Role of Galactolipids in Plastid Differentiation Before and After Light Exposure
Abstract
1. Introduction
2. Development of Etioplasts in the Dark and Their Differentiation to Chloroplasts with Light
2.1. Pchlide and Chl Biosynthesis in the Dark and in the Light
2.2. Formation of Pchlide–LPOR–NADPH Ternary Complexes in PLBs
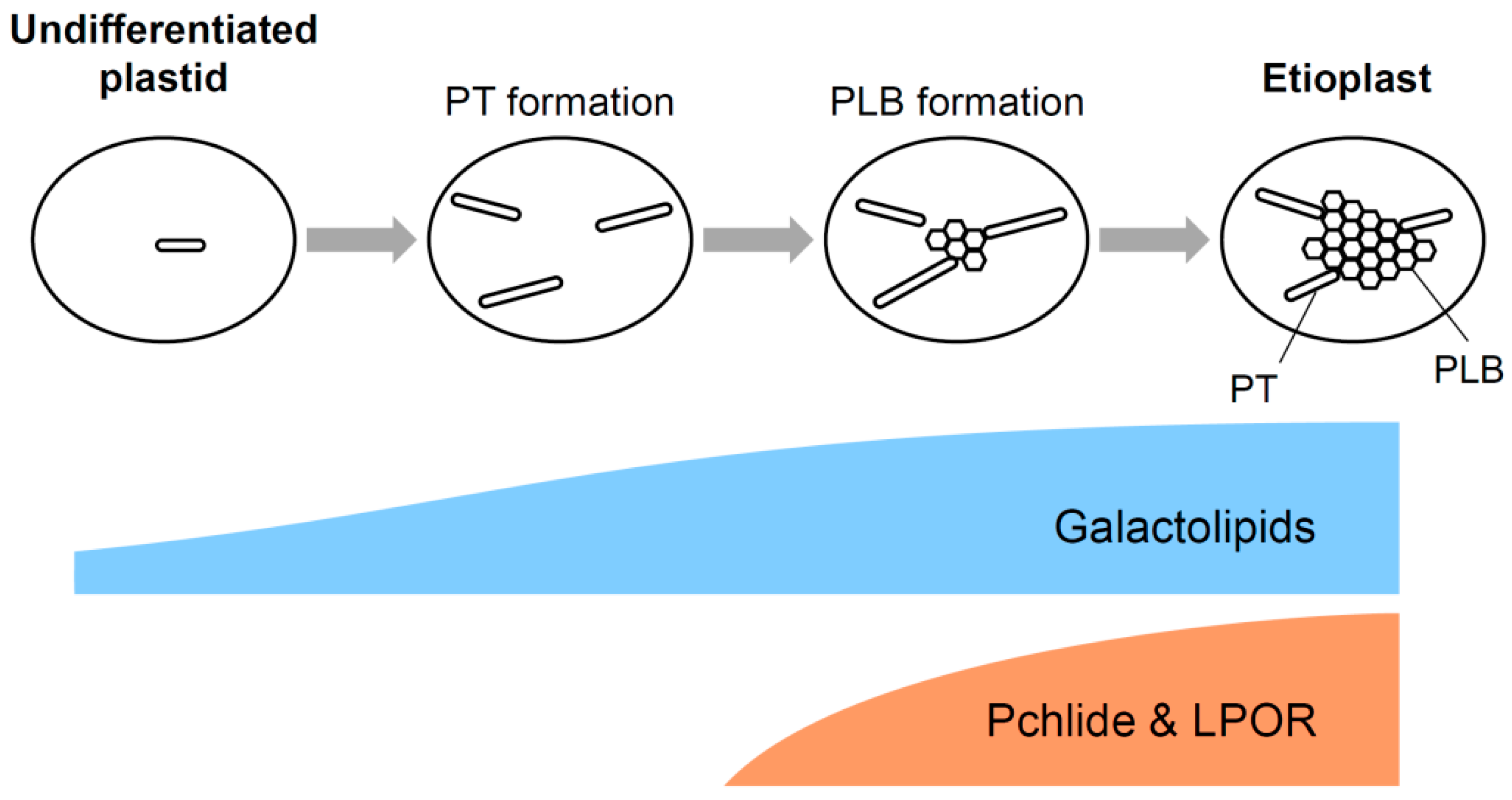
2.3. Formation of PLBs in Etioplasts
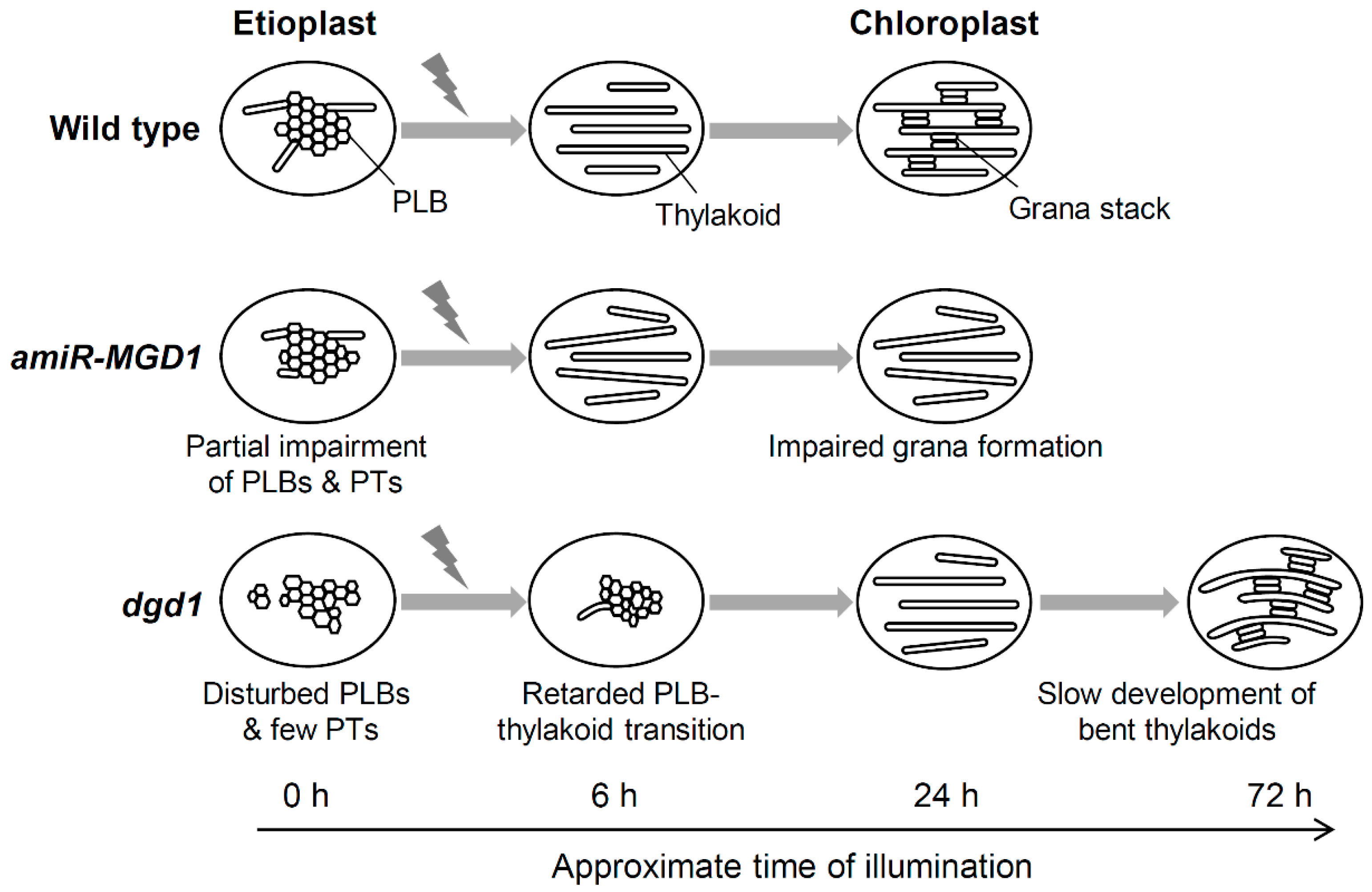
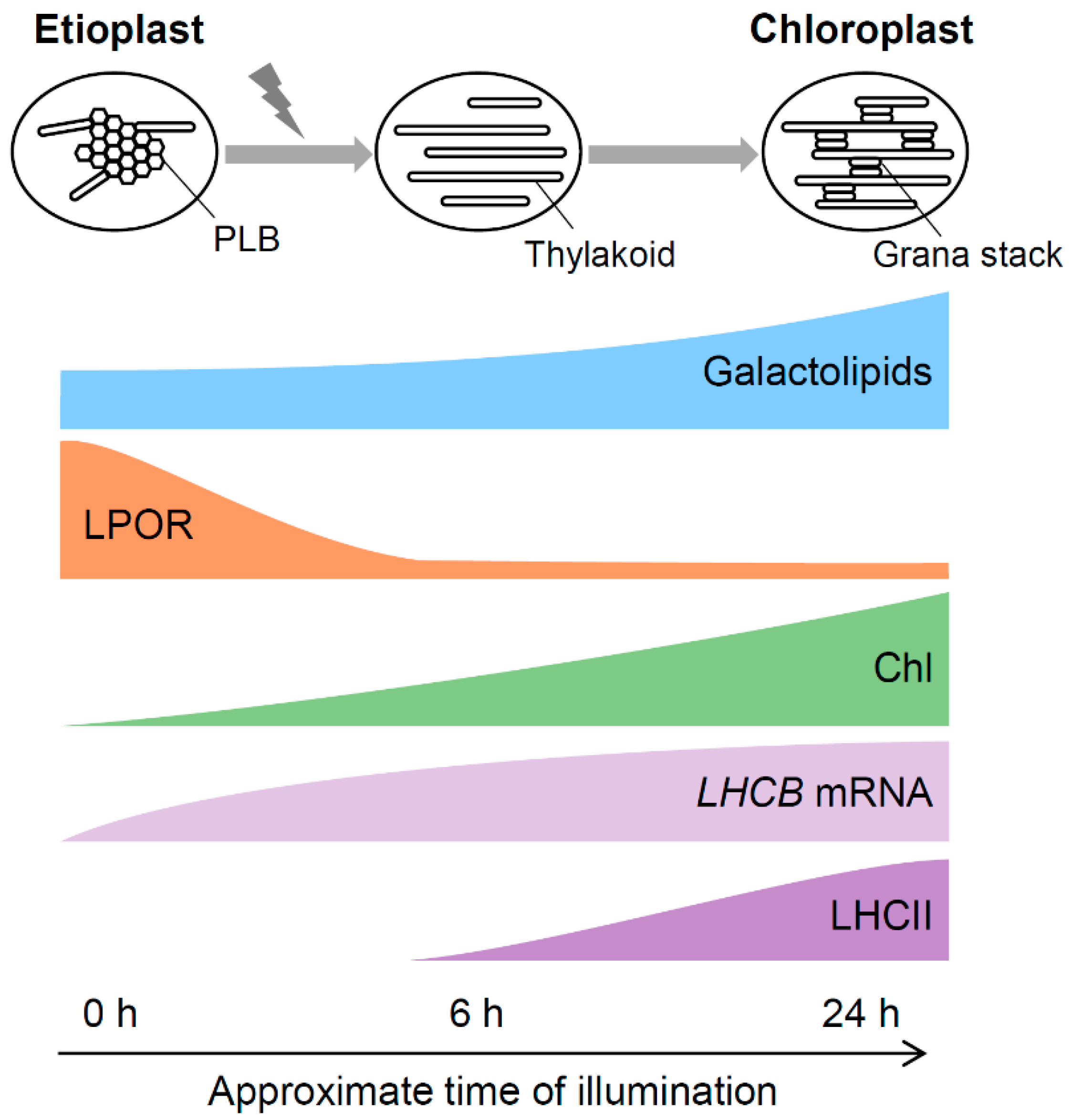
2.4. Transformation of PLBs to the Thylakoid Membrane During Etioplast-to-chloroplast Differentiation
3. Role of Galactolipids in Etioplasts
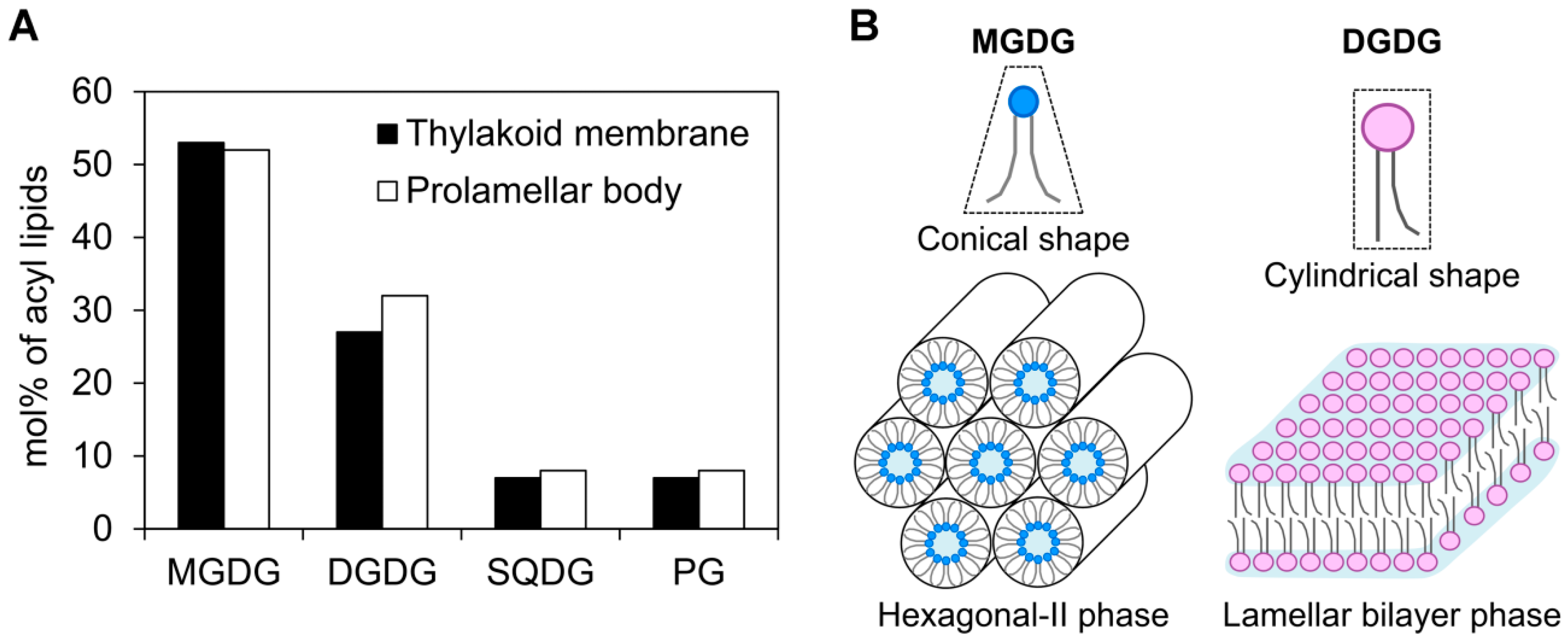
3.1. Galactolipid Synthesis in Etioplasts
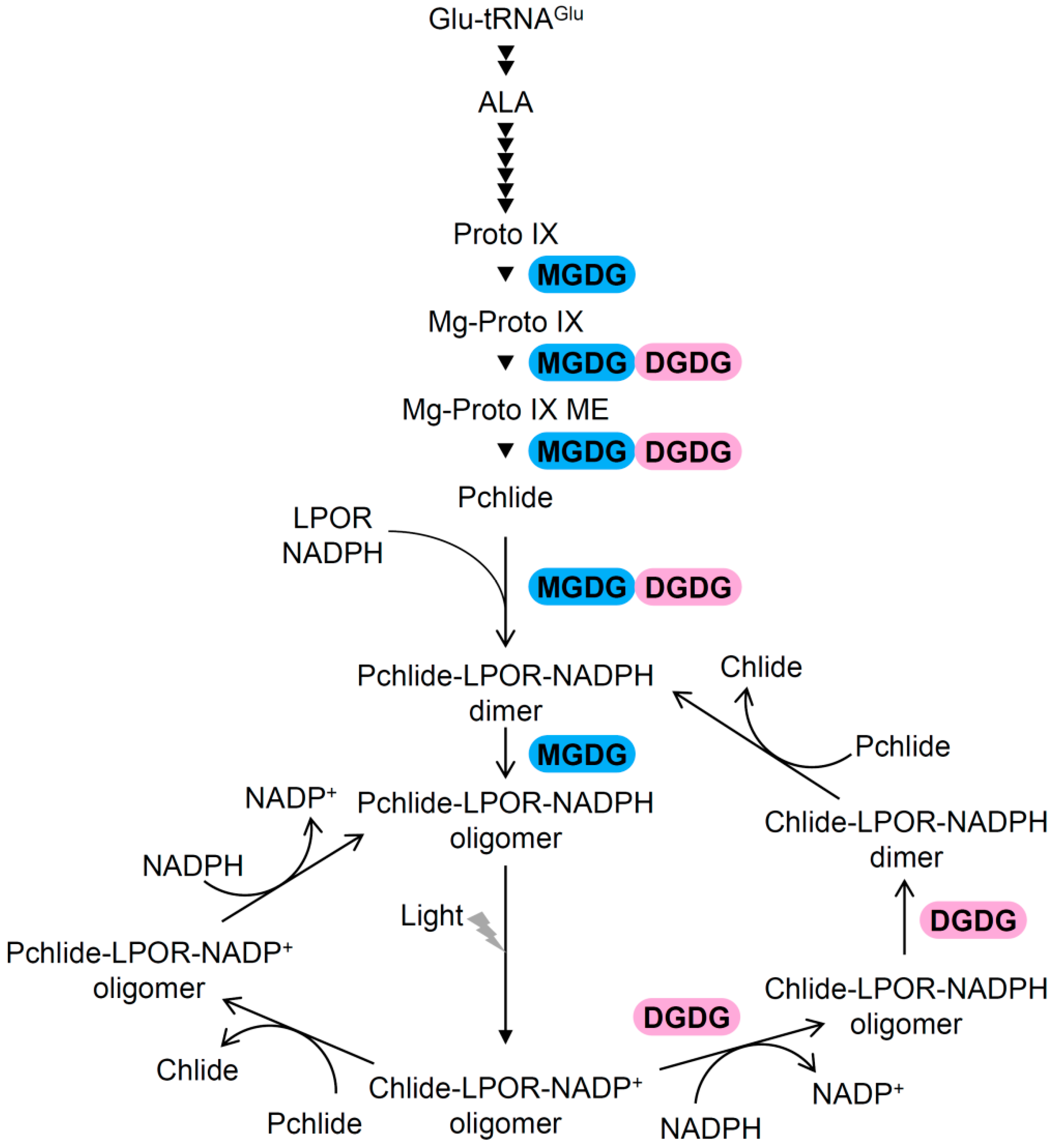
3.2. Involvement of Galactolipids in Pchlide Biosynthesis
3.3. Roles of Galactolipids in the Organization of (P)chlide–LPOR Complexes Before and After Illumination
3.4. Importance of Galactolipids in the Formation of PLBs and PTs
3.5. A Model for Galactolipid-mediated Etioplast Development
4. Role of Galactolipids During Etioplast-to-chloroplast Differentiation
4.1. Contribution of Galactolipids to Transformation of PLBs to the Thylakoid Membrane
4.2. Contribution of Galactolipids to the Development of the Thylakoid Membrane during Chloroplast Maturation
4.3. A Model for the PLB-to-thylakoid Transformation during Eitoplast-to-chloroplast Differentiation
5. Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Jarvis, P.; López-Juez, E. Biogenesis and homeostasis of chloroplasts and other plastids. Nat. Rev. Mol. Cell Biol. 2013, 14, 787–802. [Google Scholar] [CrossRef]
- Solymosi, K.; Schoefs, B. Etioplast and etio-chloroplast formation under natural conditions: The dark side of chlorophyll biosynthesis in angiosperms. Photosynth. Res. 2010, 105, 143–166. [Google Scholar] [CrossRef]
- Kobayashi, K.; Endo, K.; Wada, H. Roles of lipids in photosynthesis. Lipids Plant Algae Dev. 2016, 86, 21–49. [Google Scholar]
- Kobayashi, K. Role of membrane glycerolipids in photosynthesis, thylakoid biogenesis and chloroplast development. J. Plant Res. 2016, 129, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Shipley, G.G.; Green, J.P.; Nichols, B.W. The phase behavior of monogalactosyl, digalactosyl, and sulphoquinovosyl diglycerides. Biochim. Biophys. Acta 1973, 311, 531–544. [Google Scholar] [CrossRef]
- Demé, B.; Cataye, C.; Block, M.A.; Maréchal, E.; Jouhet, J. Contribution of galactoglycerolipids to the 3-dimensional architecture of thylakoids. FASEB J. 2014, 28, 3373–3383. [Google Scholar] [CrossRef] [PubMed]
- Selstam, E.; Sandelius, A.S. A comparison between prolamellar bodies and prothylakoid membranes of etioplasts of dark-grown wheat concerning lipid and polypeptide composition. Plant Physiol. 1984, 76, 1036–1040. [Google Scholar] [CrossRef]
