Polyphenol Compounds and Biological Activity of Caper (Capparis spinosa L.) Flowers Buds
Abstract
1. Introduction
2. Results and Discussion
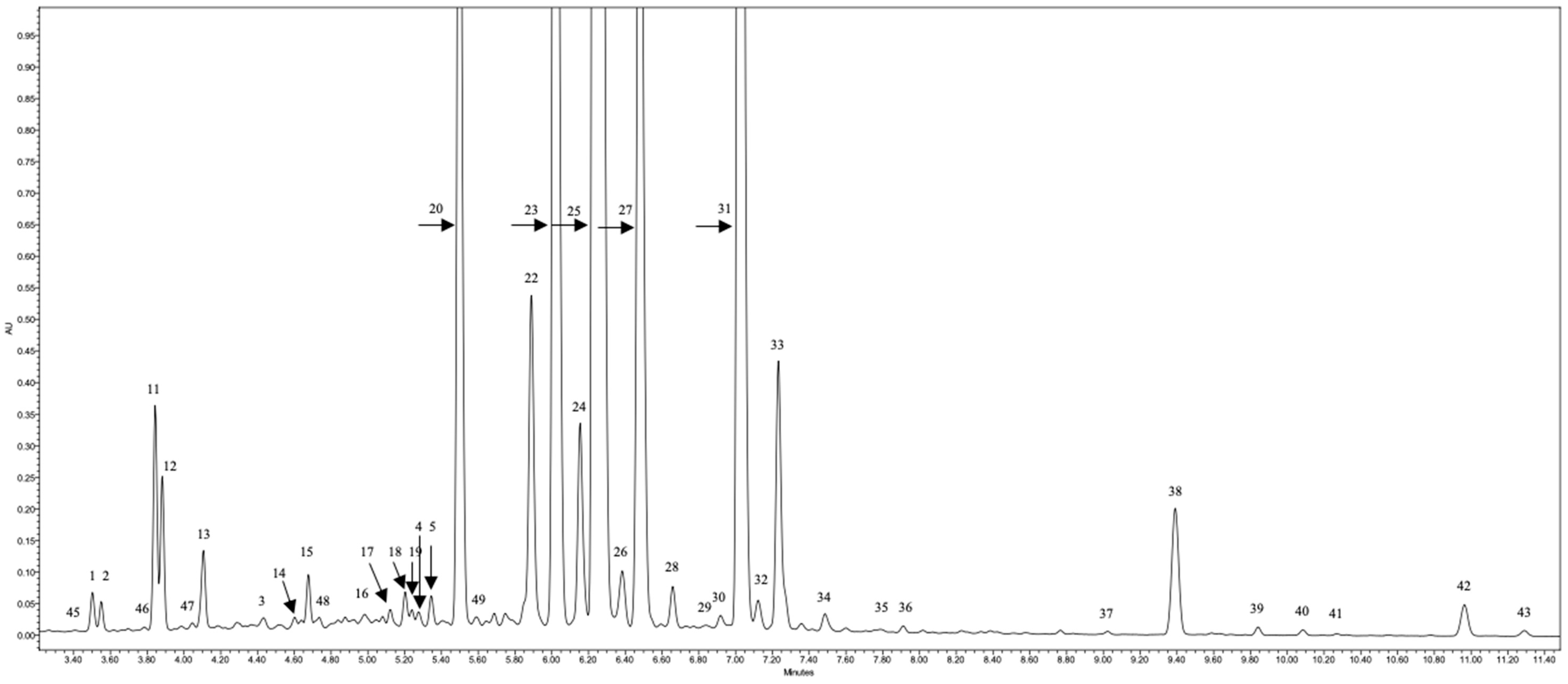
2.1. Identification of Phenolic Profile
2.1.1. Flavonol Glycosides
2.1.2. Flavan-3-ols
2.1.3. Hydroxycinnamic Acid Derivatives
2.2. Phenolic Compounds’ Quantitative Profile
2.3. Biological Potential of Caper Flowers
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. Separation, Identification, and Quantification of Polyphenolic Compounds
3.4. Determination of Biologically Activity: Anti-Oxidant, Anti-Diabetic, and Cholinesterase Inhibition
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.Z.; Che, X.N.; Pan, Q.H.; Li, X.X.; Duan, C.Q. Transcriptional activation of flavan-3-ols biosynthesis in grape berries by UV irradiation depending on developmental stage. Plant Sci. 2013, 208, 64–74. [Google Scholar] [CrossRef]
- Tlili, N.; Elfalleh, W.; Saadaoui, E.; Khaldi, A.; Triki, S.; Nasri, N. The caper (Capparis L.): Ethnopharmacology, phytochemical and pharmacological properties. Fitoterapia 2011, 82, 93–101. [Google Scholar] [CrossRef]
- Gull, T.; Anwar, F.; Sultana, B.; Alcayde, M.A.C.; Nouman, W. Capparis species: A potential source of bioactives and high-value components: A review. Ind. Crop. Prod. 2015, 67, 81–96. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Z.F. Phytochemical and pharmacological properties of Capparis spinosa as a medicinal plant. Nutrients 2018, 10, 116. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Maggi, F.; Daglia, M.; Habtemariam, S.; Rastrelli, L.; Nabavi, S.M. Pharmacological effects of Capparis spinosa L. Phytother. Res. 2016, 30, 1733–1744. [Google Scholar] [CrossRef]
- Francesca, N.; Barbera, M.; Martorana, A.; Saiano, F.; Gaglio, R.; Aponte, M.; Moschetti, G.; Settanni, L. Optimised method for the analysis of phenolic compounds from caper (Capparis spinosa L.) berries and monitoring of their changes during fermentation. Food Chem. 2016, 196, 1172–1179. [Google Scholar] [CrossRef]
- Jiménez-López, J.; Ruiz-Medina, A.; Ortega-Barrales, P.; Llorent-Martínez, E.J. Phytochemical profile and antioxidant activity of caper berries (Capparis spinosa L.): Evaluation of the influence of the fermentation process. Food Chem. 2018, 250, 54–59. [Google Scholar] [CrossRef]
- Inocencio, C.; Rivera, D.; Alcaraz, F.J.; Tomas-Barberan, F.A. Flavonoid content of commercial capers (Capparis spinosa, C. sicula and C. orientalis) produced in mediterranean countries. Eur. Food Res. Technol. 2000, 212, 70–74. [Google Scholar] [CrossRef]
- Sharaf, M.; El-Ansari, M.A.; Saleh, N.A.M. Quercetin triglycoside from Capparis spinosa. Fitoterapia 2000, 71, 46–49. [Google Scholar] [CrossRef]
