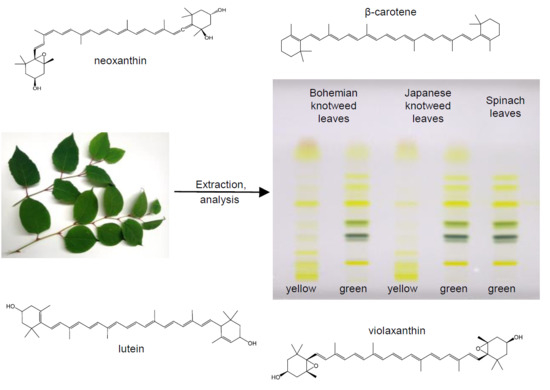
Japanese and Bohemian Knotweeds as Sustainable Sources of Carotenoids
Abstract
1. Introduction
2. Results and Discussion
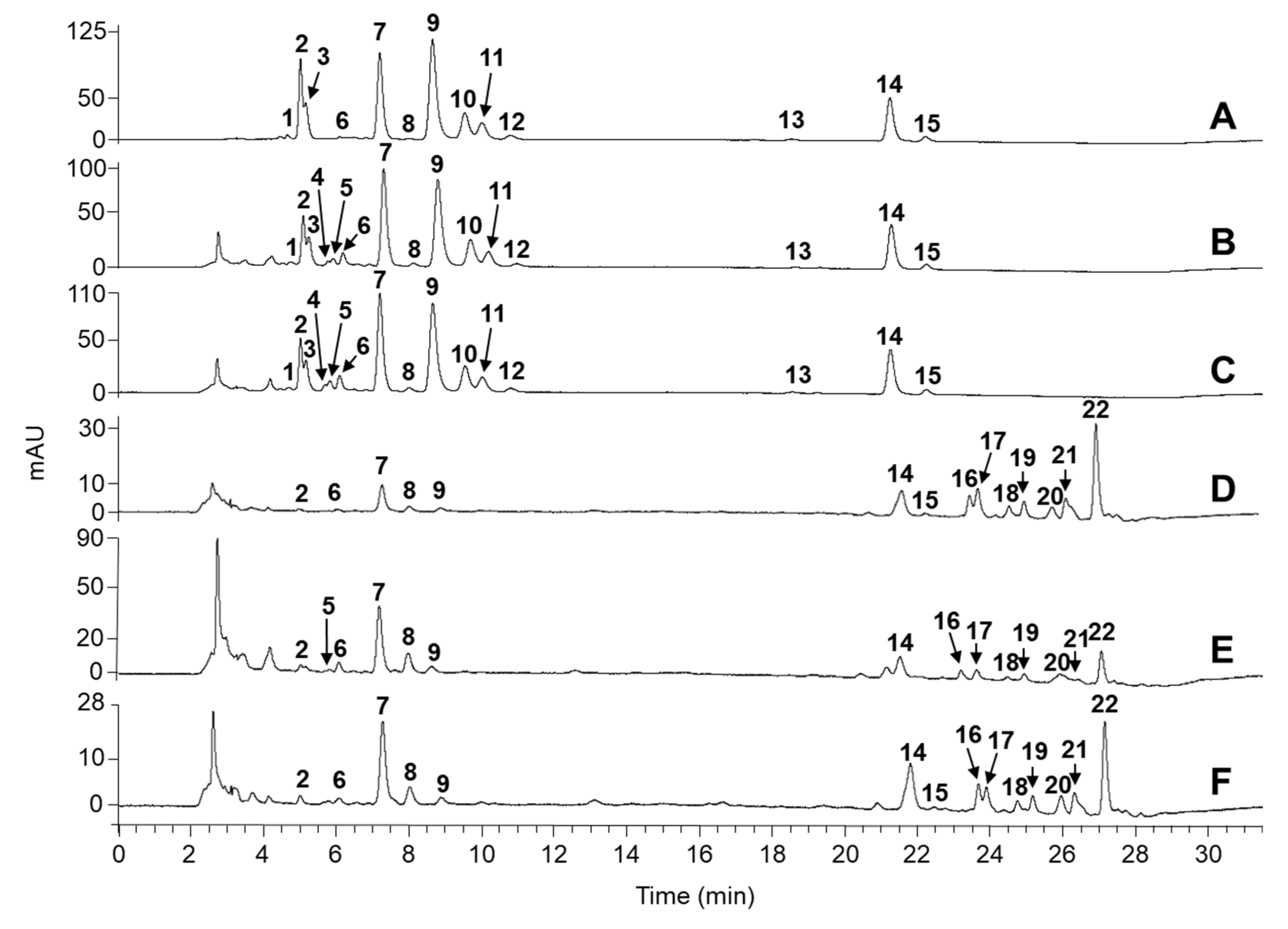
2.1. Identification of Carotenoids in Knotweed Leaf Extracts
2.2. Quantitation of Carotenoids
3. Materials and Methods
3.1. Chemicals and Standards
3.2. Preparation of Carotenoid Standard Solutions
3.3. Plant Materials and Preparation of Leaf Extract Solutions for the Analysis of Carotenoids and Fatty Acids
3.4. HPTLC with Densitometry Analysis
3.5. HPTLC–MSn Analysis
3.6. HPLC–PDA Analysis
3.7. HPLC–MSn Analysis
3.8. GC–MS Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gammon, M.A.; Grimsby, J.L.; Tsirelson, D.; Kesseli, R. Molecular and morphological evidence reveals introgression in swarms of the invasive taxa Fallopia japonica, F. sachalinensis, and F. xbohemica (Polygonaceae) in the United States. Am. J. Bot. 2007, 94, 948–956. [Google Scholar] [PubMed]
- Lavoie, C. The impact of invasive knotweed species (Reynoutria spp.) on the environment: Review and research perspectives. Biol. Invasions 2017, 19, 2319–2337. [Google Scholar] [CrossRef]
- Murrell, C.; Gerber, E.; Krebs, C.; Parepa, M.; Schaffner, U.; Bossdorf, O. Invasive knotweed affects native plants through allelopathy. Am. J. Bot. 2011, 98, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Balogh, L. Japanese, Giant and Bohemian knotweed (Fallopia japonica, Fallopia sachalinensis and Fallopia × bohemica). In The Most Important Invasive Plants in Hungary; Botta-Dukát, Z., Balogh, L., Eds.; Hungarian Academy of Sciences, Institute of Ecology and Botany: Vácrátót, Hungary, 2008; pp. 13–33. [Google Scholar]
- Delbart, E.; Mahy, G.; Weickmans, B.; Henriet, F.; Crémer, S.; Pieret, N.; Vanderhoeven, S.; Monty, A. Can land managers control Japanese knotweed? Lessons from control tests in Belgium. Environ. Manag. 2012, 50, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Bashtanova, U.B.; Beckett, K.P.; Flowers, T.J. Review: Physiological approaches to the improvement of chemical control of Japanese knotweed (Fallopia Japonica). Weed Sci. 2009, 57, 584–592. [Google Scholar] [CrossRef]
- Green, S. A review of the potential for the use of bioherbicides to control forest weeds in the UK. Forestry 2003, 76, 285–298. [Google Scholar] [CrossRef]
- Shaw, R.H.; Ellison, C.A.; Marchante, H.; Pratt, C.F.; Schaffner, U.; Sforza, R.F.H.; Deltoro, V. Weed biological control in the European Union: From serendipity to strategy. BioControl 2018, 63, 333–347. [Google Scholar] [CrossRef]
