Fertilization Improves the Yield of Sapindus saponaria by Affecting Leaf–Soil–Microbial C–N–P Content and Stoichiometry
Abstract
1. Introduction
2. Results
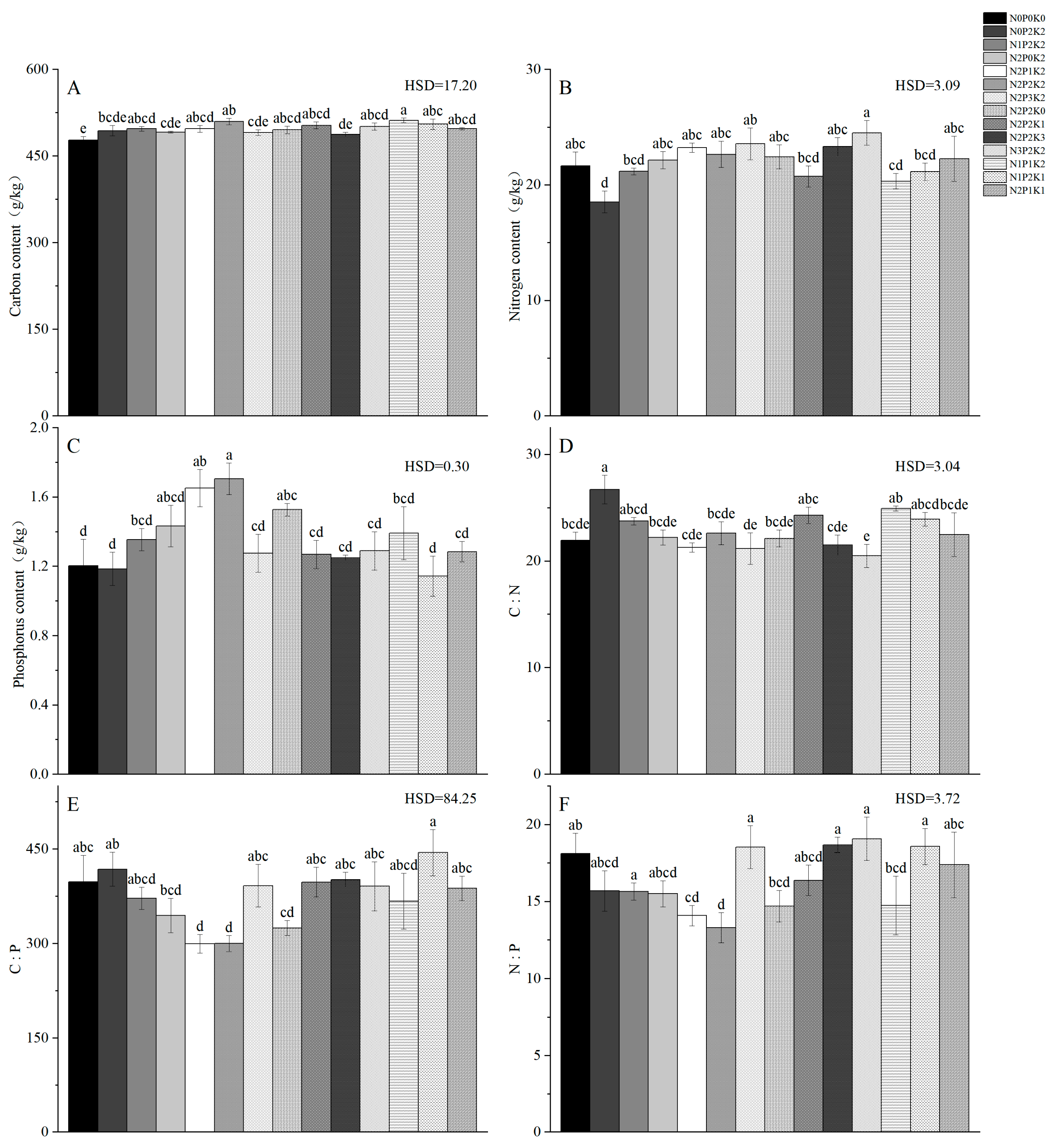
2.1. Effects of Different Fertilization Treatments on Carbon, Nitrogen, Phosphorus, and Stoichiometry of Sapindus saponaria Leaves
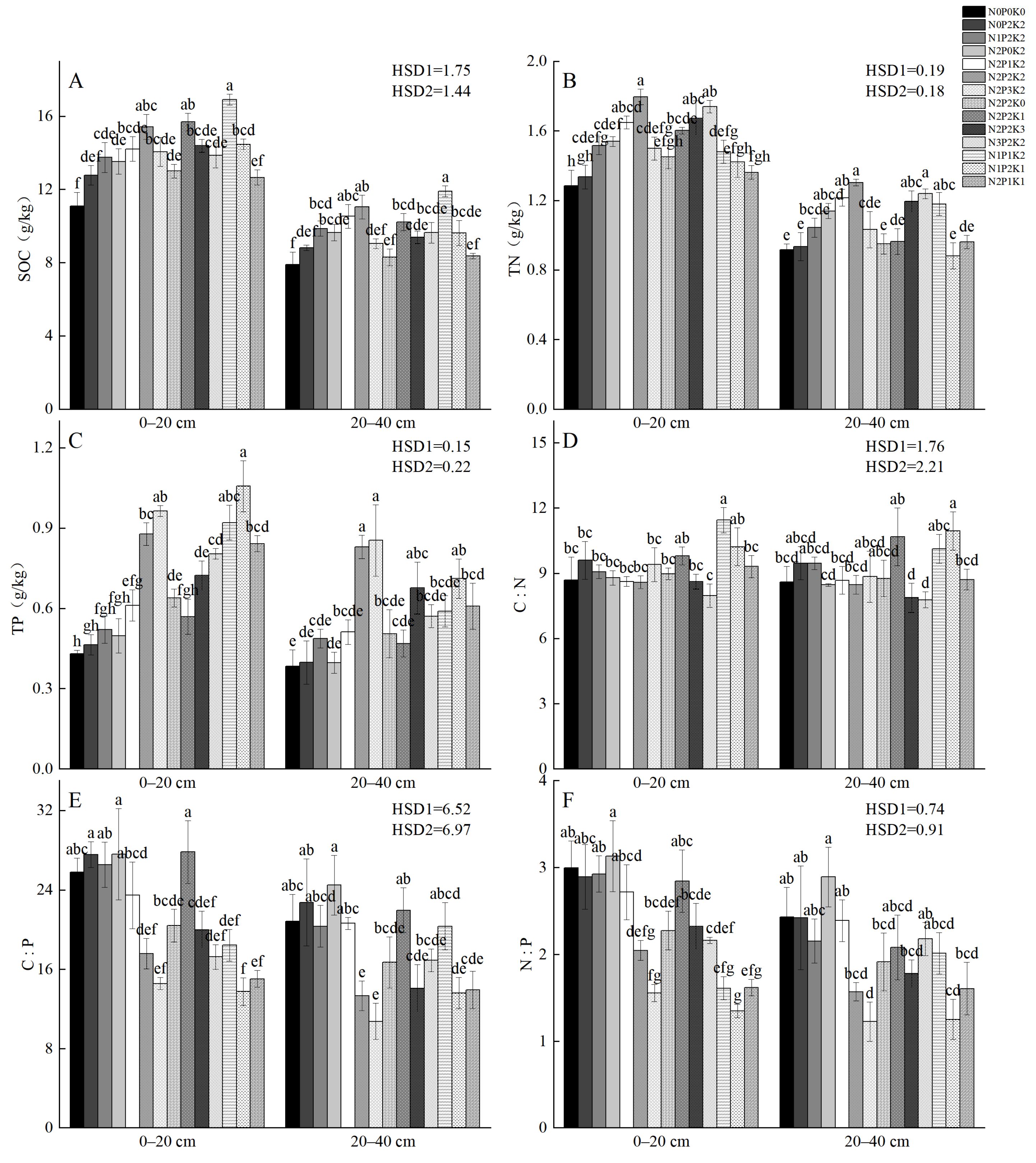