- Dorne, A.; Joyard, J.; Douce, R. Do thylakoids really contain phosphatidylcholine? Proc. Natl. Acad. Sci. USA 1990, 87, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Beale, S.I. Enzymes of chlorophyll biosynthesis. Photosynth. Res. 1999, 60, 43–73. [Google Scholar] [CrossRef]
- Masuda, T.; Fujita, Y. Regulation and evolution of chlorophyll metabolism. Photochem. Photobiol. Sci. 2008, 7, 1131–1149. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Kobayashi, K.; Masuda, T. Tetrapyrrole metabolism in Arabidopsis thaliana. Arab. Book 2011, 9, e0145. [Google Scholar] [CrossRef] [PubMed]
- Brzezowski, P.; Richter, A.S.; Grimm, B. Regulation and function of tetrapyrrole biosynthesis in plants and algae. Biochim. Biophys. Acta 2015, 1847, 968–985. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Takamiya, K. Novel insights into the enzymology, regulation and physiological functions of light-dependent protochlorophyllide oxidoreductase in angiosperms. Photosynth. Res. 2004, 81, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T. Recent overview of the Mg branch of the tetrapyrrole biosynthesis leading to chlorophylls. Photosynth. Res. 2008, 96, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Franck, F.; Sperling, U.; Frick, G.; Pochert, B.; van Cleve, B.; Apel, K.; Armstrong, G.A. Regulation of etioplast pigment-protein complexes, inner membrane architecture, and protochlorophyllide a chemical heterogeneity by light-dependent NADPH:protochlorophyllide oxidoreductases A and B. Plant Physiol. 2000, 124, 1678–1696. [Google Scholar] [CrossRef]
- La Rocca, N.; Rascio, N.; Oster, U.; Rüdiger, W. Amitrole treatment of etiolated barley seedlings leads to deregulation of tetrapyrrole synthesis and to reduced expression of Lhc and RbcS genes. Planta 2001, 213, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kreunen, S.S.; Cuttriss, A.J.; DellaPenna, D.; Pogson, B.J. Identification of the carotenoid isomerase provides insight into carotenoid biosynthesis, prolamellar body formation, and photomorphogenesis. Plant Cell 2002, 14, 321–332. [Google Scholar] [CrossRef] [PubMed]
- La Rocca, N.; Rascio, N.; Oster, U.; Rüdiger, W. Inhibition of lycopene cyclase results in accumulation of chlorophyll precursors. Planta 2007, 225, 1019–1029. [Google Scholar] [CrossRef]
- Ouazzani Chahdi, M.A.; Schoefs, B.; Franck, F. Isolation and characterization of photoactive complexes of NADPH:protochlorophyllide oxidoreductase from wheat. Planta 1998, 206, 673–680. [Google Scholar] [CrossRef]
- Schoefs, B. The protochlorophyllide-chlorophyllide cycle. Photosynth. Res. 2001, 70, 257–271. [Google Scholar] [CrossRef]
- Böddi, B.; Lindsten, A.; Ryberg, M.; Sundqvist, C. On the aggregational states of protochlorophyllide and its protein complexes in wheat etioplasts. Physiol. Plant. 1989, 76, 135–143. [Google Scholar] [CrossRef]
