Aminoacids and Flavonoids Profiling in Tempranillo Berries Can Be Modulated by the Arbuscular Mycorrhizal Fungi
Abstract
:1. Introduction
2. Results and Discussion
2.1. Plant and Berry Traits
2.2. Anthocyanins and Flavonols in Berry Skin
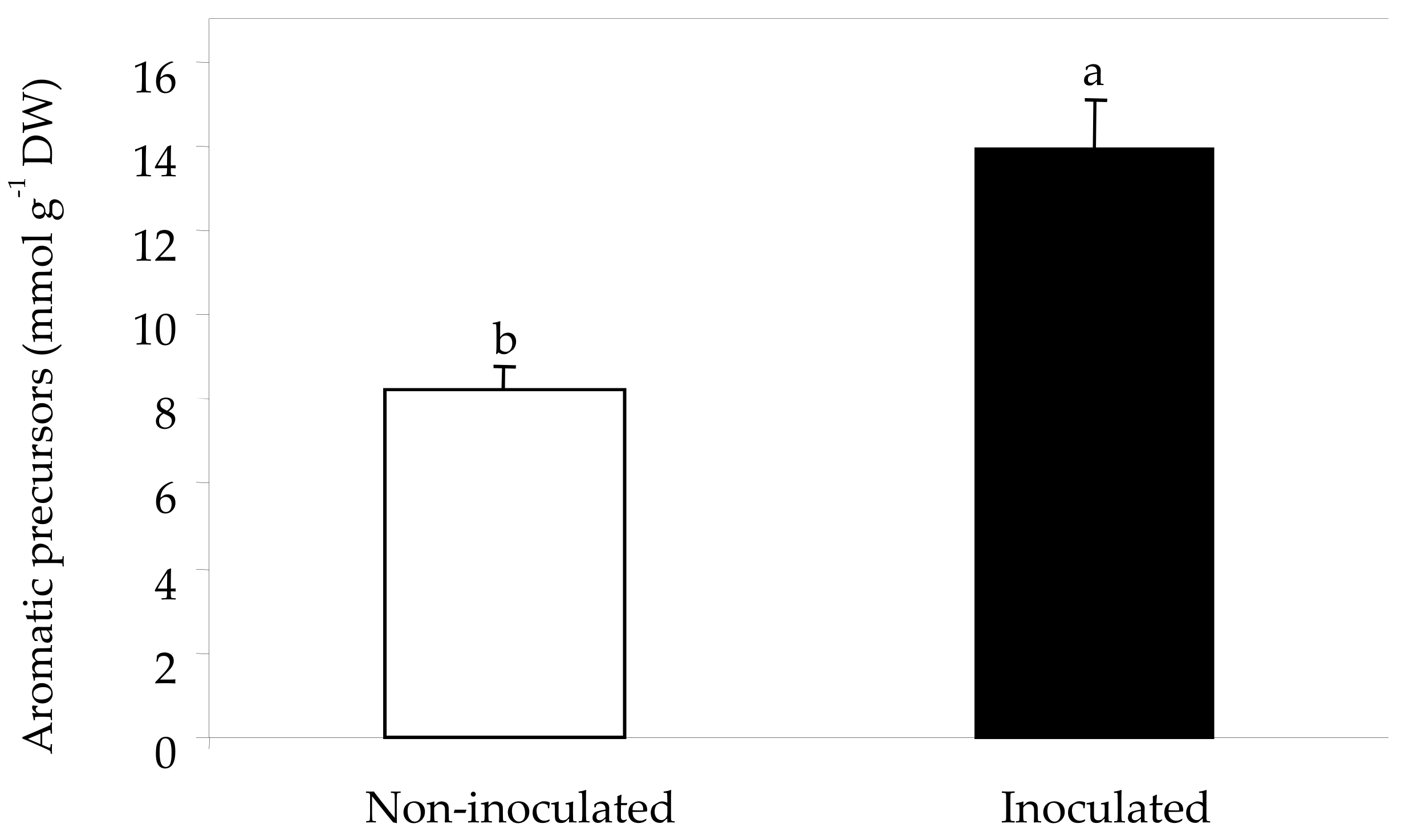
2.3. Sugars, Organic Acids and Amino Acids in Berry Skin
3. Materials and Methods
3.1. Biological Material and Growth Conditions
3.2. Determination of Plant and Berry Traits
3.2.1. Plant Traits
3.2.2. Berry Traits
3.3. Berry Skin Metabolites
3.3.1. Anthocyanins and Flavonols
3.3.2. Sugars, Organic Acids and Amino Acids
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Balestrini, R.; Magurno, F.; Walker, C.; Lumini, E.; Bianciotto, V. Cohorts of arbuscular mycorrhizal fungi (AMF) in Vitis vinifera, a typical Mediterranean fruit crop. Environ. Microbiol. Rep. 2010, 3, 594–604. [Google Scholar] [CrossRef]
- Ocete, R.; Armendáriz, I.; Cantos, M.; Álvarez, D.; Azcón, R. Ecological characterization of wild grapevine habitats focused on arbuscular mycorrhizal symbiosis. Vitis 2015, 54, 207–211. [Google Scholar]
- Torres, N.; Antolín, M.C.; Goicoechea, N. Arbuscular mycorrhizal symbiosis as a promising resource for improving berry quality in grapevines under changing environments. Front. Plant. Sci. 2018, 9, 897. [Google Scholar] [CrossRef]
