Night Temperature Affects the Growth, Metabolism, and Photosynthetic Gene Expression in Astragalus membranaceus and Codonopsis lanceolata Plug Seedlings
Abstract
1. Introduction
2. Results
2.1. Growth, Development and Morphology
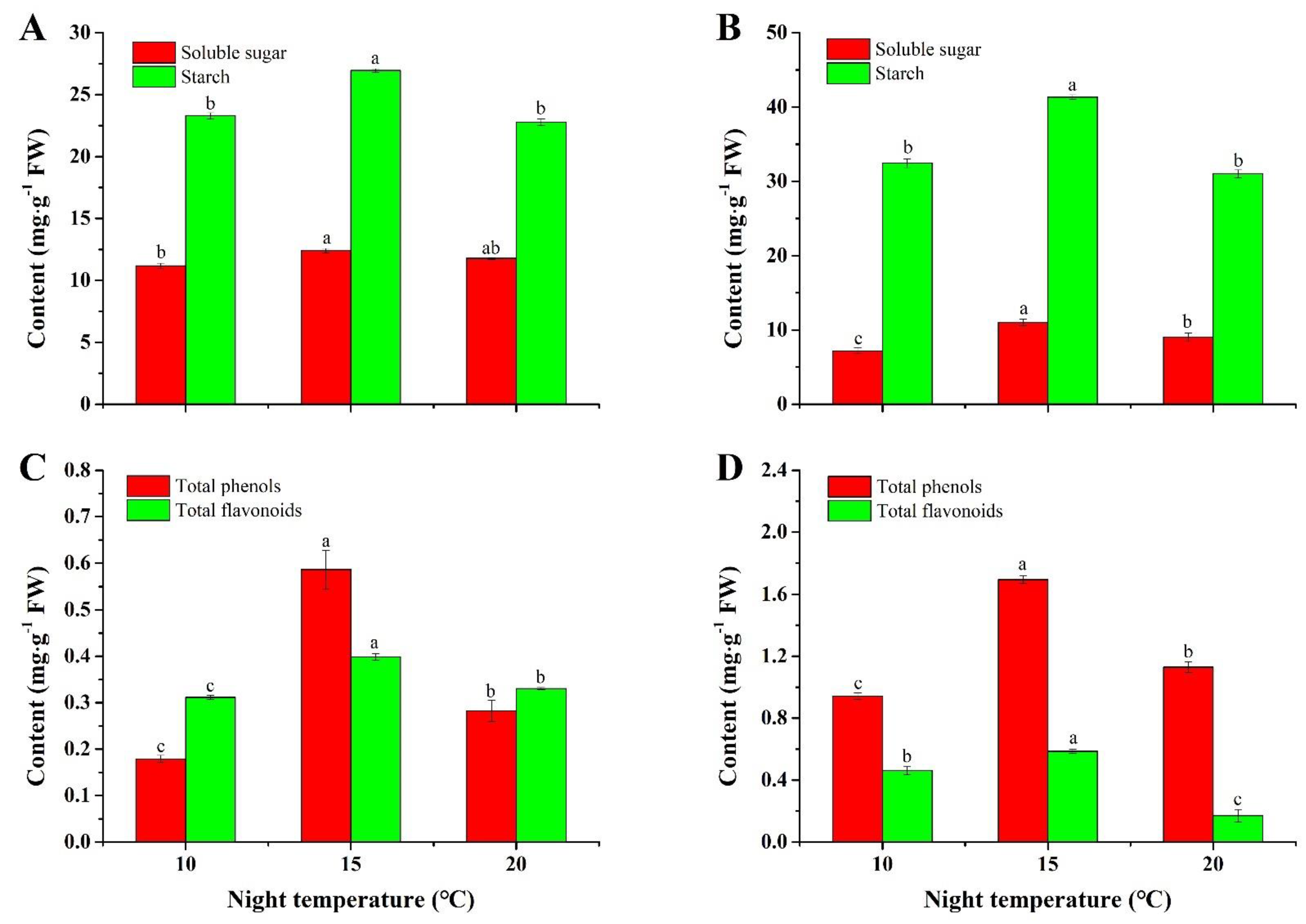
2.2. Contents of Soluble Sugar and Starch
2.3. Contents of Total Phenols and Flavonoids
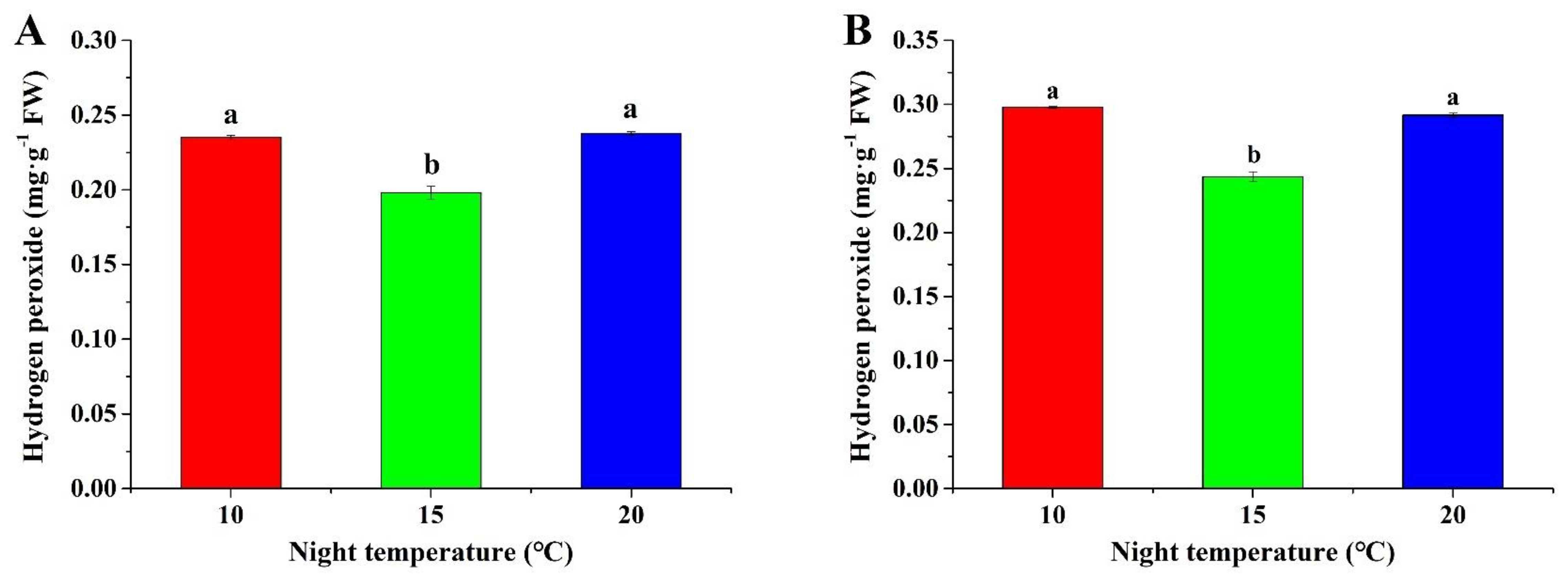
2.4. Hydrogen Peroxide Content
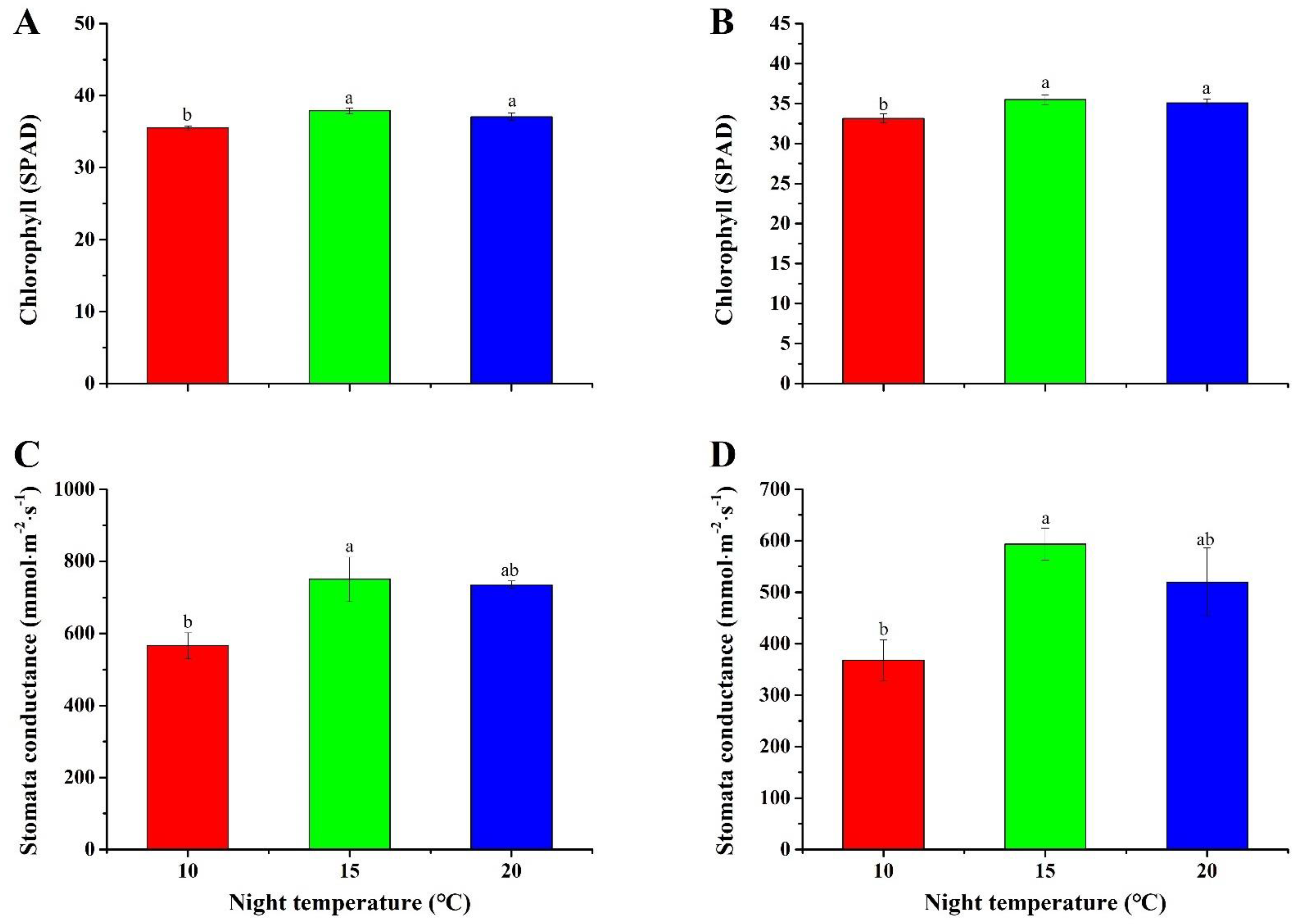
2.5. Chlorophyll Content and Stomatal Conductance
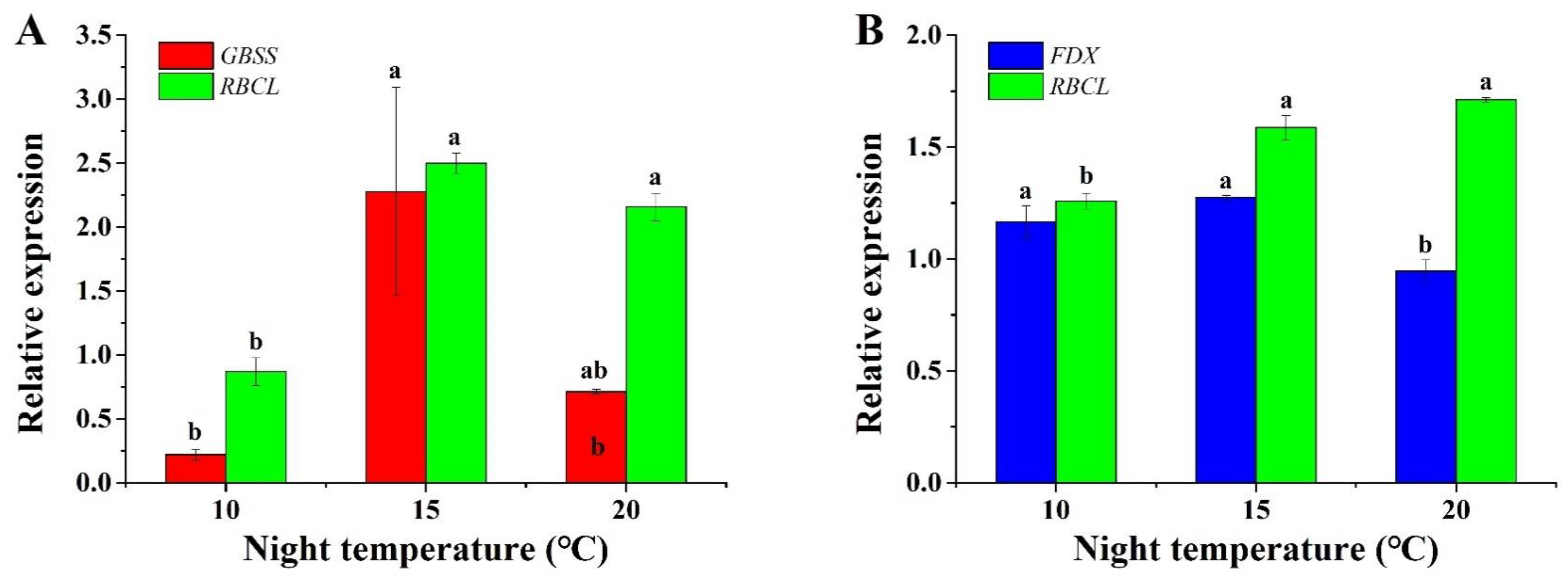
2.6. Gene Expression Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Contents of Soluble Sugar and Starch
4.3. Contents of Total Phenols and Flavonoids
4.4. Hydrogen Peroxide Content
4.5. Assessments of Chlorophyll Content and Stomatal Conductance
4.6. Analysis of the Gene Expression by Quantitative Real-Time PCR
4.7. Data Collection and Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, J.H.; Hong, J.Y.; Shin, S.R.; Yoon, K.Y. Comparison of antioxidant activity in wild plant (Adenophora triphylla) leaves and roots as a potential source of functional foods. Int. J. Food Sci. Nutr. 2009, 60, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Thwe, A.A.; Li, X.H.; Kim, Y.J.; Kim, J.K.; Arasu, M.V.; Al-Dhabi, N.A.; Park, S.U. Triterpene and flavonoid biosynthesis and metabolic profiling of hairy roots, adventitious roots, and seedling roots of Astragalus membranaceus. J. Agric. Food Chem. 2015, 63, 8862–8869. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jia, M.M.; Zhang, X.J.; Wang, P.L. Calycosin attenuates MPTP-induced Parkinson’s disease by suppressing the activation of TLR/NF-kappa B and MARK pathways. Phytother. Res. 2019, 33, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Han, A.Y.; Lee, Y.S.; Kwon, S.; Lee, H.S.; Lee, K.W.; Seol, G.H. Codonopsis lanceolata extract prevents hypertension in rats. Phytomedicine 2018, 39, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Kang, M.; Kim, Y.S. A triterpenoid saponin from Adenophora triphylla var. Japonica suppresses the growth of human gastric cancer cells via regulation of apoptosis and autophagy. Tumor Biol. 2014, 35, 12021–12030. [Google Scholar] [CrossRef] [PubMed]
- Boo, H.O.; Kim, H.H.; Barasch, D.; Nemirovski, A.; Lee, M.S.; Gorinstein, S.; Ku, Y.G. Codonopsis lanceolata and Nelumbo nucifera Gaertn. root extracts for functional food: Metabolic profiling by MS, FTIR and fluorescence and evaluation of cytotoxicity and anti-obesity properties on 3T3-L1 cell line. Eur. Food Res. Technol. 2017, 243, 689–700. [Google Scholar] [CrossRef]
