Leaf Age, Canopy Position, and Habitat Affect the Carbon Isotope Discrimination and Water-Use Efficiency in Three C3 Leguminous Prosopis Species from a Hyper-Arid Climate
Abstract
1. Introduction
2. Material and Methods
2.1. Study Sites
2.2. Leaf C and N Content
2.3. Plant Sampling and Data Collection
2.4. Data Analysis
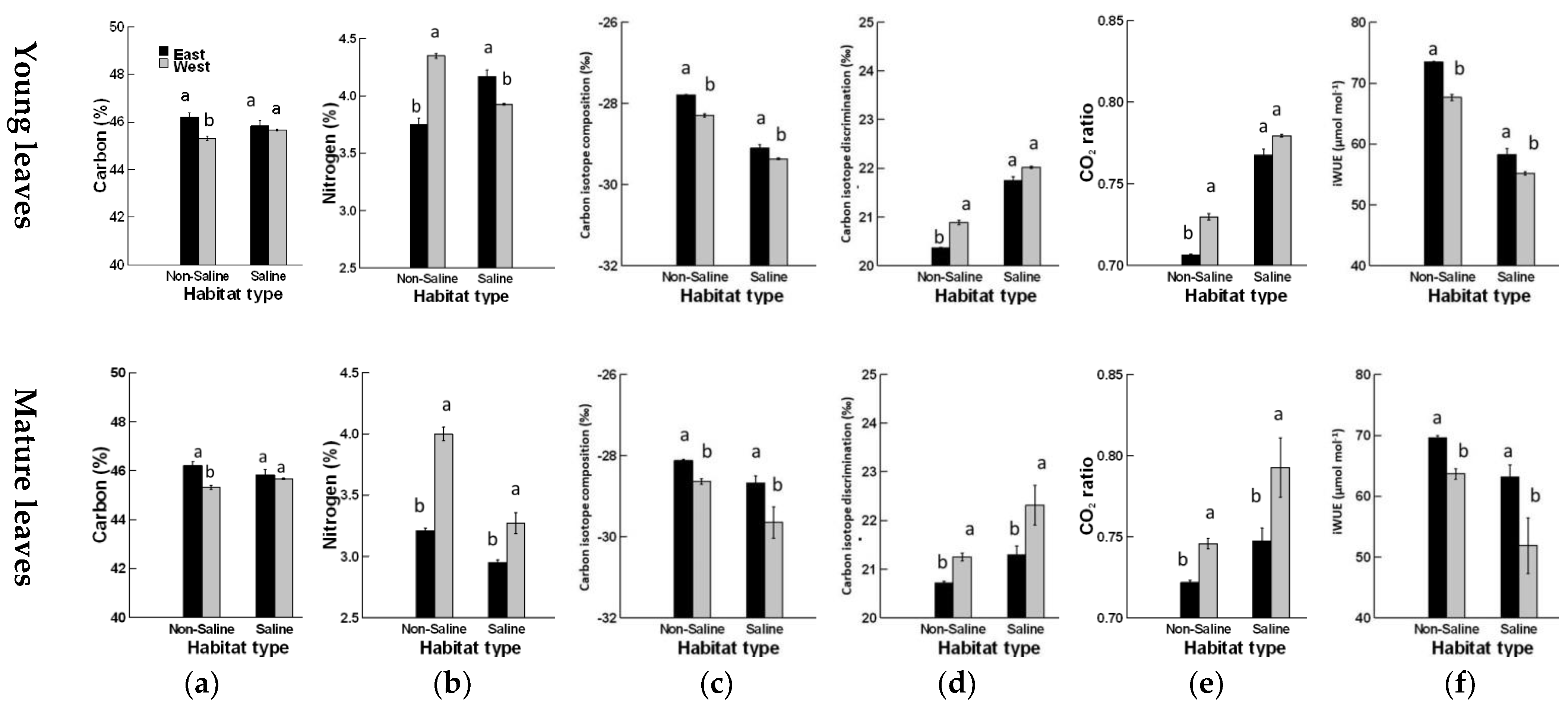
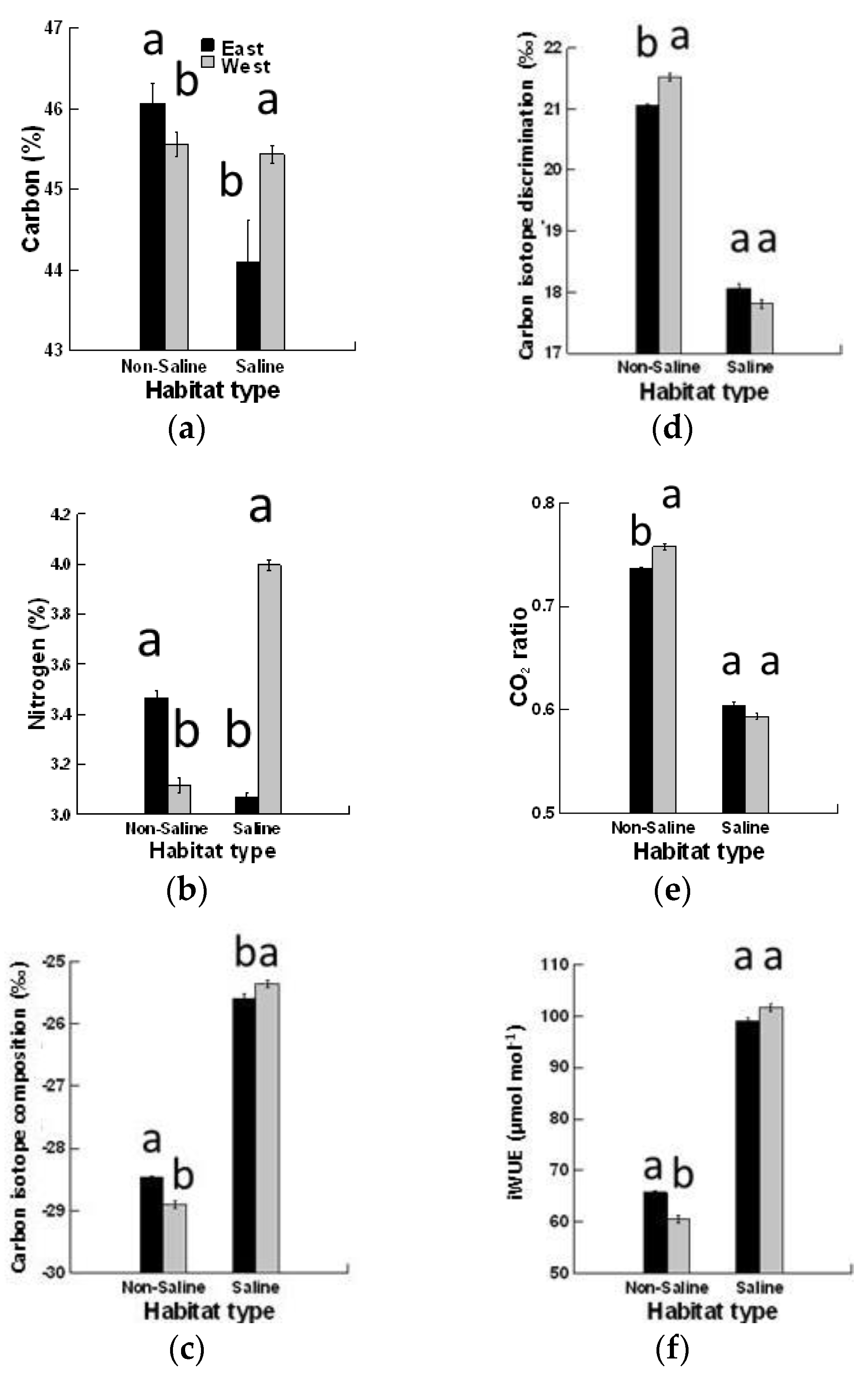
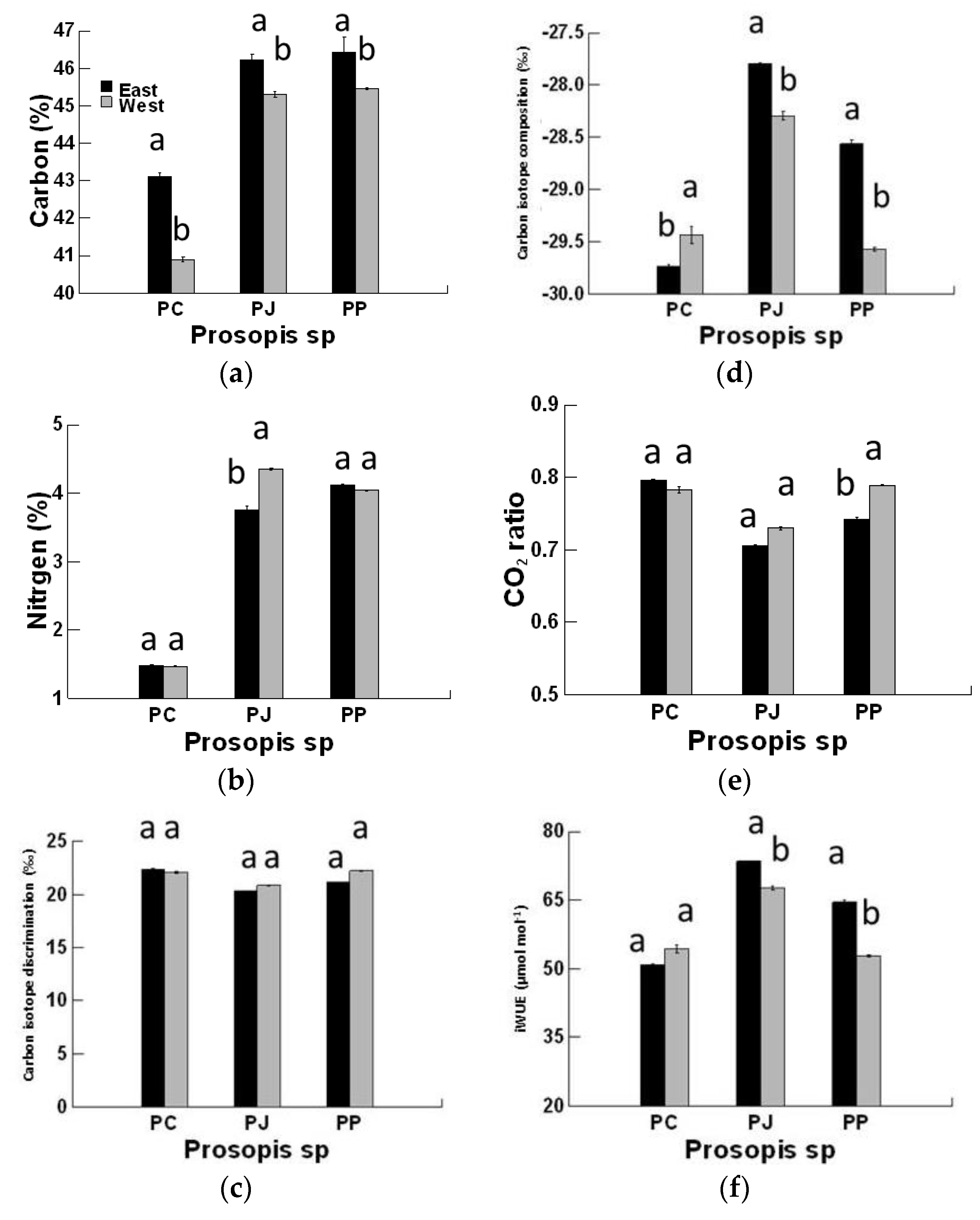
3. Results
4. Discussion
Supplementary Materials
Data accessibility
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Farquhar, G.D.; O’Leary, M.H.; Berry, J.A. On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Funct. Plant Biol. 1982, 9, 121–137. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Seibt, U.; Rajabi, A.; Griffiths, H.; Berry, J.A. Carbon isotopes and water use efficiency: Sense and sensitivity. Oecologia 2008, 155, 441–454. [Google Scholar] [CrossRef]
- Cernusak, L.A.; Winter, K.; Aranda, J.; Turner, B.L. Conifers, angiosperm trees, and lianas: Growth, whole-plant water and nitrogen use efficiency, and stable isotope composition 13C and 18O of seedlings grown in a tropical environment. Plant Physiol. 2008, 148, 642–659. [Google Scholar] [CrossRef]
- Hussain, M.I.; Reigosa, M.J. Seedling growth, leaf water status and signature of stable carbon isotopes in C3 perennials exposed to natural phytochemicals. Aust. J. Bot. 2012, 60, 676–686. [Google Scholar] [CrossRef]
- Flanagan, L.B.; Adkinson, A.C. Interacting controls on productivity in a northern Great Plains grassland and implications for response to ENSO events. Glob. Chang. Biol. 2011, 17, 3293–3311. [Google Scholar] [CrossRef]
