Global Actions for Managing Cactus Invasions
Abstract
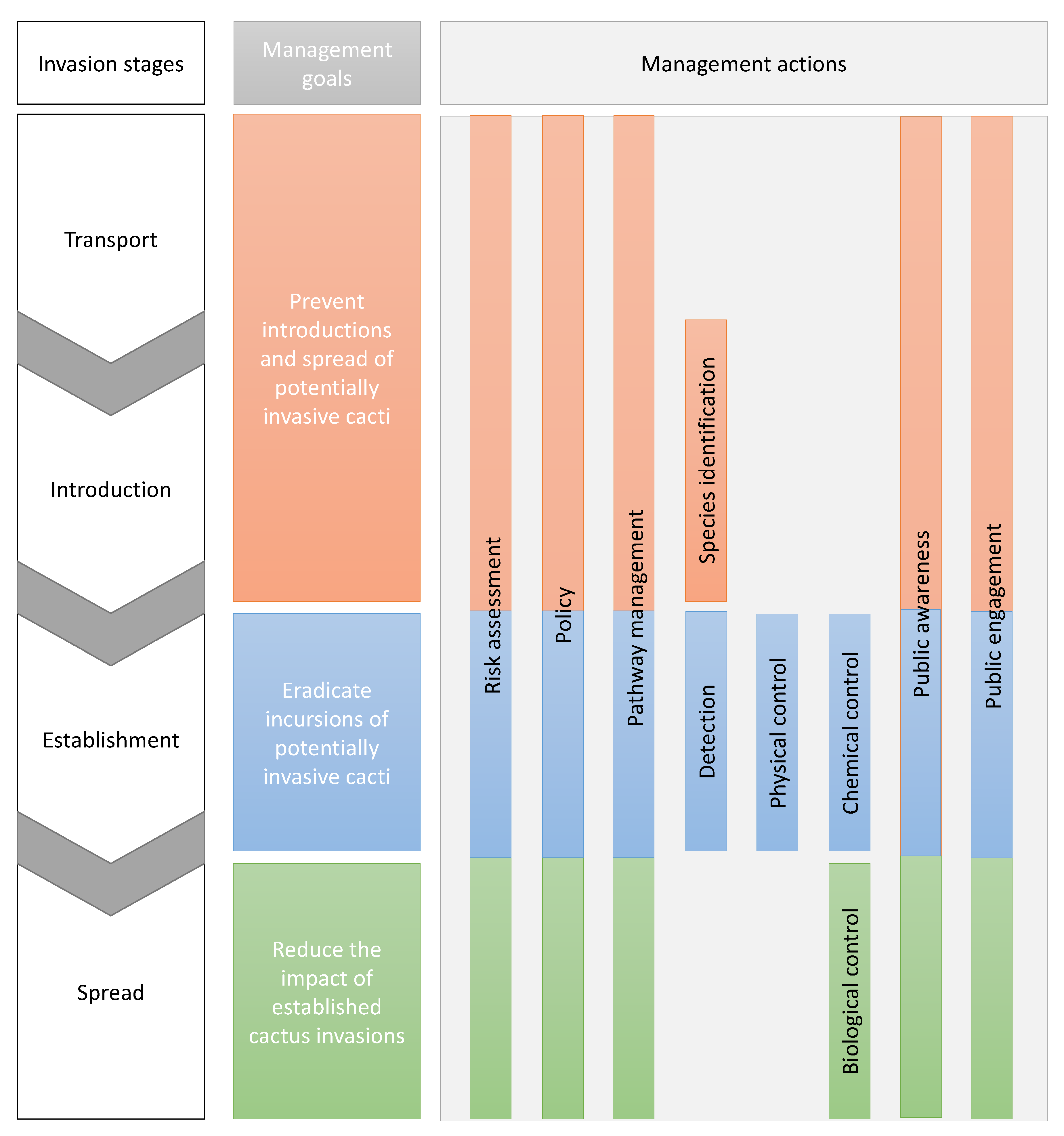
1. Introduction and Methods
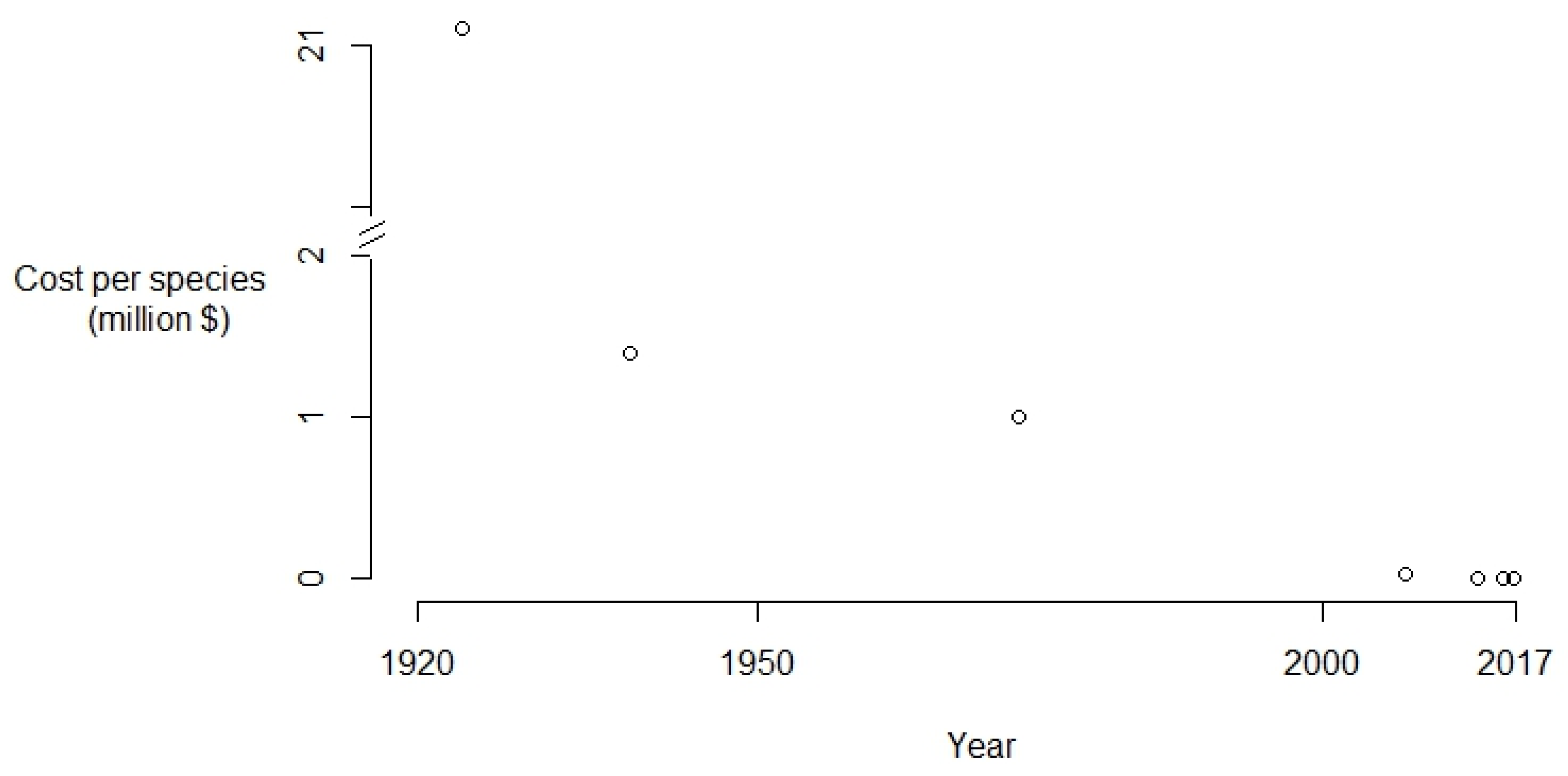
2. Results and Discussion
2.1. Risk Assessment
2.2. Policy and Legislation
2.3. Pathway Management
2.4. Species Identification
2.5. Detection of New Incursions
2.6. Physical Control
2.7. Chemical Control
2.8. Biological Control
2.9. Public Awareness
2.10. Public Engagement
3. Conclusions
- (1)
- The trade in invasive and potentially invasive cactus species SHOULD BE regulated, taking into consideration their classification in five broad risk categories: known threat, likely threat, invasion limited by climate, invasion unlikely, and species with a track record of no invasions (Table 1). This is possible as the ornamental trade is currently by far the major pathway for the introduction of new cactus species to areas outside their native range. Moreover, invasiveness in the Cactaceae appears to vary predictably, such that certain traits are of sufficient diagnostic value to allow for the identification and flagging of high-risk species (e.g., at ports of entry).