- Moon, K.G.; Um, I.S.; Jeon, S.H.; Cho, Y.S.; Kim, Y.G.; Rho, I.R. Effect of organic fertilizer application on growth characteristics and saponin content in Codonopsis lanceolata. Hortic. Environ. Biotehnol. 2018, 59, 125–130. [Google Scholar] [CrossRef]
- Ghimire, B.K.; Seong, E.S.; Yu, C.Y.; Kim, S.H.; Chung, I.M. Evaluation of phenolic compounds and antimicrobial activities in transgenic Codonopsis lanceolata plants via overexpression of the gamma-tocopherol methyltransferase (gamma-tmt) gene. S. Afr. J. Bot. 2017, 109, 25–33. [Google Scholar] [CrossRef]
- Gao, J.Q.; Liu, Z.J.; Chen, T.; Zhao, D.Q. Pharmaceutical properties of calycosin, the major bioactive isoflavonoid in the dry root extract of Radix astragali. Pharm. Biol. 2014, 52, 1217–1222. [Google Scholar] [CrossRef]
- Li, Y.; Guo, S.; Zhu, Y.; Yan, H.; Qian, D.W.; Wang, H.Q.; Yu, J.Q.; Duan, J.A. Flowers of Astragalus membranaceus var. mongholicus as a novel high potential by-product: Phytochemical characterization and antioxidant activity. Molecules 2019, 24, 434. [Google Scholar] [CrossRef]
- McClung, C.R.; Lou, P.; Hermand, V.; Kim, J.A. The importance of ambient temperature to growth and the induction of flowering. Front. Plant Sci. 2016, 7, 1266. [Google Scholar] [CrossRef]
- Rondanini, D.; Mantese, A.; Savin, R.; Hall, A.J. Responses of sunflower yield and grain quality to alternating day/night high temperature regimes during grain filling: Effects of timing, duration and intensity of exposure to stress. Field Crop. Res. 2006, 96, 48–62. [Google Scholar] [CrossRef]
- Liu, K.; Wang, X.Y.; Lu, B.L.; Tian, X.H.; Punzalan, B.; Laza, R.C.; Zhang, Y.B. Effects of high night temperature on grain quality under field chamber system condition. Philipp. J. Crop. Sci. 2017, 42, 56–62. [Google Scholar]
- Coast, O.; Ellis, R.H.; Murdoch, A.J.; Quinones, C.; Jagadish, K.S.V. High night temperature induces contrasting responses for spikelet fertility, spikelet tissue temperature, flowering characteristics and grain quality in rice. Funct. Plant Biol. 2015, 42, 149–161. [Google Scholar] [CrossRef]
- Shi, W.; Yin, X.; Struik, P.C.; Xie, F.; Schmidt, R.C.; Jagadish, K.S.V. Grain yield and quality responses of tropical hybrid rice to high night-time temperature. Field Crop. Res. 2016, 190, 18–25. [Google Scholar] [CrossRef]
- Lobell, D.B.; Ortiz-Monasterio, J.I. Impacts of day versus night temperatures on spring wheat yields: A comparison of empirical and ceres model predictions in three locations. Agron. J. 2007, 99, 469–477. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Gao, Q.; Chen, J.X. Effects of day and night temperature difference on growth, development, yield and fruit quality of tomatoes. Chin. J. Appl. Ecol. 2015, 26, 2700–2706. [Google Scholar]