- Shackleton, R.T.; Le Maitre, D.C.; Richardson, D.M. Stakeholder perceptions and practices regarding Prosopis (mesquite) invasions and management in South Africa. Ambio 2015, 44, 569–581. [Google Scholar] [CrossRef]
- Pennington, R.E.; Tischler, C.R.; Johnson, H.B.; Polley, H.W. Genetic variation for carbon isotope composition in honey mesquite (Prosopis glandulosa). Tree Physiol. 1999, 19, 583–589. [Google Scholar] [CrossRef][Green Version]
- Sun, W.; Resco, V.; Williams, D.G. Diurnal and seasonal variation in the carbon isotope composition of leaf dark-respired CO2 in velvet mesquite (Prosopis velutina). Plant Cell Environ. 2009, 32, 1390–1400. [Google Scholar] [CrossRef]
- Condon, A.G.; Richards, R.A.; Rebetzke, G.J.; Farquhar, G.D. Improving intrinsic water-use efficiency and crop yield. Crop Sci. 2002, 42, 122–131. [Google Scholar] [CrossRef]
- Hussain, M.I.; Al-Dakheel, A.J. Effect of salinity stress on phenotypic plasticity, yield stability and signature of stable isotopes of carbon and nitrogen in Safflower. Environ. Sci. Pollut. Res. 2018, 25, 23685–23694. [Google Scholar] [CrossRef]
- Hussain, M.I.; Al-Dakheel, A.J.; Reigosa, M.J. Genotypic differences in agro-physiological, biochemical and isotopic responses to salinity stress in quinoa (Chenopodium quinoa Willd.) plants: Prospects for salinity tolerance and yield stability. Plant Physiol. Biochem. 2018, 129, 411–420. [Google Scholar] [CrossRef]
- Hussain, M.I.; El-Keblawy, A.; Aljabi, A.E.; Aljabi, D.E.; Hafez, M.; Al Jasmi, A.; Schampoel, T.; Temperton, V.M. Nitrogen fixation and carbon assimilation of the desert legume Tephrosia apollinea under PEG-induced osmotic stress. Flora 2019, 251, 105–113. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Cernusak, L.A.; Barnes, B. Heavy water fractionation during transpiration. Plant Physiol. 2007, 143, 11–18. [Google Scholar] [CrossRef]