- Schreiner, R.P. Effects of native and non-native arbuscular mycorrhizal fungi on growth and nutrient uptake of ‘Pinot noir’ (Vitis vinifera L.) in two soils with contrasting levels of phosphorus. Appl. Soil Ecol. 2007, 36, 205–215. [Google Scholar] [CrossRef]
- Nikolaou, N.A.; Koukourikou, M.; Angelopoulos, K.; Karagiannidis, N. Cytokinin content and water relations of ‘Cabernet Sauvignon’ grapevine exposed to drought stress. J. Hortic. Sci. Biotechnol. 2003, 78, 113–118. [Google Scholar] [CrossRef]
- Nogales, A.; Aguirreolea, J.; María, E.S.; Camprubí, A.; Calvet, C. Response of mycorrhizal grapevine to Armillaria mellea inoculation: Disease development and polyamines. Plant. Soil 2009, 317, 177–187. [Google Scholar] [CrossRef]
- Bona, E.; Lingua, G.; Todeschini, V. Effect of Bioinoculants on the Quality of Crops. In Bioformulations: For Sustainable Agriculture; Arora, N., Mehnaz, S., Balestrini, R., Eds.; Springer: New Delhi, India, 2016; pp. 93–124. [Google Scholar]
- Avio, L.; Turrini, A.; Giovannetti, M.; Sbrana, C. Designing the ideotype mycorrhizal symbionts for the production of healthy food. Front. Plant. Sci. 2018, 9, 1089. [Google Scholar] [CrossRef]
- OIV Focus 2017. Vine Varieties Distribution in the World. Available online: http://www.oiv.int/public/medias/5336/infographie-focus-oiv-2017-new.pdf (accessed on 16 March 2018).
- OIV 2018. OIV Report on the World Vitivinicultural Situation. Available online: http://www.oiv.int/public/medias/6372/oiv-report-on-the-world-vitivinicultural-situation-2018.pdf (accessed on 18 February 2019).