- Kadir, S.; Sidhu, G.; Al-Khatib, K. Strawberry (Fragaria xananassa Duch.) growth and productivity as affected by temperature. HortScience 2006, 41, 1423–1430. [Google Scholar] [CrossRef]
- Boo, H.O.; Heo, B.G.; Gorinstein, S.; Chon, S.U. Positive effects of temperature and growth conditions on enzymatic and antioxidant status in lettuce plants. Plant Sci. 2011, 181, 479–484. [Google Scholar] [CrossRef]
- Tombesi, S.; Cincera, I.; Frioni, T.; Ughini, V.; Gatti, M.; Palliotti, A.; Poni, S. Relationship among night temperature, carbohydrate translocation and inhibition of grapevine leaf photosynthesis. Environ. Exp. Bot. 2019, 157, 293–298. [Google Scholar] [CrossRef]
- Zhou, L.H.; Yan, T.; Chen, X.; Li, Z.L.; Wu, D.Z.; Hua, S.J.; Jiang, L.X. Effect of high night temperature on storage lipids and transcriptome changes in developing seeds of oilseed rape. J. Exp. Bot. 2018, 69, 1721–1733. [Google Scholar] [CrossRef] [PubMed]
- Gaiotti, F.; Pastore, C.; Filippetti, I.; Lovat, L.; Belfiore, N.; Tomasi, D. Low night temperature at veraison enhances the accumulation of anthocyanins in corvina grapes (Vitis vinifera L.). Sci. Rep. 2018, 8, 8719. [Google Scholar] [CrossRef] [PubMed]
- Lesjak, J.; Calderini, D.F. Increased night temperature negatively affects grain yield, biomass and grain number in chilean quinoa. Front. Plant Sci. 2017, 8, 352. [Google Scholar] [CrossRef] [PubMed]
- Jing, P.; Wang, D.; Zhu, C.; Chen, J. Plant physiological, morphological and yield-related responses to night temperature changes across different species and plant functional types. Front. Plant Sci. 2016, 7, 1774. [Google Scholar] [CrossRef] [PubMed]
- Loka, D.A.; Oosterhuis, D.M. Effect of high night temperatures on cotton respiration, ATP levels and carbohydrate content. Environ. Exp. Bot. 2010, 68, 258–263. [Google Scholar] [CrossRef]
- Kanno, K.; Mae, T.; Makino, A. High night temperature stimulates photosynthesis, biomass production and growth during the vegetative stage of rice plants. Soil Sci. Plant Nutr. 2009, 55, 124–131. [Google Scholar] [CrossRef]
- Losada, M.; Arnon, D.I. Enzyme Systems in Photosynthesis. In Modern Methods of Plant Analysis/Moderne Methoden Der Pflanzenanalyse; Springer: Berlin/Heidelberg, Germany, 1964; pp. 569–615. [Google Scholar]
- Schuermann, P.; Buchanan, B.B. The ferredoxin/thioredoxin system of oxygenic photosynthesis. Antioxid. Redox Signal. 2008, 10, 1235–1273. [Google Scholar] [CrossRef]
- Sage, R.F.; Kubien, D.S. The temperature response of C-3 and C-4 photosynthesis. Plant Cell Environ. 2007, 30, 1086–1106. [Google Scholar] [CrossRef]