- Grevstad, F.; Shaw, R.; Bourchier, R.; Sanguankeo, P.; Cortat, G.; Reardon, R.C. Efficacy and host specificity compared between two populations of the psyllid Aphalara itadori, candidates for biological control of invasive knotweeds in North America. Biol. Control 2013, 65, 53–62. [Google Scholar] [CrossRef]
- Mundo, M.A.; Padilla-Zakour, O.I.; Worobo, R.W. Growth inhibition of foodborne pathogens and food spoilage organisms by select raw honeys. Int. J. Food Microbiol. 2004, 97, 1–8. [Google Scholar] [CrossRef]
- Patocka, J.; Navratilova, Z.; Ovando-Martinez, M. Review: Biologically active compounds of knotweed (Reynoutria spp.). Mil. Med. Sci. Let. 2017, 86, 17–31. [Google Scholar] [CrossRef]
- Zaki El-Readi, M.; Yehia Eid, S.; Saeed Al-Amodi, H.; Wink, M. Fallopia Japonica: Bioactive secondary metabolites and molecular mode of anticancer. J. Tradit. Med. Clin. Naturop. 2016, 5, 193–213. [Google Scholar] [CrossRef]
- Glavnik, V.; Vovk, I.; Albreht, A. High performance thin-Llayer chromatography–mass spectrometry of Japanese knotweed flavan-3-ols and proanthocyanidins on Silica Gel Plates. J. Chromatogr. A 2017, 1482, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Piola, F.; Bellvert, F.; Meiffren, G.; Rouifed, S.; Walker, V.; Comte, G.; Bertrand, C. Invasive Fallopia × Bohemica interspecific hybrids display different patterns in econdary metabolites. Écoscience 2013, 20, 230–239. [Google Scholar] [CrossRef]
- Glavnik, V.; Vovk, I. High performance thin-layer chromatography–mass spectrometry methods on diol stationary phase for the analyses of flavan-3-ols and proanthocyanidins in invasive Japanese knotweed. J. Chromatogr. A 2019, 1598, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, J. Carotenoid biosynthesis in flowering plants. Curr. Opin. Plant Biol. 2001, 4, 210–218. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef]
- Jahns, P.; Holzwarth, A.R. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 182–193. [Google Scholar] [CrossRef]
- Mares, J. Lutein and zeaxanthin isomers in eye health and disease. Annu. Rev. Nutr. 2016, 36, 571–602. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta Mol. Basis Dis. 2005, 1740, 101–107. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Z.; Sun, P.; Chen, T.; Chen, F. Microalgal carotenoids: Beneficial effects and potential in human health. Food Funct. 2014, 5, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Bonet, M.L.; Canas, J.A.; Ribot, J.; Palou, A. Carotenoids and their conversion products in the control of adipocyte function, adiposity and obesity. Arch. Biochem. Biophys. 2015, 572, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Torregrosa-Crespo, J.; Montero, Z.; Fuentes, J.L.; García-Galbis, M.R.; Garbayo, I.; Vílchez, C.; Martínez-Espinosa, R.M. Exploring the valuable carotenoids for the large-scale production by marine microorganisms. Mar. Drugs 2018, 16, 203. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.H.B.; Abdelaziz, S.; Al Yousef, H.M. Chemical composition and biological activities of the aqueous fraction of Parkinsonea Aculeata L. growing in Saudi Arabia. Arab. J. Chem. 2018, 12, 377–387. [Google Scholar] [CrossRef]
