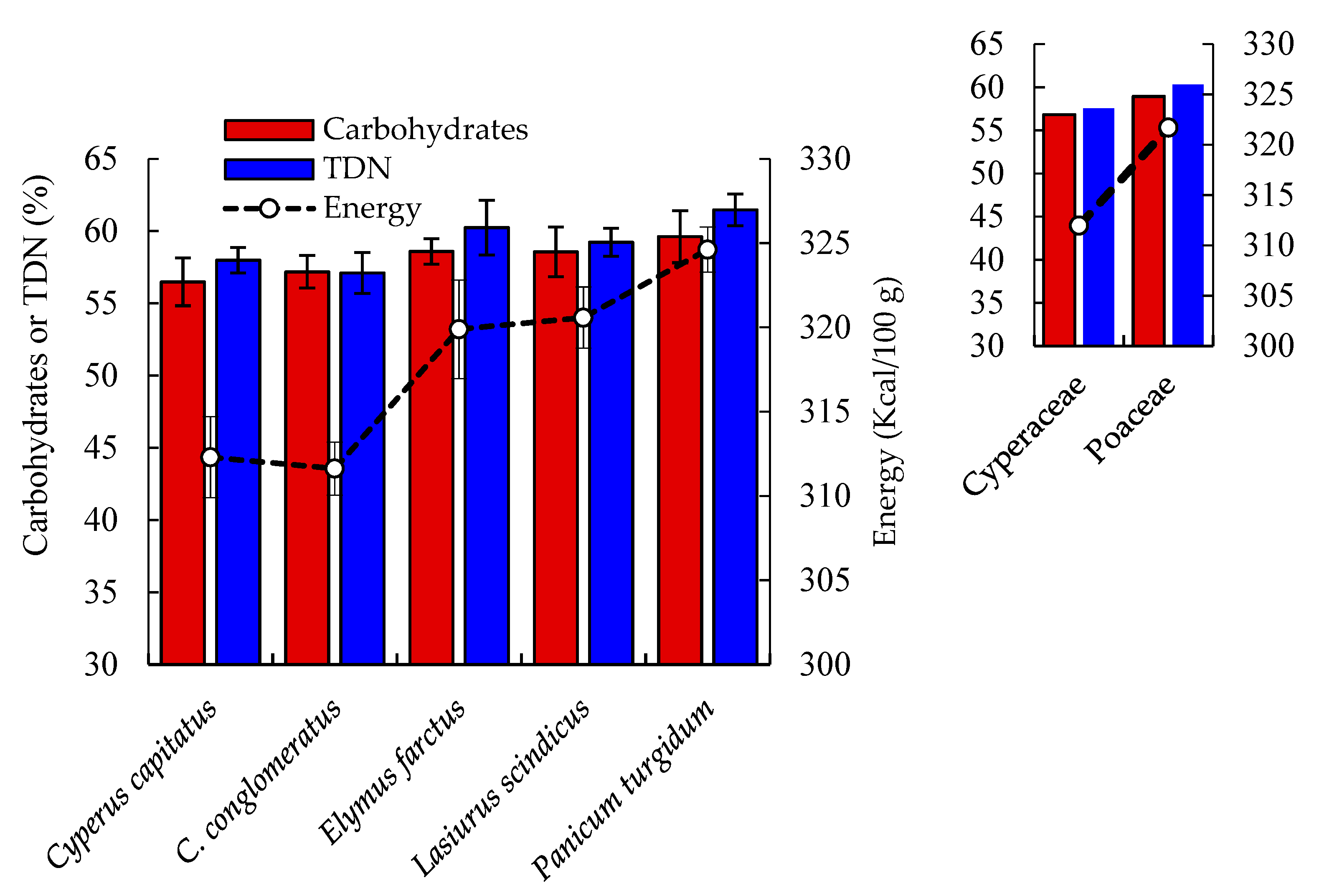
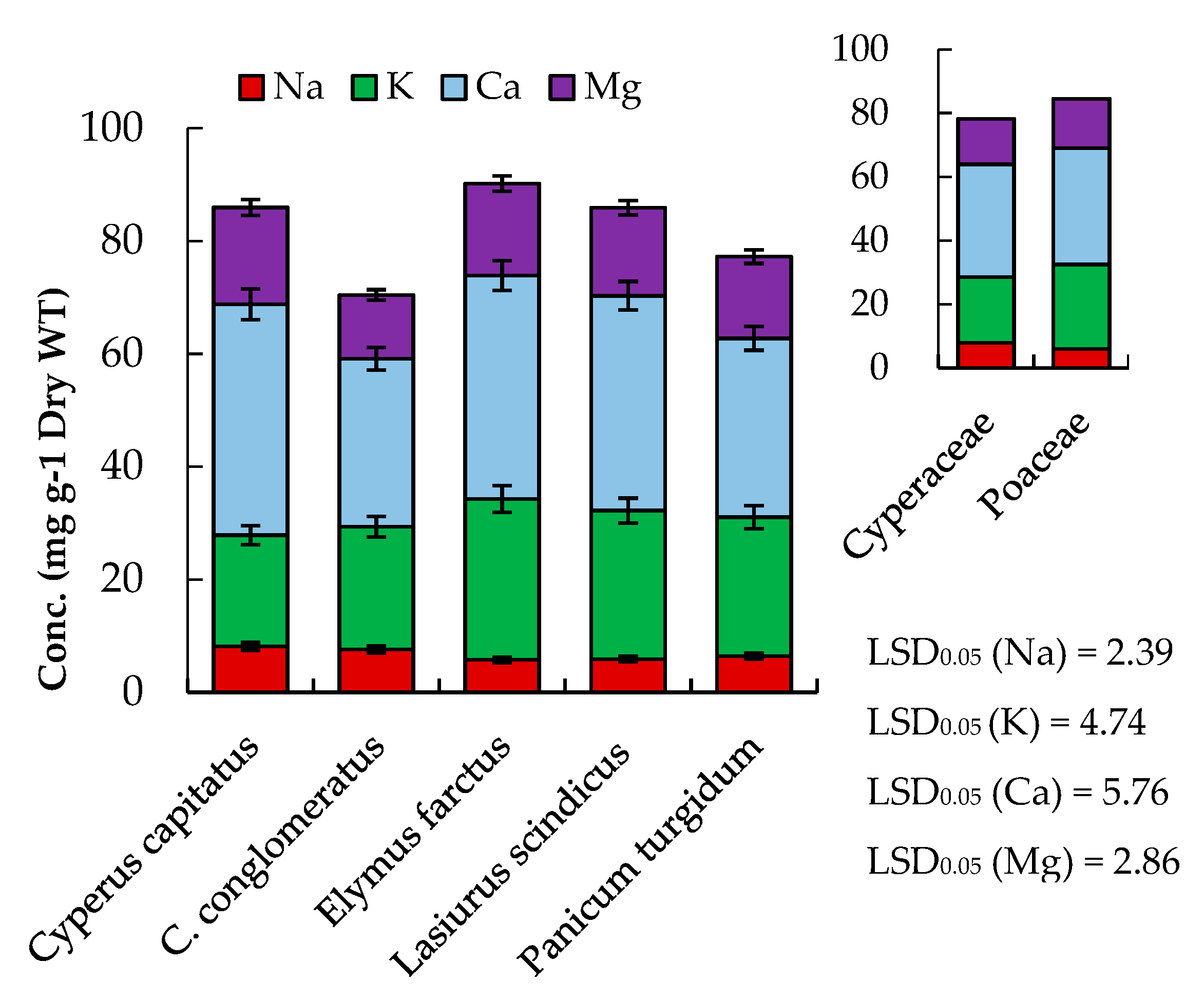
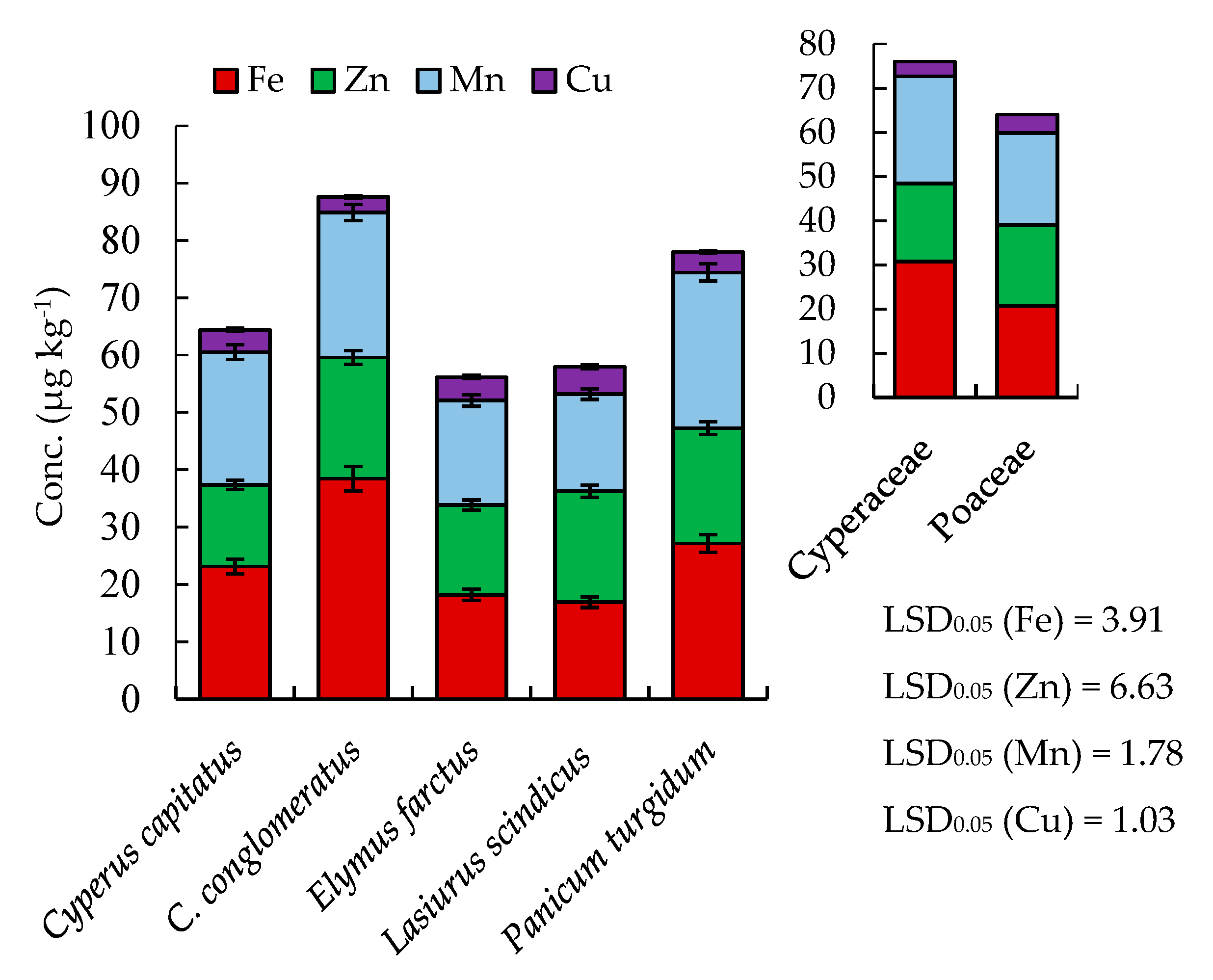
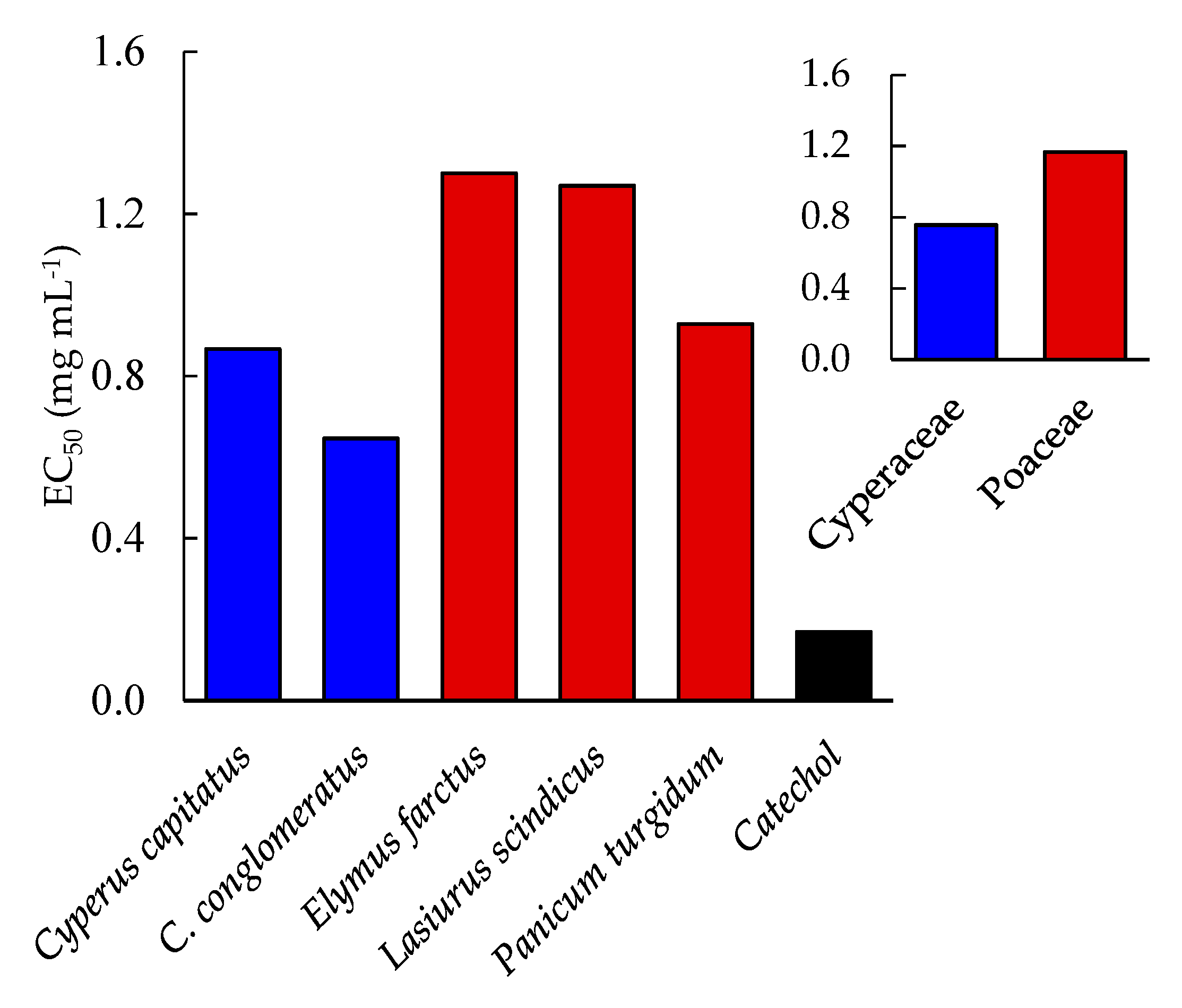
| Proximate Composition | Cyperus capitatus | Cyperus conglomeratus | Elymus farctus | Lasiurus scindicus | Panicum turgidum | p-Value |
|---|---|---|---|---|---|---|
| Dry matter % | 87.10 ± 4.94 a | 91.67 ± 3.65 a | 89.80 ± 5.09 a | 90.46 ± 3.12 a | 90.13 ± 5.11 a | 0.58 |
| Moisture % | 12.90 ± 1.29 a | 8.33 ± 0.83 a | 10.20 ± 1.02 a | 9.54 ± 0.95 a | 9.87 ± 0.99 a | 0.51 |
| Ash % | 10.75 ± 1.07 a | 10.54 ± 1.05 a | 9.39 ± 0.94 a | 9.07 ± 0.91 a | 8.62 ± 0.86 a | 0.47 |