- Merida, A.; Rodriguez-Galan, J.M.; Vincent, C.; Romero, J.M. Expression of the granule-bound starch synthase I (Waxy) gene from snapdragon is developmentally and circadian clock regulated. Plant Physiol. 1999, 120, 401–410. [Google Scholar] [CrossRef]
- Visser, R.G.F.; Stolte, A.; Jacobsen, E. Expression of a chimeric granule-bound starch synthase-gus gene in transgenic potato plants. Plant Mol. Biol. 1991, 17, 691–699. [Google Scholar] [CrossRef]
- Umemoto, T.; Nakamura, Y.; Ishikura, N. Activity of starch synthase and the amylose content in rice endosperm. Phytochemistry 1995, 40, 1613–1616. [Google Scholar] [CrossRef]
- Phan, T.T.T.; Ishibashi, Y.; Miyazaki, M.; Tran, H.T.; Okamura, K.; Tanaka, S.; Nakamura, J.; Yuasa, T.; Iwaya-Inoue, M. High temperature-induced repression of the rice sucrose transporter (OsSUT1) and starch synthesis-related genes in sink and source organs at milky ripening stage causes chalky grains. J. Agron. Crop. Sci. 2013, 199, 178–188. [Google Scholar] [CrossRef]
- Zhao, H.; Dai, T.; Jiang, D.; Cao, W. Effects of high temperature on key enzymes involved in starch and protein formation in grains of two wheat cultivars. J. Agron. Crop. Sci. 2008, 194, 47–54. [Google Scholar] [CrossRef]
- Bukhov, N.G.; Dzhibladze, T.G.; Egorova, E.A. Elevated temperatures inhibit ferredoxin-dependent cyclic electron flow around photosystem I. Russ. J. Plant Physl. 2005, 52, 578–583. [Google Scholar] [CrossRef]
- Shi, W.J.; Muthurajan, R.; Rahman, H.; Selvam, J.; Peng, S.B.; Zou, Y.B.; Jagadish, K.S.V. Source-sink dynamics and proteomic reprogramming under elevated night temperature and their impact on rice yield and grain quality. New Phytol. 2013, 197, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, L.M.; Gislerod, H.R. Night temperature drop affects the diurnal photosynthetic rhythm of Kalanchoe blossfeldiana. Eur. J. Hortic. Sci. 2018, 83, 160–165. [Google Scholar] [CrossRef]
- Liu, W.F.; Qin, Z.W.; Xin, M.; Zhou, X.Y.; Yang, J.; Wang, C.H. Analysis of cspap-fib regulation of cucumber female differentiation in response to low night temperature conditions. Sci. Hortic. 2018, 240, 81–88. [Google Scholar] [CrossRef]
- Annunziata, M.G.; Apelt, F.; Carillo, P.; Krause, U.; Feil, R.; Koehl, K.; Lunn, J.E.; Stitt, M. Response of arabidopsis primary metabolism and circadian clock to low night temperature in a natural light environment. J. Exp. Bot. 2018, 69, 4881–4895. [Google Scholar] [CrossRef] [PubMed]