- Solymosi, K.; Smeller, L.; Ryberg, M.; Sundqvist, C.; Fidy, J.; Böddi, B. Molecular rearrangement in POR macrodomains as a reason for the blue shift of chlorophyllide fluorescence observed after phototransformation. Biochim. Biophys. Acta 2007, 1768, 1650–1658. [Google Scholar] [CrossRef]
- Heyes, D.J.; Hunter, C.N. Making light work of enzyme catalysis: Protochlorophyllide oxidoreductase. Trends Biochem. Sci. 2005, 30, 642–649. [Google Scholar] [CrossRef]
- Franck, F.; Bereza, B.; Böddi, B. Protochlorophyllide-NADP+ and protochlorophyllide-NADPH complexes and their regeneration after flash illumination in leaves and etioplast membranes of dark-grown wheat. Photosynth. Res. 1999, 59, 53–61. [Google Scholar] [CrossRef]
- Schoefs, B.; Franck, F. Protochlorophyllide reduction: Mechanisms and evolution. Photochem. Photobiol. 2003, 78, 543–557. [Google Scholar] [CrossRef]
- Op den Camp, R.G.L.; Przybyla, D.; Ochsenbein, C.; Laloi, C.; Kim, C.; Danon, A.; Wagner, D.; Hideg, E.; Göbel, C.; Feussner, I.; et al. Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell 2003, 15, 2320–2332. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Murakami, S. Separation and characterization of prolamellar bodies and prothylakoids from squash etioplasts. Plant Cell Physiol. 1983, 24, 71–80. [Google Scholar] [CrossRef]
- Müller, B.; Eichacker, L.A. Assembly of the D1 precursor in monomeric photosystem II reaction center precomplexes precedes chlorophyll a–triggered accumulation of reaction center II in barley etioplasts. Plant Cell 1999, 11, 2365–2377. [Google Scholar]
- Frick, G.; Su, Q.; Apel, K.; Armstrong, G.A. An Arabidopsis porB porC double mutant lacking light-dependent NADPH:protochlorophyllide oxidoreductases B and C is highly chlorophyll-deficient and developmentally arrested. Plant J. 2003, 35, 141–153. [Google Scholar] [CrossRef]
- Masuda, T.; Fusada, N.; Oosawa, N.; Takamatsu, K.; Yamamoto, Y.Y.; Ohto, M.; Nakamura, K.; Goto, K.; Shibata, D.; Shirano, Y.; et al. Functional analysis of isoforms of NADPH:Protochlorophyllide oxidoreductase (POR), PORB and PORC, in Arabidopsis thaliana. Plant Cell Physiol. 2003, 44, 963–974. [Google Scholar] [CrossRef]
- Sperling, U.; Franck, F.; van Cleve, B.; Frick, G.; Apel, K.; Armstrong, G.A. Etioplast differentiation in arabidopsis: Both PORA and PORB restore the prolamellar body and photoactive protochlorophyllide-F655 to the cop1 photomorphogenic mutant. Plant Cell 1998, 10, 283–296. [Google Scholar]
- Sperling, U.; van Cleve, B.; Frick, G.; Apel, K.; Armstrong, G.A. Overexpression of light-dependent PORA or PORB in plants depleted of endogenous POR by far-red light enhances seedling survival in white light and protects against photooxidative damage. Plant J. 1997, 12, 649–658. [Google Scholar] [CrossRef]