| Fiber % | 12.51 ± 1.25 a | 11.97 ± 0.98 a | 13.40 ± 1.34 a | 12.89 ± 1.29 a | 13.84 ± 0.88 a | 0.23 |
| Fat % | 3.89 ± 0.28 cd | 3.19 ± 0.23 d | 5.13 ± 0.38 ab | 4.61 ± 0.34 bc | 5.87 ± 0.43 a | 0.0013 |
| Protein % | 16.38 ± 1.20 ab | 17.13 ± 1.25 a | 13.50 ± 0.99 cd | 14.88 ± 1.09 bc | 12.06 ± 0.88 d | 0.0004 |
| Sucrose % | 1.89 ± 0.14 a | 1.98 ± 0.15 a | 1.74 ± 0.13 a | 1.81 ± 0.13 a | 1.52 ± 0.11 a | 0.98 |
| Glucose % | 0.72 ± 0.05 a | 0.94 ± 0.07 a | 0.78 ± 0.06 a | 0.82 ± 0.06 a | 0.68 ± 0.05 a | 0.78 |
| Secondary Compounds | Cyperus capitatus | Cyperus conglomeratus | Elymus farctus | Lasiurus scindicus | Panicum turgidum |
|---|---|---|---|---|---|
| Total phenolics | 16.02 ± 0.86 b | 26.34 ± 1.41 a | 13.57 ± 0.73 bc | 9.59 ± 0.51 d | 10.69 ± 0.57 cd |
| Alkaloids | 15.15 ± 0.81 b | 21.84 ± 1.17 a | 16.10 ± 0.86 b | 6.89 ± 0.37 c | 6.08 ± 0.33 c |
| Total flavonoid | 10.57 ± 0.57 b | 18.27 ± 0.98 a | 8.02 ± 0.43 c | 5.31 ± 0.28 d | 5.82 ± 0.31 d |
| Saponins | 19.59 ± 1.05 b | 41.16 ± 2.21 a | 18.60 ± 1.00 c | 10.13 ± 0.54 d | 11.41 ± 0.61 d |
| Tannins | 12.42 ± 0.67 b | 26.10 ± 1.40 a | 12.80 ± 0.69 b | 5.46 ± 0.29 c | 4.13 ± 0.22 c |