- Torres, N.; Antolín, M.C.; Garmendia, I.; Goicoechea, N. Nutritional properties of Tempranillo grapevine leaves are affected by clonal diversity, mycorrhizal symbiosis and air temperature regime. Plant. Physiol. Biochem. 2018, 130, 542–554. [Google Scholar] [CrossRef]
- Torres, N.; Goicoechea, N.; Morales, F.; Antolín, M.C. Berry quality and antioxidant properties in Vitis vinifera L. cv. Tempranillo as affected by clonal variability, mycorrhizal inoculation and temperature. Crop. Pasture Sci. 2016, 67, 961–977. [Google Scholar] [CrossRef]
- Torres, N.; Goicoechea, N.; Antolín, M.C. Influence of irrigation strategy and mycorrhizal inoculation on fruit quality in different clones of Tempranillo grown under elevated temperatures. Agric. Water Manag. 2018, 202, 285–298. [Google Scholar] [CrossRef]
- Torres, N.; Zamarreño, A.; Goicoechea, N.; Antolín, M.C. Changes in ABA conjugation/catabolism could account for the effects of AMF inoculation on Tempranillo (Vitis vinifera L.) fruit quality under climate change scenarios. Plant. Sci. 2018, 274, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.; Maltese, F.; Choi, Y.H.; Verpoorte, R. Metabolic constituents of grapevine and grape-derived products. Phytochem. Rev. 2010, 9, 357–378. [Google Scholar] [CrossRef] [PubMed]
- Mollavali, M.; Perner, H.; Rohn, S.; Riehle, P.; Hanschen, F.S.; Schwarz, D. Nitrogen form and mycorrhizal inoculation amount and timing affect flavonol biosynthesis in onion (Allium cepa L.). Mycorrhiza 2018, 28, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Kallithraka, S.; Aliaj, L.; Makris, D.P.; Kefalas, P. Anthocyanin profiles of major red grape (Vitis vinifera L.) varieties cultivated in Greece and their relationship with in vitro antioxidant characteristics. Int. J. Food Sci. Technol. 2009, 44, 2385–2393. [Google Scholar] [CrossRef]
- Niculcea, M.; Martínez-Lapuente, L.; Guadalupe, Z.; Sánchez-Díaz, M.; Ayestarán, B.; Antolín, M.C. Characterization of phenolic composition of Vitis vinifera L. “Tempranillo” and “Graciano” subjected to deficit irrigation during berry development. Vitis 2015, 54, 9–16. [Google Scholar]
- Torres, N.; Hilbert, G.; Luquin, J.; Goicoechea, N.; Antolín, M.C. Flavonoid and amino acid profiling on Vitis vinifera L. cv Tempranillo subjected to deficit irrigation under elevated temperatures. J. Food Comp. Anal. 2017, 62, 51–62. [Google Scholar] [CrossRef]
- Jiménez, A.; Lisa-Santamaría, P.; García-Marino, M.; Escribano-Bailón, M.T.; Rivas-Gonzalo, J.C.; Revuelta, J.L. The biological activity of the wine anthocyanins delphinidin and petunidin is mediated through Msn2 and Msn4 in Saccharomyces cerevisiae. FEMS Yeast Res. 2010, 10, 858–869. [Google Scholar] [CrossRef]
- Castellanos-Morales, V.; Villegas, J.; Wendelin, S.; Vierheiling, H.; Eder, R.; Cárdenas-Navarro, R. Root colonisation by the arbuscular mycorrhizal fungus Glomus intraradices alters the quality of strawberry fruits (Fragaria × ananassa Duch.) at different nitrogen levels. J. Sci. Food Agric. 2010, 90, 1774–1782. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Torres, N.; Hilbert, G.; Richard, T.; Sánchez-Díaz, M.; Delrot, S.; Aguirreolea, J.; Pascual, I.; Gòmes, E. Ultraviolet-B radiation modifies the quantitative and qualitative profile of flavonoids and amino acids in grape berries. Phytochemistry 2014, 102, 106–114. [Google Scholar] [CrossRef]