- Morita, S.; Yonemaru, J.; Takanashi, J. Grain growth and endosperm cell size under high night temperatures in rice (Oryza sativa L.). Ann. Bot. 2005, 95, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Sakai, H.; Yagi, K.; Hasegawa, T. Interactions of elevated CO2 and night temperature on rice growth and yield. Agric. For. Meteorol. 2009, 149, 51–58. [Google Scholar] [CrossRef]
- Glaubitz, U.; Li, X.; Schaedel, S.; Erban, A.; Sulpice, R.; Kopka, J.; Hincha, D.K.; Zuther, E. Integrated analysis of rice transcriptomic and metabolomic responses to elevated night temperatures identifies sensitivity- and tolerance-related profiles. Plant Cell Environ. 2017, 40, 121–137. [Google Scholar] [CrossRef]
- Loka, D.A.; Oosterhuis, D.M. Increased night temperatures during cotton’s early reproductive stage affect leaf physiology and flower bud carbohydrate content decreasing flower bud retention. J. Agron. Crop. Sci. 2016, 202, 518–529. [Google Scholar] [CrossRef]
- Peraudeau, S.; Lafarge, T.; Roques, S.; Quinones, C.O.; Clement-Vidal, A.; Ouwerkerk, P.B.F.; Van Rie, J.; Fabre, D.; Jagadish, K.S.V.; Dingkuhn, M. Effect of carbohydrates and night temperature on night respiration in rice. J. Exp. Bot. 2015, 66, 3931–3944. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.R.; Cothren, J.T.; Tarpley, L. High night temperature and abscisic acid affect rice productivity through altered photosynthesis, respiration and spikelet fertility. Crop. Sci. 2013, 53, 2603–2612. [Google Scholar] [CrossRef]
- Ryu, S.; Han, H.H.; Jeong, J.H.; Kwon, Y.; Han, J.H.; Do, G.R.; Choi, I.M.; Lee, H.J. Night temperatures affect fruit coloration and expressions of anthocyanin biosynthetic genes in ’hongro’ apple fruit skins. Eur. J. Hortic. Sci. 2017, 82, 232–238. [Google Scholar] [CrossRef]
- Li, X.A.; Li, M.L.; Han, C.; Jin, P.; Zheng, Y.H. Increased temperature elicits higher phenolic accumulation in fresh-cut pitaya fruit. Postharvest Biol. Technol. 2017, 129, 90–96. [Google Scholar] [CrossRef]
- Yamori, W.; Kondo, E.; Sugiura, D.; Terashima, I.; Suzuki, Y.; Makino, A. Enhanced leaf photosynthesis as a target to increase grain yield: Insights from transgenic rice lines with variable rieske fes protein content in the cytochrome b6/f complex. Plant Cell Environ. 2016, 39, 80–87. [Google Scholar] [CrossRef]
- Jamsheer, K.M.; Jindal, S.; Laxmi, A. Evolution of TOR-SnRK dynamics in green plants and its integration with phytohormone signaling networks. J. Exp. Bot. 2019, 70, 2239–2259. [Google Scholar] [CrossRef]
- Wang, X.; Peng, F.; Li, M.; Yang, L.; Li, G. Expression of a heterologous SnRK1 in tomato increases carbon assimilation, nitrogen uptake and modifies fruit development. J. Plant Physiol. 2012, 169, 1173–1182. [Google Scholar] [CrossRef]
