Ecophysiological Plasticity and Cold Stress Adaptation in Himalayan Alpine Herbs: Bistorta affinis and Sibbaldia procumbens
Abstract
:1. Introduction
2. Results
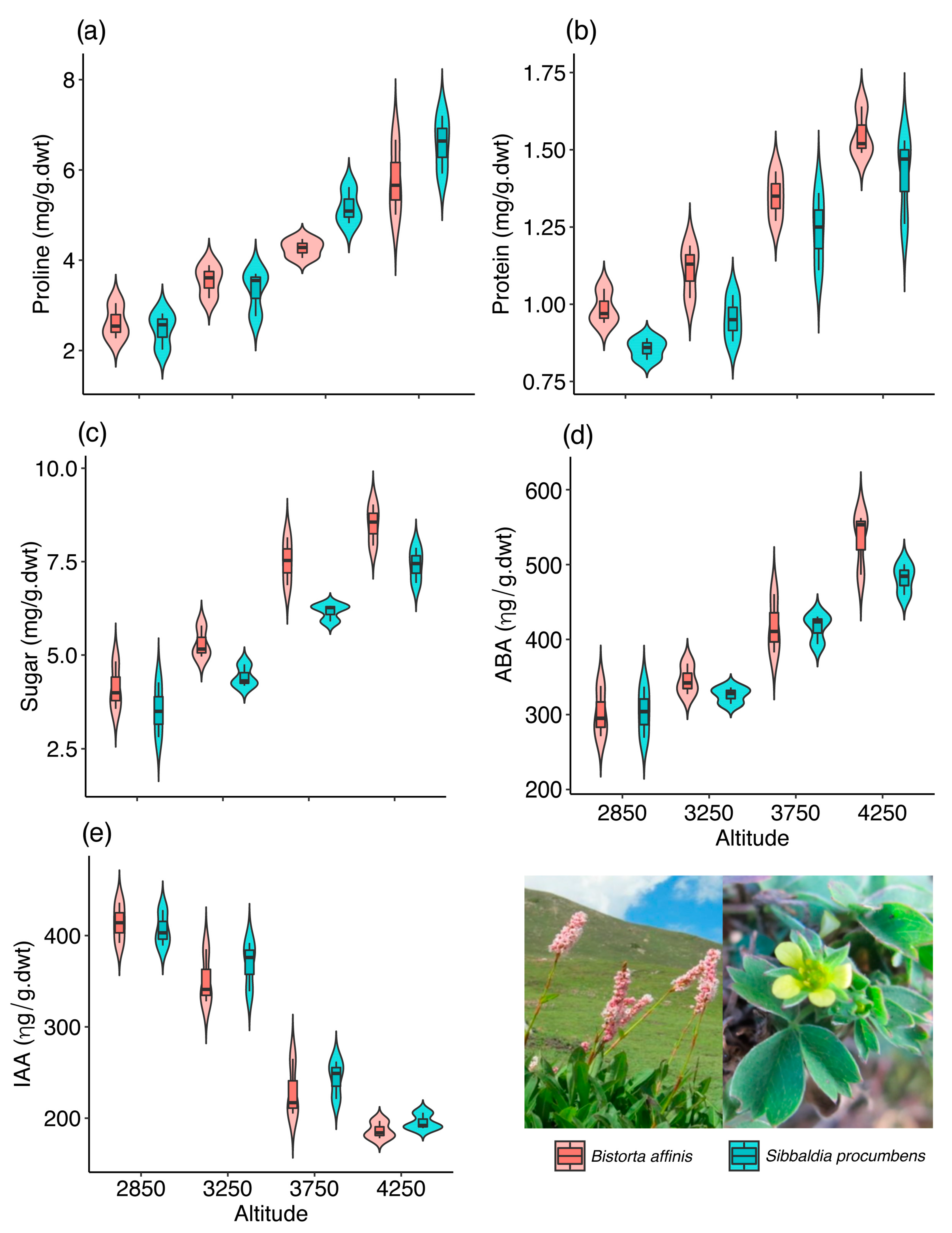
2.1. Ecophysiological Plasticity in Bistorta affinis
2.2. Ecophysiological Plasticity in Sibbaldia procumbens
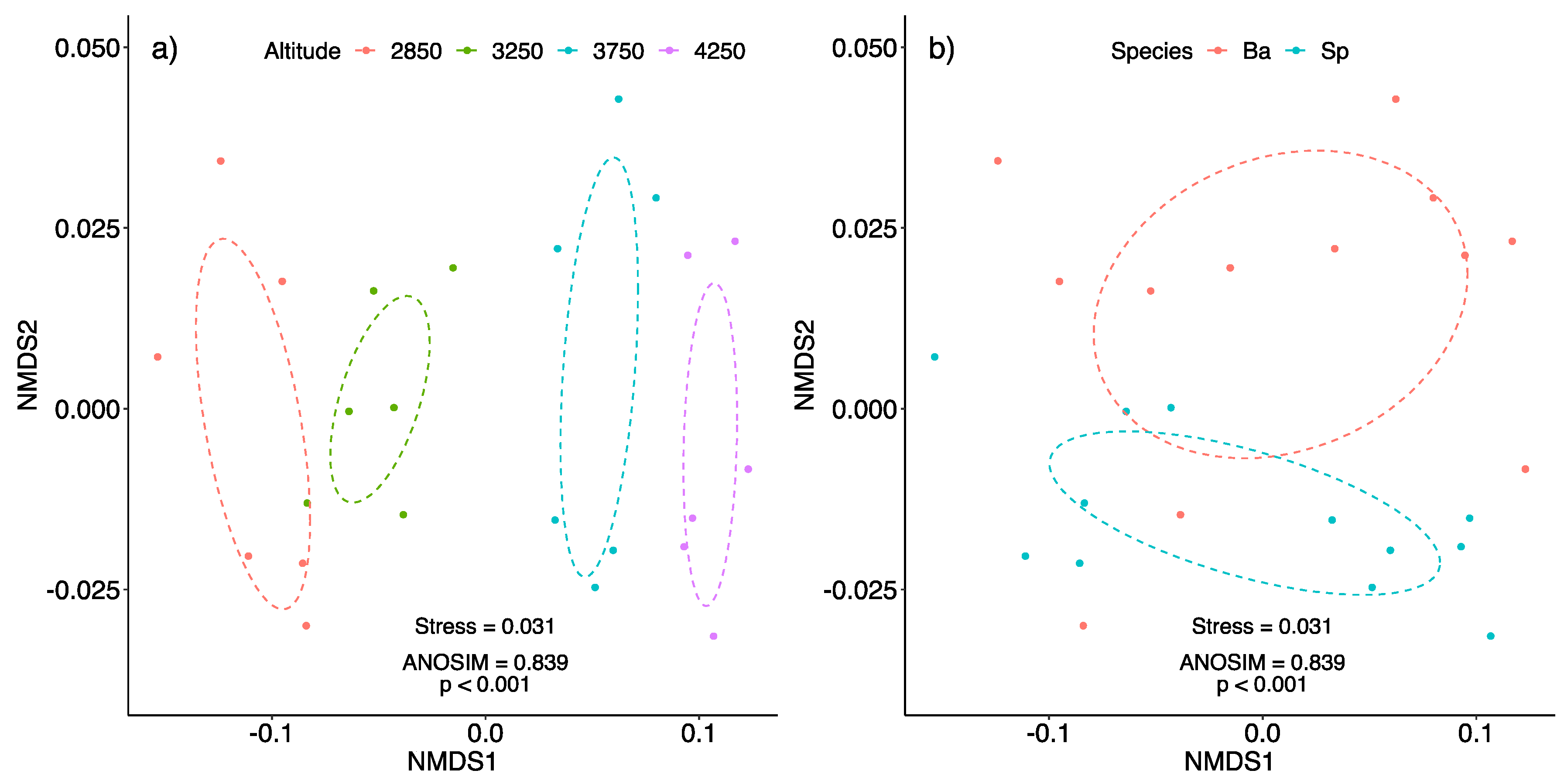
2.3. Non-Metric Multidimensional Scaling (NMDS)
3. Discussion
4. Materials and Methods
4.1. Study Site, Experimental Design and Environmental Gradients
4.2. Investigational Species, Sample Collection and Physiological Attributes
4.3. Statistical Analyses
4.4. Ethical Approval
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thomashow, M.F. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant. Phys. 1999, 50, 571–599. [Google Scholar] [CrossRef] [PubMed]
- Guy, C.L.; Huber, J.L.; Huber, S.C. Sucrose phosphate synthase and sucrose accumulation at low temperature. Plant. Physiol. 1992, 100, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Kandler, O.; Hopf, H. Oligo saccharides based on sucrose. In Encyclopedia of Plant Physiology; New series 13 A; Loewus, A.F., Tanner, W., Eds.; Springer: Berlin, Germany, 1982; pp. 348–382. [Google Scholar]
- Guy, C.L. Cold acclimation and freezing stress tolerance: Role of protein metabolism. Annu Rev. Plant. Physiol. Plant. Mol. Biol. 1990, 41, 187–223. [Google Scholar] [CrossRef]
- Galiba, G.; Sutka, J.; Snape, J.W.; Tuberosa, R.; Quarrie, S.A.; Sarkadi, L.; Veisz, O. The association of forest resistance gene Frl with stress induced osmolyte and ABA accumulation in wheat. In Proceedings of the Workshop on Crop Adaptation to Cool Climates, Hamburg, Germany, 12–14 October 1994. [Google Scholar]
- Hansen, H.; Grossmann, K. Auxin-induced ethylene triggers abscisic acid biosynthesis and growth inhibition. Plant. Physiol. 2000, 124, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophy. 2005, 444, 139–158. [Google Scholar] [CrossRef]