- Del Castillo-Alonso, M.A.; Diago, M.P.; Tomás-Las-Heras, R.; Monforte, L.; Soriano, G.; Martínez-Abaigar, J.; Núñez-Olivera, E. Effects of ambient solar UV radiation on grapevine leaf physiology and berry phenolic composition along one entire season under Mediterranean field conditions. Plant. Physiol. Biochem. 2016, 109, 374–386. [Google Scholar] [CrossRef]
- Boulton, R. The copigmentation of anthocyanins and its role in the color of red wine. A critical review. Am. J. Enol. Vitic. 2001, 52, 67–87. [Google Scholar]
- Mollavali, M.; Bolandnazar, S.A.; Schwarz, D.; Rohn, S.; Riehle, P.; Nahandi, F.Z. Flavonol glucoside and antioxidant enzyme biosynthesis affected by mycorrhizal fungi in various cultivars of onion (Allium cepa L.). J. Agric. Food Chem. 2016, 64, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Zouari, I.; Salvioli, A.; Chialva, M.; Novero, M.; Miozzi, L.; Tenore, G.C.; Bagnaresi, P.; Bonfante, P. From root to fruit: RNA-Seq analysis shows that arbuscular mycorrhizal symbiosis may affect tomato fruit metabolism. BMC Genomics 2014, 15, 221. [Google Scholar] [CrossRef] [PubMed]
- Portu, J.; López, R.; González-Arenzana, L.; López-Alfaro, I.; Santamaría, P.; Garde-Cerdán, T. Amino acid content in red wines obtained from grapevine nitrogen foliar treatments: Consumption during the alcoholic fermentation. Wine Studies 2014, 3, 4475. [Google Scholar] [CrossRef]
- Hannam, K.D.; Neilsen, G.H.; Neilsen, D.; Midwood, A.J.; Millard, P.; Zhang, Z.; Thornton, B.; Steinkes, D. Amino acid composition of grape (Vitis vinifera L.) juice in response to applications of urea to the soil or foliage. Am. J. Enol. Vitic. 2016, 67, 47–55. [Google Scholar] [CrossRef]
- Scandellari, F. Arbuscular mycorrhizal contribution to nitrogen uptake of grapevines. Vitis 2017, 56, 147–154. [Google Scholar]
- Tian, C.; Kasiborski, B.; Koul, R.; Lammers, P.J.; Bucking, H.; Shachar-Hill, Y. Regulation of the nitrogen transfer pathway in the arbuscular mycorrhizal symbiosis: Gene characterization and the coordination of expression with nitrogen flux. Plant. Physiol. 2010, 153, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, M.D.; Garcia, M.O.; Treseder, K.K. Amino acid uptake in arbuscular mycorrhizal plants. PLoS ONE 2012, 7, e47643. [Google Scholar] [CrossRef] [PubMed]
- Salvioli, A.; Zouari, I.; Chalot, M.; Bonfante, P. The arbuscular mycorrhizal status has an impact on the transcriptome profile and amino acid composition of tomato fruit. BMC Plant. Biol. 2012, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Orte, P.; Cacho, J.; Ferreira, V. Relationship between varietal amino acid profile of grapes and wine aromatic composition: Experiments with model solutions and chemometric study. J. Agric. Food Chem. 2002, 50, 2891–2899. [Google Scholar] [CrossRef]