- Zhang, Y.; Primavesi, L.F.; Jhurreea, D.; Andralojc, P.J.; Mitchell, R.A.C.; Powers, S.J.; Schluepmann, H.; Delatte, T.; Wingler, A.; Paul, M.J. Inhibition of SNF1-related protein kinase1 activity and regulation of metabolic pathways by trehalose-6-phosphate. Plant Physiol. 2009, 149, 1860–1871. [Google Scholar] [CrossRef]
- Szymanska, R.; Slesak, I.; Orzechowska, A.; Kruk, J. Physiological and biochemical responses to high light and temperature stress in plants. Environ. Exp. Bot. 2017, 139, 165–177. [Google Scholar] [CrossRef]
- Jin, S.H.; Li, X.Q.; Zheng, B.S.; Wang, J.G. Response of the photosynthesis and antioxidant systems to high-temperature stress in Euonymus japonicus seedlings. Science 2010, 56, 172–180. [Google Scholar]
- Bertamini, M.; Zulini, L.; Muthuchelian, K.; Nedunchezhian, N. Low night temperature effects on photosynthetic performance on two grapevine genotypes. Biol. Plant. 2007, 51, 381–385. [Google Scholar] [CrossRef]
- Bertamini, M.; Muthuchelian, K.; Rubinigg, M.; Zorer, R.; Nedunchezhian, N. Low-night temperature (LNT) induced changes of photosynthesis in grapevine (Vitis vinifera L.) plants. Plant Physiol. Biochem. 2005, 43, 693–699. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Djanaguiraman, M. High night temperature decreases leaf photosynthesis and pollen function in grain sorghum. Funct. Plant Biol. 2011, 38, 993–1003. [Google Scholar] [CrossRef]
- Yadav, S.K.; Tiwari, Y.K.; Singh, V.; Patil, A.A.; Shanker, A.K.; Jyothi Lakshmi, N.; Vanaja, M.; Maheswari, M. Physiological and biochemical basis of extended and sudden heat stress tolerance in maize. Proc. Nat. Acad. Sci. India Sect. B Biol. Sci. 2018, 88, 249–263. [Google Scholar] [CrossRef]
- Li, S.; Yang, Y.; Zhang, Q.; Liu, N.F.; Xu, Q.G.; Hu, L.X. Differential physiological and metabolic response to low temperature in two zoysiagrass genotypes native to high and low latitude. PLoS ONE 2018, 13, e0198885. [Google Scholar] [CrossRef]
- Liu, J.L.; Wang, W.X.; Wang, L.Y.; Sun, Y. Exogenous melatonin improves seedling health index and drought tolerance in tomato. Plant Growth Regul. 2015, 77, 317–326. [Google Scholar] [CrossRef]
- Van der Tol, C.; Verhoef, W.; Rosema, A. A model for chlorophyll fluorescence and photosynthesis at leaf scale. Agric. For. Meteorol. 2009, 149, 96–105. [Google Scholar] [CrossRef]
- Machado, D.F.S.; Ribeiro, R.V.; da Silveira, J.A.G.; Magalhaes, J.R.; Machado, E.C. Rootstocks induce contrasting photosynthetic responses of orange plants to low night temperature without affecting the antioxidant metabolism. Theor. Exp. Plant Phys. 2013, 25, 26–35. [Google Scholar] [CrossRef][Green Version]