2.2. Soil Carbon, Nitrogen, and Phosphorus Contents, and Stoichiometric Ratios Under Different Fertilization Treatments
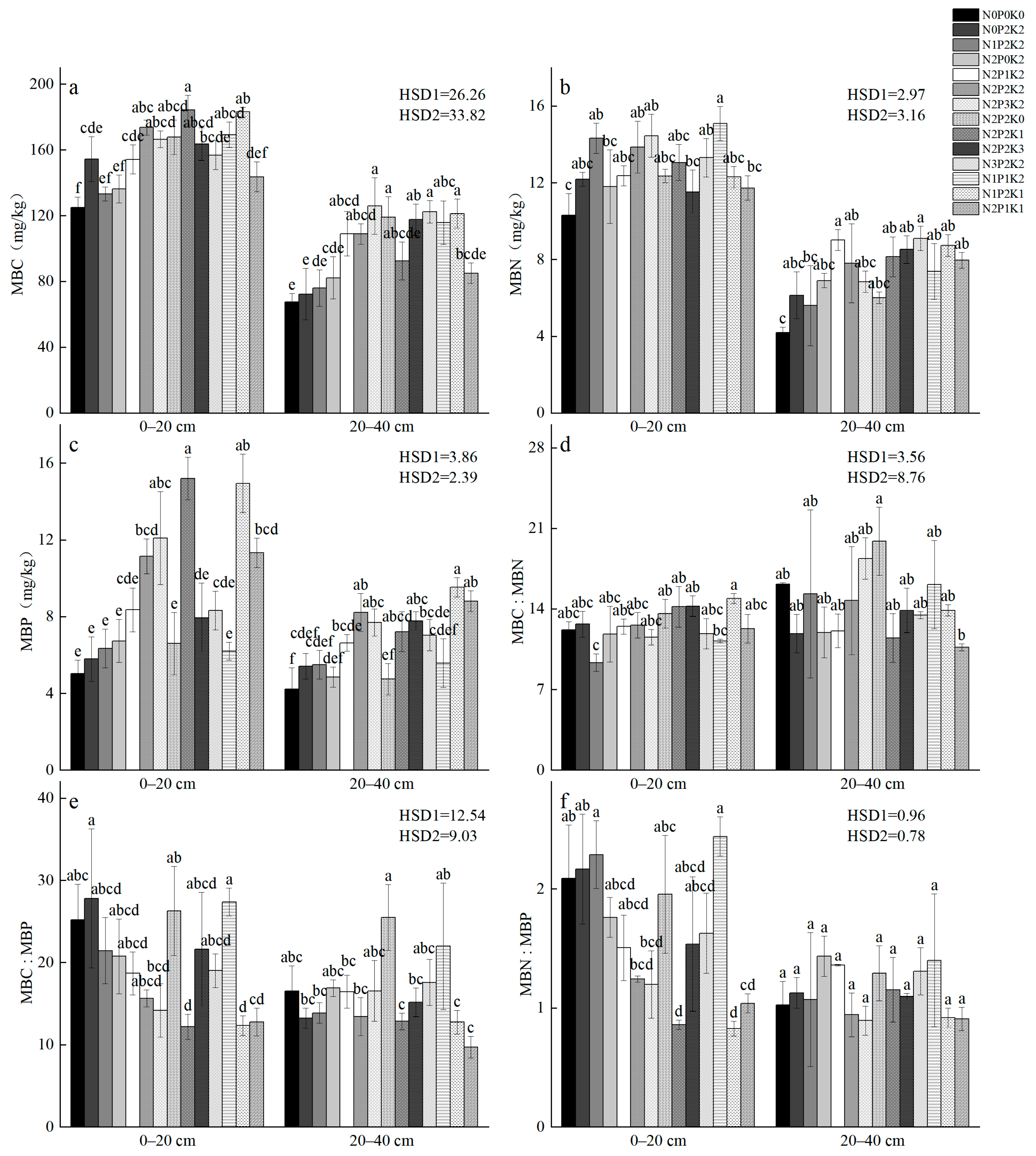
2.3. Microbial Carbon, Nitrogen, and Phosphorus Contents, and Stoichiometric Ratios Under Different Fertilization Treatments
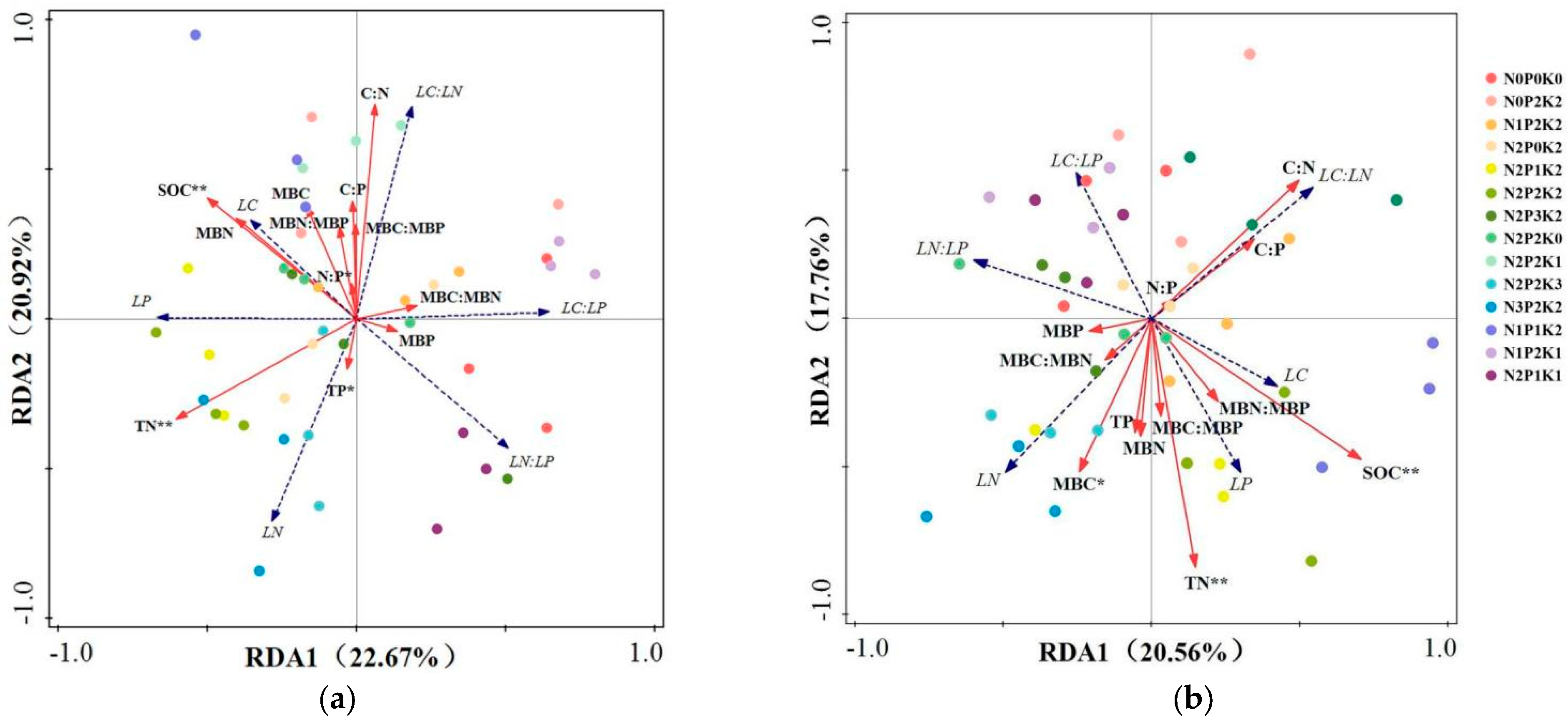
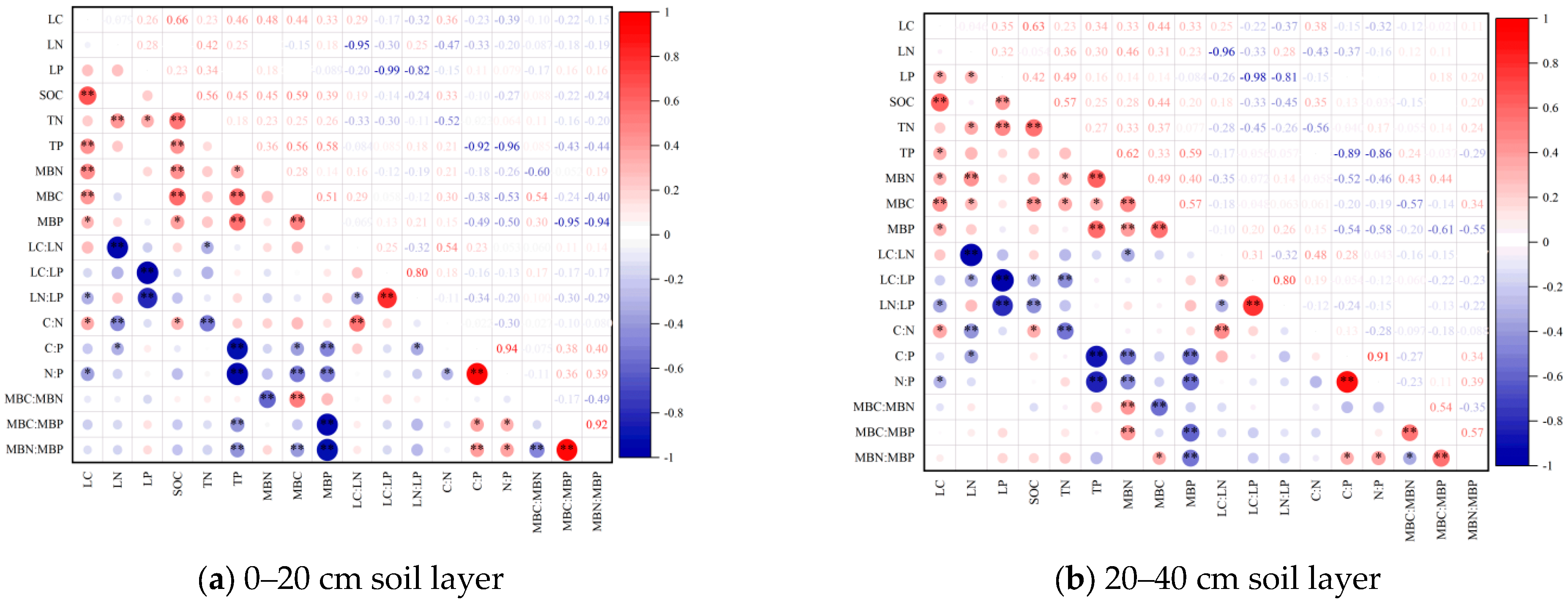
2.4. Leaf–Soil–Microbial Biomass C, N, P Contents, and Stoichiometric Characteristics