- Zhang, B. MicroRNA: A new target for improving plant tolerance to abiotic stress. J. Exp. Bot. 2015, 66, 1749–1761. [Google Scholar] [CrossRef]
- Davies, W.J.; Zhang, J. Root signals and the regulation of growth and development of plants in drying soil. Ann. Rev. Plant. Biol. 1991, 42, 55–76. [Google Scholar] [CrossRef]
- Fahad, S.; Bano, A. Ethnobotanical and physiological studies of some endangered plant species collected from two different altitudes in Gilgit Baltistan. Pak. J. Bot. 2012, 44, 165–170. [Google Scholar]
- Khan, S.M. Plant Communities and Vegetation Ecosystem Services in the Naran Valley, Western Himalaya. Ph.D. Thesis, University of Leicester, Leicester, UK, 2012. [Google Scholar]
- Chen, B.X.; Zhang, X.Z.; Tao, J.; Wu, J.S.; Wang, J.S.; Shi, P.L.; Zhanga, Y.; Yua, C. The impact of climate change and anthropogenic activities on alpine grassland over the Qinghai-Tibet Plateau. Agric. For. Met. 2014, 189, 11–18. [Google Scholar] [CrossRef]
- Ahmad, K.S.; Hameed, M.; Fatima, S.; Ashraf, M.; Ahmad, F.; Naseer, M.; Akhtar, N. Morpho-anatomical and physiological adaptations to high altitude in some Aveneae grasses from Neelum Valley, Western Himalayan Kashmir. Acta Physiol. Plant. 2016, 38. [Google Scholar] [CrossRef]
- Rahman, I.U.; Hart, R.; Afzal, A.; Iqbal, Z.; Abd_Allah, E.F.; Alqarawi, A.A.; Ijaz, F.; Ali, N.; Kausar, R.; Muzammil, S.; et al. Phenological plasticity in Berberis lycium Royle along temporal and altitudinal gradients. Appl. Ecol. Environ. Res. 2019, 17, 331–341. [Google Scholar] [CrossRef]
- Paleg, L.; Aspinall, D. The Physiology and Biochemistry of Drought Resistance in Plants; Academic Press: New York, NY, USA, 1981. [Google Scholar]
- Smirnoff, N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 2006, 125, 27–58. [Google Scholar] [CrossRef]
- Manivannan, P.; Jaleel, C.A.; Sankar, B.; Kishorekumar, A.; Somasundaram, R.; Lakshmanan, G.; Panneerselvam, R. Growth, biochemical modifications and proline metabolism in Helianthus annuus L. as induced by drought stress. Colloids Surf. B Biointerfaces 2007, 59, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Galiba, G. In vitro adaptation for drought and cold hardiness in wheat. Plant. Breed. Rev. 1994, 12, 115–162. [Google Scholar]