- (2)
- National strategies and action plans consider developing efforts to detect cactus invasions early in the invasion process and eradicate them using physical or chemical control before they become widespread. The use of herbicide is often the most cost-effective management option for such small and localised populations.
- (3)
- Managers with land with extensive and/or abundant cactus invasions should use biological control agents to reduce the abundance, density and impacts of widespread cactus invasions in a manner sensitive to native species and the needs of cactus users.
- (4)
- Policies and actions are implemented to promote public awareness and engagement activities to integrate available knowledge and all perspectives in the process of developing and implementing management actions, and to deal with potential conflicts of interest, especially in the agricultural and ornamental horticulture sectors.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Van Kleunen, M.; Dawson, W.; Essl, F.; Pergl, J.; Winter, M.; Weber, E.; Kreft, H.; Weigelt, P.; Kartesz, J.; Nishino, M.; et al. Global exchange and accumulation of non-native plants. Nature 2015, 525, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Pyšek, P.; Pergl, J.; Essl, F.; Lenzner, B.; Dawson, W.; Kreft, H.; Weigelt, P.; Winter, M.; Kartesz, J.; Nishino, M.; et al. Naturalized alien flora of the world: Species diversity, taxonomic and phylogenetic patterns, geographic distribution and global hotspots of plant invasion. Preslia 2017, 89, 203–274. [Google Scholar] [CrossRef]
- Pimentel, D.; Lach, L.; Zuniga, R.; Morrison, D. Environmental and economic costs of nonindigenous species in the United States. Bioscience 2000, 50, 53–65. [Google Scholar] [CrossRef]
- Richardson, D.M. Fifty Years of Invasion Ecology: The Legacy of Charles Elton; John Wiley & Sons Ltd.: Oxford, UK, 2011; ISBN 9781444335859. [Google Scholar]
- Simberloff, D.; Rejmánek, M. Encyclopedia of Biological Invasions; University of California Press: Berkeley, CA, USA, 2011; ISBN 9780520264212. [Google Scholar]
- Vilà, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarošík, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pyšek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef]
- Bacher, S.; Blackburn, T.M.; Essl, F.; Genovesi, P.; Heikkilä, J.; Jeschke, J.M.; Jones, G.; Keller, R.; Kenis, M.; Kueffer, C.; et al. Socio-economic impact classification of alien taxa (SEICAT). Methods Ecol. Evol. 2018, 9, 159–168. [Google Scholar] [CrossRef]
- Brondizio, E.S.; Settele, J.; Díaz, S.; Ngo, H.T. Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2019; ISBN 978-3-947851-08-9. [Google Scholar]
- Pyšek, P.; Richardson, D.M. Invasive species, environmental change and management, and health. Annu. Rev. Environ. Resour. 2010, 35, 25–55. [Google Scholar] [CrossRef]
- Hyatt, M.W. Investigation of Crayfish Control Technology; Arizona Game and Fish Department: Phoenix, AZ, USA, 2004; ISBN 1448-20181-02-J850.
- van Wilgen, B.W.; Dyer, C.; Hoffmann, J.H.; Ivey, P.; Le Maitre, D.C.; Moore, J.L.; Richardson, D.M.; Rouget, M.; Wannenburgh, A.; Wilson, J.R.U. National-scale strategic approaches for managing introduced plants: Insights from Australian acacias in South Africa. Divers. Distrib. 2011, 17, 1060–1075. [Google Scholar] [CrossRef]
- Novoa, A.; Richardson, D.M.; Pyšek, P.; Meyerson, L.A.; Bacher, S.; Canavan, S.; Caford, J.A.; Čuda, J.; Essl, F.; Foxcroft, L.; et al. Invasion syndromes: A systematic approach for predicting biological invasions and facilitating effective management. Biol. Inv. 2019, in press. [Google Scholar]
- Richardson, D.M.; van Wilgen, B.W.; Nunez, M.A. Alien conifer invasions in South America: Short fuse burning? Biol. Inv. 2008, 10, 573–577. [Google Scholar] [CrossRef]
- Simberloff, D.; Nunez, M.A.; Ledgard, N.J.; Pauchard, A.; Richardson, D.M.; Sarasola, M.; Van Wilgen, B.W.; Zalba, S.M.; Zenni, R.D.; Bustamante, R.; et al. Spread and impact of introduced conifers in South America: Lessons from other southern hemisphere regions. Austral. Ecol. 2010, 35, 489–504. [Google Scholar] [CrossRef]
- Novoa, A.; Le Roux, J.J.; Robertson, M.P.; Wilson, J.R.U.; Richardson, D.M. Introduced and invasive cactus species—A global review. AoB Plants 2015, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Edwards, E.J.; Nyffeler, R.; Donoghue, M.J. Basal cactus phylogeny: Implications of Pereskia (Cactaceae) paraphyly for the transition to the cactus life form. Am. J. Bot. 2005, 92, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Rebman, J.P.; Pinkava, D.J. Opuntia cacti of North America: An overview. Fla. Entomol. 2001, 84, 474–483. [Google Scholar] [CrossRef]
- Novoa, A.; Kaplan, H.; Wilson, J.R.U.; Richardson, D.M. Resolving a prickly situation: Involving stakeholders in invasive cactus management in South Africa. Environ. Manag. 2016, 57, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Walters, M.; Figueiredo, E.; Crouch, N.R.; Winter, P.J.; Smith, G.; Zimmermann, H.G.; Mashope, B.K. Naturalised and Invasive Succulents of Southern Africa; ABC Taxa and the Belgian Development Cooperation: Brussels, Belgium, 2011; ISSN 1784-1283. [Google Scholar]
- Novoa, A.; Kumschick, S.; Richardson, D.M.; Rouget, M.; Wilson, J.R.U. Native range size and growth form in Cactaceae predict invasiveness and impact. NeoBiota 2016, 30, 75–90. [Google Scholar] [CrossRef]
- Winston, R.L.; Schwarzländer, M.; Hinz, H.L.; Day, M.D.; Cock, M.J.W.; Julien, M.H. Biological Control of Weeds: A World Catalogue of Agents and Their Target Weeds, 5th ed.; USDA Forest Service, Forest Health Technology Enterprise Team: Morgantown, VA, USA, 2014; ISBN 20153151446.
