Exogenously-Sourced Ethylene Modulates Defense Mechanisms and Promotes Tolerance to Zinc Stress in Mustard (Brassica juncea L.)
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Material, Growth Conditions, and Treatments
2.2. Measurements of Photosynthetic Traits and Plant Dry Mass Accumulation
2.3. Determination of Oxidative Stress Markers
2.3.1. Lipid Peroxidation
2.3.2. Electrolyte leakage
2.3.3. Methylglyoxal Content
2.3.4. Lipoxygenase Activity
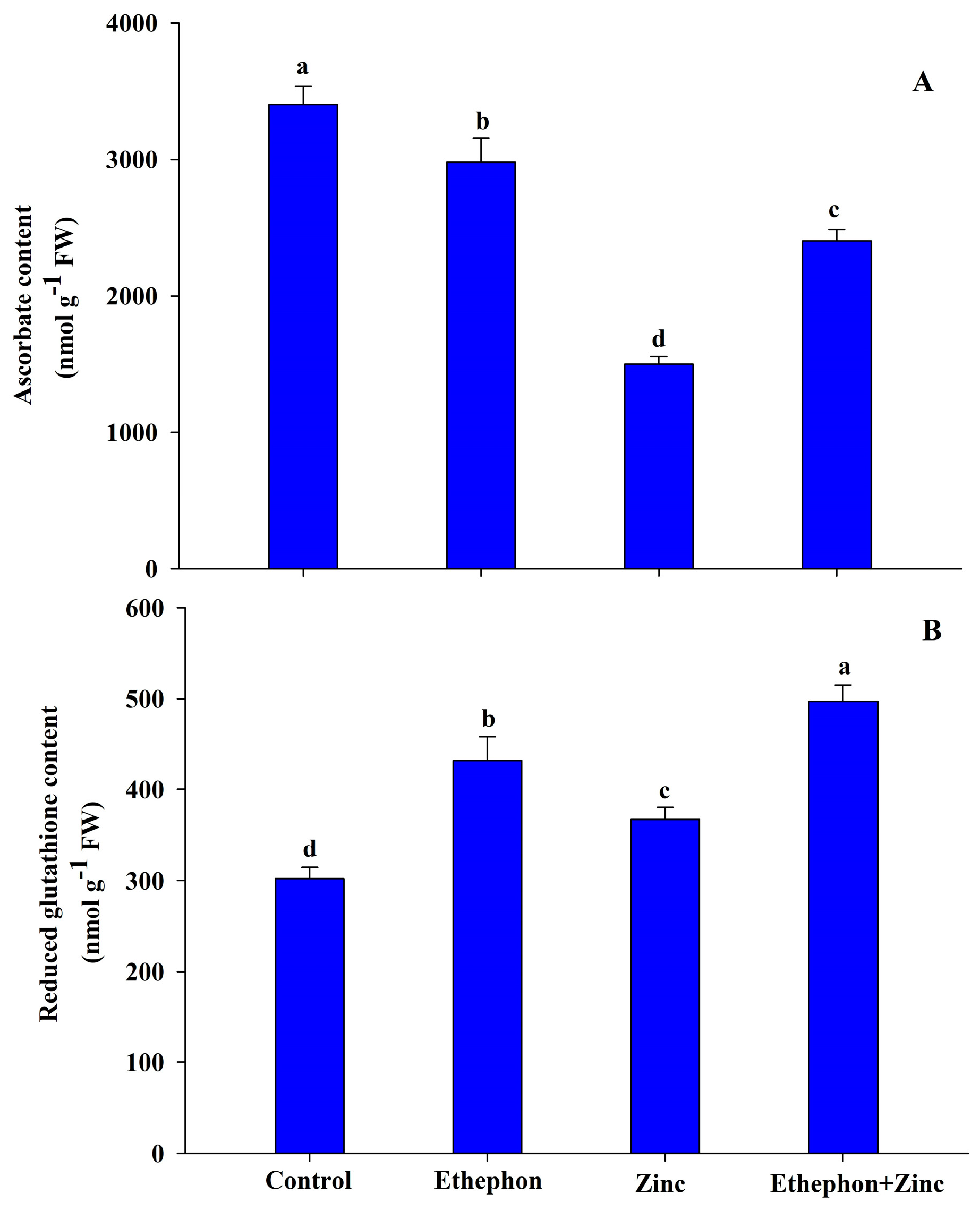
2.4. Measurement of Ascorbate and Glutathione Content
2.4.1. Ascorbate Content
2.4.2. Reduced Glutathione Content
2.5. Extraction and Determination of Antioxidant Enzymes
2.5.1. SOD
2.5.2. APX
2.5.3. GR
2.5.4. GPX
2.5.5. GST
2.5.6. MDHAR
2.5.7. DHAR
2.6. Extraction and Assay of Glyoxalase Systems’ Enzymes
2.6.1. Gly I Activity
2.6.2. Gly II Activity
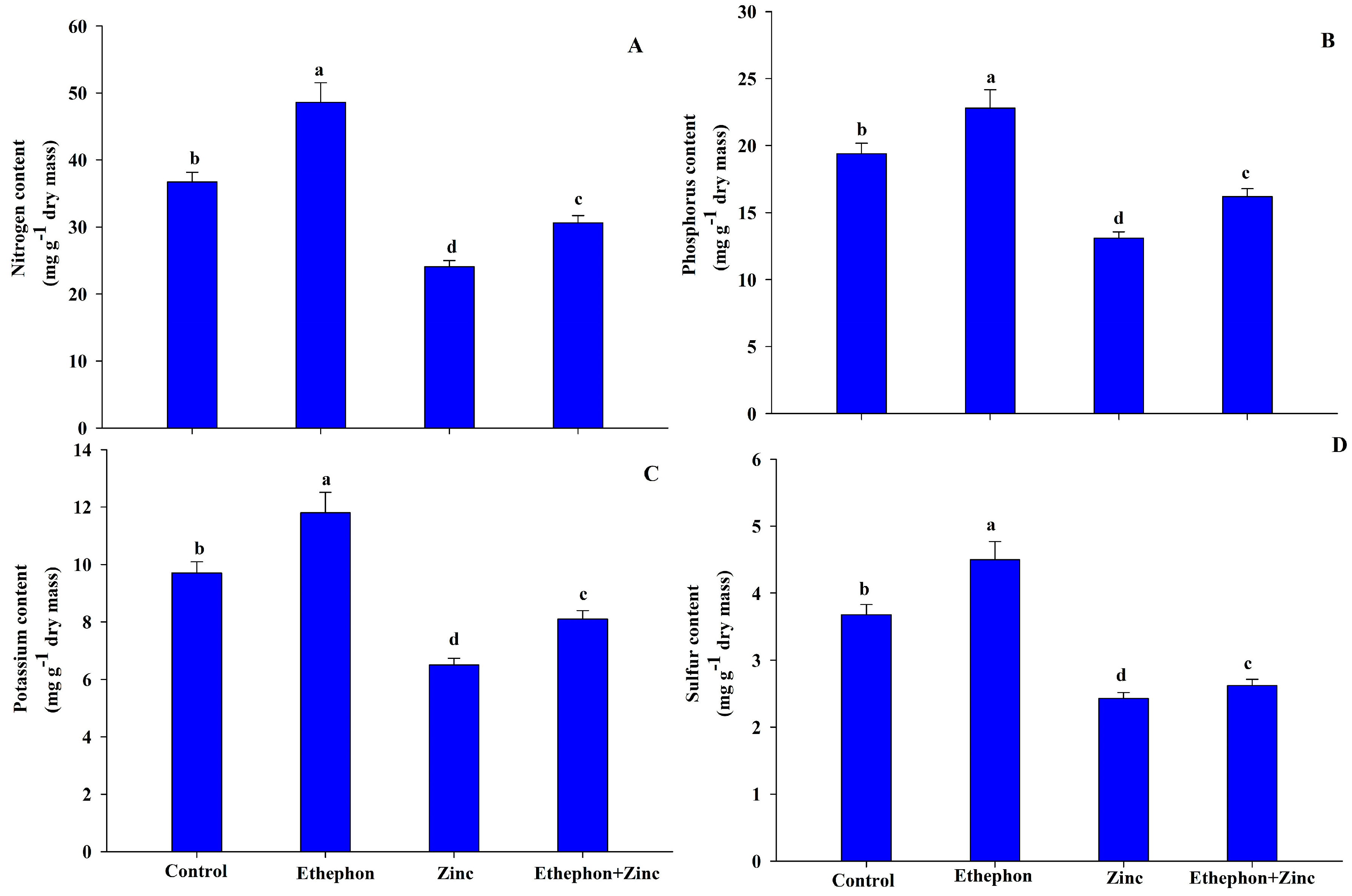
2.7. Determination of Nutrient Content
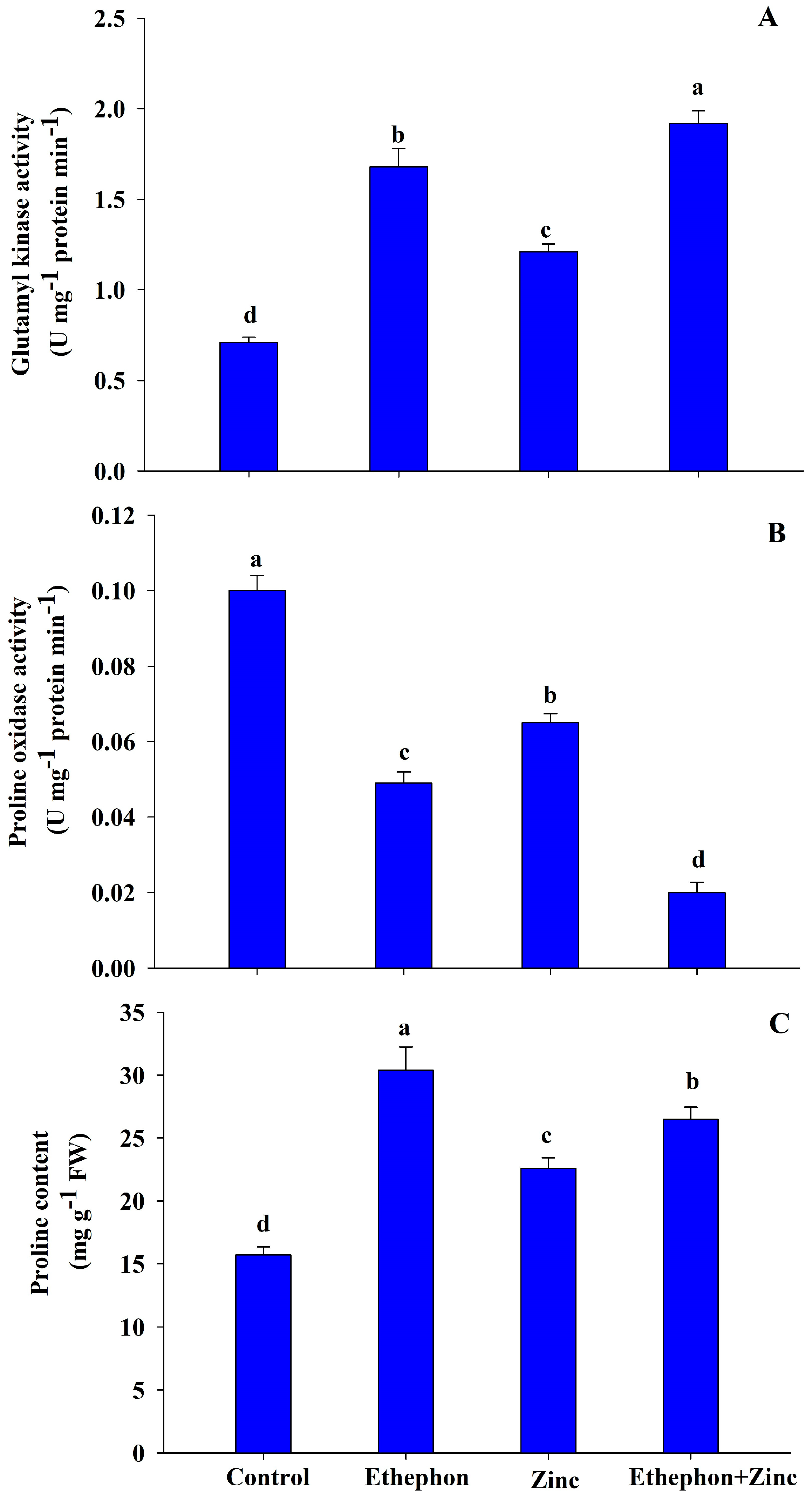
2.8. Determination of Proline Content and Activity of Proline Metabolizing Enzymes
2.8.1. Proline Content
2.8.2. GK Activity
2.8.3. POX Activity
2.9. Water Potential and Osmotic Potential
2.10. Statistical Analysis
3. Results
3.1. Ethephon Reverses Effects of Zn Stress on Photosynthetic and Growth Attributes
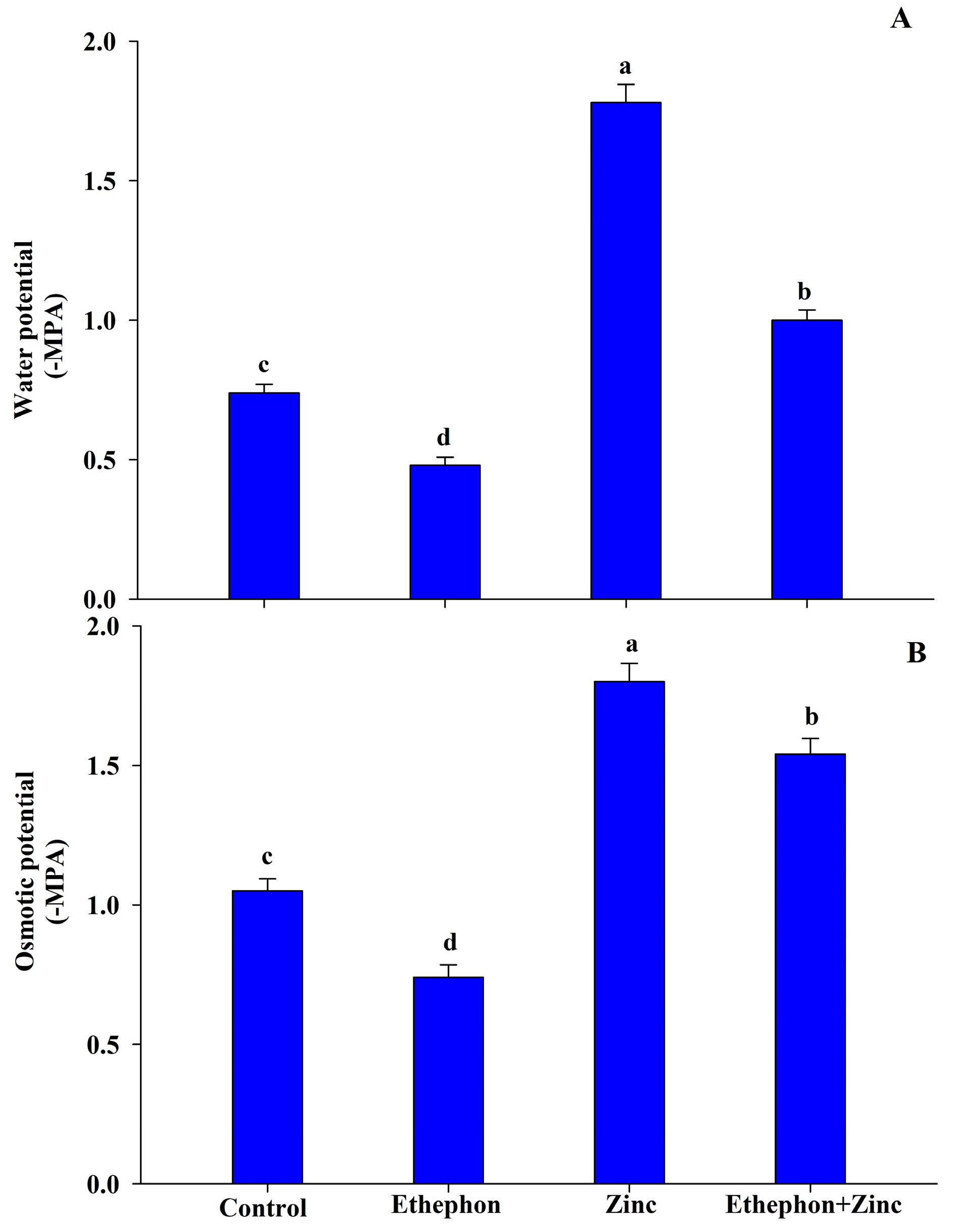
3.2. Influence of Ethephon on Water Relations under Zn Stress
3.3. Ethephon Reduces Zn-Induced Oxidative Stress
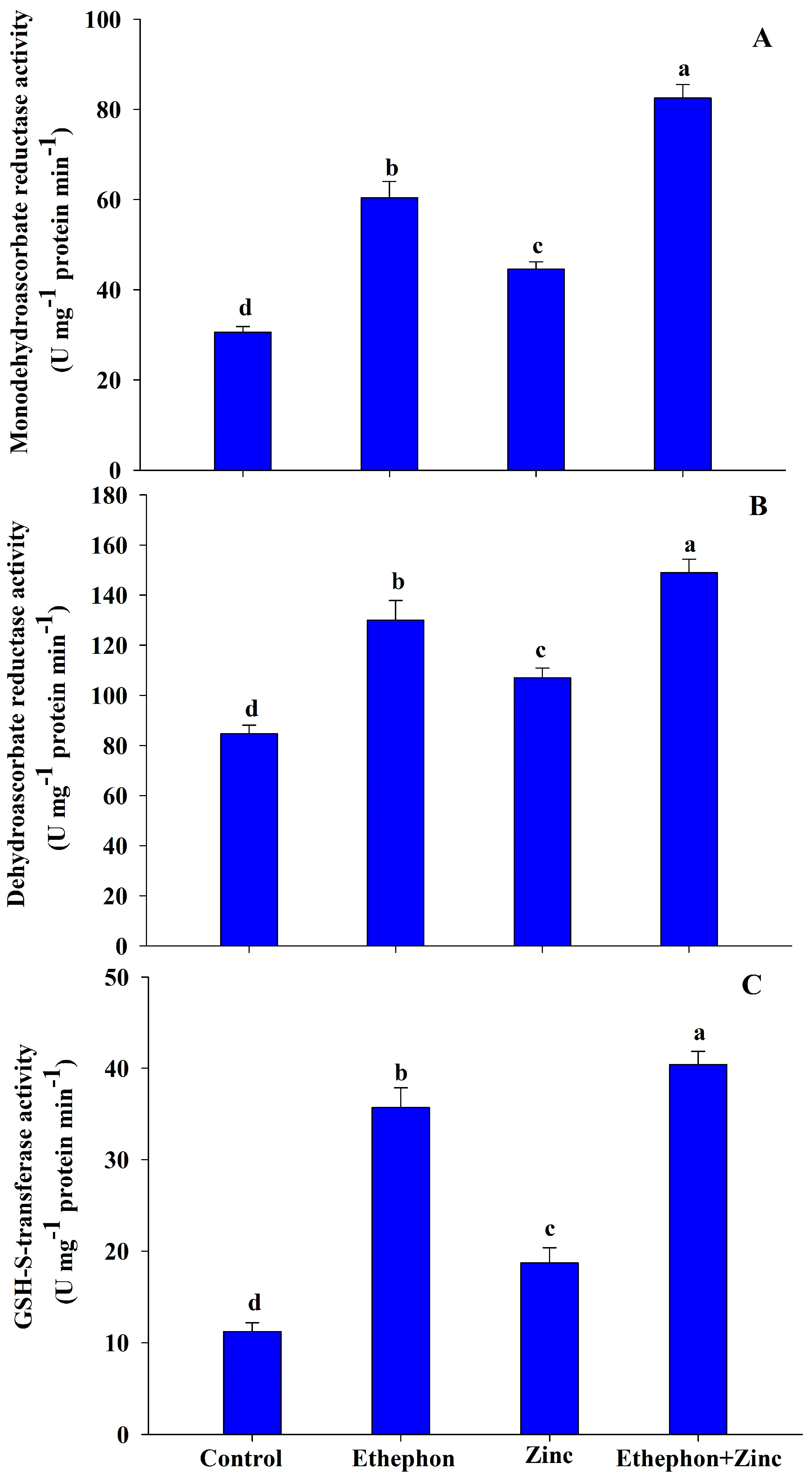
3.4. Application of Ethephon Enhanced Antioxidant Systems under Zn Stress
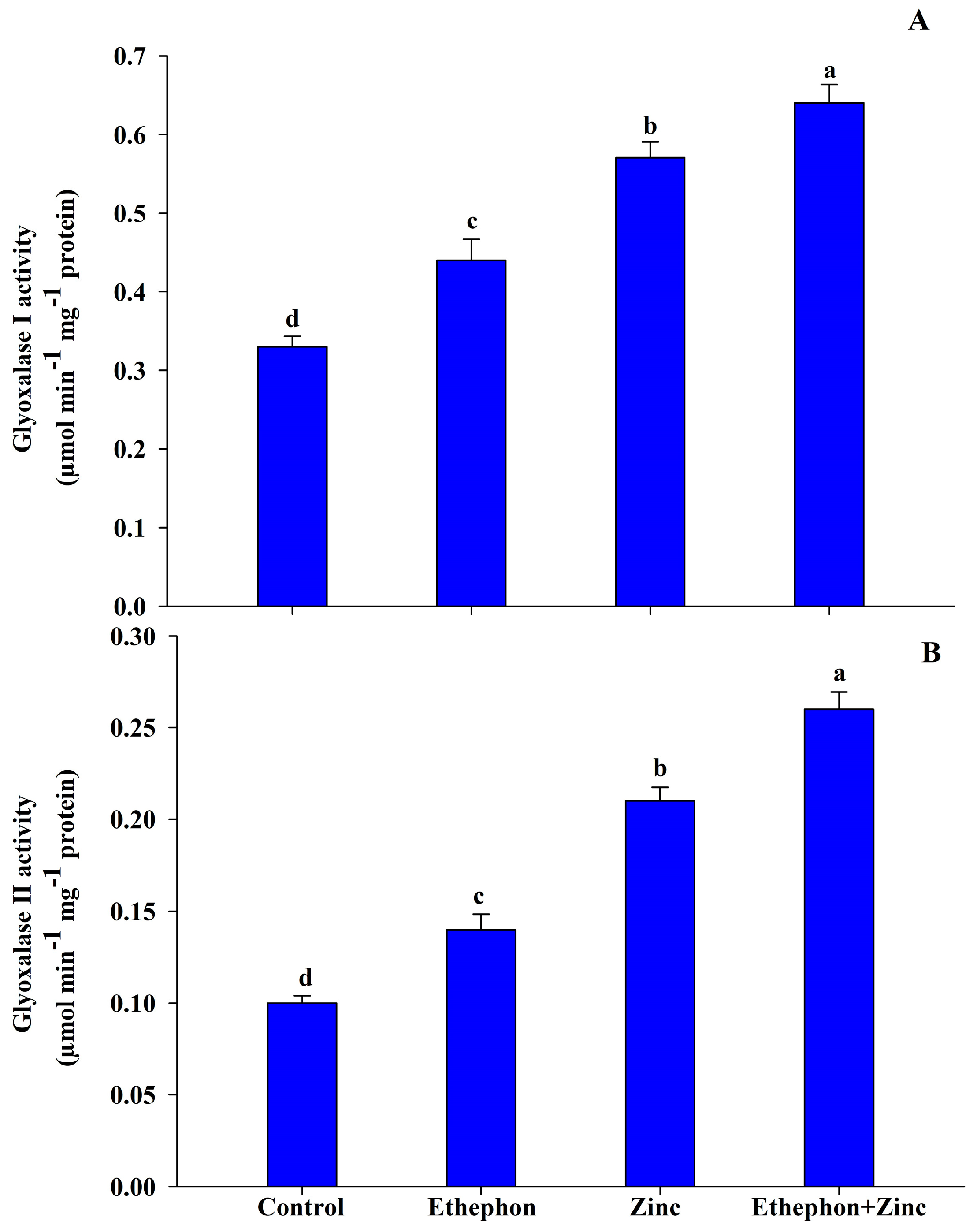
3.5. Ethephon Application Enhances Glyoxalase System
3.6. Ethephon Increases Proline Metabolism under Zn Stress
3.7. Ethephon Supplementation Maintained Nutrient Contents under Zn Stress
4. Discussion
5. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Rizwan, M.; Ali, S.; Rehman, M.Z.; Maqbool, A. A critical review on the effects of zinc at toxic levels of cadmium in plants. Environ. Sci. Pollut. Res. 2019, 26, 6279–6289. [Google Scholar] [CrossRef]
- Salla, V.; Hardaway, C.J.; Sneddon, J. Preliminary investigation of Spartina alterniflora for phytoextraction of selected heavy metals in soils from Southwest Louisiana. Microchem. J. 2011, 97, 207–212. [Google Scholar] [CrossRef]