- Cardini, F. Carotenoids in ripe green and in autumn senescing leaves of apple tree: II—Seasonal changes of free carotenoids and xanthophyll esters and relationship between their content and senescing stage. Plant Biosyst. 1983, 117, 75–97. [Google Scholar] [CrossRef]
- Deli, J.; Ősz, E. Carotenoid 5,6-, 5,8- and 3,6-epoxides. Arkivoc 2004, 7, 150–168. [Google Scholar]
- Curl, A.L.; Bailey, G.F. Carotenoid epoxide detection: An improved test for carotenoid epoxides. J. Agric. Food Chem. 1961, 9, 403–405. [Google Scholar] [CrossRef]
- Kopec, R.E.; Cooperstone, J.L.; Chichon, M.J.; Schwartz, S.J. Analysis methods of carotenoids. In Analysis of Antioxidant-Rich Phytochemicals, 1st ed.; Xu, Z., Howard, L.R., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 105–148. [Google Scholar]
- Bell, C.M.; Sander, L.C.; Wise, S.A. Temperature dependence of carotenoids on C18, C30, and C34 bonded stationary hases. J. Chromatogr. A 1997, 757, 29–39. [Google Scholar] [CrossRef]
- Jing, C.; Qun, X.; Rohrer, J. HPLC separation of all-trans-β-carotene and its iodine-induced isomers using a C30 column. Thermo Scientific 2012, 391, 2–6. [Google Scholar]
- Pérez-Gálvez, A.; Mínguez-Mosquera, M.I. Esterification of xanthophylls and its effect on chemical behavior and bioavailability of carotenoids in the human. Nutr. Res. 2005, 25, 631–640. [Google Scholar] [CrossRef]
- Khoo, H.E.; Prasad, K.N.; Kong, K.W.; Jiang, Y.; Ismail, A. Carotenoids and their isomers: Color pigments in fruits and vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz, S.; Oszmiański, J.; Wojdyło, A.; Cebulak, T.; Hirnle, L.; Siewiński, M. UPLC-PDA-Q/TOF-MS identification of bioactive compounds and on-line UPLC-ABTS assay in Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) leaves and rhizomes grown in Poland. Eur. Food Res. Technol. 2019, 245, 691–706. [Google Scholar] [CrossRef]
- Amorim-Carrilho, K.T.; Cepeda, A.; Fente, C.; Regal, P. Review of methods for analysis of carotenoids. TrAC Trends Anal. Chem. 2014, 56, 49–73. [Google Scholar] [CrossRef]
- Rodić, Z.; Simonovska, B.; Albreht, A.; Vovk, I. Determination of lutein by high-performance thin-layer chromatography using densitometry and screening of major dietary carotenoids in food supplements. J. Chromatogr. A 2012, 1231, 59–65. [Google Scholar] [CrossRef]