- Bano, A.; Rehman, A.; Winiger, M. Altitudinal variation in the content of protein, proline, sugar and abscisic acid (ABA) in the alpine herbs from Hunza valley, Pakistan. Pak. J. Bot. 2009, 41, 1593–1602. [Google Scholar]
- Korner, C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Quarrie, S.A.; Galiba, G.; Sutka, J.; Snape, J.W.; Semikhodski, A.; Steed, A.; Gulli, M.; Calestani, C. Association of a major vernalization gene of wheat with stress induced ABA production. In Proceedings of the Workshop on Crop Adaptation to Cool Climates, Hamburg, Germany, 12–14 October 1994. [Google Scholar]
- Neumann, D.; Nover, L.; Parthier, B.; Rieger, R.; Scharf, K.D.; Wollgiehn, R. Heat shock and other stress response systems of plants. Results Probl. Cell Diff. 1989, 16, 1–155. [Google Scholar]
- Kacperska-Palacz, A. Mechanism of cold acclimation in herbaceous plants. In Plant Cold Hardiness and Freezing Stress; Li, P.H., Sakai, A., Eds.; Academic Press: New York, NY, USA, 1978; pp. 139–152. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.F.; Farr, A.L.; Rohdoll, R.I. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Dubois, S.M.; Giles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1983, 28, 350. [Google Scholar] [CrossRef]
- Bates, I.S.; Waldern, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant. Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Kettner, J.; Doerffling, K. Biosynthesis and metabolism of abscisic acid in tomato leaves infected with Botrytis cinerea. Planta 1995, 196, 627–634. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; Mcglinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package, R package version 2.5-3; World Agroforestry Centre: Nairobi, Kenya, 2018. [Google Scholar]
| Compound | Factor | χ2 | df | p-Value |
|---|---|---|---|---|