- Chen, W.H.; Tseng, Y.C.; Liu, Y.C.; Chuo, C.M.; Chen, P.T.; Tseng, K.M.; Yeh, Y.C.; Ger, M.J.; Wang, H.L. Cool-night temperature induces spike emergence and affects photosynthetic efficiency and metabolizable carbohydrate and organic acid pools in Phalaenopsis aphrodite. Plant Cell Rep. 2008, 27, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Ball, J.T.; Woodrow, I.E.; Berry, J.A. A Model Predicting Stomatal Conductance and its Contribution to the Control of Photosynthesis Under Different Environmental Conditions. In Progress in Photosynthesis Research: Volume 4 Proceedings of the VIIth International Congress on Photosynthesis, Providence, RI, USA, 10–15 August 1986; Biggins, J., Ed.; Springer: Dordrecht, The Netherlands, 1987; pp. 221–224. [Google Scholar]
- Gago, J.; Daloso, D.D.M.; Figueroa, C.M.; Flexas, J.; Fernie, A.R.; Nikoloski, Z. Relationships of leaf net photosynthesis, stomatal conductance, and mesophyll conductance to primary metabolism: A multispecies meta-analysis approach. Plant Physiol. 2016, 171, 265. [Google Scholar] [CrossRef] [PubMed]
- Dickson, A.; Leaf, A.L.; Hosner, J.F. Quality appraisal of white spruce and white pine seedling stock in nurseries. For. Chron. 1960, 36, 10–13. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, X.; Jeong, H.; Wei, H.; Jeong, B. Growth and physiological responses of Adenophora triphylla (Thunb.) A.DC. plug seedlings to day and night temperature regimes. Agronomy 2018, 8, 173. [Google Scholar] [CrossRef]
- Manivannan, A.; Soundararajan, P.; Park, Y.G.; Jeong, B.R. In vitro propagation, phytochemical analysis, and evaluation of free radical scavenging property of scrophularia kakudensis franch tissue extracts. Biomed Res. Int. 2015, 2015. [Google Scholar] [CrossRef]
- Ren, X.; Liu, Y.; Jeong, H.; Jeong, B. Supplementary light source affects the growth and development of codonopsis lanceolata seedlings. Int. J. Mol. Sci. 2018, 19, 3074. [Google Scholar] [CrossRef]

| Night Temperature (°C) | Length (cm) | Dry Weight (mg) | Leaf Area (cm−2) | Stem Diameter (mm) | Root DW: Shoot DW Ratio | Shoot Dry Weight per Shoot Length (g·m−1) | Root Dry Weight per Root Length (g·m−1) | Dickson’s Quality Index (×10−4) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | |||||||
| 10 | 10.8 ± 0.4 b z | 4.1 ± 0.3 b | 54.9 ± 7.8 b | 8.7 ± 1.2 b | 4.3 ± 0.2 a | 0.83 ± 0.03 b | 0.16 ± 0.01 b | 0.51 ± 0.07 | 0.21 ± 0.03 b | 4.6 ± 0.6 b |
| 15 | 14.0 ± 0.4 a | 4.5 ± 0.5 b | 78.2 ± 4.3 a | 17.8 ± 1.7 a | 4.8 ± 0.2 a | 1.06 ± 0.04 a | 0.23 ± 0.02 a | 0.56 ± 0.03 | 0.42 ± 0.06 a | 7.1 ± 0.4 a |