- Rivera, S.M.; Christou, P.; Canela-Garayoa, R. Identification of carotenoids using mass spectrometry. Mass Spectrom. Rev. 2014, 33, 353–372. [Google Scholar] [CrossRef]
- Petry, F.C.; Mercadante, A.Z. Composition by LC-MS/MS of new carotenoid esters in mango and citrus. J. Agric. Food Chem. 2016, 64, 8207–8224. [Google Scholar] [CrossRef]
- Aparicio-Ruiz, R.; Riedl, K.M.; Schwartz, S.J. Identification and quantification of metallo-chlorophyll complexes in bright green table olives by high-performance liquid chromatrography-mass spectrometry quadrupole/time-of-flight. J. Agric. Food Chem. 2011, 59, 11100–11108. [Google Scholar] [CrossRef]
- Wen, X.; Hempel, J.; Schweiggert, R.M.; Ni, Y.; Carle, R. Carotenoids and carotenoid esters of red and yellow Physalis (Physalis alkekengi L. and P. Pubescens L.) fruits and calyces. J. Agric. Food Chem. 2017, 65, 6140–6151. [Google Scholar] [CrossRef]
- Simonovska, B.; Vovk, I.; Glavnik, V.; Černelič, K. Effects of extraction and high-performance liquid chromatographic conditions on the determination of lutein in spinach. J. Chromatogr. A 2013, 1276, 95–101. [Google Scholar] [CrossRef]
- Ahamed, M.N.; Saleemullah, M.; Shah, H.U.; Khalil, I.A.; Saljoq, A.U.R. Determination of beta carotene content in fresh vegetables using high performance liquid chromatography. Sarhad J. Agric. 2007, 23, 767–770. [Google Scholar]
- Bunea, A.; Andjelkovic, M.; Socaciu, C.; Bobis, O.; Neacsu, M.; Verhé, R.; Van Camp, J. Total and individual carotenoids and phenolic acids content in fresh, refrigerated and processed spinach (Spinacia oleracea, L.). Food Chem. 2008, 108, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Chandra-Hioe, M.V.; Rahman, H.H.; Arcot, J. Lutein and β-carotene in selected Asian leafy vegetables. J. Food Chem. Nanotechnol. 2017, 3, 93–97. [Google Scholar] [CrossRef]
- Kidmose, U.; Edelenbos, M.; Christensen, L.P.; Hegelung, E. Chromatographic determination of changes in pigments in spinach (Spinacia oleracea L.) during processing. J. Chromatogr. Sci. 2005, 43, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Ligor, M.; Buszewski, B. Study of xanthophyll concentration in spinach leaves by means of HPLC coupled with UV-VIS and corona CAD detectors. Food Anal. Methods 2012, 5, 388–395. [Google Scholar] [CrossRef]
- Perry, A.; Rasmussen, H.; Johnson, E.J. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J. Food Compos. Anal. 2009, 22, 9–15. [Google Scholar] [CrossRef]
- Becerra-Moreno, A.; Alanís-Garza, P.A.; Mora-Nieves, J.L.; Mora-Mora, J.P.; Jacobo-Velázquez, D.A. Kale: An excellent source of vitamin C, pro-vitamin A, lutein and glucosinolates. CyTA J. Food 2014, 12, 298–303. [Google Scholar] [CrossRef]