| ABA | Altitude | 173.00 | 3 | <0.001 |
| Species | 2.23 | 1 | 0.1353 | |
| IAA | Altitude | 409.57 | 3 | <0.001 |
| Species | 0.97 | 1 | 0.3246 | |
| Proline | Altitude | 149.944 | 3 | <0.001 |
| Species | 2.273 | 1 | 0.1316 | |
| Protein | Altitude | 165.446 | 3 | <0.001 |
| Species | 14.938 | 1 | <0.001 | |
| Sugar | Altitude | 245.805 | 3 | <0.001 |
| Species | 22.658 | 1 | <0.001 |
| Altitude (m) | Proline Content (mg/g.dwt) | Protein Content (mg/g.dwt) | Sugar Content (mg/g.dwt) | ABA (ηg/g.dwt) | IAA (ηg/g.dwt) |
|---|---|---|---|---|---|
| 2850 | 2.62 ± 0.32C | 0.98 ± 0.05C | 4.13 ± 0.52C | 301.33 ± 27.7C | 414.00 ± 17.9A |
| 3250 | 3.55 ± 0.3BC | 1.11 ± 0.07C | 5.30 ± 0.35B | 345.67 ± 16.9C | 351.33 ± 24.3B |
| 3750 | 4.26 ± 0.17B | 1.35 ± 0.06B | 7.51 ± 0.52A | 418.33 ± 32.2B | 229.00 ± 25.9C |
| 4250 | 5.78 ± 0.68A | 1.55 ± 0.06A | 8.50 ± 0.45A | 534.67 ± 33.9A | 186.33 ± 7.9C |
| LSD(0.05) | 0.95 | 0.14 | 1.07 | 65.68 | 46.87 |
| CV | 12.55 | 6.11 | 9.00 | 8.2 | 8.43 |
| Altitude (m) | Proline Content (mg/g.dwt) | Protein Content (mg/g.dwt) | Sugar Content (mg/g.dwt) | ABA (ηg/g.dwt) | IAA (ηg/g.dwt) |
|---|---|---|---|---|---|
| 2850 | 2.47 ± 0.33C | 0.85 ± 0.03B | 3.52 ± 0.6C | 303.33 ± 27.7C | 406.67 ± 16.1A |
| 3250 | 3.33 ± 0.41C | 0.95 ± 0.06B | 4.41 ± 0.24C | 326.00 ± 9.1C | 369.00 ± 22.2A |
| 3750 | 5.17 ± 0.33B | 1.24 ± 0.1A | 6.16 ± 0.19B | 416.33 ± 16.1B | 244.00 ± 17.1B |
| 4250 | 6.58 ± 0.52A | 1.42 ± 0.1A | 7.41 ± 0.38A | 482.00 ± 16.87A | 195.67 ± 7.4C |
| LSD(0.05) | 0.94 | 0.19 | 0.89 | 43.07 | 38.24 |
| CV | 11.38 | 9.24 | 8.80 | 5.99 | 6.69 |
| Environmental Variables | Altitudinal Ranges (m.a.s.l.) | |||
|---|---|---|---|---|
| 2850 | 3250 | 3750 | 4250 | |
| Latitude | 34.73111 | 34.71972 | 34.80139 | 34.80889 |
| Longitude | 73.6675 | 73.6525 | 73.66528 | 73.61639 |
| Aspect | N | N | N | N |
| Slope Angle | 40 | 38 | 38 | 28 |
| Temperature (°C) | 18.4 | 15.4 | 5.2 | 4.7 |
| Humidity | 53.85 | 55.3 | 56.9 | 51.2 |