- Mollica, A.; Stefanucci, A.; Macedonio, G.; Locatelli, M.; Luisi G Novellino, E.; Zengin, G. Chemical composition and biological activity of Capparis spinosa L. from Lipari Island. S. Afr. J. Bot. 2019, 120, 135–140. [Google Scholar] [CrossRef]
- Afsharypuor, S.; Jeiran, K.; Jazy, A.A. First investigation of the flavour profiles of the leaf, ripe fruit and root of Capparis spinosa var. mucronifolia from Iran. Pharm. Acta Helv. 1998, 72, 307–309. [Google Scholar] [CrossRef]
- Dahot, M.U. Chemical evaluation of the nutritive value of flowers and fruits of Capparis decidua. J. Chem. Soc. Pak. 1993, 15, 78–81. [Google Scholar]
- Abderrahmane, B.; Ameni, D.; Boumerfeg, S.; Adjadj, M.; Djarmouni, M.; Charef, N.; Khennouf, S.; Arrar, L. Studies of antioxidants and xanthine oxidase inhibitory potentials of root and aerial parts of medicinal plant Capparis spinosa L. Am. J. Med. Med. Sci. 2012, 2, 25–32. [Google Scholar]
- Maldini, M.; Foddai, M.; Natella, F.; Addis, R.; Chessa, M.; Petretto, G.L.; Tuberoso, C.I.G.; Pintore, G. Metabolomic study of wild and cultivated caper (Capparis spinosa L.) from different areas of Sardinia and their comparative evaluation. J. Mass. Spectrom. 2016, 51, 716–728. [Google Scholar] [CrossRef]
- Pérez Pulido, R.; Omar, N.B.; Abriouel, H.; López, R.L.; Cañamero, M.M.; Gálvez, A. Microbiological study of lactic acid fer- mentation of caper berries by molecular and culture- dependent methods. App. Environ. Microbiol. 2005, 71, 7872–7879. [Google Scholar] [CrossRef]
- Ferreres, F.; Gil-Izquierdo, A.; Vinholes, J.; Silva, S.T.; Valentão, P.; Andrade, P.B. Bauhinia forficata Link authenticity using flavonoids profile: Relation with their biological properties. Food Chem. 2012, 134, 894–904. [Google Scholar] [CrossRef]
- Siracusa, L.; Kulisic-Bilusic, T.; Politeo, O.; Krause, I.; Dejanovic, B.; Ruberto, G. Phenolic Composition and antioxidant activity of aqueous infusions from Capparis spinosa L. and Crithmum maritimum L. before and after submission to a two-step in vitro digestion model. J. Agric. Food Chem. 2011, 59, 12453–12459. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Gil-Izquierdo, Á.; Medina, S.; Ferreres, F. Phenolic composition profiling of different edible parts and by-products of date palm (Phoenix dactylifera L.) by using HPLC-DAD-ESI/MSn. Food Res. Inter. 2017, 100, 494–500. [Google Scholar] [CrossRef]
- Ferreres, F.; Grosso, C.; Gil-Izquierdo, A.; Fernandes, A.; Valentão, P.; Andrade, P.B. Comparing the phenolic profile of Pilocarpus pennatifolius Lem. by HPLC–DAD–ESI/MSn with respect to authentication and enzyme inhibition potential. Ind. Crops. Prod. 2015, 77, 391–401. [Google Scholar] [CrossRef]