- Masuda, S.; Ikeda, R.; Masuda, T.; Hashimoto, H.; Tsuchiya, T.; Kojima, H.; Nomata, J.; Fujita, Y.; Mimuro, M.; Ohta, H.; et al. Prolamellar bodies formed by cyanobacterial protochlorophyllide oxidoreductase in Arabidopsis. Plant J. 2009, 58, 952–960. [Google Scholar] [CrossRef]
- Paddock, T.N.; Mason, M.E.; Lima, D.F.; Armstrong, G.A. Arabidopsis protochlorophyllide oxidoreductase A (PORA) restores bulk chlorophyll synthesis and normal development to a porB porC double mutant. Plant Mol. Biol. 2010, 72, 445–457. [Google Scholar] [CrossRef]
- Kowalewska, Ł.M.; Mazur, R.; Suski, S.; Garstka, M.; Mostowska, A. Three-dimensional visualization of the internal plastid membrane network during runner bean chloroplast biogenesis. Plant Cell 2016, 28, 875–891. [Google Scholar] [CrossRef]
- Oster, U.; Bauer, C.E.; Rüdiger, W. Characterization of chlorophyll a and bacteriochlorophyll a synthases by heterologous expression in Escherichia coli. J. Biol. Chem. 1997, 272, 9671–9676. [Google Scholar] [CrossRef]
- Schoefs, B.; Bertrand, M. The formation of chlorophyll from chlorophyllide in leaves containing proplastids is a four-step process. FEBS Lett. 2000, 486, 243–246. [Google Scholar] [CrossRef]
- Peter, G.F.; Thornber, J.P. Biochemical composition and organization of higher plant photosystem II light-harvesting pigment-proteins. J. Biol. Chem. 1991, 266, 16745–16754. [Google Scholar]
- Apitz, J.; Schmied, J.; Lehmann, M.J.; Hedtke, B.; Grimm, B. GluTR2 complements a hema1 mutant lacking glutamyl-tRNA reductase 1, but is differently regulated at the post-translational level. Plant Cell Physiol. 2014, 55, 645–657. [Google Scholar] [CrossRef]
- Bang, W.Y.; Jeong, I.S.; Kim, D.W.; Im, C.H.; Ji, C.; Hwang, S.M.; Kim, S.W.; Son, Y.S.; Jeong, J.; Shiina, T.; et al. Role of Arabidopsis CHL27 protein for photosynthesis, chloroplast development and gene expression profiling. Plant Cell Physiol. 2008, 49, 1350–1363. [Google Scholar] [CrossRef]
- Pontier, D.; Albrieux, C.; Joyard, J.; Lagrange, T.; Block, M.A. Knock-out of the magnesium protoporphyrin IX methyltransferase gene in Arabidopsis. Effects on chloroplast development and on chloroplast-to-nucleus signaling. J. Biol. Chem. 2007, 282, 2297–2304. [Google Scholar] [CrossRef]
- Kirchhoff, H.; Mukherjee, U.; Galla, H.J. Molecular architecture of the thylakoid membrane: Lipid diffusion space for plastoquinone. Biochemistry 2002, 41, 4872–4882. [Google Scholar] [CrossRef]
- Fujii, S.; Nagata, N.; Masuda, T.; Wada, H.; Kobayashi, K. Galactolipids are essential for internal membrane transformation during etioplast-to-chloroplast differentiation. Plant Cell Physiol. 2019, 60, 1224–1238. [Google Scholar] [CrossRef]
- Blomqvist, L.A.; Ryberg, M.; Sundqvist, C. Proteomic analysis of highly purified prolamellar bodies reveals their significance in chloroplast development. Photosynth. Res. 2008, 96, 37–50. [Google Scholar] [CrossRef]