- Ghashghaie, J.; Badeck, F.W.; Lanigan, G.J.; Nogues, S.; Tcherkez, G.; Deleens, E.; Cornic, G.; Griffiths, H. Carbon isotope fractionation during dark respiration and photorespiration in C3 plants. Phytochem. Rev. 2003, 2, 145–161. [Google Scholar] [CrossRef]
- Barbour, M.M.; McDowell, N.G.; Tcherkez, G.; Bickford, C.P.; Hanson, D.T. A new measurement technique reveals rapid post-illumination changes in the carbon isotope composition of leaf-respired CO2. Plant Cell Environ. 2007, 30, 469–482. [Google Scholar] [CrossRef]
- Gessler, A.; Tcherkez, G.; Peuke, A.D.; Ghashghaie, J.; Farquhar, G.D. Experimental evidence for diel variations of the carbon isotope composition in leaf, stem and phloem sap organic matter in Ricinus communis. Plant Cell Environ. 2008, 31, 941–953. [Google Scholar] [CrossRef]
- Bahn, M.; Schmitt, M.; Siegwolf, R.; Richter, A.; Brüggemann, N. Does photosynthesis affect grassland soil-respired CO2 and its carbon isotope composition on a diurnal timescale? New Phytol. 2009, 182, 451–460. [Google Scholar] [CrossRef]
- Jones, H.G. What is water use efficiency. In Water Use Efficiency in Plant Biology; Bacon, M.A., Ed.; Blackwell Publishing-CRC Press: Boca Raton, FL, USA, 2004; pp. 27–41. [Google Scholar]
- Condon, A.G.; Richards, R.A.; Rebetzke, G.J.; Farquhar, G.D. Breeding for high water-use efficiency. J. Exp. Bot. 2004, 55, 2447–2460. [Google Scholar] [CrossRef]