- Raghu, S.; Walton, C. Understanding the ghost of Cactoblastis past: Historical clarifications on a poster child of classical biological control. Bioscience 2007, 57, 699–705. [Google Scholar] [CrossRef]
- Paterson, I.D.; Hoffmann, J.H.; Klein, H.; Mathenge, C.W.; Neser, S.; Zimmermann, H.G. Biological Control of Cactaceae in South Africa. Afr. Entomol. 2011, 19, 230–246. [Google Scholar] [CrossRef]
- Kaplan, H.; Wilson, J.R.U.; Klein, H.; Henderson, L.; Zimmermann, H.G.; Manyama, P.; Ivey, P.; Richardson, D.M.; Novoa, A. A proposed national strategic framework for the management of Cactaceae in South Africa. Bothalia 2017, 47, 1–12. [Google Scholar] [CrossRef]
- Daehler, C.C.; van Kleunen, M.; Pyšek, P.; Richardson, D.M. EMAPi 2015: Highlighting links between science and management of alien plant invasions. NeoBiota 2016, 30, 1–3. [Google Scholar] [CrossRef]
- Pyšek, P.; Brundu, G.; Brock, J.; Child, L.; Wade, M. Twenty-five years of conferences on the Ecology and Management of Alien Plant invasions: The history of EMAPi 1992–2017. Biol. Inv. 2018, 1–18. [Google Scholar] [CrossRef]
- Pheloung, P.C.; Williams, P.A.; Halloy, S.R. A weed risk assessment model for use as a biosecurity tool evaluating plant introductions. J. Environ. Manag. 1999, 57, 239–251. [Google Scholar] [CrossRef]
- Kumschick, S.; Richardson, D.M. Species-based risk assessments for biological invasions: Advances and challenges. Divers. Distrib. 2013, 19, 1095–1105. [Google Scholar] [CrossRef]
- Weber, E.; Gut, D. Assessing the risk of potentially invasive plant species in central Europe. J. Nat. Conserv. 2004, 12, 171–179. [Google Scholar] [CrossRef]
- Andreu, J.; Vila, M. Risk analysis of potential invasive plants in Spain. J. Nat. Conserv. 2010, 18, 34–44. [Google Scholar] [CrossRef]
- Ducatillion, C.; Badeau, V.; Bellanger, R.; Buchlin, S.; Diadema, K.; Gili, A.; Thevenet, J. Détection précoce du risque d’invasion par des espèces végétales exotiques introduites en arboretum forestier dans le Sud-Est de la France. Émergence des espèces du genre Hakea. Mesures de gestion. Rev. Ecol. Terre Vie 2015, 70, 139–150. [Google Scholar]
- Anderson, E.F. The Cactus Family; Timber Press: Portland, OR, USA, 2001; ISBN 978-0881924985. [Google Scholar]
- Novoa, A.; Kaplan, H.; Kumschick, S.; Wilson, J.R.U.; Richardson, D.M. Soft touch or heavy hand? Legislative approaches for preventing invasions: Insights from Cacti in South Africa. Invasive Plant. Sci. Manag. 2015, 8, 307–316. [Google Scholar] [CrossRef]
- Novoa, A.; Le Roux, J.J.; Richardson, D.M.; Wilson, J.R.U. Level of environmental threat posed by horticultural trade in Cactaceae. Conserv. Biol. 2017, 31, 1066–1075. [Google Scholar] [CrossRef]
- Dehnen-Schmutz, K. Determining non-invasiveness in ornamental plants to build green lists. J. Appl. Ecol. 2011, 48, 1374–1380. [Google Scholar] [CrossRef]
- Batianoff, G.N.; Butler, D.W. Assessment of invasive naturalized plants in south-east Queensland. Plant Prot. Q. 2002, 17, 27–34. [Google Scholar]
- Blackburn, T.M.; Pyšek, P.; Bacher, S.; Carlton, J.T.; Duncan, R.P.; Jarošík, V.; Richardson, D.M. A proposed unified framework for biological invasions. Trends Ecol. Evolut. 2011, 26, 333–339. [Google Scholar] [CrossRef]
- Wilson, J.R.U.; Gairifo, C.; Gibson, M.R.; Arianoutsou, M.; Bakar, B.B.; Baret, S.; Celesti-Grapow, L.; DiTomaso, J.M.; Dufour-Dror, J.M.; Kueffer, C.; et al. Risk assessment, eradication, and biological control: Global efforts to limit Australian acacia invasions. Divers. Distrib. 2011, 17, 1030–1046. [Google Scholar] [CrossRef]
- Novoa, A.; Shackleton, R.; Canavan, S.; Cybele, C.; Davies, S.J.; Dehnen-Schmutz, K.; Fried, J.; Gaertner, M.; Geerts, S.; Griffiths, C.L.; et al. A framework for engaging stakeholders on the management of alien species. J. Environ. Manag. 2018, 205, 286–297. [Google Scholar] [CrossRef] [PubMed]
- McGeoch, M.A.; Genovesi, P.; Bellingham, P.J.; Costello, M.J.; McGrannachan, C.; Sheppard, A. Prioritizing species, pathways, and sites to achieve conservation targets for biological invasion. Biol. Inv. 2016, 18, 299–314. [Google Scholar] [CrossRef]
- Foxcroft, L.C.; Spear, D.; van Wilgen, N.J.; McGeoch, M.A. Assessing the association between pathways of alien plant invaders and their impacts in protected areas. NeoBiota 2019, 43, 1–25. [Google Scholar] [CrossRef]
- Howard, R.A.; Touw, M. The cacti of the Lesser Antilles and the typification of the genus Opuntia Miller. Cactus Succul. J. 1981, 53, 233–237. [Google Scholar]
- Le Houérou, H.N. The role of cacti (Opuntia spp.) in erosion control, land reclamation, rehabilitation and agricultural development in the Mediterranean Basin. J. Arid Environ. 1996, 33, 135–159. [Google Scholar] [CrossRef]
- Griffith, M.P. The Origins of an Important Cactus Crop, Opuntia ficus-indica (Cactaceae): New Molecular Evidence. Am. J. Bot. 2004, 11, 1915–1921. [Google Scholar] [CrossRef]
- Nefzaoui, A.P.; El Mourid, M. Cactus: A crop to meet the challenges of climate change in dry area. In Proceedings of the 10th International Conference on Development of Drylands, Cairo, Egypt, 12–15 December 2010. [Google Scholar]
- Zimmermann, H.G.; Granata, G. Insect pests and diseases. In Cacti: Biology and Uses; Nobel, P.S., Ed.; University of California Press: Berkeley, CA, USA, 2002; pp. 235–254. ISBN 0-520-23157-0. [Google Scholar]
- Anderson, N.O.; Olsen, R.T. A vast array of beauty: The accomplishments of the father of American ornamental breeding, Luther Burbank. HortScience 2015, 50, 161–188. [Google Scholar] [CrossRef]
- Novoa, A.; Flepu, V.; Boatwright, J.S. Is spinelessness a stable character in cactus pear cultivars? Implications for invasiveness. J. Arid Environ. 2019, 160, 11–16. [Google Scholar] [CrossRef]
- Essl, F.; Kobler, J. Spiny invaders—Patterns and determinants of cacti invasion in Europe. Flora 2009, 204, 485–494. [Google Scholar] [CrossRef]
- Burt, J.W.; Muir, A.A.; Piovia-Scott, J.; Veblen, K.E.; Chang, A.L.; Grossman, J.D.; Weiskel, H.W. Preventing horticultural introductions of invasive plants: Potential efficacy of voluntary initiatives. Biol. Inv. 2007, 9, 909–923. [Google Scholar] [CrossRef]