2.5. Multi-Objective Decision-Making and Evaluation Processes Leveraging the Entropy-Weighted TOPSIS Approach
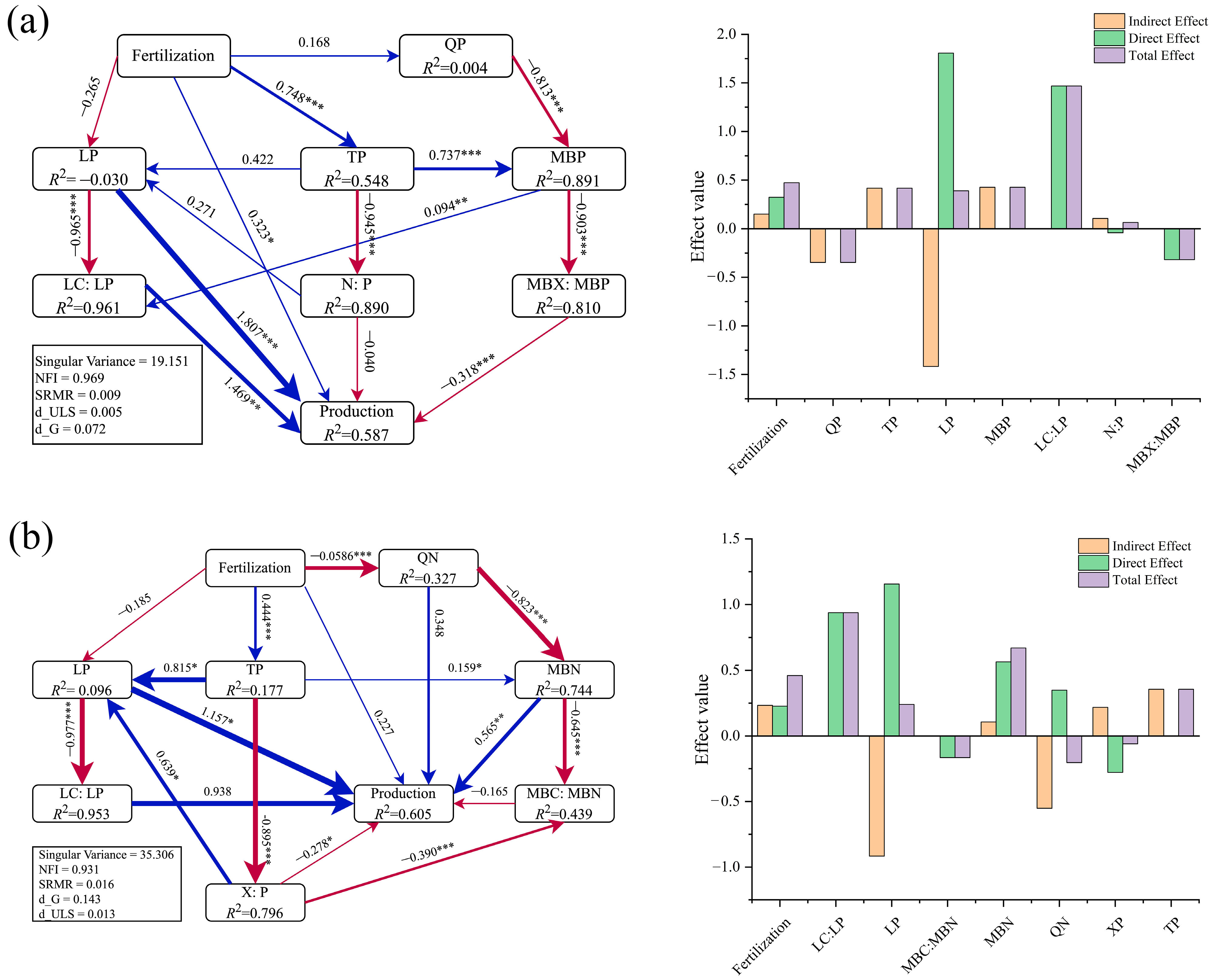
2.6. Analysis Using Partial Least Squares Path Model (PLS–PM)
3. Discussion
3.1. Characterization of C, N, and P Contents and Their Stoichiometric Ratios in Leaves
3.2. Characterization of Soil–Microbial C, N, and P Contents and Stoichiometric Ratios
3.3. Influence of Fertilization Regimes on Fruit Yield of Sapindus saponaria
3.4. Plant–Soil–Microbe Coupling
4. Materials and Methods
4.1. Overview of the Experimental Site
4.2. Experimental Design
4.3. Sample Collection and Measurement
4.4. Calculation of Leaf–Soil–Microbe-Related Indicators
4.5. Multi-Objective Decision Making and Evaluation Based on Entropy-Weighted TOPSIS Approach
- (1)
- An evaluation index matrix of leaf and soil C, N, and P contents, as well as yield attributes of Sapindus indica under different fertilization treatments, was established as follows.
- (2)
- Standardization of evaluation indicators to harmonize the types and dimensions of each indicator, with the following formula.
- For negative indicators, use the following formula:where Xij is the j indicator value for the i treatment (i = 1, …, n; j = 1, …, m) and X’ij is the normalized Xij.