- Ju, Y.-I.; Xu, G.; Yue, X.; Zhao, X.; Tu, T.; Zhang, J.; Fang, Y. Effects of regulated deficit irrigation on amino acid profiles and their derived volatile compounds in Cabernet Sauvignon (Vitis vinifera L.) grapes and wines. Molecules 2018, 23, 1983. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; Prats-Moya, M.S. Free amino acids and biogenic amines in Alicante Monastrell wines. Food Chem. 2012, 135, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-Y.; Yang, Y.-P.; Peng, Q.; Han, Y. Biogenic amines in wine: A review. Int. J. Food Sci. Technol. 2015, 50, 1523–1532. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Ye, D.Q.; Zhu, B.Q.; Wu, G.F.; Duan, C.Q. Rapid HPLC analysis of amino acids and biogenic amines in wines during fermentation and evaluation of matrix effect. Food Chem. 2014, 163, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Valdés, M.E.; Talaverano, M.I.; Moreno, D.; Prieto, M.H.; Mancha, L.A.; Uriarte, D.; Vilanova, M. Effect of the timing of water deficit on the must amino acid profile of Tempranillo grapes grown under the semiarid conditions of SW Spain. Food Chem. 2019, 292, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Mullins, M.G. Test-plants for investigations of the physiology of fruiting in Vitis vinifera L. Nature 1966, 209, 419–420. [Google Scholar] [CrossRef]
- Ollat, N.; Gény, L.; Soyer, J.P. Les boutures fructifères de vigne: Validation d’un modèle d’étude de la physiologie de la vigne. I. Principales caractéristiques de l’appareil végétatif. J. Int. Sci. Vigne Vin. 1998, 32, 1–9. [Google Scholar]
- Antolín, M.C.; Santesteban, H.; Ayari, M.; Aguirreolea, J.; Sánchez-Díaz, M. Grapevine fruiting cuttings: An experimental system to study grapevine physiology under water deficit conditions. In Methodologies and Results in Grapevine Research; Delrot, S., Medrano Gil, H., Or, E., Bavaresco, L., Grando, S., Dordrecht, B.V., Eds.; Springer Science & Business Media: Amsterdam, The Netherlands, 2010; pp. 151–163. [Google Scholar]
- Agnolucci, M.; Battini, F.; Cristani, C.; Giovannetti, M. Diverse bacterial communities are recruited on spores of different arbuscular mycorrhizal fungal isolates. Biol. Fertil. Soils 2015, 51, 379–389. [Google Scholar] [CrossRef]
- Trotel-Aziz, P.; Abou-Mansour, E.; Courteaux, B.; Rabenoelina, F.; Clément, C.; Fontaine, F.; Aziz, A. Bacillus subtilis PTA-271 counteracts botryosphaeria dieback in grapevine, triggering immune responses and detoxification of fungal phytotoxins. Front. Plant. Sci. 2019, 10, 25. [Google Scholar] [CrossRef]
- AEMET (Agencia Estatal de Meteorología). Ministerio de Agricultura, Alimentación y Medio Ambiente, Spain. Available online: http://www.aemet.es/es/ (accessed on 15 January 2017).
- Passioura, J.B. The perils of pot experiments. Funct. Plant. Biol. 2006, 33, 1075–1079. [Google Scholar] [CrossRef]
- Poorter, H.; Bühler, J.; van Dusschoten, D.; Climent, J.; Postma, J.A. Pot size matters: A meta-analysis of the effects of rooting volume on plant growth. Funct. Plant. Biol. 2012, 39, 839–850. [Google Scholar] [CrossRef]
- Coombe, B.G. Adoption of a system for identifying grapevine growth stages. Aust. J. Grape Wine Res. 1995, 1, 104–110. [Google Scholar] [CrossRef]