- Goettsch, B.; Hilton-Taylor, C.; Cruz-Piñón, G.; Duffy, J.P.; Frances, A.; Hernández, H.M.; Inger, R.; Pollock, C.; Schipper, J.; Superina, M.; et al. High proportion of cactus species threatened with extinction. Nat. Plants 2015, 1, 15142. [Google Scholar] [CrossRef] [PubMed]
- Olmos-Lau, V.R.; Mandujano, M.C. An open door for illegal trade: Online sale of Strombocactus disciformis (Cactaceae). J. Nat. Conserv. 2016, 15, 1–9. [Google Scholar] [CrossRef]
- Armstrong, K.F.; Ball, S.L. DNA barcodes for biosecurity: Invasive species identification. Philos Trans. R Soc. Lond. B Biol. Sci. 2005, 360, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Dunning, L.T.; Savolainen, V. Broad-scale amplification of matK for DNA barcoding plants, a technical note. Bot. J. Linn. Soc. 2010, 164, 1–9. [Google Scholar] [CrossRef]
- Adrienne, E.; Sandoval, E.; Murphy, T.M. Identification and Individualization of Lophophora using DNA Analysis of the trn L/trn F Region and rbc L Gene. J. Forensic Sci. 2016, 61, S226–S229. [Google Scholar] [CrossRef]
- Gathier, G.; van der Niet, T.; Peelen, T.; van Vugt, R.R.; Eurlings, M.C.; Gravendeel, B. Forensic identification of CITES protected slimming cactus (Hoodia) using DNA barcoding. J. Forensic Sci. 2013, 58, 1467–1471. [Google Scholar] [CrossRef]
- Novoa, A.; Rodríguez, J.; López-Nogueira, A.; Richardson, D.M.; González, L. Seed characteristics in Cactaceae: Useful diagnostic features for screening species for invasiveness? S. Afr. J. Bot. 2016, 105, 61–65. [Google Scholar] [CrossRef]
- Henderson, L.; Wilson, J.R.U. Changes in the composition and distribution of alien plants in South Africa: An update from the Southern African Plant Invaders Atlas (SAPIA). Bothalia 2017, 47, a2172. [Google Scholar] [CrossRef]
- Deltoro, V.; Gomez-Serrano, M.A.; Laguna, E.; Novoa, A. Bases Para el Control Del Cactus Invasor Cylindropuntia Pallida; Conselleria d’Infraestructures, Territori i Medi Ambient, Generalitat Valenciana: Valencia, Spain, 2014; ISBN 978-84-482-5983-9. [Google Scholar]
- Hui, C.; Foxcroft, L.C.; Richardson, D.M.; MacFadyen, S. Defining optimal sampling effort for large-scale monitoring of invasive alien plants: A Bayesian method for estimating abundance and distribution. J. Appl. Ecol. 2011, 48, 768–776. [Google Scholar] [CrossRef]
- Jarošík, V.; Pyšek, P.; Foxcroft, L.C.; Richardson, D.M.; Rouget, M.; MacFadyen, S. Predicting incursion of plant invaders into Kruger National Park, South Africa: The interplay of general drivers and species-specific factors. PLoS ONE 2011, 6, e28711. [Google Scholar] [CrossRef] [PubMed]
- Mafanya, M.; Tsela, P.; Botai, J.O.; Manyama, P.; Swart, B.; Monate, T. Evaluating pixel and object based image classification techniques for mapping plant invasions from UAV derived aerial imagery: Harrisia pomanensis as a case study. ISPRS J. Photogramm. Remote Sens. 2017, 129, 1–11. [Google Scholar] [CrossRef]
- Pocock, M.J.; Roy, H.E.; Preston, C.D.; Roy, D.B. The Biological Records Centre: A pioneer of citizen science. Biol. J. Linn. Soc. 2015, 115, 475–493. [Google Scholar] [CrossRef]
- Cardoso, A.C.; Tsiamis, K.; Gervasini, E.; Schade, S.; Taucer, F.; Adriaens, T.; Copas, K.; Flevaris, S.; Galiay, P.; Jennings, E.; et al. Citizen science and open data: A model for invasive alien species in Europe. Res. Ideas Outcomes 2017, 3, e14811. [Google Scholar] [CrossRef]
- Dehnen-Schmutz, K.; Conroy, J. Working with gardeners to identify potential invasive ornamental garden plants: Testing a citizen science approach. Biol. Inv. 2018, 20, 3069–3077. [Google Scholar] [CrossRef]
- Marchante, H.; Morais, M.C.; Gamela, A.; Marchante, E. Using a WebMapping platform to engage volunteers to collect data on invasive plants distribution. Trans. GIS 2017, 21, 238–252. [Google Scholar] [CrossRef]
- European and Mediterranean Plant Protection Organization Organisation. Guidelines for the management of plant health risks of biowaste of plant origin. EPPO Bull. 2008, 38, 4–9. [Google Scholar] [CrossRef]
- Zimmermann, H.G. Global invasions of cacti (Opuntia sp.): Control, management and conflicts of interest. In Crop Ecology, Cultivation and Uses of Cactus Pear; Inglese, P., Mondragon, C., Nefzaoui, A., Saenz, C., Eds.; Food and Agriculture Organization of the United Nations and International Center for Agricultural Research in the Dry Areas: Rome, Italy, 2017; pp. 171–184. ISBN 978-92-5-109860-8. [Google Scholar]
- Grobler, M. Control of Unwanted Plants; XACT Information: Pretoria, South Africa, 2005. [Google Scholar]
- Ashton, F.M.; Crafts, A.S. Mode of Action of Herbicides; John Wiley and Sons: New York, NY, USA, 1981; ISBN 047104847X. [Google Scholar]
- Dodd, A.P. The Biological Campaign Against Prickly Pear; Commonwealth Prickly Pear Board Bulletin; Government Printer: Brisbane, Australia, 1940; ISBN 1-86849-176-5.
- Annecke, D.P.; Moran, V.C. Critical reviews of biological pest control in South Africa. The prickly pear, Opuntia ficus-indica (L) Miller. J. Entomol. Soc. S. Afr. 1978, 41, 161–188. [Google Scholar] [CrossRef]
- Moran, V.C.; Annecke, D.P. Critical reviews of biological pest control in South Africa. The jointed cactus, Opuntia aurantiaca Lindley. J. Entomol. Soc. S. Afr. 1979, 42, 299–329. [Google Scholar] [CrossRef]
- Guyton, K.Z.; Loomis, D.; Grosse, Y.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Scoccianti, C.; Mattock, H.; Straif, K.; International Agency for Research on Cancer Monograph Working Group, I.L.F. Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet Oncol. 2015, 16, 490–491. [Google Scholar] [CrossRef]
- Osunkoya, O.O.; Froese, J.G.; Nicol, S. Management feasibility of established invasive plant species in Queensland, Australia: A stakeholders’ perspective. J. Environ. Manag. 2019, 246, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Zachariades, C.; Paterson, I.D.; Strathie, L.W.; Hill, M.P.; Van Wilgen, B.W. Assessing the status of biological control as a management tool for suppression of invasive alien plants in South Africa. Bothalia 2017, 47, 142. [Google Scholar] [CrossRef]
- Zachariades, C. Biological Control of Invasive Alien Plants in South Africa: A List of All Insects, Mites and Pathogens Released as Biological Control Agents from 1913–2018. Available online: http://www.arc.agric.za/arc-ppri/Documents/Table2-NaturalEnemiesReleased.pdf (accessed on 23 August 2019).