| 20 | 13.4 ± 0.4 a | 5.9 ± 0.2 a | 72.8 ± 5.0 a | 14.8 ± 1.3 a | 3.5 ± 0.2 b | 0.98 ± 0.03 a | 0.20 ± 0.01 a | 0.54 ± 0.03 | 0.25 ± 0.03 b | 6.2 ± 0.5 a |
| F-test | *** | ** | * | ** | ** | ** | ** | NS | ** | * |
| Night Temperature (°C) | Length (cm) | Dry Weight (mg) | Leaf Area (cm−2) | Stem Diameter (mm) | Root DW: Shoot DW Ratio | Shoot Dry Weight per Shoot Length (g·m−1) | Root Dry Weight per Root Length (g·m−1) | Dickson’s Quality Index (×10−4) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | Leaf | |||||||
| 10 | 15.1 ± 0.6 b z | 3.3 ± 0.3 b | 33.4 ± 2.6 b | 4.1 ± 0.4 b | 7.1 ± 0.9 b | 6.6 ± 0.6 | 1.46 ± 0.05 b | 0.19 ± 0.02 b | 0.22 ± 0.02 | 0.13 ± 0.02 b | 3.4 ± 0.3 b |
| 15 | 17.5 ± 0.7 a | 3.9 ± 0.1 b | 57.6 ± 3.9 a | 9.0 ± 0.6 a | 11.7 ± 0.8 a | 7.7 ± 0.6 | 1.81 ± 0.05 a | 0.29 ± 0.02 a | 0.34 ± 0.03 | 0.23 ± 0.02 a | 6.5 ± 0.5 a |
| 20 | 17.8 ± 0.7 a | 4.9 ± 0.3 a | 51.0 ± 5.6 a | 6.9 ± 1.3 a | 9.4 ± 1.0 ab | 8.0 ± 0.5 | 1.80 ± 0.04 a | 0.23 ± 0.01 b | 0.29 ± 0.04 | 0.15 ± 0.03 b | 5.5 ± 0.8 a |
| F-test | * | ** | ** | *** | ** | NS | *** | ** | NS | ** | ** |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| 18S | 5′-CTCAACCATAAA CGATGCCGACC-3′ | 5′-AGTTTCAGCCTTGCGACCATACTCC-3′ |
| AmGBSS | 5′-ATAACATAGCGTATCAGGG-3′ | 5′-CTCGGTCAGATTCTAACACT-3′ |
| AmRBCL | 5′-TGGCTGTTCCTATCGTCA-3′ | 5′-AAGTAATCTCCCTTTCTCCT-3′ |
| β-Actin | 5′-CGAGAAGAGCTACGAGCTACCCGATGG-3′ | 5′-CTCGGTGCTAGGGCAGTGATCTCTTTGCT-3′ |
| ClFDX | 5′-CTTCGGCGTTTCTTCGT-3′ | 5′-CTGCCAAACCCTTGATAACT-3′ |
| ClRBCL | 5′-GCTTACCCATTAGACCTTT-3′ | 5′-GGGACGACCATACTTGTT-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Ren, X.; Jeong, B.R. Night Temperature Affects the Growth, Metabolism, and Photosynthetic Gene Expression in Astragalus membranaceus and Codonopsis lanceolata Plug Seedlings. Plants 2019, 8, 407. https://doi.org/10.3390/plants8100407
Liu Y, Ren X, Jeong BR. Night Temperature Affects the Growth, Metabolism, and Photosynthetic Gene Expression in Astragalus membranaceus and Codonopsis lanceolata Plug Seedlings. Plants. 2019; 8(10):407. https://doi.org/10.3390/plants8100407
Chicago/Turabian StyleLiu, Ya, Xiuxia Ren, and Byoung Ryong Jeong. 2019. "Night Temperature Affects the Growth, Metabolism, and Photosynthetic Gene Expression in Astragalus membranaceus and Codonopsis lanceolata Plug Seedlings" Plants 8, no. 10: 407. https://doi.org/10.3390/plants8100407
APA StyleLiu, Y., Ren, X., & Jeong, B. R. (2019). Night Temperature Affects the Growth, Metabolism, and Photosynthetic Gene Expression in Astragalus membranaceus and Codonopsis lanceolata Plug Seedlings. Plants, 8(10), 407. https://doi.org/10.3390/plants8100407