- Shahid, M.; Khalid, S.; Abbas, G.; Shahid, N.; Nadeem, M.; Sabir, M. Heavy Metal Stress and Crop Productivity. In Crop Production and Global Environmental Issues; Hakeem, K.R., Ed.; Springer: Cham, Switzerland, 2015; pp. 1–25. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: New York, NY, USA, 1995. [Google Scholar]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef] [PubMed]
- Feigl, G.; Lehotai, N.; Molnár, Á.; Ördög, A.; Rodríguez-Ruiz, M.; Palma, J.M.; Corpas, F.J.; Erdei, L.; Kolbert, Z. Zinc induces distinct changes in the metabolism of reactive oxygen and nitrogen species (ROS and RNS) in the roots of two Brassica species with different sensitivity to zinc stress. Ann. Bot. 2015, 116, 613–625. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, Y.; Ding, H.; Ge, C. Epibrassinolide confers zinc stress tolerance by regulating antioxidant enzyme responses, osmolytes, and hormonal balance in Solanum melongena seedlings. Braz. J. Bot. 2016, 39, 295–303. [Google Scholar] [CrossRef]
- Cakmak, I. Possible Roles of Zinc in Protecting Plant Cells from Damage by Reactive Oxygen Species. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef]
- Zhao, H.; Zheng, W. Effects of zinc stress on growth and antioxidant enzyme responses of Kandelia obovata seedlings. Toxicol. Environ. Chem. 2015, 97, 1190–1201. [Google Scholar]
- Anjum, N.A.; Singh, H.P.; Khan, M.I.R.; Masood, A.; Per, T.S.; Negi, A.; Ahmad, I. Too much is bad-an appraisal of phytotoxicity of elevated plant-beneficial heavy metal ions. Environ. Sci. Pollut. Res. 2015, 22, 3361–3382. [Google Scholar] [CrossRef]
- Anwaar, S.A.; Ali, S.; Ali, S.; Ishaque, W.; Farid, M.; Farooq, M.A.; Najeeb, U.; Abbas, F.; Sharif, M. Silicon (Si) alleviates cotton (Gossypium hirsutum L.) from zinc (Zn) toxicity stress by limiting Zn uptake and oxidative damage. Environ. Sci. Pollut. Res. 2015, 22, 3441–3450. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Khan, N. Reactive Oxygen Species and Antioxidant System in Plants: Role and Regulation under Abiotic Stress; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Suzuki, T.; Fujita, M. Polyamines-induced aluminum tolerance in mung bean: A study on antioxidant defense and methylglyoxal detoxification systems. Ecotoxicology 2017, 26, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Sharma, S.; Singla-Pareek, S.L.; Sopory, S.K. Methylglyoxal detoxification in plants: Role of glyoxalase pathway. Indian J. Plant Physiol. 2016, 21, 377–390. [Google Scholar] [CrossRef]