- (3)
- The proportion of the j indicator represented by the i treatment (Pij) is calculated as follows:
- (4)
- The entropy value ej of the j indicator is calculated as follows:
- (5)
- The coefficient of variation gj for the j indicator is calculated as follows:
- (6)
- The weight Wj of the j indicator is calculated as follows:
- (7)
- A weighted normalized decision matrix (R) is formed from the normalized decision matrix X = (X’ij)14 × 10 and the weight vector W = (w1, w2, w3, …, w14).
- (8)
- Determine the optimal solution Zij+ and the worst solution Zij− to form the optimal vector Z+ and the worst vector Z−, respectively.
- (9)
- Determination of Euclidean spatial distances D+ and D− between 14 fertilization treatments and the worst solution.
- (10)
- Calculate the comprehensive evaluation value Ci of each treatment, i.e., calculate the closeness of the evaluation object to the optimal program as follows:
4.6. Statistics and Analysis of Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carnicer, J.; Sardans, J.; Stefanescu, C.; Ubach, A.; Bartrons, M.; Asensio, D.; Peñuelas, J. Global Biodiversity, Stoichiometry and Ecosystem Function Responses to Human-Induced C–N–P Imbalances. J. Plant Physiol. 2015, 172, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Feng, F.; Lu, C.; Fu, Y. C:N:P Stoichiometry of Different Soil Components after the Transition of Temperate Primary Coniferous and Broad-Leaved Mixed Forests to Secondary Forests. Soil Tillage Res. 2022, 216, 105260. [Google Scholar] [CrossRef]
- Xu, H.; Qu, Q.; Li, G.; Liu, G.; Geissen, V.; Ritsema, C.J.; Xue, S. Impact of Nitrogen Addition on Plant-Soil-Enzyme C–N–P Stoichiometry and Microbial Nutrient Limitation. Soil Biol. Biochem. 2022, 170, 108714. [Google Scholar] [CrossRef]
- Ding, D.; Arif, M.; Liu, M.; Li, J.; Hu, X.; Geng, Q.; Yin, F.; Li, C. Plant-Soil Interactions and C:N:P Stoichiometric Homeostasis of Plant Organs in Riparian Plantation. Front. Plant Sci. 2022, 13, 979023. [Google Scholar] [CrossRef]
- Gao, D.; Bai, E.; Wang, S.; Zong, S.; Liu, Z.; Fan, X.; Zhao, C.; Hagedorn, F. Three-dimensional Mapping of Carbon, Nitrogen, and Phosphorus in Soil Microbial Biomass and Their Stoichiometry at the Global Scale. Glob. Change Biol. 2022, 28, 6728–6740. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.Y.H. Plant Mixture Balances Terrestrial Ecosystem C:N:P Stoichiometry. Nat. Commun. 2021, 12, 4562. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Chen, X.; Liu, S.; Lu, X.; Chen, H.Y.H.; Ruan, H. Phosphorus Additions Imbalance Terrestrial Ecosystem C:N:P Stoichiometry. Glob. Change Biol. 2022, 28, 7353–7365. [Google Scholar] [CrossRef]
- Zhang, P.; Lü, X.-T.; Li, M.-H.; Wu, T.; Jin, G. N Limitation Increases along a Temperate Forest Succession: Evidences from Leaf Stoichiometry and Nutrient Resorption. J. Plant Ecol. 2022, 15, 1021–1035. [Google Scholar] [CrossRef]
- Hu, B.; Xie, M.; Li, H.; Zhao, W.; Hu, J.; Jiang, Y.; Ji, W.; Li, S.; Hong, Y.; Yang, M.; et al. Stoichiometry of Soil Carbon, Nitrogen, and Phosphorus in Farmland Soils in Southern China: Spatial Pattern and Related Dominates. Catena 2022, 217, 106468. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Cai, G.; Sauheitl, L.; Xiao, M.; Shibistova, O.; Ge, T.; Guggenberger, G. Meta-Analysis on the Effects of Types and Levels of N, P, and K Fertilization on Organic Carbon in Cropland Soils. Geoderma 2023, 437, 116580. [Google Scholar] [CrossRef]
- Han, B.; Yao, Y.; Wang, Y.; Su, X.; Ma, L.; Chen, X.; Li, Z. Microbial Traits Dictate Soil Necromass Accumulation Coefficient: A Global Synthesis. Glob. Ecol. Biogeogr. 2023, 33, 151–161. [Google Scholar] [CrossRef]
- Barłóg, P.; Grzebisz, W.; Łukowiak, R. Fertilizers and Fertilization Strategies Mitigating Soil Factors Constraining Efficiency of Nitrogen in Plant Production. Plants 2022, 11, 1855. [Google Scholar] [CrossRef] [PubMed]