- Kuttkat, A.; Edhofer, I.; Eichacker, L.A.; Paulsen, H. Light-harvesting chlorophyll a/b-binding protein stably inserts into etioplast membranes supplemented with Zn-pheophytin a/b. J. Biol. Chem. 1997, 272, 20451–20455. [Google Scholar] [CrossRef]
- Kanervo, E.; Singh, M.; Suorsa, M.; Paakkarinen, V.; Aro, E.; Battchikova, N.; Aro, E.M. Expression of protein complexes and individual proteins upon transition of etioplasts to chloroplasts in pea (Pisum sativum). Plant Cell Physiol. 2008, 49, 396–410. [Google Scholar] [CrossRef]
- Pribil, M.; Labs, M.; Leister, D. Structure and dynamics of thylakoids in land plants. J. Exp. Bot. 2014, 65, 1955–1972. [Google Scholar] [CrossRef]
- Labate, M.T.V.; Ko, K.; Ko, Z.W.; Costa Pinto, L.S.R.; Real, M.J.U.D.; Romano, M.R.; Barja, P.R.; Granell, A.; Friso, G.; van Wijk, K.J.; et al. Constitutive expression of pea Lhcb1-2 in tobacco affects plant development, morphology and photosynthetic capacity. Plant Mol. Biol. 2004, 55, 701–714. [Google Scholar] [CrossRef]
- Kim, E.H.; Li, X.P.; Razeghifard, R.; Anderson, J.M.; Niyogi, K.K.; Pogson, B.J.; Chow, W.S. The multiple roles of light-harvesting chlorophyll a/b-protein complexes define structure and optimize function of Arabidopsis chloroplasts: A study using two chlorophyll b-less mutants. Biochim. Biophys. Acta 2009, 1787, 973–984. [Google Scholar] [CrossRef]
- Cui, Y.L.; Jia, Q.S.; Yin, Q.Q.; Lin, G.N.; Kong, M.M.; Yang, Z.N. The GDC1 gene encodes a novel ankyrin domain-containing protein that is essential for grana formation in Arabidopsis. Plant Physiol. 2011, 155, 130–141. [Google Scholar] [CrossRef][Green Version]
- Pietrzykowska, M.; Suorsa, M.; Semchonok, D.A.; Tikkanen, M.; Boekema, E.J.; Aro, E.M.; Jansson, S. The light-harvesting chlorophyll a/b binding proteins Lhcb1 and Lhcb2 play complementary roles during state transitions in Arabidopsis. Plant Cell 2014, 26, 3646–3660. [Google Scholar] [CrossRef]
- Armbruster, U.; Labs, M.; Pribil, M.; Viola, S.; Xu, W.; Scharfenberg, M.; Hertle, A.P.; Rojahn, U.; Jensen, P.E.; Rappaport, F.; et al. Arabidopsis CURVATURE THYLAKOID1 proteins modify thylakoid architecture by inducing membrane curvature. Plant Cell 2013, 25, 2661–2678. [Google Scholar] [CrossRef]
- Awai, K.; Maréchal, E.; Block, M.A.; Brun, D.; Masuda, T.; Shimada, H.; Takamiya, K.; Ohta, H.; Joyard, J. Two types of MGDG synthase genes, found widely in both 16:3 and 18:3 plants, differentially mediate galactolipid syntheses in photosynthetic and nonphotosynthetic tissues in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2001, 98, 10960–10965. [Google Scholar] [CrossRef]
- Kelly, A.A.; Kalisch, B.; Hölzl, G.; Schulze, S.; Thiele, J.; Melzer, M.; Roston, R.L.; Benning, C.; Dörmann, P. Synthesis and transfer of galactolipids in the chloroplast envelope membranes of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2016, 113, 10714–10719. [Google Scholar] [CrossRef]