- Per, T.S.; Khan, N.A.; Reddy, P.S.; Masood, A.; Hasanuzzaman, M.; Khan, M.I.R.; Anjum, N.A. Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: Phytohormones, mineral nutrients and transgenics. Plant Physiol. Biochem. 2017, 115, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.; Saxena, P. Effect of salicylic acid on proline metabolism in lentil grown under salinity stress. Plant Sci. 2009, 177, 181–189. [Google Scholar] [CrossRef]
- Khan, N.A.; Khan, M.I.R.; Ferrante, A.; Poor, P. Editorial: Ethylene: A key regulatory molecule in plants. Front. Plant Sci. 2017, 8, 1782. [Google Scholar] [CrossRef]
- Pahwa, K.; Ghai, N.; Kaur, J.; Singh, I.; Singh, S.; Dhingra, M. Influence of ethylene and cobalt chloride on photosynthetic parameters and pedicel anatomy of pigeon pea (Cajanus cajan L.) genotypes. J. Environ. Biol. 2017, 38, 367–374. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, N.A.; Nazar, R.; Silva, J.A. Ethylene-stimulated photosynthesis results from increased nitrogen and sulfur assimilation in mustard types that differ in photosynthetic capacity. Environ. Exp. Bot. 2012, 78, 84–90. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Iqbal, N.; Masood, A.; Per, T.S.; Khan, N.A. Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Signal. Behav. 2013, 8, e26374. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Nazir, F.; Asgher, M.; Per, T.S.; Khan, N.A. Selenium and sulfur influence ethylene formation and alleviate cadmium-induced oxidative stress by improving proline and glutathione production in wheat. J. Plant Physiol. 2015, 173, 9–18. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Khan, N.A. Ethylene reverses photosynthetic inhibition by nickel and zinc in mustard through changes in PS II activity, photosynthetic nitrogen use efficiency, and antioxidant metabolism. Protoplasma 2014, 251, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Usuda, H. The activation state of ribulose 1, 5-bisphosphate carboxylase in maize leaves in dark and light. Plant Cell Physiol. 1985, 26, 1455–1463. [Google Scholar]