- Mann, J. Cactus−Feeding Insects and Mites; United States National Museum: Washington, DC, USA, 1969; ISBN 1333075251. [Google Scholar]
- Moran, V.C. Interactions between phytophagous insects and their Opuntia hosts. Ecol. Entomol. 1980, 5, 153–164. [Google Scholar] [CrossRef]
- Olckers, T.; Hill, M.P. Biological Control of Weeds in South Africa, 1990–1998 (African Entomology Memoir); Entomological Society of Southern Africa: Pretoria, South Africa, 1999; ISBN 0620242914. [Google Scholar]
- Lounsbury, C.P. Plant killing insects: The Indian cochineal. Agric. J. Union S. Afr. 1915, 1, 537–543. [Google Scholar]
- Zimmermann, H.G.; Moran, V.C.; Hoffmann, J.H. Invasive cactus species (Cactaceae). In Biological Control of Tropical Weeds Using Arthropods; Muniappan, R., Reddy, G.V.P., Raman, A., Eds.; Cambridge University Press: Cambridge, UK, 2009; pp. 108–129. ISBN 9780521877916. [Google Scholar]
- Hosking, J.R. Opuntia spp. In Biological Control of Weeds in Australia; Julien, M., McFadyen, R., Cullen, J., Eds.; CSIRO Publishing: Collingwood, Australia, 2012; pp. 431–436. ISBN 9780643104204. [Google Scholar]
- Page, A.R.; Lacey, K.L. Economic Impact assessment of Australian Weed Biological Control; AEC group and Australian Weed Management: Adelaide, Australia, 2006; ISBN 9781920932558.
- Klein, H. A catalogue of the insects, mites and pathogens that have been used or rejected, or are under consideration, for the biological control of invasive alien plants in South Africa. Afr. Entomol. 2011, 19, 515–549. [Google Scholar] [CrossRef]
- Barbetta, M. Classical Weed Biological Control Outcomes: A Catalogue-based Analysis of Success Rates and Their Correlates. Master’s Thesis, University of Toronto, Toronto, ON, Canada, 2018. [Google Scholar]
- van Wilgen, B.W.; De Wit, M.P.; Anderson, H.J.; Le Maitre, D.C.; Kotze, I.M.; Ndala, S.; Brown, B.; Rapholo, M.B. Costs and benefits of biological control of invasive alien plants: Case studies from South Africa. S. Afr. J. Sci. 2004, 100, 113–122. [Google Scholar] [CrossRef]
- Zimmermann, H.G.; Moran, V.C. Biological control of prickly pear, Opuntia ficus-indica (Cactaceae), in South Africa. Agr. Ecosyst. Environ. 1991, 37, 29–35. [Google Scholar] [CrossRef]
- Paterson, I.D.; Manheimmer, C.A.; Zimmermann, H.G. Prospects for biological control of cactus weeds in Namibia. Biocontrol. Sci. Techn. 2019, 29, 393–399. [Google Scholar] [CrossRef]
- Henderson, L. The Southern African Plant Invaders Atlas (SAPIA) and its contribution to biological weed control. Afr. Entomol. Memoir. 1999, 1, 159–163. [Google Scholar]
- Whittaker, A. Hypogeococcus Pungens (Cactus Mealybug). Available online: https://www.cabi.org/isc/datasheet/110614 (accessed on 23 August 2019).
- Marchante, H.; Marchante, E. Engaging society to fight invasive alien plants in Portugal—One of the main threats to biodiversity. In Biodiversity and Education for Sustainable Development; Castro, P., Azeiteiro, U.M., Bacelar-Nicolau, P., Leal Filho, W., Azul, A.M., Eds.; Springer: Basel, Switzerland, 2016; pp. 107–122. ISBN 3319323180, 9783319323183. [Google Scholar]
- Sheehan, M.R.; Potter, S. Managing Opuntioid Cacti in Australia: Best Practice Control Manual for Austrocylindropuntia, Cylindropuntia and Opuntia Species; Department of Primary Industries and Regional Development: Perth, Australia, 2017; ISBN 9780992308377.
- Shackleton, R.T.; Adriaens, T.; Brundu, G.; Dehnen-Schmutz, K.; Estévez, R.; Fried, J.; Larson, B.M.H.; Liu, S.; Marchante, E.; Marchante, H.; et al. Stakeholder engagement in the study and management of invasive alien species. J. Environ. Manag. 2018, 229, 88–101. [Google Scholar] [CrossRef]
- Faulkner, K.T.; Hurley, B.P.; Robertson, M.P.; Rouget, M.; Wilson, J.R.U. The balance of trade in alien species between South Africa and the rest of Africa. Bothalia 2017, 47, a2157. [Google Scholar] [CrossRef]
- Latombe, G.; Pyšek, P.; Jeschke, J.M.; Blackburn, T.M.; Bacher, S.; Capinha, C.; Costello, M.J.; Fernández, M.; Gregory, R.D.; Hobern, D.; et al. A vision for global monitoring of biological invasions. Biol. Cons. 2017, 213, 295–308. [Google Scholar] [CrossRef]
- Packer, J.G.; Meyerson, L.A.; Richardson, D.M.; Brundu, G.; Allen, W.J.; Bhattarai, G.P.; Brix, H.; Canavan, S.; Castiglione, S.; Cicatelli, A.; et al. Global networks for invasion science: Benefits, challenges and guidelines. Biol. Inv. 2017, 19, 1081–1096. [Google Scholar] [CrossRef]
| Categories | Criteria | Recommendations |
|---|---|---|
| Known threat | Species is known to be invasive AND The introduced range has a suitable climate |
|
| Likely threat | Species is not currently recorded as invasive AND Species has detachable segments and/or spines and a large native range AND The introduced range has a suitable climate |
|
| Invasion limited by climate | [Species is known to be invasive AND/OR Species has detachable segments and/or spines and a large native range] AND The introduced range does not have a suitable climate |
|
| Invasion unlikely | Species is not currently recorded as invasive AND Species does not have detachable segments and/or spines or does not have a large native range AND The introduced range does not have a suitable climate |
|
| Species with a track record of no invasions | Species is not currently recorded as invasive despite having been planted in climatically suitable introduced ranges for over 50 years |
|
| Country | Area | Taxa Regulated (Name as in the Text) | Policy | Notes |
|---|---|---|---|---|
| Australia | National | Opuntia spp. (with the exception of O. ficus-indica), Cylindropuntia spp. and Austrocylindropuntia spp. | Weeds of National Significance (WoNS) | Indicates that the state and territory governments are responsible for their legislation, regulation and administration |
| Australia | Queensland | Opuntia spp. (with the exception of O. ficus-indica), Cylindropuntia spp. and Austrocylindropuntia spp. | Land Protection (Pest and Stock Route Management) Act 2002 | Regulates their introduction, use and management |
| Australia | New South Wales | Opuntia spp. (with the exception of O. ficus-indica), Cylindropuntia spp. and Austrocylindropuntia spp. | Biosecurity Act 2015 | Regulates their introduction, use and management |
| Australia | Northern Territory | Opuntia spp. (with the exception of O. ficus-indica), Cylindropuntia spp. and Austrocylindropuntia spp. | Weeds Management Act 2013 | Regulates their introduction, use and management |