- Kelly, A.A.; Froehlich, J.E.; Dörmann, P. Disruption of the two digalactosyldiacylglycerol synthase genes DGD1 and DGD2 in Arabidopsis reveals the existence of an additional enzyme of galactolipid synthesis. Plant Cell 2003, 15, 2694–2706. [Google Scholar] [CrossRef]
- Kobayashi, K.; Nakamura, Y.; Ohta, H. Type A and type B monogalactosyldiacylglycerol synthases are spatially and functionally separated in the plastids of higher plants. Plant Physiol. Biochem. 2009, 47, 518–525. [Google Scholar] [CrossRef]
- Kobayashi, K.; Awai, K.; Nakamura, M.; Nagatani, A.; Masuda, T.; Ohta, H. Type-B monogalactosyldiacylglycerol synthases are involved in phosphate starvation-induced lipid remodeling, and are crucial for low-phosphate adaptation. Plant J. 2009, 57, 322–331. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kondo, M.; Fukuda, H.; Nishimura, M.; Ohta, H. Galactolipid synthesis in chloroplast inner envelope is essential for proper thylakoid biogenesis, photosynthesis, and embryogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 17216–17221. [Google Scholar] [CrossRef]
- Fujii, S.; Kobayashi, K.; Nagata, N.; Masuda, T.; Wada, H. Monogalactosyldiacylglycerol facilitates synthesis of photoactive protochlorophyllide in etioplasts. Plant Physiol. 2017, 174, 2183–2198. [Google Scholar] [CrossRef]
- Fujii, S.; Kobayashi, K.; Nagata, N.; Masuda, T.; Wada, H. Digalactosyldiacylglycerol is essential for organization of the membrane structure in etioplasts. Plant Physiol. 2018, 177, 1487–1497. [Google Scholar] [CrossRef]
- Jensen, P.E.; Gibson, L.C.; Henningsen, K.W.; Hunter, C.N. Expression of the chlI, chlD, and chlH genes from the cyanobacterium Synechocystis PCC6803 in Escherichia coli and demonstration that the three cognate proteins are required for magnesium-protoporphyrin chelatase activity. J. Biol. Chem. 1996, 271, 16662–16667. [Google Scholar] [CrossRef] [PubMed]
- Papenbrock, J.; Gräfe, S.; Kruse, E.; Hänel, F.; Grimm, B. Mg-chelatase of tobacco: Identification of a Chl D cDNA sequence encoding a third subunit, analysis of the interaction of the three subunits with the yeast two-hybrid system, and reconstitution of the enzyme activity by co-expression of recombinant CHL D, CHL H and CHL I. Plant J. 1997, 12, 981–990. [Google Scholar] [PubMed]
- Block, M.A.; Tewari, A.K.; Albrieux, C.; Maréchal, E.; Joyard, J. The plant S-adenosyl-l-methionine:Mg-protoporphyrin IX methyltransferase is located in both envelope and thylakoid chloroplast membranes. Eur. J. Biochem. 2002, 269, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.E.; Canniffe, D.P.; Barnett, S.F.H.; Hollingshead, S.; Brindley, A.A.; Vasilev, C.; Bryant, D.A.; Hunter, C.N. Complete enzyme set for chlorophyll biosynthesis in Escherichia coli. Sci. Adv. 2018, 4, eaaq1407. [Google Scholar] [CrossRef] [PubMed]