- Ferreres, F.; Llorach, R.; Gil-Izquierdo, A. Characterization of the interglycosidic linkage in di-, tri-, tetra- and pentaglycosylated flavonoids and differentiation of positional isomers by liquid chromatography/electrospray ionization tandem mass spectrometry. J. Mass. Spectrom. 2004, 39, 312–321. [Google Scholar] [CrossRef]
- Rodrigo, M.; Lazaro, M.J.; Alvarruiz, A.; Giner, V. Composition of capers (Capparis spinosa): Influence of cultivar, size and harvest date. J. Food Sci. 1992, 57, 1152–1154. [Google Scholar] [CrossRef]
- Sharaf, M.; El-Ansari, M.A.; Saleh, N.A.M. Flavonoids of four Cleome and three Capparis species. Biochem. Syst. Ecol. 1997, 25, 161–166. [Google Scholar] [CrossRef]
- Zhou, H.F.; Xie, C.; Jian, R.; Kang, J.; Li, Y.; Zhuang, C.L.; Yang, F.; Zhang, L.; Lai, L.; Wu, T.; et al. Biflavonoids from caper (Capparis spinosa L.) fruits and their effects in inhibiting NF-kappa B activation. J. Agric. Food Chem. 2011, 59, 3060–3065. [Google Scholar] [CrossRef]
- Parveen, I.; Threadgill, M.D.; Hauck, B.; Donnison, I.; Winters, A. Isolation, identification and quantitation of hydroxycinnamic acid conjugates, potential platform chemicals, in the leaves and stems of Miscanthus×giganteus using LC–ESI-MSn. Phytochemistry 2011, 72, 2376–2384. [Google Scholar] [CrossRef]
- Jaiswal, R.; Müller, H.; Müller, A.; Karar, M.G.E.; Kuhnert, N. Identification and characterization of chlorogenic acids, chlorogenic acid glycosides and flavonoids from Lonicera henryi L. (Caprifoliaceae) leaves by LC–MSn. Phytochemistry 2014, 108, 252–263. [Google Scholar] [CrossRef]
- Mollica, A.; Zengin, G.; Locatelli, M.; Stefanucci, A.; Mocan, A.; Macedonio, G.; Carradori, S.; Onaolapo, O.; Onaolapo, A.; Adegoke, J.; et al. Anti-diabetic and anti-hyperlipidemic properties of Capparis spinosa L.: In vivo and in vitro evaluation of its nutraceutical potential. J. Funct. Foods 2017, 35, 32–42. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zheng, W. Effect of plant growth temperature on antioxidant capacity in strawberry. J. Agric. Food Chem. 2001, 49, 4977–4982. [Google Scholar] [CrossRef]
- Solovchenko, A.; Schmitz-Eiberger, M. Significance of skin flavonoids for UV-B-protection in apple fruits. J. Exp. Bot. 2003, 54, 1977–1984. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.Y.; Kim, K.N.; Kim, H.S. Quantitative analysis of two major flavonoid aglycones in acid hydrolyzed samples of Angelica keiskei by HPLC. Food Sci. Biotech. 2003, 12, 415–418. [Google Scholar]
- Jaakola, L.; Hohtola, A. Effect of latitude on flavonoid biosynthesis in plants. Plant Cell Environ. 2010, 33, 1239–1247. [Google Scholar] [CrossRef]
- Wojdyło, A.; Jáuregui, N.N.; Carbonell-Barrachina, A.A.; Oszmiański, J.; Golis, T. Variability of physicochemical properties and the content of bioactive compounds in the Lonicera caerulea L. var. kamtschatica berries. J. Agric. Food Chem. 2013, 61, 12072–12084. [Google Scholar] [CrossRef]