- Lesfsrud, M.; Kopsell, D.; Sams, C.; Wills, J.; Both, A.J. Dry matter content and stability of carotenoids in kale and spinach during drying. HortScience 2008, 43, 1731–1736. [Google Scholar] [CrossRef]
- Siriamornpun, S.; Kaisoon, O.; Meeso, N. Changes in colour, antioxidant activities and carotenoids (lycopene, β-carotene, lutein) of marigold flower (Tagetes erecta L.) resulting from different drying processes. J. Funct. Foods 2012, 4, 757–766. [Google Scholar] [CrossRef]
- Britton, G. UV/Visible Spectroscopy. In Carotenoids. Volume 1B: Spectroscopy; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser Verlag: Basel, Switzerland, 1995; pp. 13–62. [Google Scholar]
- Rivera, S.; Canela, R. Influence of sample processing on the analysis of carotenoids in maize. Molecules 2012, 17, 11255–11268. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Craft, N.E.; Soares, J.H. Relative solubility, stability, and absorptivity of lutein and β-carotene in organic solvents. J. Agric. Food Chem. 1992, 40, 431–434. [Google Scholar] [CrossRef]

| Japanese Knotweed | Bohemian Knotweed | Spinach | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak no. | tR (min) | Compound | Precursor Ion (m/z) | Fragment Ions (m/z) | UV/vis Absorption Maxima (nm) | Green Leaves | Yellow Leaves | Green–Yellowish Leaves | Green Leaves | Yellow Leaves | Green Leaves | Ref. |
| 1 | 4.6 | violaxanthin (cis or trans) | 601 | 583, 565, 509, 491 | 475, 440, 415 | + | + | + | [37,38,39] | |||
| 2 | 5.1 | violaxanthin (cis or trans) | 601 | 583, 565, 517, 495 | 475, 442, 416 | + | + | + | + | + | + | [37,38,39] |
| 3 | 5.3 | neoxanthin | 601 | 583, 565, 413 | 473, 438, 412 | + | + | + | [37,38,39] | |||
| 4 | 5.5 | luteoxanthin (cis or trans) | 601 | 583, 565, 509, 491 | 450, 425, 400 | + | + | [39] | ||||
| 5 | 5.8 | luteoxanthin (cis or trans) | 601 | 583, 565, 491 | 450, 425, 400 | + | + | + | [39] | |||
| 6 | 6.1 | antheraxnthin | 586 | 567, 554, 536, 493 | 470, 445, 421 | + | + | + | + | + | + | [37,38,39] |
| 7 | 7.2 | (all-trans)-lutein | 569 | 551, 533, 495 | 475, 450, 424 | + | + | + | + | + | + | [37,38,39] |
| 8 | 8.1 | (all-trans)-zeaxanthin | 569 | 551, 495, 449 | 480, 455, 435 | + | + | + | + | + | + | [37,38,39] |
| 9 | 8.8 | chlorophyll b | 907 | 629, 597, 569 | 645, 595, 455 | + | + | + | + | + | + | [37,40,41] |
| 10 | 9.7 | chlorophyll a | 893 | 615, 555, 538 | 660, 614, 430 | + | + | + | [37,40,41] | |||
| 11 | 10.0 | chlorophyll b’ | 907 | 629, 607, 569 | 645, 455, 430 | + | + | + | [40] | |||
| 12 | 10.7 | chlorophyll a’ | 893 | 615, 555, 535, 409 | 660, 615, 430 | + | + | + | [40] | |||