- Dincă, L.C.; Grenni, P.; Onet, C.; Onet, A. Fertilization and Soil Microbial Community: A Review. Appl. Sci. 2022, 12, 1198. [Google Scholar] [CrossRef]
- Rutkowski, K.; Łysiak, G.P. Effect of Nitrogen Fertilization on Tree Growth and Nutrient Content in Soil and Cherry Leaves (Prunus cerasus L.). Agriculture 2023, 13, 578. [Google Scholar] [CrossRef]
- Fang, Z.; Yu, H.; Li, C.; Wang, B.; Huang, J. Soil Microbial Biomass C:N:P Stoichiometry Is Driven More by Climate, Soil Properties and Plant Traits than by N Enrichment in a Desert Steppe. Catena 2022, 216, 106402. [Google Scholar] [CrossRef]
- Jia, Y.; Cheng, Z.; Peng, Y.; Yang, G. Microplastics Alter the Equilibrium of Plant-Soil-Microbial System: A Meta-Analysis. Ecotoxicol. Environ. Saf. 2024, 272, 116082. [Google Scholar] [CrossRef]
- Song, S.; Xiong, K.; Chi, Y. Ecological Stoichiometric Characteristics of Plant–Soil–Microorganism of Grassland Ecosystems under Different Restoration Modes in the Karst Desertification Area. Agronomy 2023, 13, 2016. [Google Scholar] [CrossRef]
- Song, Z.; Zuo, X.; Zhao, X.; Qiao, J.; Ya, H.; Li, X.; Yue, P.; Chen, M.; Wang, S.; Medina-Roldán, E. Plant Functional Traits Mediate the Response Magnitude of Plant-Litter-Soil Microbial C:N:P Stoichiometry to Nitrogen Addition in a Desert Steppe. Sci. Total Environ. 2024, 915, 169915. [Google Scholar] [CrossRef]
- Li, C.; Zhao, C.; Zhao, X.; Wang, Y.; Lv, X.; Zhu, X.; Song, X. Beneficial Effects of Biochar Application with Nitrogen Fertilizer on Soil Nitrogen Retention, Absorption and Utilization in Maize Production. Agronomy 2023, 13, 113. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, H.; Yi, H. Impact of Fertilizer on Crop Yield and C:N:P Stoichiometry in Arid and Semi-Arid Soil. Int. J. Environ. Res. Public Health 2021, 18, 4341. [Google Scholar] [CrossRef]
- Sardans, J.; Rivas-Ubach, A.; Peñuelas, J. The C:N:P Stoichiometry of Organisms and Ecosystems in a Changing World: A Review and Perspectives. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 33–47. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, C.; Su, Y.; Peng, W.; Lu, R.; Liu, Y.; Huang, H.; He, X.; Yang, M.; Zhu, S. Soil Acidification Caused by Excessive Application of Nitrogen Fertilizer Aggravates Soil-Borne Diseases: Evidence from Literature Review and Field Trials. Agric. Ecosyst. Environ. 2022, 340, 108176. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, R.; Xia, S.; Wang, L.; Liu, C.; Zhang, R.; Fan, Z.; Chen, F.; Liu, Y. Interactions between N, P and K Fertilizers Affect the Environment and the Yield and Quality of Satsumas. Glob. Ecol. Conserv. 2019, 19, e00663. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, X.; Wei, D.; Zhao, B.; Ma, M.; Chen, S.; Cao, F.; Shen, D.; Guan, D.; Li, J. Consistent Effects of Nitrogen Fertilization on Soil Bacterial Communities in Black Soils for Two Crop Seasons in China. Sci. Rep. 2017, 7, 3267. [Google Scholar] [CrossRef]
- Lin, Y.; Lai, Y.; Tang, S.; Qin, Z.; Liu, J.; Kang, F.; Kuang, Y. Climatic and Edaphic Variables Determine Leaf C, N, P Stoichiometry of Deciduous Quercus Species. Plant Soil 2022, 474, 383–394. [Google Scholar] [CrossRef]
- Fan, H.; Wu, J.; Liu, W.; Yuan, Y.; Hu, L.; Cai, Q. Linkages of Plant and Soil C:N:P Stoichiometry and Their Relationships to Forest Growth in Subtropical Plantations. Plant Soil 2015, 392, 127–138. [Google Scholar] [CrossRef]
- Liu, J.; Wang, D.; Yan, X.; Jia, L.; Chen, N.; Liu, J.; Zhao, P.; Zhou, L.; Cao, Q. Effect of Nitrogen, Phosphorus and Potassium Fertilization Management on Soil Properties and Leaf Traits and Yield of Sapindus mukorossi. Front. Plant Sci. 2024, 15, 1300683. [Google Scholar] [CrossRef]
- Tuo, Y.; Wang, Z.; Zheng, Y.; Shi, X.; Liu, X.; Ding, M.; Yang, Q. Effect of Water and Fertilizer Regulation on the Soil Microbial Biomass Carbon and Nitrogen, Enzyme Activity, and Saponin Content of Panax Notoginseng. Agric. Water Manag. 2023, 278, 108145. [Google Scholar] [CrossRef]