- Krall, J.P.; Edwards, G.E. Relationship between photosystem II activity and CO2 fixation in leaves. Physiol. Plant. 1992, 86, 180–187. [Google Scholar] [CrossRef]
- Dhindsa, R.H.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence correlated with increased level of membrane permeability, lipid peroxidation and decreased level of SOD and CAT. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Wild, R.; Ooi, L.; Srikanth, V.; Münch, G. A quick, convenient and economical method for the reliable determination of methylglyoxal in millimolar concentrations: The N-acetyl-L-cysteine assay. Anal. Bioanal. Chem. 2012, 403, 2577–2581. [Google Scholar] [CrossRef]
- Doderer, A.; Kokkelink, I.; van der Veen, S.; Valk, B.; Schram, A.; Douma, A. Purification and characterization of two Lipoxygenase isoenzymes from germinating barley. Biochim. Biophys. Acta 1992, 112, 97–104. [Google Scholar] [CrossRef]
- Law, M.E.; Charles, S.A.; Halliwell, B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts: The effect of hydrogen peroxide and of paraquat. Biochem. J. 1983, 210, 899–903. [Google Scholar] [CrossRef]
- Anjum, N.A.; Umar, S.; Iqbal, M.; Khan, N.A. Cadmium causes oxidative stress in mung bean by affecting the antioxidant enzyme system and ascorbate-glutathione cycle metabolism. Russ. J. Plant Physiol. 2011, 58, 92–99. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of GSH disulphide using GSH reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–211. [Google Scholar] [CrossRef]
- Masood, A.; Khan, M.I.R.; Fatma, M.; Asgher, M.; Per, T.S.; Khan, N.A. Involvement of ethylene in gibberellic acid-induced sulfur assimilation, photosynthetic responses, and alleviation of cadmium stress in mustard. Plant Physiol. Biochem. 2016, 104, 1–10. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the Quantitation of micro-gram quantities of proteins utilising the principle of protein–dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutase. I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Exogenous selenium pretreatment protects rapeseed seedlings from cadmium-induced oxidative stress by up regulating antioxidant defense and methylglyoxal detoxification systems. Biol. Trace Elem. Res. 2012, 149, 248–261. [Google Scholar] [CrossRef]
- Booth, J.; Boyland, E.; Sims, P. An enzyme from rat liver catalyzing conjugation. Biochem. J. 1961, 79, 516–524. [Google Scholar]