- Vahid, H.; Rakhshandeh, H.; Ghorbani, A. Antidiabetic properties of Capparis spinosa L. and its components. Biomed. Pharm. 2017, 92, 293–302. [Google Scholar] [CrossRef]
- Brimijoin, S.; Chen, V.P.; Pang, Y.P.; Geng, L.; Gao, Y. Physiological roles for butyrylcholinesterase: A BChE–Ghrelin Axis. Chem. Biol. Interact 2016, 259, 271–275. [Google Scholar] [CrossRef]
- Fan, P.; Hay, A.E.; Marston, A.; Hostettmann, K. Acetylcholinesterase-Inhibitory Activity of Linarin from Buddleja davidii, structure-activity relationships of related flavonoids, and chemical investigation of Buddleja nitida. Pharm. Biol. 2008, 46, 596–601. [Google Scholar] [CrossRef]
- Fan, P.; Terrier, L.; Hay, A.E.; Marston, A.; Hostettmann, K. Antioxidant and enzyme inhibition activities and chemical profiles of Polygonum sachalinensis F. Schmidt ex Maxim (Polygonaceae). Fitoterapia 2010, 81, 124–131. [Google Scholar]
- Sawasdee, P.; Sabphon, C.; Sitthiwongwanit, D.; Kokpol, U. Anticholinesterase activity of 7-methoxyflavones isolated from Kaempferia parviflora. Phytother. Res. 2009, 23, 1792–1794. [Google Scholar] [CrossRef]
- García-Rollán, M. Claves de la Flora de España Vol 1 (Península y Baleares), Pteridofitas, Gimnospermas, Dicotiledóneas (AJ); Ediciones Mundi-Prensa: Madrid, Spain, 1981. [Google Scholar]
- Wojdyło, A.; Nowicka, P.; Oszmiański, J.; Golis, T. Phytochemical compounds and biological effects of Actinidia fruits. J. Funct. Foods 2017, 30, 194–202. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of antioxidant activities of common vegetables employing Oxygen Radical Absorbance Capacity (ORAC) and Ferric Reducing Antioxidant Power (FRAP) Assays: A Comparative Study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Carbonell-Barrachina, Á.A.; Hernández, F. Phenolic compounds, antioxidant and antidiabetic activity of different cultivars of Ficus carica L. fruits. J. Funct. Foods 2016, 25, 421–432. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Bąbelewski, P. Phenolic and carotenoid profile of new goji cultivars and their antihyperglycemic, anti-aging and antioxidant properties. J. Funct. Foods 2018, 48, 632–642. [Google Scholar] [CrossRef]

| No. | Name of Compounds | Rt | λmax | MS [M − H]–(m/z) | MS/MS [M − H]−(m/z) | |||
|---|---|---|---|---|---|---|---|---|
| Hydroxycinnamic Acid | ||||||||
| 1 | p-Coumaric acid | 3.41 | 310 | 163.04 | 119.05 | |||
| 2 | 5-Caffeoylquinic acid | 3.51 | 285, 326 | 353.04 | 191.06/179.03/173.05 | |||
| 3 | 4-Caffeoylquinic acid | 4.43 | 285, 326 | 353.01 | ||||
| 4 | trans-5-p-Coumaroylquinic acid | 5.29 | 311 | 337.04 | 163.05 | |||
| 5 | cis-5-p-Coumaroylquinic acid | 5.34 | 311 | 337.04 | 163.05 | |||
| 6 | 3-Feruloylquinic acid | 5.59 | 325 | 367.05 | 193.03/191.02/173.09 | |||