| Australia | South Australia | Opuntia spp. (with the exception of O. ficus-indica), Cylindropuntia spp. and Austrocylindropuntia spp. | Natural Resources Management Act 2004 | Regulates their introduction, use and management |
| Australia | Victoria | Opuntia spp. (with the exception of O. ficus-indica), Cylindropuntia spp. and Austrocylindropuntia spp. | Catchment and Land Protections Act 1994 | Regulates their introduction, use and management |
| Australia | Western Australia | Opuntia spp. (with the exception of O. ficus-indica), Cylindropuntia spp. and Austrocylindropuntia spp. | Biosecurity and Agricultural Management Act 2007 | Regulates their introduction, use and management |
| Botswana | National | Opuntia aurantiaca and Opuntia imbricata (Haw) | Noxious Weeds Order (Chapter 35:04). Consolidated version of S.I. No. 49 of 1968 as at 31 December 2013 and amended by S.I. No. 84 of 1976 | Declared as noxious weeds |
| Kenya | National | Opuntia inermis and Opuntia stricta | Plant Protection Order, 1961. Consolidated version of 2012 of L.N.744/19661 as amended last by L.N. 130/1990 | Declared as pest plants |
| Italy | Tuscany region | Opuntia ficus-indica | Regional Act No. 56 making provision for the conservation and the protection of natural and seminatural habitats, flora and wildlife and laying down amendments to Regional Act No. 7 of 23 January 1998 and to Regional Act No. 49 of 11 April 1995. | Opuntia ficus-indica cannot be planted in the natural environment |
| Portugal | National | All cacti except Opuntia ficus-indica (L.) Miller | Decree-Law No. 565/99 regulating the introduction of exotic flora and fauna species | States that the regulated species cannot be introduced without undertaking a detailed risk assessment showing the lack of risk of invasion |
| Portugal | National | Opuntia elata Salm - Dyck, Opuntia maxima Miller and Opuntia subulata (Muehlenpf.) Engelm (= Austrocylindropuntia subulata) are listed as invasive (National List of Invasive Species) in the Mainland and Madeira and Azores Archipelagos; Opuntia tuna (L.) Mill. is listed only in Madeira. Opuntia ficus-indica (L.) Miller is listed under an exception regime whereby growers and nursery workers must comply with the duties of care and reporting, as well as control plans. The introduction into the wild of any other cactus species is subject to authorisation by the nature conservation authority (ICNF). | Decree-Law No. 92/2019 regulates the control, keeping, introduction into nature and restocking of exotic species and implementation at the national level of Regulation (EU) No. 1143/2014, on the prevention and management of the introduction and spread of invasive alien species. | Species listed as invasive, except Opuntia ficus-indica, which is included in an exceptional regime, cannot be detained, cultivated, traded, introduced into the wild and repopulated. |
| South Africa | National | Thirty-five cactus species are regulated as invasive in the country [33], as well as most of their congeners | National Environmental Management: Biodiversity Act: List of invasive species (No. 599 of 2014). It implements the National Environmental Management Biodiversity Act, 2004 (No. 10 of 2004). 2004-05-31 “Alien and Invasive Species Regulations” | Regulates their introduction and use |
| Spain | National | Cylindropuntia spp., Opuntia dillenii (Ker-Gawler) Haw Opuntia maxima Miller., and Opuntia stricta (Haw.) | Real Decreto No. 630/2013 - Regula el Catálogo español de especies exóticas invasoras. | Regulates their introduction and use |
| Uganda | National | Opuntia spp. | Plant Protection (Importation of Plants) Order (S.I. 31-3); consolidated version of Statutory Instrument 3 of Cap. 31, History: S.I. 244-3 | Prohibited plants and seeds |
| USA | National | Opuntia aurantiaca Lindley | Noxious Weed Regulations (7 CFR 360.100-360.600); consolidated version as at 1 January 2018 | Designated as a noxious weed (to prevent their introduction into the United States or their dissemination within the United States). |
| Vanuatu | National | Opuntia spp. | Prevention of Spread of Noxious Weeds Act (Cap. 44). | Declared as a noxious weed |
| Zambia | National | Opuntia spp., including spineless cactus, vegetative material, seed and fruit of, for propagation | Plant Pests and Diseases (Importation) Regulations (Cap. 233); consolidated version of F.G.N. No. 144 of 1960 as at 2006 and amended last by G.N. No. 497 of 1964 | These Regulations provide for the control of the importation of plants and related items for purposes of plant health. It puts a total ban on Opuntia spp. |
| Zimbabwe | National | Opuntia aurantiaca Lindl. | Environmental Management Act (Chapter 20:27); consolidated version of Act No. 13 of 2002 as amended by Act No. 6 of 2005 with effect as from 1 July 2005 | Declared as a noxious weed |
| Herbicide | Concentration of the Active Ingredient | Dilution | Application | Notes |
|---|---|---|---|---|
| Amitrole | 250 g/L amitrole and 220 g/L ammonium thiocyanate | 1:25 in water | Foliar spray | Expensive but efficient |
| Glyphosate | 450 g/L glyphosate | 1:3 in water | Stem injection | Inject 4 mL per cladode |
| Metsulfuron-methyl | 600 g/L metsulfuron-methyl | 0.03:100 in water | Foliar spray | |
| MSMA | 800 g/L MSMA | 2.5:100 in water | Foliar spray | |
| No dilution | Stem injection | Inject 4 mL per meter of plant height per stem branch | ||
| Triclopyr | 600 g/L Triclopyr | 3:100 in water or 1.5:100 in diesel | Foliar spray | The diesel mix often yields better results |
| Triclopyr and Picloram | 240 g/L Triclopyr and 120 g/L Picloram | 1:60 in diesel | Foliar spray | |
| Triclopyr and Picloram | 300 g/L Triclopyr and 100 g/L Picloram | 1:100 in water | Foliar spray | |
| Triclopyr, Picloram and Aminopyralid | 300 g/L Triclopyr, 100 g/L Picloram and 9g/L Aminopyralid | 1:100 in water | Foliar spray | |
| Triclopyr and Fluroxipir | 30 g/L Fluroxipir + 90 g/L Triclopir | 1:100 in water | Foliar spray | Efficiency declines with cactus size. Requires several applications for a complete kill of large specimens. Expensive. |
| Host Species | Biocontrol Agent | Feeding Guild | Establishment | Country of Release | Severity of Damage to the Host Plant |
|---|---|---|---|---|---|
| Acanthocereus tetragonus (L.) Hummelinck | Hypogeococcus festerianus (Lizer y Trelles) | Stem sucker | Yes | Australia | Moderate |
| Nealcidion cereicola (Fisher) | Stem borer | No | Australia | NA | |
| Austrocylindropuntia subulata (Muehlenpf.) Backeb | Cactoblastis cactorum (Berg) | Cladode borer | Yes | South Africa | Unknown |