- Hossain, M.A.; Hossain, M.Z.; Fujita, M. Stress induced changes of methylglyoxal level and glyoxalase I activity in pumpkin seedlings and cDNA cloning of glyoxalase I gene. Aust. J. Crop Sci. 2009, 3, 53–64. [Google Scholar]
- Hossain, M.A.; Nakano, Y.; Asada, K. Monodehydroascorbate reductase in spinach chloroplasts and its participation in the regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol. 1984, 25, 385–395. [Google Scholar]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Khan, M.I.R.; Fujita, M. Silicon-mediated regulation of antioxidant defense and glyoxalase systems confers drought stress tolerance in Brassica napus L. S. Afr. J. Bot. 2018, 115, 50–57. [Google Scholar] [CrossRef]
- Principato, G.B.; Rosi, G.; Talesa, V.; Giovanni, E.; Uotila, L. Purification and characterization of two forms of glyoxalase II from the liver and brain of wistar rats. Biochim. Biophys. Acta 1987, 911, 349–355. [Google Scholar] [CrossRef]
- Lindner, R.C. Rapid analytical methods for some of the more common inorganic constituents of plant tissues. Plant Physiol. 1944, 19, 70–89. [Google Scholar] [CrossRef] [PubMed]
- Fiske, C.H.; Subba Row, Y. The colorimetric determination of phosphorus. J. Biol. Chem. 1925, 66, 375–400. [Google Scholar]
- Bates, L.E.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Hayzer, D.J.; Leisinger, T.H. The gene-enzyme relationships of proline biosynthesis in Escherichia coli. J. Gen. Microbial. 1980, 118, 287–293. [Google Scholar] [CrossRef]
- Huang, A.H.C.; Cavalieri, A.J. Proline oxidase and water stress induced proline accumulation in spinach leaves. Plant Physiol. 1979, 63, 531–535. [Google Scholar] [CrossRef]
- Mishra, S.; Dubey, R. Heavy Metal Toxicity induced Alterations in Photosynthetic Metabolism in Plants. In Handbook of Photosynthesis; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 827–844. [Google Scholar]
- Chen, Y.; Wu, N.; Zhang, Z.W.; Yuan, M.; Yuan, S. Perspective of Monitoring Heavy Metals by Moss Visible Chlorophyll Fluorescence Parameters. Front. Plant Sci. 2019, 10, 35. [Google Scholar] [CrossRef]
- Bączek-Kwinta, R.; Antonkiewicz, J.; Łopata-Stasiak, A.; Kępka, W. Smoke compounds aggravate stress inflicted on Brassica seedlings by unfavorable soil conditions. Photosynthetica 2019, 57, 1–8. [Google Scholar] [CrossRef]