- Koske, R.E.; Gemma, J.N. A modified procedure for staining roots to detect VA mycorrhizas. Mycol. Res. 1989, 92, 486–488. [Google Scholar] [CrossRef]
- Giovanetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytol. 1980, 87, 489–500. [Google Scholar] [CrossRef]
- OIV 2018. Compendium of International Methods of Analysis of Wines and Musts; Organisation of Vine and Wine Paris: Paris, France, 2018; Volume I, ISBN 979-10-91799-80-5. [Google Scholar]
- Revilla, E.; Ryan, J.-M.; Martín-Ortega, G. Comparison of several procedures used for the extraction of anthocyanins from red grapes. J. Agric. Food Chem. 1998, 46, 4592–4597. [Google Scholar] [CrossRef]
- Lima, A.; Bento, A.; Baraldi, I.; Malheiro, R. Selection of grapevine leaf varieties for culinary process based on phytochemical composition and antioxidant properties. Food Chem. 2016, 212, 291–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acevedo De la Cruz, A.; Hilbert, G.; Rivière, C.; Mengin, V.; Ollat, N.; Bordenave, L.; Decroocq, S.; Delaunay, J.-C.; Delrot, S.; Mérillon, J.-M.; et al. Anthocyanin identification and composition of wild Vitis spp. accessions by using LC–MS and LC–NMR. Anal. Chim. Acta 2012, 732, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Hilbert, G.; Temsamani, H.; Bordenave, L.; Pedrot, E.; Chaher, N.; Cluzet, S.; Delaunay, J.C.; Ollat, N.; Delrot, S.; Mérillon, J.M.; et al. Flavonol profiles in berries of wild Vitis accessions using liquid chromatography coupled to mass spectrometry and nuclear magnetic resonance spectrometry. Food Chem. 2015, 169, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Bobeica, N.; Poni, S.; Hilbert, G.; Renaud, C.; Gomès, E.; Delrot, S.; Dai, Z. Differential responses of sugar, organic acids and anthocyanins to source sink modulation in Cabernet Sauvignon and Sangiovese grapevines. Front. Plant. Sci. 2015, 6, 382. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.E.; Gaudillere, J.P.; Pieri, P.; Hilbert, G.; Maucourt, M.; Deborde, C.; Moing, A.; Roin, D. Microclimate influence on mineral and metabolic profiles of grape berries. J. Agric. Food Chem. 2006, 54, 6765–6775. [Google Scholar] [CrossRef] [PubMed]
- Hilbert, G.; Soyer, J.P.; Molot, C.; Giraudon, J.; Milin, S.; Gaudillère, J.P. Effects of nitrogen supply on must quality and anthocyanin accumulation in berries of cv. Merlot. Vitis 2003, 42, 69–76. [Google Scholar]
- Habran, A.; Commisso, M.; Helwi, P.; Hilbert, G.; Negri, S.; Ollat, N.; Gomès, E.; van Leeuwen, C.; Guzzo, F.; Delrot, S. Roostocks/Scion/Nitrogen interactions affect secondary metabolism in the grape berry. Front. Plant. Sci. 2016, 7, 1134. [Google Scholar] [CrossRef] [PubMed]

| Non-Inoculated | Inoculated | |
|---|---|---|
| Plant traits | ||
| Mycorrhization (%) | - | 31.5 |
| Leaf area (m2 plant−1) | 0.79 a | 0.84 a |
| Bunch mass (g plant−1) | 182.3 a | 198.5 a |
| Berry traits | ||
| Berry mass (g berry−1) | 1.24 a | 1.22 a |
| Relative skin mass (% berry FW) | 33.3 a | 23.9 b |
| Total soluble solids (°Brix) | 22.8 a | 21.9 a |
| Must pH | 3.7 a | 3.6 a |
| Titratable acidity (g L−1) | 7.11 a | 6.94 a |
| Total phenolic compounds (mg g−1 DW) | 62.24 b | 84.17 a |