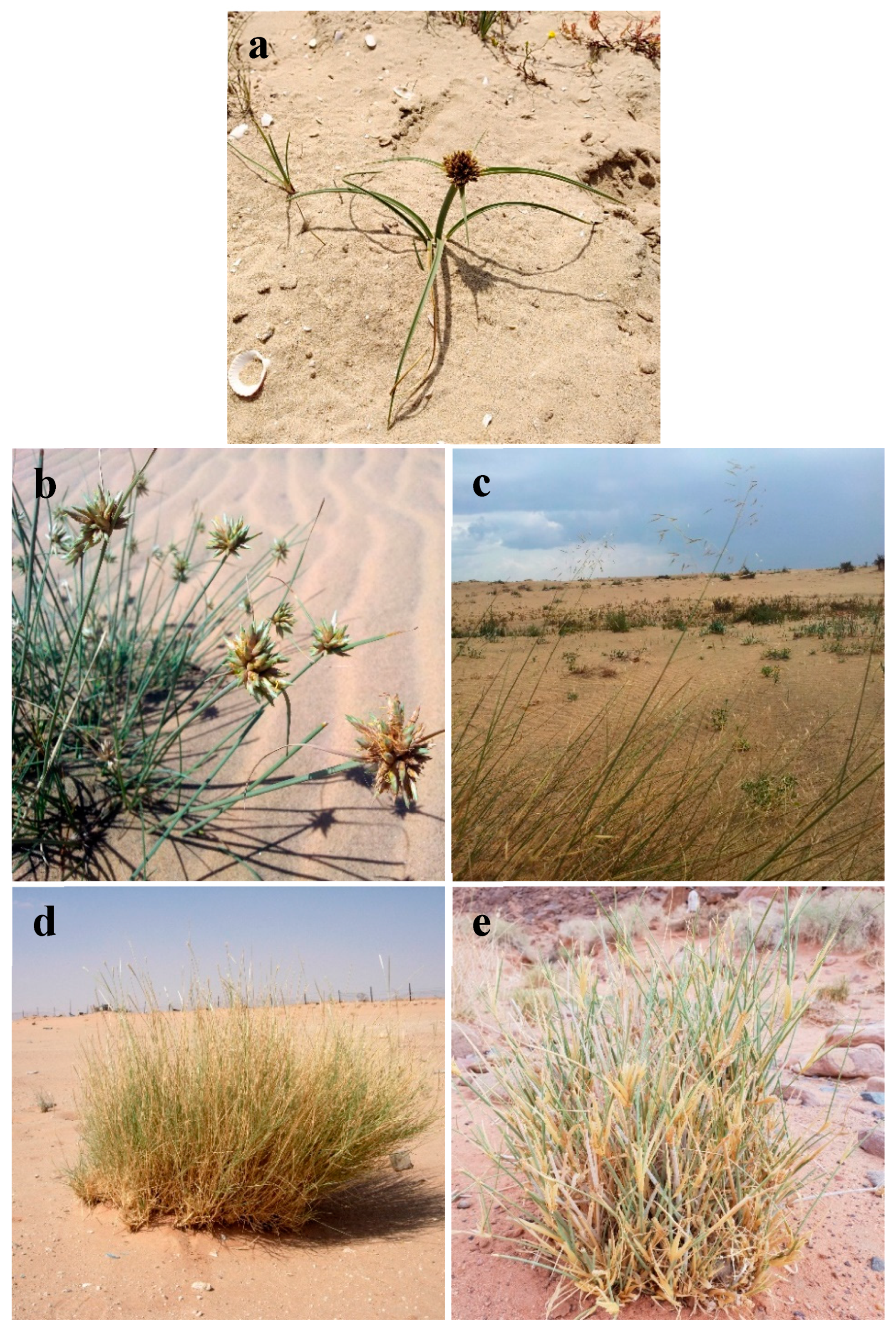
| Botanical Name | Family | Common Name | Duration | Chorotype | Voucher Code |
|---|---|---|---|---|---|
| Cyperus capitatus Vand. | Cyperaceae | Seed | Perennial | ME | Mans.030303012 |
| Cyperus conglomeratus Rottb. | Cyperaceae | Seed, Oshb | Perennial | SA-SI + S-Z | Mans.030303008 |
| Elymus farctus (Viv.) Runem. ex Melderis | Poaceae | Gazzoof | Perennial | ME | Mans.160506018 |
| Lasiurus scindicus Henrard. | Poaceae | Sammat | Perennial | SA-SI + S-Z | Mans.161219003 |
| Panicum turgidum Forssk. | Poaceae | Thommam, Shoosh | Perennial | SA-SI | Mans.161620007 