- Zolfaghar, S.; Villalobos-Vega, R.; Zeppel, M.; Cleverly, J.; Rumman, R.; Hingee, M.; Boulain, N.; Li, Z.; Eamus, D. Transpiration of Eucalyptus woodlands across a natural gradient of depth-to-groundwater. Tree Physiol. 2017, 37, 961–975. [Google Scholar] [CrossRef]
- Ubierna, N.; Holloway-Phillips, M.M.; Farquhar, G.D. Using stable carbon isotopes to study C3 and C4 photosynthesis: Models and calculations. In Photosynthesis; Humana Press: New York, NY, USA, 2018; pp. 155–196. [Google Scholar]
- Shatil-Cohen, A.; Attia, Z.; Moshelion, M. Bundle-sheath cell regulation of xylem-mesophyll water transport via aquaporins under drought stress: A target of xylem-borne ABA? Plant J. 2011, 67, 72–80. [Google Scholar] [CrossRef]
- Trifiló, P.; Raimondo, F.; Savi, T.; Lo Gullo, M.A.; Nardini, A. The contribution of vascular and extra-vascular water pathways to drought-induced decline of leaf hydraulic conductance. J. Exp. Bot. 2016, 67, 5029–5039. [Google Scholar] [CrossRef]
- Patnaik, P.; Abbasi, T.; Abbasi, S.A. Prosopis (Prosopis juliflora): Blessing and bane. Trop. Ecol. 2017, 58, 455–483. [Google Scholar]
- Meroni, M.; Ng, W.T.; Rembold, F.; Leonardi, U.; Atzberger, C.; Gadain, H.; Shaiye, M. Mapping Prosopis juliflora in West Somaliland with Landsat 8 satellite imagery and ground information. Land Degrad. Dev. 2017, 28, 494–506. [Google Scholar] [CrossRef]
- Yoda, K.Y.; Tsuji, W.; Inoune, T.; Saito, T.; Abd Elbasit, M.A.M.; Eldoma, A.M.; Magzoub, M.K.; Hoshino, B.; Nawata, H.; Yasuda, H. Evaluation of the effect of a rain pulse on the initial growth of Prosopis seedlings. Arid Land Res. Manag. 2015, 29, 210–221. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Abdelfatah, M.A. Impacts of native and invasive exotic Prosopis congeners on soil properties and associated flora in the arid United Arab Emirates. J. Arid Environ. 2014, 100, 1–8. [Google Scholar] [CrossRef]