- Gabruk, M.; Mysliwa-Kurdziel, B.; Kruk, J. MGDG, PG and SQDG regulate the activity of light-dependent protochlorophyllide oxidoreductase. Biochem. J. 2017, 474, 1307–1320. [Google Scholar] [CrossRef]
- Brentel, I.; Selstam, E.; Lindblom, G. Phase equilibria of mixtures of plant galactolipids. The formation of a bicontinuous cubic phase. Biochim. Biophys. Acta 1985, 812, 816–826. [Google Scholar] [CrossRef]
- Aronsson, H.; Sundqvist, C.; Dahlin, C. POR—Import and membrane association of a key element in chloroplast development. Physiol. Plant. 2003, 118, 1–9. [Google Scholar] [CrossRef]
- Jarvis, P.; Dörmann, P.; Peto, C.A.; Lutes, J.; Benning, C.; Chory, J. Galactolipid deficiency and abnormal chloroplast development in the Arabidopsis MGD synthase 1 mutant. Proc. Natl. Acad. Sci. USA 2000, 97, 8175–8179. [Google Scholar] [CrossRef]
- Yamaryo, Y.; Kanai, D.; Awai, K.; Shimojima, M.; Masuda, T.; Shimada, H.; Takamiya, K.; Ohta, H. Light and cytokinin play a co-operative role in MGDG synthesis in greening cucumber cotyledons. Plant Cell Physiol. 2003, 44, 844–855. [Google Scholar] [CrossRef]
- Selldén, G.; Selstam, E. Changes in chloroplast lipids during the development of photosynthetic activity in barley etio-chloroplasts. Physiol. Plant. 1976, 37, 35–41. [Google Scholar] [CrossRef]
- Appelqvist, L.Å.; Boynton, J.E.; Henningsen, K.W.; Stumpf, P.K.; von Wettstein, D. Lipid biosynthesis in chloroplast mutants of barley. J. Lipid Res. 1968, 9, 513–524. [Google Scholar] [PubMed]
- Murphy, D.J. The importance of non-planar bilayer regions in photosynthetic membranes and their stabilisation by galactolipids. FEBS Lett. 1982, 150, 19–26. [Google Scholar] [CrossRef]
- Seiwert, D.; Witt, H.; Ritz, S.; Janshoff, A.; Paulsen, H. The nonbilayer lipid MGDG and the major light-harvesting complex (LHCII) promote membrane stacking in supported lipid bilayers. Biochemistry 2018, 57, 2278–2288. [Google Scholar] [CrossRef] [PubMed]
- Gounaris, K.; Sundby, C.; Andersson, B.; Barber, J. Lateral heterogeneity of polar lipids in the thylakoid membranes of spinach chloroplasts. FEBS Lett. 1983, 156, 170–174. [Google Scholar] [CrossRef]
- Fujii, S.; Kobayashi, K.; Nakamura, Y.; Wada, H. Inducible knockdown of MONOGALACTOSYLDIACYLGLYCEROL SYNTHASE1 reveals roles of galactolipids in organelle differentiation in Arabidopsis cotyledons. Plant Physiol. 2014, 166, 1436–1449. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Ping, W.; Wu, H.; Li, M.; Gu, D.; Xu, Y. Monogalactosyldiacylglycerol deficiency in tobacco inhibits the cytochrome b6f-mediated intersystem electron transport process and affects the photostability of the photosystem II apparatus. Biochim. Biophys. Acta 2013, 1827, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Mazur, R.; Mostowska, A.; Szach, J.; Gieczewska, K.; Wójtowicz, J.; Bednarska, K.; Garstka, M.; Kowalewska, Ł. Galactolipid deficiency disturbs spatial arrangement of the thylakoid network in Arabidopsisthaliana plants. J. Exp. Bot. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kanduč, M.; Schlaich, A.; de Vries, A.H.; Jouhet, J.; Maréchal, E.; Demé, B.; Netz, R.R.; Schneck, E. Tight cohesion between glycolipid membranes results from balanced water-headgroup interactions. Nat. Commun. 2017, 8, 14899. [Google Scholar] [CrossRef]