- Sirhindi, G.; Mir, M.A.; Abd-Allah, E.F.; Ahmad, P.; Gucel, S. Jasmonic acid modulates the physio-biochemical attributes, antioxidant enzyme activity, and gene expression in Glycine max under nickel toxicity. Front. Plant Sci. 2016, 7, 591. [Google Scholar] [CrossRef]
- Thao, N.P.; Khan, M.I.R.; Thu, N.B.A.; Hoang, X.L.T.; Asgher, M.; Khan, N.A.; Tran, L.S.P. Role of ethylene and its cross talk with other signaling molecules in plant responses to heavy metal stress. Plant Physiol. 2015, 169, 73–84. [Google Scholar] [CrossRef]
- Khan, N.A.; Mir, M.R.; Nazar, R.; Singh, S. The application of ethephon (an ethylene releaser) increases growth, photosynthesis and nitrogen accumulation in mustard (Brassica juncea L.) under high nitrogen levels. Plant Biol. 2008, 10, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Asgher, M.; Per, T.S.; Verma, S.; Pandith, S.A.; Masood, A.; Khan, N.A. Ethylene supplementation increases PSII Efficiency and alleviates chromium-inhibited photosynthesis through increased nitrogen and sulfur assimilation in mustard. J. Plant Growth Regul. 2018, 37, 1300–1317. [Google Scholar] [CrossRef]
- Molassiotis, A.; Sotiropoulos, T.; Tanou, G.; Diamantidis, G.; Therios, I. Boron induced oxidative damage and antioxidant and nucleolytic responses in shoot tips culture of the apple rootstock EM9 (Malus domestica Borkh). Environ. Exp. Bot. 2006, 56, 54–62. [Google Scholar] [CrossRef]
- Khan, N.A.; Asgher, M.; Per, T.S.; Masood, A.; Fatma, M.; Khan, M.I.R. Ethylene potentiates sulfur-mediated reversal of cadmium inhibited photosynthetic responses in mustard. Front. Plant Sci. 2016, 7, 1628. [Google Scholar] [CrossRef] [PubMed]
- Álvarez Viveros, M.F.; Inostroza-Blancheteau, C.; Timmermann, T.; González, M.; Arce-Johnson, P. Overexpression of Gly I and Gly II genes in transgenic tomato (Solanum ycopersicum Mill.) plants confers salt tolerance by decreasing oxidative stress. Mol. Biol. Rep. 2013, 40, 3281–3290. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Hossain, M.A.; Fujita, M. Trehalose pretreatment induces salt tolerance in rice (Oryza sativa L.) seedlings: Oxidative damage and co-induction of antioxidant defense and glyoxalase systems. Protoplasma 2015, 252, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984, 219, 1–14. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Alam, M.M.; Nahar, K.; Mohsin, S.M.; Bhuyan, M.B.; Parvin, K.; Hawrylak-Nowak, B.; Fujita, M. Silicon-induced antioxidant defense and methylglyoxal detoxification works coordinately in alleviating nickel toxicity in Oryza sativa L. Ecotoxicology 2019, 28, 261–276. [Google Scholar] [CrossRef]
- Alia; Saradhi, P.P.; Mohanty, P. Involvement of proline in protecting thylakoid membranes against free radical-induced photodamage. J. Photochem. Photobiol. 1997, 38, 253–257. [Google Scholar] [CrossRef]
- Hare, P.D.; Cress, W.A. Metabolism implications of stress-induced proline accumulation in plants. Plant Growth Regul. 1997, 21, 79–102. [Google Scholar] [CrossRef]
- Szabados, L.; Savoure, A. Proline: Multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Umar, S.; Khan, N.A. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J. Plant Physiol. 2015, 178, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Antonkiewicz, J.; Czesława, J.; Małgorzata, K.; Renata, S. Nickel bioaccumulation by the chosen plant species. Acta Physiol. Plant. 2016, 38, 40. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Khan, A.M.; Rengel, Z. Zinc-biofortified wheat accumulates more cadmium in grains than standard wheat when grown on cadmium-contaminated soil regardless of soil and foliar zinc application. Sci. Total Environ. 2019, 654, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Agarwal, R.M. Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L) as influenced by potassium supplementation. Plant Physiol. Biochem. 2017, 115, 449–460. [Google Scholar] [CrossRef]

| Control | Ethephon | Zinc | Ethephon + Zinc | |
|---|---|---|---|---|
| Chlorophyll content (SPAD value) | 31.4 ± 1.3 b | 39.5 ± 1.6 a | 21.3 ± 0.8 d | 26.4 ± 0.9 c |
| Net photosynthesis (µmol CO2 m−2s−1) | 19.8 ± 0.8 b | 25.2 ± 0.9 a | 11.2 ± 0.7 d | 16.2 ± 0.6 c |
| Intracellular CO2 concentration (µmol CO2 mol−1) | 272 ± 10.9 b | 319 ± 12.7 a | 187 ± 6.7 d | 230 ± 8.2 c |
| Stomatal conductance (mmol CO2 m−2 s−1) | 380 ± 10.7 b | 458 ± 12.3 a | 280 ± 10.1 d | 330 ± 11.8 c |
| Rubisco activity (µmol CO2 m−2 s−1) | 48.6 ± 1.9 b | 62.6 ± 2.2 a | 32.5 ± 1.17 d | 40.6 ± 1.5 c |
| Actual PSII efficiency (Φ PS II) | 0.62 ± 0.03 ab | 0.68 ± 0.04 a | 0.44 ± 0.02 c | 0.57 ± 0.02 b |