- Shiferaw, H.; Teketay, D.; Nemomissa, S.; Assefa, F. Some biological characteristics that foster the invasion of Prosopis juliflora (Sw.) D.C. at Middle Awash Rift Valley Area, north-eastern Ethiopia. J. Arid Environ. 2004, 58, 135–154. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Al-Rawai, A. Impacts of the invasive exotic Prosopis juliflora (Sw.) D.C. on the native flora and soils of the UAE. Plant Ecol. 2007, 190, 23–35. [Google Scholar] [CrossRef]
| Source of Variation | df | Carbon (%) | Nitrogen (%) | C Isotope Composition | C Isotope Discrimination | Ci/Ca Ratio | iWUE |
|---|---|---|---|---|---|---|---|
| Habitat (H) | 1 | 93.13*** | 55.98*** | 80.37*** | 80.13*** | 79.35*** | 80.18*** |
| Canopy Position (C) | 1 | 0.839 | 122.08*** | 25.97*** | 25.89*** | 25.93*** | 25.92*** |
| Age (A) | 1 | 152.71*** | 438.32*** | 1.483 | 1.475 | 1.470 | 1.483 |
| H*C | 1 | 16.37** | 97.85*** | 0.254 | 0.258 | 0.220 | 0.261 |
| H*A | 1 | 89.82*** | 54.78*** | 3.498 | 3.481 | 3.457 | 3.468 |
| C*A | 1 | 14.07** | 32.90*** | 2.658 | 2.656 | 2.666 | 2.666 |
| H*C*A | 1 | 0.637 | 7.88* | 2.505 | 2.498 | 2.562 | 2.509 |
| Error | 16 |
| Source of Variation | df | Carbon (%) | Nitrogen (%) | C Isotope Composition | C Isotope Discrimination | Ci/Ca Ratio | iWUE |
|---|---|---|---|---|---|---|---|
| Habitat (H) | 1 | 12.54** | 90.88*** | 3097*** | 3105*** | 3025*** | 3105*** |
| Canopy Position (C) | 1 | 2.026 | 130.96*** | 3.155 | 3.233 | 3.422 | 3.233 |
| H*C | 1 | 9.672* | 639.06*** | 34.65*** | 34.81*** | 33.58*** | 34.81*** |
| Error | 8 |