| Cereus hildmannianus K. Schum. | Hypogeococcus festerianus (Lizer y Trelles) | Stem sucker | Yes | South Africa | Extensive |
| Nealcidion cereicola (Fisher) | Stem borer | Yes | South Africa | Considerable | |
| Cereus hildmannianus K. Schum subsp. Uruguayensis | Hypogeococcus festerianus (Lizer y Trelles) | Stem sucker | Yes | Australia | Too early to determine |
| Cereus jamacaru DC. | Hypogeococcus festerianus (Lizer y Trelles) | Stem sucker | Yes | South Africa | Considerable |
| Nealcidion cereicola (Fisher) | Stem borer | Yes | South Africa | Considerable | |
| Cylindropuntia fulgida (Engelm.) F.M. Knuth var. fulgida | Dactylopius tomentosus (Lamark), “cholla” biotype | Cladode sucker | Yes | South Africa Zimbabwe | Extensive |
| Dactylopius tomentosus (Lamark), “imbricata” biotype | Cladode sucker | Yes | South Africa | Trivial | |
| Cylindropuntia fulgida (Engelm.) F.M. Knuth var. mamillata | Dactylopius tomentosus (Lamark), “cholla” biotype | Cladode sucker | Yes | Australia Namibia South Africa Zimbabwe | Extensive |
| Cylindropuntia imbricata (Haw.) F.M. Knuth | Cactoblastic cactorum (Berg) | Cladode sucker | Yes | South Africa | Trivial |
| Dactylopius tomentosus (Lamark), “imbricata” biotype | Cladode sucker | Yes | Botswana Namibia South Africa | Considerable | |
| Australia | Considerable | ||||
| Dactylopius tomentosus “cylindropuntia sp.” biotype | Cladode sucker | Unknown | Australia | Too early to determine | |
| Metamasius spinolae (Gyllenhal) | Stem borer | No | South Africa | - | |
| Cylindropuntia kleiniae (DC.) F.M. Knuth | Dactylopius tomentosus (Lamark), “imbricata” biotype | Cladode sucker | Yes | Australia | Considerable |
| Cylindropuntia leptocaulis (DC.) F.M. Knuth | Dactylopius tomentosus (Lamark), “imbricata” biotype | Cladode sucker | Yes | Australia | Considerable |
| South Africa | Extensive | ||||
| Cylindropuntia rosea (DC.) Backeb = Cylindropuntia pallida (Rose) F.M. Knuth | Dactylopius tomentosus (Lamark), “imbricata” biotype | Cladode sucker | Yes | Australia | Trivial |
| Dactylopius tomentosus “califórnica var. parkeri” biotype | Cladode sucker | Yes | Australia | Too early to determine | |
| Cylindropuntia prolifera | Dactylopius tomentosus “califórnica var. parkeri” biotype | Cladode sucker | Yes | Australia | Too early to determine |
| Cylindropuntia spinosior | Dactylopius tomentosus “bigelovii” biotype | Cladode sucker | Unknown | Australia | Too early to determine |
| Harrisia balansae (K. Schum.) N.P. Taylor & Zappi = Harrisia bonplandii (Pfeiff.) Britton and Rose | Hypogeococcus festerianus (Lizer y Trelles) | Stem sucker | Yes | South Africa | Considerable |
| Harrisia martinii (Labour.) Britton and Rose | Eriocereophaga humeridens O’Brien | Cactus feeder | No | Australia | - |
| Hypogeococcus festerianus (Lizer y Trelles) | Stem sucker | Yes | Australia South Africa | Considerable | |
| Nealcidion cereicola (Fisher) | Stem borer | Yes | South Africa | Moderate | |
| Harrisia pomanensis | Hypogeococcus festerianus (Lizer y Trelles) | Stem sucker | Yes | South Africa | Considerable |
| Harrisia regelii (Weing.) Borg | Hypogeococcus festerianus (Lizer y Trelles) | Stem sucker | Yes | Australia | Considerable |
| Nealcidion cereicola (Fisher) | Stem borer | Yes | Australia | None | |
| Harrisia tortuosa (J. Forbes ex Otto and A. Dietr.) Britton and Rose | Hypogeococcus festerianus (Lizer y Trelles) | Stem sucker | Yes | Australia South Africa | Considerable |
| Nealcidion cereicola (Fisher) | Stem borer | Yes | Australia | None | |
| Hylocereus undata (Haw.) Britton and Rose | Hypogeococcus festerianus (Lizer y Trelles) | Stem galler | Unknown | South Africa | Unknown |
| Opuntia aurantiaca Lindl. | Cactoblastis cactorum (Berg) | Cladode sucker | Yes | Australia South Africa | Moderate |
| Dactylopius austrinus De Lotto | Cladode sucker | Yes | Australia South Africa | Considerable | |
| Dactylopius ceylonicus (Green) | Cladode sucker | No | Australia | - | |
| Melitara prodenialis Walker | Cladode borer | No | Australia | - | |
| Mimorista pulchellalis Dyar | Cladode borer | No | South Africa | - | |
| Nanaia sp. | Cladode borer | No | South Africa | - | |
| Zophodia tapiacola (Dyar) | Cladode borer | Yes | Australia | Moderate | |
| No | South Africa | - | |||
| Tucumania tapiacola Dyar | Cladode borer | Yes | Australia | Trivial | |
| No | South Africa | - | |||
| Opuntia elata Link and Otto ex S-D | Dactylopius ceylonicus (Green) | Cladode sucker | Yes | Australia | Trivial |
| Opuntia elatior Mill. | Dactylopius ceylonicus (Green) | Cladode sucker | No | India | - |
| Dactylopius opuntiae (Cockerell) | Cladode sucker | Yes | India Indonesia | Extensive | |
| Opuntia engelmannii Salm = Dyck ex Engelm. | Cactoblastis cactorum (Berg) | Cladode sucker | Yes | Antigua South Africa | Extensive |
| No | Federation of St Kitts and Nevis | - | |||
| Dactylopius opuntiae (Cokerell), “ficus-indica” biotype | Cladode sucker | Yes | Australia South Africa | Trivial to moderate | |
| No | Federation of St Kitts and Nevis | - | |||
| Opuntia ficus-indica (L.) Mill. | Lagocheirus funestus (Thomson) | Stem borer | Yes | Hawaii (USA) | Considerable |
| South Africa | Trivial | ||||
| Cactoblastis cactorum (Berg) | Cladode borer | Yes | Australia Hawaii (USA) Mauritius Puerto Rico (USA) South Africa U.S. Virgin Islands (USA) | Considerable | |
| Dactylopius opuntiae (Cokerell), “ficus-indica” biotype | Cladode sucker | Yes | Hawaii (USA) South Africa Spain | Considerable | |
| Australia | Moderate | ||||
| Fusarium oxysporum Schlecktendahl | Unknown | Yes | Hawaii (USA) | Unknown | |
| Lagocheirus funestus (Thompson) | Stem borer | Yes | Hawaii (USA) South Africa | Trivial | |
| Melitara dentata (Grote) | Cactus feeder | No | Hawaii (USA) | - | |
| Melitara prodenialis Walker | Cladode borer | No | Hawaii (USA) | - | |
| Metamasius spinolae (Gyllenhal) | Stem borer | Yes | South Africa | Considerable | |
| Moneilema armatum LeConte | Cladode and root borer | No | Hawaii (USA) | - | |
| Opuntia humifusa (Raf.) Raf. | Cactoblastis cactorum (Berg) | Cladode borer | Yes | South Africa | Trivial |
| Dactylopius opuntiae (Cokerell), “stricta” biotype | Cladode sucker | Yes | South Africa | Extensive | |
| Opuntia littoralis (Engelm.) Cockerell | Chelinidea tabulata (Burmeister) | Unknown | Yes | USA | None |
| Chelinidea vittiger Uhler | Unknown | Yes | USA | None | |
| Dactylopius confusus (Cockerell) | Cladode sucker | No | USA | - | |