| Maximum PSII efficiency (Fv/Fm) | 0.77 ± 0.03 a | 0.84 ± 0.03 a | 0.57 ± 0.02 c | 0.67 ± 0.03 b |
| Intrinsic PSII efficiency (Fv’/Fm’) | 0.71 ± 0.03 b | 0.75 ± 0.03 a | 0.55 ± 0.02 c | 0.65 ± 0.02 b |
| Photochemical quenching (qP) | 0.83 ± 0.04 a | 0.87 ± 0.03 a | 0.63 ± 0.02 c | 0.73 ± 0.03 b |
| Non-photochemical quenching (NPQ) | 0.54 ± 0.02 bc | 0.49 ± 0.03 c | 0.86 ± 0.03 a | 0.62 ± 0.03 b |
| Electron transport rate (ETR) | 154 ± 6.2 a | 167 ± 5.1 a | 116 ± 4.2 c | 135 ± 5.3 b |
| Plant dry mass (g plant−1) | 6.11 ± 0.6 ab | 7.54 ± 0.4 a | 3.58 ± 0.4 c | 5.2 ± 0.5 b |
| Leaf area (cm2 plant−1) | 140 ± 5.7 b | 178 ± 6.1 a | 97.5 ± 3.8 d | 121 ± 4.6 c |
| Control | Ethephon | Zinc | Ethephon + Zinc | |
|---|---|---|---|---|
| Electrolyte leakage (%) | 1.24 ± 0.07 c | 0.81 ± 0.09 d | 3.93 ± 0.34 a | 2.08 ± 0.15 b |
| TBARS content (nmol g−1 FW) | 13.24 ± 0.6 c | 8.23 ± 0.4 d | 25.84 ± 0.8 a | 18.56 ± 0.7 b |
| Methylglyoxal content (mol g−1 FW) | 45.7 ± 1.7 d | 55.9 ± 2.1 c | 73.7 ± 3.0 a | 64.8 ± 2.1 b |
| Lipoxygenase activity (µmol min−1 mg−1 protein) | 10.2 ± 0.6 c | 7.9 ± 0.6 d | 15.4 ± 0.6 a | 13.5 ± 0.6 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.I.R.; Jahan, B.; Alajmi, M.F.; Rehman, M.T.; Khan, N.A. Exogenously-Sourced Ethylene Modulates Defense Mechanisms and Promotes Tolerance to Zinc Stress in Mustard (Brassica juncea L.). Plants 2019, 8, 540. https://doi.org/10.3390/plants8120540
Khan MIR, Jahan B, Alajmi MF, Rehman MT, Khan NA. Exogenously-Sourced Ethylene Modulates Defense Mechanisms and Promotes Tolerance to Zinc Stress in Mustard (Brassica juncea L.). Plants. 2019; 8(12):540. https://doi.org/10.3390/plants8120540
Chicago/Turabian StyleKhan, M. Iqbal R., Badar Jahan, Mohamed F Alajmi, Md Tabish Rehman, and Nafees A. Khan. 2019. "Exogenously-Sourced Ethylene Modulates Defense Mechanisms and Promotes Tolerance to Zinc Stress in Mustard (Brassica juncea L.)" Plants 8, no. 12: 540. https://doi.org/10.3390/plants8120540
APA StyleKhan, M. I. R., Jahan, B., Alajmi, M. F., Rehman, M. T., & Khan, N. A. (2019). Exogenously-Sourced Ethylene Modulates Defense Mechanisms and Promotes Tolerance to Zinc Stress in Mustard (Brassica juncea L.). Plants, 8(12), 540. https://doi.org/10.3390/plants8120540