| Source of Variation | df | Carbon (%) | Nitrogen (%) | C Isotope Composition | C Isotope Discrimination | Ci/Ca Ratio | iWUE |
|---|---|---|---|---|---|---|---|
| Species (Sp) | 2 | 286.73*** | 7737*** | 743.1*** | 742.12*** | 722.91*** | 742.12*** |
| Canopy Position (C) | 1 | 80.92*** | 74.1*** | 147.99*** | 147.64*** | 145.23*** | 147.64*** |
| SP * C | 2 | 7.948** | 118.83*** | 130.26*** | 130.28*** | 125.79*** | 130.28*** |
| Error | 12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, M.I.; El-Keblawy, A.; Mitterand Tsombou, F. Leaf Age, Canopy Position, and Habitat Affect the Carbon Isotope Discrimination and Water-Use Efficiency in Three C3 Leguminous Prosopis Species from a Hyper-Arid Climate. Plants 2019, 8, 402. https://doi.org/10.3390/plants8100402
Hussain MI, El-Keblawy A, Mitterand Tsombou F. Leaf Age, Canopy Position, and Habitat Affect the Carbon Isotope Discrimination and Water-Use Efficiency in Three C3 Leguminous Prosopis Species from a Hyper-Arid Climate. Plants. 2019; 8(10):402. https://doi.org/10.3390/plants8100402
Chicago/Turabian StyleHussain, M. Iftikhar, Ali El-Keblawy, and François Mitterand Tsombou. 2019. "Leaf Age, Canopy Position, and Habitat Affect the Carbon Isotope Discrimination and Water-Use Efficiency in Three C3 Leguminous Prosopis Species from a Hyper-Arid Climate" Plants 8, no. 10: 402. https://doi.org/10.3390/plants8100402
APA StyleHussain, M. I., El-Keblawy, A., & Mitterand Tsombou, F. (2019). Leaf Age, Canopy Position, and Habitat Affect the Carbon Isotope Discrimination and Water-Use Efficiency in Three C3 Leguminous Prosopis Species from a Hyper-Arid Climate. Plants, 8(10), 402. https://doi.org/10.3390/plants8100402