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Rowaily, S.L.; Abd-ElGawad, A.M.; Alghanem, S.M.; Al-Taisan, W.A.; El-Amier, Y.A. Nutritional Value, Mineral Composition, Secondary Metabolites, and Antioxidant Activity of Some Wild Geophyte Sedges and Grasses. Plants 2019, 8, 569. https://doi.org/10.3390/plants8120569
Al-Rowaily SL, Abd-ElGawad AM, Alghanem SM, Al-Taisan WA, El-Amier YA. Nutritional Value, Mineral Composition, Secondary Metabolites, and Antioxidant Activity of Some Wild Geophyte Sedges and Grasses. Plants. 2019; 8(12):569. https://doi.org/10.3390/plants8120569
Chicago/Turabian StyleAl-Rowaily, Saud L., Ahmed M. Abd-ElGawad, Suliman M. Alghanem, Wafa’a A. Al-Taisan, and Yasser A. El-Amier. 2019. "Nutritional Value, Mineral Composition, Secondary Metabolites, and Antioxidant Activity of Some Wild Geophyte Sedges and Grasses" Plants 8, no. 12: 569. https://doi.org/10.3390/plants8120569
APA StyleAl-Rowaily, S. L., Abd-ElGawad, A. M., Alghanem, S. M., Al-Taisan, W. A., & El-Amier, Y. A. (2019). Nutritional Value, Mineral Composition, Secondary Metabolites, and Antioxidant Activity of Some Wild Geophyte Sedges and Grasses. Plants, 8(12), 569. https://doi.org/10.3390/plants8120569