| 13 | 18.6 | (13-cis)-β-carotenea | 537 | 481, 413, 399 | 475, 450, 335 | + | + | + | [41] | |||
| 14 | 21.6 -21.8 | (all-trans)-β-caroteneb | 537 | 481, 413, 399, 347 | 665, 480, 455, 410 | + | + | + | + | + | + | [37,38,39] |
| 15 | 22.3 | (9-cis)-β-carotenea | 537 | 481, 413, 399, 347 | 475, 450, 425, 335 | + | + | + | + | + | + | [41] |
| 16 | 23.6 | violaxanthin palmitate oleate | 1103 | 1085, 543, 826, 547 | 470, 440, 415 | + | + | + | [39] | |||
| 17 | 23.9 | antheraxanthin dilaurate | 949 | 932, 547 | 465, 440, 415 | + | + | + | [39] | |||
| 18 | 24.7 | luteoxanthin dimyristate | 1006 | 988, 826, 549 | 450, 425, 400 | + | + | + | [39] | |||
| 19 | 25.2 | luteoxanthin palmitate oleate | 1103 | 1085, 826, 547 | 450, 425, 400, 395 | + | + | + | [39] | |||
| 20 | 25.9 | zeinoxanthin palmitate oleate or β-cryptoxanthin palmitate oleate | 790 | 697, 535 | 475, 450, 422 | + | + | + | [39] | |||
| 21 | 26.3 | violaxanthin palmitate stearate | 1100 | 1081, 844, 804, 565 | 465, 435, 411 | + | + | + | [39] | |||
| 22 | 27.0 | violaxanthin myristate | 811 | 794, 533 | 475, 445, 423 | + | + | + | [39] | |||
| Japanese Knotweed | Bohemian Knotweed | Spinach | |||||
|---|---|---|---|---|---|---|---|
| Green Leaves | Yellow Leaves | Green–Yellowish Leaves | Green Leaves | Yellow Leaves | Green Leaves | ||
| Recovery (%) | |||||||
| (all-trans)-lutein | 86 ± 2 | 93 ± 4 | 81 ± 1 | 93 ± 3 | 88 ± 5 | 94 ± 4 | |
| (all-trans)-β-carotene | 91 ± 4 | 56 ± 5 | 72 ± 4 | 89 ± 1 | 54 ± 1 | 85 ± 2 | |
| Peak no. | Compound | Content (mg/100 g DW) | |||||
| 1 | violaxanthin (cis or trans) | 4.9 ± 0.9 | < LOQ | < LOQ | 3.9 ± 0.2 | < LOQ | 7.1 ± 0.4 |
| 2 | violaxanthin (cis or trans) | 58.3 ± 7.0 | < LOQ | 4.2 ± 1.2 | 39.9 ± 0.9 | 1.5 ± 0.2 | 96.8 ± 1.8 |
| 3 | neoxanthin | 38.2 ± 6.5 | < LOQ | 3.3 ± 1.3 | 24.4 ± 0.6 | < LOQ | 44.3 ± 2.6 |
| 4 | luteoxanthin (cis or trans) | 2.9 ± 0.3 | < LOQ | < LOQ | 2.2 ± 0.1 | < LOQ | < LOQ |
| 5 | luteoxanthin (cis or trans) | 6.3 ± 1.1 | < LOQ | 1.1 ± 0.1 | 5.4 ± 0.4 | < LOQ | < LOQ |
| 6 | antheraxnthin | 10.3 ± 0.5 | 1.0 ± 0.1 | 6.4 ± 0.1 | 12.8 ± 0.7 | 2.0 ± 0.1 | 3.6 ± 0.1 |
| 7 | (all-trans)-lutein | 144.3 ± 8.7 | 9.4 ± 1.2 | 55.8 ± 7.7 | 97.1 ± 4.0 | 28.6 ± 2.8 | 127.9 ± 1.4 |
| 8 | (all-trans)-zeaxanthin | 3.4 ± 0.2 | 1.8 ± 0.1 | 5.1 ± 1.3 | 2.7 ± 0.1 | 6.1 ± 1.8 | < LOQ |
| 13 | (13-cis)-β-carotene | 1.4 ± 0.5 | < LOQ | < LOQ | 0.9 ± 0.2 | < LOQ | 1.9 ± 0.8 |
| 14 | (all-trans)-β-carotene | 97.3 ± 1.7 | 8.0 ± 0.5 | 23.2 ± 4.7 | 68.7 ± 0.4 | 12.7 ± 2.4 | 97.4 ± 0.7 |
| 15 | (9-cis)-β-carotene | 8.6 ± 1.7 | < LOQ | < LOQ | 6.1 ± 0.6 | < LOQ | 9.8 ± 1.0 |
| 16 | violaxanthin palmitate oleate | < LOQ | 6.6 ± 0.9 | 10.9 ± 0.5 | < LOQ | 4.7 ± 0.1 | < LOQ |