| 7 | 5-Feruloylquinic acid | 9.39 | 325 | 367.05 | 191.02/135.06 | |||
| 8 | 4-Feruloylquinic acid | 9.54 | 325 | 367.05 | 193.03/173.09/134.09 | |||
| 9 | Coumaric acid-O-hexoside | 10.26 | 275, 314 | 325.03 | 163.11/119.12 | |||
| 10 | Sinapic acid | 10.95 | 337 | 223.06 | 205.13/179.03/164.06 | |||
| Flavonols | −308 | −162 | −146 | aglycone | ||||
| 11 | Quercetin-3-O-rutinoside-7-hexoside | 3.85 | 285, 352 | 771.06 | 463.06 | 609.06 | 301.02 | |
| 12 | Quercetin-3-O-rutinoside-hexoside-7-O-rhamnoside | 3.93 | 917.11 | 609.11 | 755.11 | 463.04 | 30.,02 | |
| 13 | Kaempferol-3-O-rutinoside-hexoside-7-O-rhamnoside | 4.25 | 901.12 | 593.12 | 739.12 | 285.95 | ||
| 14 | Isorhamnetin-3-O-rutinoside-hexoside-7-O-rhamnoside | 4.45 | 931.08 | 607.08 | 623.08 | 315.05 | ||
| 15 | Quercetin-3-O-rutinoside-7-O-hexoside | 4.50 | 202, 256, 351 | 771.06 | 463.06 | 609.06 | 301.02 | |
| 16 | Quercetin-3-O-rutinoside-7-O-hexoside | 4.90 | 266, 352 | 771.06 | 463.06 | 609.06 | 625.06 | 301.02 |
| 17 | Kaempferol-3-O-rutinoside-7-O-hexoside | 5.04 | 342 | 755.07 | 593.07 | 285.95 | ||
| 18 | Isorhamnetin-3-O-rutinoside | 5.09 | 271, 337 | 623.03 | 315.03 | 315.00 | ||
| 19 | Isorhamnetin-3-O-rutinoside-7-O-hexoside | 5.21 | 264, 326 | 785.07 | 477.07 | 623.07 | 315.05 | |
| 20 | Myricetin-3-O-rutinoside | 5.29 | 274, 355 | 625.02 | 317.02 | 317.09 | ||
| 21 | Myricetin-3-O-rutinoside (isomer) | 5.46 | 274, 355 | 625.03 | 317.03 | 317.09 | ||
| 22 | Quercetin-3-O-rutinoside-7-O-rhamnoside | 5.56 | 755.07 | 447.07 | 609.07 | 301.02 | ||
| 23 | Isorhamnetin-3-O-rutinoside-7-O-hexoside | 5.69 | 785.08 | 477.08 | 623.08 | 315.08 | ||
| 24 | Quercetin-3-O-rutinoside-7-O-rhamnoside (isomer) | 5.90 | 254, 353 | 755.07 | 447.07 | 609.07 | 301.02 | |
| 25 | Quercetin-3-O-rutinoside | 6.33 | 252, 348 | 609.03 | 301.03 | 301.02 | ||
| 26 | Kaempferol-3-O-(2-rhamnoside)-rutinoside | 6.41 | 264, 347 | 739.08 | 431.08 | 593.08 | 285.95 | |
| 27 | Isorhamnetin-3-O-(2-rhamnoside)-rutinoside | 6.50 | 253, 347 | 769.08 | 461.08 | 623.08 | 315.95 | |
| 28 | Quercetin-3-O-rutinoside (rutin) | 6.66 | 256, 354 | 609.03 | 301.03 | 301.02 | ||
| 29 | Quercetin-3-O-hexoside-7-O-hexoside | 6.80 | 253, 330 | 625.02 | 463.02 | 301.02 | ||
| 30 | Kaempferol-3-O-rutinoside | 6.90 | 254, 346 | 593.03 | 285.03 | 285.95 | ||
| 31 | Isorhamnetin-3-O-rutinoside | 7.02 | 255, 266sh, 351 | 623.04 | 315.04 | 461.04 | 315.03 | |
| 32 | Kaempferol-3-O-rutionoside-7-O-hexoside | 7.28 | 264, 348 | 755.07 | 447.07 | 593.07 | 285.95 | |
| 33 | Kaempferol-3-O-rutinoside (isomer) | 7.38 | 254, 348 | 593.03 | 285.03 | 285.95 | ||
| 34 | Isorhamnetin-3-O-rutinoside (isomer) | 7.55 | 253, 352 | 623.04 | 315.04 | 315.03 | ||
| 35 | Isorhamnetin-3-O-hexoside) | 7.83 | 254, 336, 365 | 477.04 | 315.05 | 315.03 | ||