| Color density (AU) | 20.9 a | 20.4 a |
| Tonality index | 0.53 a | 0.52 a |
| Non-Inoculated | Inoculated | |
|---|---|---|
| Secondary metabolites | ||
| Anthocyanins (mg g−1 DW) | 36.3 a | 34.8 a |
| Flavonols (mg g−1 DW) | 1.01 a | 0.96 a |
| Primary metabolites | ||
| Glucose (mg g−1 DW) | 98.9 b | 147.3 a |
| Fructose (mg g−1 DW) | 100.1 a | 129.5 a |
| Malic acid (mg g−1 DW) | 19.9 a | 28.4 a |
| Tartaric acid (mg g−1 DW) | 22.8 a | 29.4 a |
| Total amino acids (mmol g−1 DW) | 89.4 b | 129.3 a |
| Concentration (mg g−1 DW) | ||
|---|---|---|
| Non-Inoculated | Inoculated | |
| Anthocyanins | ||
| 3-Monoglucosides | ||
| Delphinidin | 8.79 a | 7.90 a |
| Cyanidin | 3.62 a | 2.99 a |
| Petunidin | 5.88 a | 5.43 a |
| Peonidin | 4.42 a | 4.82 a |
| Malvidin | 11.16 a | 11.02 a |
| 3-Acetyl-glucosides | ||
| Delphinidin | 0.36 a | 0.26 b |
| Petunidin | 0.25 a | 0.19 a |
| Malvidin | 0.37 a | 0.36 a |
| 3 p-Coumaroyl-glucosides | ||
| Peonidin | 0.28 a | 0.38 a |
| Malvidin | 1.49 a | 1.39 a |
| Flavonols | ||
| Myricetin-3-O-glucoside | 0.43 a | 0.48 a |
| Quercetin-3-O-galactoside | 0.02 b | 0.04 a |
| Quercetin-3-O-glucoside | 0.13 a | 0.08 b |
| Laricitrin-3-O-glucoside | 0.05 a | 0.06 a |
| Kaempferol-3-O-glucoside | 0.22 a | 0.22 a |
| Isorhamnetin-3-O-glucoside | 0.09 a | 0.08 a |
| Precursor | Amino Acid | Concentration (mmol g−1 DW) | |
|---|---|---|---|
| Non-Inoculated | Inoculated | ||
| 3-Phosphoglycerate | Glycine | 0.31 a | 0.26 a |
| Serine | 1.89 b | 2.91 a | |
| Phosphoenolpyruvate | Tyrosine | 0.21 b | 0.49 a |
| Phenylalanine | 0.29 b | 0.57 a | |
| Oxaloacetate | Aspartic acid | 2.33 a | 3.86 a |
| Asparagine | 2.35 b | 5.03 a | |
| Threonine | 4.65 b | 7.49 a | |
| Methionine | 0.01 a | 0.01 a | |
| Isoleucine | 0.20 b | 0.42 a | |
| α-ketoglutarate | Glutamic acid | 2.62 b | 5.53 a |
| Glutamine | 6.84 a | 8.16 a | |
| Histidine | 0.91 a | 0.99 a | |
| Arginine | 25.97 b | 39.65 a | |
| γ-aminobutyric acid | 3.40 b | 5.10 a | |
| Proline | 38.35 a | 43.53 a | |
| Pyruvate | Alanine | 3.88 b | 6.83 a |
| Valine | 0.54 b | 1.12 a | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, N.; Hilbert, G.; Antolín, M.C.; Goicoechea, N. Aminoacids and Flavonoids Profiling in Tempranillo Berries Can Be Modulated by the Arbuscular Mycorrhizal Fungi. Plants 2019, 8, 400. https://doi.org/10.3390/plants8100400
Torres N, Hilbert G, Antolín MC, Goicoechea N. Aminoacids and Flavonoids Profiling in Tempranillo Berries Can Be Modulated by the Arbuscular Mycorrhizal Fungi. Plants. 2019; 8(10):400. https://doi.org/10.3390/plants8100400
Chicago/Turabian StyleTorres, Nazareth, Ghislaine Hilbert, María Carmen Antolín, and Nieves Goicoechea. 2019. "Aminoacids and Flavonoids Profiling in Tempranillo Berries Can Be Modulated by the Arbuscular Mycorrhizal Fungi" Plants 8, no. 10: 400. https://doi.org/10.3390/plants8100400
APA StyleTorres, N., Hilbert, G., Antolín, M. C., & Goicoechea, N. (2019). Aminoacids and Flavonoids Profiling in Tempranillo Berries Can Be Modulated by the Arbuscular Mycorrhizal Fungi. Plants, 8(10), 400. https://doi.org/10.3390/plants8100400