| 17 | antheraxanthin dilaurate | < LOQ | 10.8 ± 0.2 | 5.4 ± 0.1 | < LOQ | 5.6 ± 0.6 | < LOQ |
| 18 | antheraxanthin dimyristate | < LOQ | 3.8 ± 0.2 | 0.9 ± 0.1 | < LOQ | 1.9 ± 0.1 | < LOQ |
| 19 | luteoxanthin palmitate oleate | < LOQ | 5.1 ± 0.3 | 1.2 ± 0.1 | < LOQ | 3.9 ± 0.1 | < LOQ |
| 20 | zeinoxanthin palmitate oleate or β-cryptoxanthin palmitate oleate | < LOQ | 1.1 ± 0.3 | < LOQ | < LOQ | 0.9 ± 0.1 | < LOQ |
| 21 | violaxanthin palmitate stearate | < LOQ | 16.1 ± 3.3 | 7.0 ± 1.1 | < LOQ | 10.1 ± 0.3 | < LOQ |
| 22 | violaxanthin myristate | < LOQ | 6.4 ± 3.3 | 2.8 ± 1.1 | < LOQ | 4.0 ± 0.3 | < LOQ |
| Total carotenoids (mg LE/100 g DW) | 378 ± 17 | 67 ± 4 | 127 ± 9 | 260 ± 4 | 70 ± 4 | 384 ± 4 | |
| Plant Source | (all-trans)-β-carotene (mg/100 g DW) | (all-trans)-lutein (mg/100 g DW) | Ref. |
|---|---|---|---|
| Green leaves of Japanese knotweed | 97 | 144 | This study |
| Green leaves of Bohemian knotweed | 69 | 97 | This study |
| Spinach leaves | 97 | 128 | This study |
| Green leaves of Japanese knotweed | 63 | 24 | [35] |
| Spinach leaves | 9–75 | 19–83 | [43,44,45,46,47,48] |
| Kale leaves | 30–40 | 36–55 | [49,50] |
| Marigold petals | 16 | 280 | [51] |
| Carrot roots | 11–56 | 10–31 | [43,48] |
| White cabbage leaves | 2–57 | 4–25 | [43,45,48] |
| Broccoli crown | 5–33 | 7–20 | [45,48] |
| Cucumber fruits | 5–17 | 1–24 | [43,48] |
| Sample | m [mg] | Vadd((all-trans)-β-carotene) [µL]a | Vadd((all-trans)-lutein) [µL]a | Vadd(acetone) [µL] |
|---|---|---|---|---|
| Spinach leaves | 20 | 200 | 200 | 9600 |
| Japanese knotweed green leaves | 20 | 200 | 200 | 9600 |
| Japanese knotweed green–yellowish leaves | 30 | 100 | 30 | 9870 |
| Japanese knotweed yellow leaves | 30 | 100 | 50 | 9850 |
| Bohemian knotweed green leaves | 20 | 200 | 200 | 9600 |
| Bohemian knotweed yellow leaves | 30 | 100 | 30 | 9870 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metličar, V.; Vovk, I.; Albreht, A. Japanese and Bohemian Knotweeds as Sustainable Sources of Carotenoids. Plants 2019, 8, 384. https://doi.org/10.3390/plants8100384
Metličar V, Vovk I, Albreht A. Japanese and Bohemian Knotweeds as Sustainable Sources of Carotenoids. Plants. 2019; 8(10):384. https://doi.org/10.3390/plants8100384
Chicago/Turabian StyleMetličar, Valentina, Irena Vovk, and Alen Albreht. 2019. "Japanese and Bohemian Knotweeds as Sustainable Sources of Carotenoids" Plants 8, no. 10: 384. https://doi.org/10.3390/plants8100384
APA StyleMetličar, V., Vovk, I., & Albreht, A. (2019). Japanese and Bohemian Knotweeds as Sustainable Sources of Carotenoids. Plants, 8(10), 384. https://doi.org/10.3390/plants8100384