- Dörmann, P.; Hoffmann-Benning, S.; Balbo, I.; Benning, C. Isolation and characterization of an Arabidopsis mutant deficient in the thylakoid lipid digalactosyl diacylglycerol. Plant Cell 1995, 7, 1801–1810. [Google Scholar]
- Hölzl, G.; Witt, S.; Gaude, N.; Melzer, M.; Schöttler, M.A.; Dörmann, P. The role of diglycosyl lipids in photosynthesis and membrane lipid homeostasis in Arabidopsis. Plant Physiol. 2009, 150, 1147–1159. [Google Scholar] [CrossRef]
- Simidjiev, I.; Stoylova, S.; Amenitsch, H.; Javorfi, T.; Mustardy, L.; Laggner, P.; Holzenburg, A.; Garab, G. Self-assembly of large, ordered lamellae from non-bilayer lipids and integral membrane proteins in vitro. Proc. Natl. Acad. Sci. USA 2000, 97, 1473–1476. [Google Scholar] [CrossRef] [PubMed]
- Seiwert, D.; Witt, H.; Janshoff, A.; Paulsen, H. The non-bilayer lipid MGDG stabilizes the major light-harvesting complex (LHCII) against unfolding. Sci. Rep. 2017, 7, 5158. [Google Scholar] [CrossRef] [PubMed]
- Schaller, S.; Latowski, D.; Jemioła-Rzemińska, M.; Dawood, A.; Wilhelm, C.; Strzałka, K.; Goss, R. Regulation of LHCII aggregation by different thylakoid membrane lipids. Biochim. Biophys. Acta 2011, 1807, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Park, M.J.; Gwon, G.H.; Silkov, A.; Xu, Z.Y.; Yang, E.C.; Song, S.; Song, K.; Kim, Y.; Yoon, H.S.; et al. An ankyrin repeat domain of AKR2 drives chloroplast targeting through coincident binding of two chloroplast lipids. Dev. Cell 2014, 30, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Wada, H.; Kobayashi, K. Expression of photosynthesis-associated genes depends on galactolipid synthesis during chloroplast differentiation. Manuscript in preparation.

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujii, S.; Wada, H.; Kobayashi, K. Role of Galactolipids in Plastid Differentiation Before and After Light Exposure. Plants 2019, 8, 357. https://doi.org/10.3390/plants8100357
Fujii S, Wada H, Kobayashi K. Role of Galactolipids in Plastid Differentiation Before and After Light Exposure. Plants. 2019; 8(10):357. https://doi.org/10.3390/plants8100357
Chicago/Turabian StyleFujii, Sho, Hajime Wada, and Koichi Kobayashi. 2019. "Role of Galactolipids in Plastid Differentiation Before and After Light Exposure" Plants 8, no. 10: 357. https://doi.org/10.3390/plants8100357
APA StyleFujii, S., Wada, H., & Kobayashi, K. (2019). Role of Galactolipids in Plastid Differentiation Before and After Light Exposure. Plants, 8(10), 357. https://doi.org/10.3390/plants8100357