| Heat index | 19.2 | 14.2 | 5.5 | 3.85 |
| Wind speed (m/s) | 2.5 | 4.5 | 4.5 | 6.5 |
| Dew point | 15.2 | 14.2 | 11.8 | 11 |
| Wet bulb | 14.72 | 15.2 | 12.3 | 13.82 |
| Barometric Pressure | 731 | 697.4 | 651.4 | 614.8 |
| pH | 5.9 | 5.3 | 4.7 | 5.1 |
| Electric conductivity (EC) | 1.51 | 1.37 | 3.68 | 2.82 |
| Organic matter (OM) | 1.03 | 1.74 | 1.79 | 1.92 |
| Calcium carbonate (CaCO3) | 7.129 | 5.549 | 6.349 | 7.82 |
| Potassium (K) (mg/kg) | 202.93 | 199 | 203 | 204 |
| Phosphorous (P) (mg/kg) | 14 | 14.11 | 12 | 9.3 |
| Sand (%) | 33.82 | 41 | 50.23 | 53 |
| Silt (%) | 42.54 | 27 | 22.76 | 27 |
| Clay (%) | 23.64 | 32 | 27.01 | 20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, I.U.; Hart, R.; Afzal, A.; Iqbal, Z.; Alqarawi, A.A.; Abd_Allah, E.F.; Hashem, A.; Ijaz, F.; Ali, N.; Calixto, E.S. Ecophysiological Plasticity and Cold Stress Adaptation in Himalayan Alpine Herbs: Bistorta affinis and Sibbaldia procumbens. Plants 2019, 8, 378. https://doi.org/10.3390/plants8100378
Rahman IU, Hart R, Afzal A, Iqbal Z, Alqarawi AA, Abd_Allah EF, Hashem A, Ijaz F, Ali N, Calixto ES. Ecophysiological Plasticity and Cold Stress Adaptation in Himalayan Alpine Herbs: Bistorta affinis and Sibbaldia procumbens. Plants. 2019; 8(10):378. https://doi.org/10.3390/plants8100378
Chicago/Turabian StyleRahman, Inayat Ur, Robbie Hart, Aftab Afzal, Zafar Iqbal, Abdulaziz A. Alqarawi, Elsayed Fathi Abd_Allah, Abeer Hashem, Farhana Ijaz, Niaz Ali, and Eduardo Soares Calixto. 2019. "Ecophysiological Plasticity and Cold Stress Adaptation in Himalayan Alpine Herbs: Bistorta affinis and Sibbaldia procumbens" Plants 8, no. 10: 378. https://doi.org/10.3390/plants8100378
APA StyleRahman, I. U., Hart, R., Afzal, A., Iqbal, Z., Alqarawi, A. A., Abd_Allah, E. F., Hashem, A., Ijaz, F., Ali, N., & Calixto, E. S. (2019). Ecophysiological Plasticity and Cold Stress Adaptation in Himalayan Alpine Herbs: Bistorta affinis and Sibbaldia procumbens. Plants, 8(10), 378. https://doi.org/10.3390/plants8100378