| Dactylopius opuntiae (Cockerell) | Cladode sucker | Yes | USA | Extensive | |
| Dactylopius tomentosus (Lamark) | Cladode sucker | No | USA | - | |
| Melitara prodenialis Walker | Cladode borer | No | USA | - | |
| Olycella junctolineella (Hulst) | Cladode borer | No | USA | - | |
| Opuntia monocantha Haw. | Cactoblastis cactorum (Berg) | Cladode borer | Yes | Cuba Mauritius | Considerable |
| South Africa | Trivial | ||||
| Dactylopius ceylonicus (Green) | Cladode sucker | Yes | Australia India Madagascar Mauritius South Africa Sri Lanka Tanzania | Extensive | |
| Kenya | Moderate | ||||
| Dactylopius confusus (Cockerell) | Cladode sucker | No | Australia India South Africa | - | |
| Dactylopius opuntiae (Cockerell) | Cladode sucker | Yes | Mauritius | Considerable | |
| Opuntia oricola Philbrick | Chelinidea tabulata (Burmeister) | Unknown | Yes | USA | None |
| Chelinidea vittiger Uhler | Unknown | Yes | USA | None | |
| Dactylopius confusus (Cockerell) | Cladode sucker | No | USA | - | |
| Dactylopius opuntiae (Cockerell) | Cladode sucker | Yes | USA | Moderate | |
| Dactylopius tomentosus (Lamark) | Cladode sucker | No | USA | - | |
| Melitara prodenialis Walker | Cladode borer | No | USA | - | |
| Olycella junctolineella (Hulst) | Cladode borer | No | USA | - | |
| Opuntia robusta J.C.Wendl. ex Pfeiff | Cactoblastis cactorum (Berg) | Cladode borer | Yes | Australia | Considerable |
| South Africa | |||||
| Dactylopius opuntiae (Cokerell), “ficus-indica” biotype | Cladode sucker | Yes | Australia | Considerable | |
| South Africa | Moderate | ||||
| Opuntia salmiana J. Parm. ex Pfeiff. | Cactoblastis cactorum (Berg) | Cladode borer | Yes | South Africa | Trivial |
| Opuntia spinulifera Salm-Dyck | Cactoblastis cactorum (Berg) | Cladode borer | Yes | South Africa | Unknown |
| Opuntia streptacantha Lem. | Cactoblastis cactorum (Berg) | Cladode borer | Yes | Australia | Trivial |
| Chelinidea tabulata (Burmeister) | Unknown | Yes | Australia | None | |
| Chelinidea vittiger Uhler | Unknown | No | Australia | - | |
| Dactylopius opuntiae (Cockerell) “ficus-indica” biotype | Cladode sucker | Yes | Australia | Considerable | |
| Lagocheirus funestus Thomson | Stem borer | Yes | Australia | Trivial | |
| Moneilema blapsides (Newman) subsp. ulkei Horn | Cladode and root borer | Yes | Australia | Trivial | |
| Opuntia stricta (Haw.) Haw. | Cactoblastis cactorum (Berg) | Cladode borer | Yes | New Caledonia | Considerable |
| Antigua Cayman Islands Cuba Federation of St Kitts and Nevis Guadeloupe Jamaica Montserrat U.S. Virgin Islands | Extensive | ||||
| Namibia South Africa | Moderate | ||||
| Australia Kenya | Trivial | ||||
| Bahamas | Unknown | ||||
| Cactoblastis doddi Heinrich | Cladode borer | No | Australia | - | |
| Chelinidea tabulata (Burmeister) | Unknown | Yes | Australia | None | |
| Chelinidea vittiger Uhler | Unknown | Yes | Australia | Unknown | |
| Dactylopius austrinus De Lotto | Cladode sucker | No | Federation of St Kitts and Nevis | - | |
| Dactylopius ceylonicus (Green) | Cladode sucker | No | India | - | |
| Dactylopius coccus | Cladode sucker | No | Australia | - | |
| Dactylopius confusus (Cockerell) | Cladode sucker | Yes | Australia | None | |
| Dactylopius opuntiae (Cokerell), “stricta” biotype | Cladode sucker | Yes | Sri Lanka | Considerable | |
| Australia India Kenya Saudi Arabia South Africa | Extensive | ||||
| Namibia | Moderate | ||||
| Kenya | Unknown | ||||
| No | Federation of St Kitts and Nevis | - | |||
| Lagocheirus funestus Thomson | Stem borer | Yes | Australia | Trivial | |
| Loxomorpha flavidissimalis (Grote) | Cactus feeder | No | Australia | - | |
| Melitara dentata (Grote) | Cactus feeder | No | Australia | - | |
| Melitara prodenialis Walker | Cladode borer | No | Australia | - | |
| Moneilema blapsides (Newman) subsp. ulkei Horn | Cladode and root borer | Yes | Australia | Trivial | |
| Moneilema variolare Thomson | Cladode and root borer | Yes | Australia | None | |
| Olycella junctolineella (Hulst) | Cladode borer | Yes | Australia | None | |
| Opuntia tomentosa Salm-Dyck | Cactoblastis cactorum (Berg) | Cladode borer | Yes | Australia | Trivial |
| Dactylopius opuntiae (Cokerell), “ficus-indica” biotype | Cladode sucker | Yes | Australia | Moderate | |
| South Africa | Considerable | ||||
| Opuntia triacantha (Willd.) Sweet | Cactoblastis cactorum (Berg) | Cladode borer | Yes | Antigua Cayman Islands Cuba Federation of St Kitts and Nevis Guadeloupe Montserrat U.S. Virgin Islands | Extensive |
| Puerto Rico | Unknown | ||||
| Dactylopius austrinus De Lotto | Cladode sucker | No | Federation of St Kitts and Nevis | - | |
| Dactylopius opuntiae (Cokerell) | Cladode sucker | No | Federation of St Kitts and Nevis | - | |
| Opuntia tuna (L.) Mill. | Cactoblastis cactorum (Berg) | Cladode borer | Yes | Mauritius | Extensive |
| Dactylopius ceylonicus (Green) | Cladode sucker | No | Mauritius | - | |
| Dactylopius opuntiae (Cokerell) “ficus-indica” biotype | Cladode sucker | Yes | Mauritius | Trivial | |
| Peniocereus serpentinus (Lag. and Rodr.) N.P. Taylor | Hypogeococcus festerianus (Lizer y Trelles) | Stem sucker | Yes | South Africa | Unknown |
| Pereskia aculeata Mill. | Catorhintha schaffneri (Brailovsky & Garcia) | Stem wilter | Yes | South Africa | Unknown |
| Phenrica guerini Bechyné | Leaf feeder | Yes | South Africa | Moderate |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novoa, A.; Brundu, G.; Day, M.D.; Deltoro, V.; Essl, F.; Foxcroft, L.C.; Fried, G.; Kaplan, H.; Kumschick, S.; Lloyd, S.; et al. Global Actions for Managing Cactus Invasions. Plants 2019, 8, 421. https://doi.org/10.3390/plants8100421
Novoa A, Brundu G, Day MD, Deltoro V, Essl F, Foxcroft LC, Fried G, Kaplan H, Kumschick S, Lloyd S, et al. Global Actions for Managing Cactus Invasions. Plants. 2019; 8(10):421. https://doi.org/10.3390/plants8100421
Chicago/Turabian StyleNovoa, Ana, Giuseppe Brundu, Michael D. Day, Vicente Deltoro, Franz Essl, Llewellyn C. Foxcroft, Guillaume Fried, Haylee Kaplan, Sabrina Kumschick, Sandy Lloyd, and et al. 2019. "Global Actions for Managing Cactus Invasions" Plants 8, no. 10: 421. https://doi.org/10.3390/plants8100421
APA StyleNovoa, A., Brundu, G., Day, M. D., Deltoro, V., Essl, F., Foxcroft, L. C., Fried, G., Kaplan, H., Kumschick, S., Lloyd, S., Marchante, E., Marchante, H., Paterson, I. D., Pyšek, P., Richardson, D. M., Witt, A., Zimmermann, H. G., & Wilson, J. R. U. (2019). Global Actions for Managing Cactus Invasions. Plants, 8(10), 421. https://doi.org/10.3390/plants8100421