| 36 | Kaempferol-3-O-rutinoside (isomer) | 7.85 | 264, 346 | 593.03 | 285.03 | 285.95 | ||
| 37 | Kaempferol-3-O-rutinoside-7-O-rhamnoside | 8.97 | 753.09 | 445.09 | 591.09 | 607.09 | 285.05 | |
| 38 | Myricetin-3-O-hexoside | 9.62 | 355 | 479.11 | 317.11 | 317.11 | ||
| 39 | Quercetin-3-O-(2-rhamnoside)-hexoside | 9.87 | 366 | 609.05 | 447.05 | 463.05 | 301.05 | |
| 40 | Myricetin-3-O-rhamnoside | 10.30 | 355 | 463.10 | 317,1 | 317.08 | ||
| 41 | Quercetin-3-O-(2-rhamnoside)-hexoside | 10.47 | 316 | 609.64 | 447.64 | 463.64 | 301.02 | |
| 42 | Myricetin-3-O-rhamnoside (isomer) | 10.98 | 355 | 463.10 | 317.10 | 317.08 | ||
| 43 | Kaempferol | 11.43 | 285.95 | 285.95 | ||||
| 44 | Quercetin | 11.71 | 256, 268sh, 352 | 301.04 | 301.02 | |||
| Flavan-3-ols | ||||||||
| 45 | (+)-Catechin | 3.41 | 278 | 289.06 | 245.14 | |||
| 46 | Procyanidin B2 | 3.73 | 280 | 577.03 | 289.04/245.14 | |||
| 47 | Procanidin C1 | 4.14 | 280 | 865.07 | 577.08/289.04/245.14 | |||
| 48 | (−)-Epicatechin | 4.61 | 278 | 289.06 | 245.14 | |||
| 49 | Procyanidin dimer | 5.56 | 280 | 577.04 | 289.04/245.14 | |||
| Cultivars | Stages of Development | Hydroxycinnamic Acid | Flavonols | F-3-ols | ∑ Total polyphenols | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dpCA | dCA | dpQCA | dFQA | SA | dQ | dK | dISO | dM | ||||
| ORI.7 | nonpareilles | 46.4 ± 2.3 | 42.4 ± 2.4 | 18.5 ± 1.4 | 67.2 ± 2.1 | 8.3 ± 1.5 | 6254.9 ± 56 | 1511.0 ± 14 | 619.2 ± 12 | 1643.8 ± 23 | 513.2 ± 23 | 10724.9 |
| surfines | 33.3 ± 2.1 | 30.1 ± 1.3 | 19.4 ± 1.6 | 90.1 ± 3.8 | 11.2 ± 1.1 | 5218.3 ± 57 | 2331.3 ± 15 | 618.3 ± 13 | 1607.4 ± 32 | 304.3 ± 19 | 10263.6 | |
| capucines | 19.3 ± 1.4 | 19.2 ± 2.1 | 20.8 ± 2.5 | 34.6 ± 2.6 | 9.6 ± 1.5 | 2245.6 ± 34 | 2133.5 ± 23 | 437.0 ± 21 | 1084.0 ± 11 | 670.7 ± 32 | 6674.3 | |
| capotes | 31.4 ± 2.1 | 16.6 ± 1.7 | 16.5 ± 1.7 | 24.3 ± 3.1 | 11.6 ± 1.6 | 2711.6 ± 21 | 1694.1 ± 14 | 355.2 ± 15 | 1046.1 ± 18 | 507.7 ± 26 | 6415.1 | |
| fines | 18.9 ± 1.6 | 20.2 ± 1.6 | 11.5 ± 1.4 | 25.4 ± 1.6 | 1.4 ± 0.4 | 1657.6 ± 32 | 815.0 ± 24 | 125.5 ± 17 | 361.6 ± 25 | 305.0 ± 29 | 3342.2 | |
| gruesas | 10.8 ± 0.9 | 22.9 ± 2.1 | 3.5 ± 2.6 | 13.7 ± 1.3 | 2.0 ± 0.2 | 1453.2 ± 19 | 979.1 ± 21 | 119.3 ± 10 | 317.7 ± 21 | 315.0 ± 32 | 3237.3 | |
| ORI.10 | nonpareilles | 32.9 ± 3.1 | 15.6 ± 2.7 | 5.4 ± 0.8 | 47.0 ± 1.9 | 5.0 ± 0.7 | 2375.6 ± 26 | 1885.1 ± 27 | 229.2 ± 9 | 760.6±25 | 208.0 ± 37 | 5564.5 |
| surfines | 29.5 ± 2.7 | 14.8 ± 2.5 | 7.5 ± 1.1 | 51.1 ± 2.7 | 8.9 ± 1.1 | 2528.9 ± 21 | 1753.0 ± 31 | 230.8 ± 11 | 804.9 ± 31 | 344.0 ± 35 | 5773.4 | |
| capucines | 34.0 ± 4.1 | 17.2 ± 1.9 | 6.9 ± 0.5 | 14.4 ± 3.1 | 13.0 ± 0.9 | 2660.7 ± 22 | 1302.6 ± 16 | 141.0 ± 11 | 802.9 ± 27 | 366.8 ± 28 | 5359.4 | |
| capotes | 29.4 ± 2.5 | 15.3 ± 2.7 | 5.4 ± 0.9 | 21.5 ± 2.6 | 13.1 ± 1.1 | 2603.6 ± 32 | 1052.3 ± 14 | 225.7 ± 16 | 1018.7 ± 28 | 511.9 ± 26 | 5497.0 | |
| fines | 10.9 ± 1.8 | 15.6 ± 2.3 | 2.5 ± 0.3 | 14.7 ± 2.9 | 0.5 ± 0.2 | 2257.8 ± 15 | 503.4 ± 19 | 200.2 ± 14 | 430.1 ± 34 | 547.2 ± 21 | 3982.9 | |
| gruesas | 6.4 ± 0.4 | 11.5 ± 1.3 | 1.8 ± 0.4 | 14.4 ± 1.1 | 0.7 ± 0.1 | 2124.8 ± 19 | 532.5 ± 17 | 202.2 ± 18 | 322.6 ± 18 | 476.9 ± 22 | 3693.9 | |
| Stage of development | nonpareilles | a | a | a | a | b | a | c | a | a | a | a |
| surfines | b | b | a | a | a | b | a | a | a | b | a | |
| capucines | c | bc | ab | b | ab | c | b | b | b | a | b | |
| capotes | d | c | b | c | ab | c | d | c | b | b | b | |
| fines | de | c | c | d | c | c | e | c | c | c | c | |
| Cultivars | ORI.7 | a | a | a | a | a | a | a | a | ab | a | a |
| ORI.10 | b | b | b | b | b | b | b | b | b | b | b | |
| Cultivars | Stages of Development | Antioxidant Activity [mmol Trolox/100 g] | Anti-Diabetic Activity [IC50; mg/mL] | Cholinesterase’s Inhibition [% of Inhibition] | ||||
|---|---|---|---|---|---|---|---|---|
| ABTS | FRAP | ORAC | α-amylase | α-glucosidase | AChE | BuChE | ||
| ORI.7 | nonpareilles | 6.92 ± 0.54 | 7.51 ± 0.11 | 27.66 ± 1.43 | 3.15 ± 0.11 | 2.98 ± 0.11 | 18.3 ± 0.1 | 31.0 ± 2.4 |
| surfines | 6.89 ± 0.12 | 7.23 ± 0.47 | 25.52 ± 1.11 | 2.10 ± 0.05 | 2.47 ± 0.13 | 15.9 ± 0.3 | 20.1 ± 1.8 | |
| capucines | 6.53 ± 0.32 | 7.03 ± 0.72 | 22.97 ± 0.99 | 1.94 ± 0.21 | 2.32 ± 0.14 | 14.5 ± 0.7 | 28.4 ± 3.8 | |
| capotes | 6.05 ± 0.14 | 6.35 ± 0.32 | 22.25 ± 0.57 | 1.72 ± 0.13 | 2.20 ± 0.11 | 13.8 ± 1.7 | 20.5 ± 1.1 | |
| fines | 5.16 ± 0.21 | 6.76 ± 0.54 | 20.79 ± 1.17 | 1.30 ± 0.11 | 1.89 ± 0.11 | 12.1 ± 1.0 | 18.0 ± 1.2 | |
| gruesas | 3.56 ± 0.11 | 4.48 ± 0.38 | 16.77 ± 1.21 | 0.93 ± 0.07 | 1.52 ± 0.09 | 10.5 ± 0.9 | 11.4 ± 0.9 | |
| ORI.10 | nonpareilles | 6.82 ± 0.21 | 7.64 ± 0.51 | 19.27 ± 0.99 | 3.74 ± 0.99 | 4.46 ± 0.15 | 28.1 ± 2.0 | 33.8 ± 2.3 |
| surfines | 5.83 ± 0.11 | 6.45 ± 0.58 | 18.55 ± 0.60 | 3.23 ± 0.21 | 3.68 ± 0.19 | 17.8 ± 1.3 | 25.2 ± 2.1 | |
| capucines | 5.45 ± 0.43 | 6.54 ± 0.32 | 16.45 ± 2.32 | 2.67 ± 0.15 | 2.38 ± 0.10 | 15.4 ± 0.9 | 23.4 ± 1.6 | |
| capotes | 2.43 ± 0.11 | 3.68 ± 0.24 | 15.29 ± 1.12 | 2.14 ± 0.21 | 2.34 ± 0.13 | 13.5 ± 1.3 | 20.4 ± 1.4 | |
| fines | 2.01 ± 0.09 | 2.83 ± 0.44 | 13.59 ± 1.43 | 1.71 ± 0.32 | 2.02 ± 0.06 | 13.4 ± 1.4 | 19.2 ± 1.8 | |
| gruesas | 0.54 ± 0.04 | 1.69 ± 0.37 | 10.09 ± 2.01 | 1.45 ± 0.15 | 1.97 ± 0.89 | 10.4 ± 1.2 | 8.6 ± 1.2 | |
| Stage of development | nonpareilles | a | a | a | a | a | a | a |
| surfines | a | a | ab | a | b | b | ab | |
| capucines | a | ab | ab | ab | c | bc | ab | |
| capotes | b | b | b | b | c | c | b | |
| fines | b | b | b | bc | d | c | b | |
| Cultivars | ORI.7 | a | a | a | a | a | a | a |
| ORI.10 | b | b | b | b | b | b | b | |
| Accession | ABTS | FRAP | ORAC | AChE | BuChE | α-amylase | α-glucosidase |
|---|---|---|---|---|---|---|---|
| ∑ Flavonols | 0.666 | 0.598 | 0.805 | 0.393 | 0.547 | 0.497 | 0.357 |
| ∑ Quercetin derivatives | 0.474 | 0.419 | 0.684 | 0.285 | 0.408 | −0.301 | −0.220 |
| ∑ Kaempferol derivatives | 0.855 | 0.791 | 0.737 | 0.550 | 0.641 | 0.025 | −0.438 |
| ∑ Isorhamnetin derivatives | 0.556 | 0.466 | 0.786 | 0.226 | 0.423 | −0.422 | −0.181 |
| ∑ Myricetin derivatives | 0.652 | 0.585 | 0.790 | 0.348 | 0.571 | −0.217 | −0.403 |
| ∑ Aglycone of flavonols | 0.460 | 0.425 | 0.635 | −0.170 | 0.278 | −0.140 | −0.257 |
| ∑ Mono-O-glycosides of flavonols | 0.546 | 0.513 | 0.677 | 0.490 | 0.601 | 0.638 | 0.464 |
| ∑ Di-O-glycosides of flavonols | 0.480 | 0.425 | 0.689 | 0.291 | 0.412 | 0.434 | 0.258 |
| ∑ Tri-O-glycosides of flavonols | 0.546 | 0.468 | 0.806 | 0.183 | 0.379 | 0.224 | 0.105 |
| ∑ Tetra-O-glycosides of flavonols | 0.479 | 0.420 | 0.784 | 0.027 | 0.251 | 0.092 | −0.023 |
| ∑ Phenolic acid | 0.571 | 0.582 | 0.287 | 0.866 | 0.765 | 0.925 | 0.871 |
| ∑ p-CA derivatives | 0.737 | 0.730 | 0.694 | 0.605 | 0.732 | 0.085 | −0.607 |
| ∑ CA derivatives | 0.506 | 0.487 | 0.772 | 0.120 | 0.309 | −0.458 | −0.398 |
| ∑ p-QCA derivatives | 0.747 | 0.699 | 0.906 | 0.102 | 0.445 | −0.286 | −0.371 |
| ∑ FQA derivatives | 0.676 | 0.628 | 0.753 | 0.498 | 0.496 | −0.268 | −0.233 |
| SA | 0.496 | 0.460 | 0.428 | 0.204 | 0.434 | 0.270 | −0.516 |
| ∑ flavan-3-ols | −0.239 | −0.307 | −0.009 | −0.403 | 0.005 | −0.268 | 0.153 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojdyło, A.; Nowicka, P.; Grimalt, M.; Legua, P.; Almansa, M.S.; Amorós, A.; Carbonell-Barrachina, Á.A.; Hernández, F. Polyphenol Compounds and Biological Activity of Caper (Capparis spinosa L.) Flowers Buds. Plants 2019, 8, 539. https://doi.org/10.3390/plants8120539
Wojdyło A, Nowicka P, Grimalt M, Legua P, Almansa MS, Amorós A, Carbonell-Barrachina ÁA, Hernández F. Polyphenol Compounds and Biological Activity of Caper (Capparis spinosa L.) Flowers Buds. Plants. 2019; 8(12):539. https://doi.org/10.3390/plants8120539
Chicago/Turabian StyleWojdyło, Aneta, Paulina Nowicka, Mar Grimalt, Pilar Legua, Maria Soledad Almansa, Asunción Amorós, Ángel Antonio Carbonell-Barrachina, and Francisca Hernández. 2019. "Polyphenol Compounds and Biological Activity of Caper (Capparis spinosa L.) Flowers Buds" Plants 8, no. 12: 539. https://doi.org/10.3390/plants8120539
APA StyleWojdyło, A., Nowicka, P., Grimalt, M., Legua, P., Almansa, M. S., Amorós, A., Carbonell-Barrachina, Á. A., & Hernández, F. (2019). Polyphenol Compounds and Biological Activity of Caper (Capparis spinosa L.) Flowers Buds. Plants, 8(12), 539. https://doi.org/10.3390/plants8120539