- Yan, F.; Liu, X.; Bai, W.; Fan, J.; Zhang, F.; Xiang, Y.; Hou, X.; Pei, S.; Dai, Y.; Zeng, H.; et al. Multi-Objective Optimization of Water and Nitrogen Regimes for Drip-Fertigated Sugar Beet in a Desert Climate. Field Crops Res. 2022, 288, 108703. [Google Scholar] [CrossRef]
- Liu, X.; Peng, Y.; Yang, Q.; Wang, X.; Cui, N. Determining Optimal Deficit Irrigation and Fertilization to Increase Mango Yield, Quality, and WUE in a Dry Hot Environment Based on TOPSIS. Agric. Water Manag. 2021, 245, 106650. [Google Scholar] [CrossRef]
- Han, P.; Zhang, W.; Wang, G.; Sun, W.; Huang, Y. Changes in Soil Organic Carbon in Croplands Subjected to Fertilizer Management: A Global Meta-Analysis. Sci. Rep. 2016, 6, 27199. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, L.; Wang, D.; Gong, Y.; Yan, X.; Cao, Q.; Wu, S.; Weng, J.; Zhang, G.; Jia, L. The Effect of Balanced N, P and K Fertilization on Fine Root Traits and Soil Properties in Sapindus mukorossi. Forests 2024, 15, 94. [Google Scholar] [CrossRef]
- Zhao, G.; Gao, Y.; Gao, S.; Xu, Y.; Liu, J.; Sun, C.; Gao, Y.; Liu, S.; Chen, Z.; Jia, L. The Phenological Growth Stages of Sapindus mukorossi According to BBCH Scale. Forests 2019, 10, 462. [Google Scholar] [CrossRef]
- Ågren, G.I.; Wetterstedt, J.Å.M.; Billberger, M.F.K. Nutrient Limitation on Terrestrial Plant Growth—Modeling the Interaction between Nitrogen and Phosphorus. New Phytol. 2012, 194, 953–960. [Google Scholar] [CrossRef]
- Li, Y.; Niu, S.; Yu, G. Aggravated Phosphorus Limitation on Biomass Production under Increasing Nitrogen Loading: A Meta-analysis. Glob. Change Biol. 2016, 22, 934–943. [Google Scholar] [CrossRef]
- Tessier, J.T.; Raynal, D.J. Use of Nitrogen to Phosphorus Ratios in Plant Tissue as an Indicator of Nutrient Limitation and Nitrogen Saturation. J. Appl. Ecol. 2003, 40, 523–534. [Google Scholar] [CrossRef]
- Han, W.; Fang, J.; Guo, D.; Zhang, Y. Leaf Nitrogen and Phosphorus Stoichiometry across 753 Terrestrial Plant Species in China. New Phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef]
- Raven, J.A.; Handley, L.L.; Andrews, M. Global Aspects of C/N Interactions Determining Plant-Environment Interactions. J. Exp. Bot. 2003, 55, 11–25. [Google Scholar] [CrossRef]
- Thuille, A.; Laufer, J.; Höhl, C.; Gleixner, G. Carbon Quality Affects the Nitrogen Partitioning between Plants and Soil Microorganisms. Soil Biol. Biochem. 2015, 81, 266–274. [Google Scholar] [CrossRef]
- Elser, J.J.; Fagan, W.F.; Denno, R.F.; Dobberfuhl, D.R.; Folarin, A.; Huberty, A.; Interlandi, S.; Kilham, S.S.; McCauley, E.; Schulz, K.L.; et al. Nutritional Constraints in Terrestrial and Freshwater Food Webs. Nature 2000, 408, 578–580. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J. Global Patterns of Plant Leaf N and P in Relation to Temperature and Latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef] [PubMed]
- Bindraban, P.S.; Dimkpa, C.O.; Pandey, R. Exploring Phosphorus Fertilizers and Fertilization Strategies for Improved Human and Environmental Health. Biol. Fertil. Soils 2020, 56, 299–317. [Google Scholar] [CrossRef]
- Feng, J.; Chen, L.; Xia, T.; Ruan, Y.; Sun, X.; Wu, T.; Zhong, Y.; Shao, X.; Tang, Z. Microbial Fertilizer Regulates C:N:P Stoichiometry and Alleviates Phosphorus Limitation in Flue-Cured Tobacco Planting Soil. Sci. Rep. 2023, 13, 10276. [Google Scholar] [CrossRef] [PubMed]
- Francis, B.; Aravindakumar, C.T.; Brewer, P.B.; Simon, S. Plant Nutrient Stress Adaptation: A Prospect for Fertilizer Limited Agriculture. Environ. Exp. Bot. 2023, 213, 105431. [Google Scholar] [CrossRef]
- He, M.; Dijkstra, F.A.; Zhang, K.; Li, X.; Tan, H.; Gao, Y.; Li, G. Leaf Nitrogen and Phosphorus of Temperate Desert Plants in Response to Climate and Soil Nutrient Availability. Sci. Rep. 2014, 4, 6932. [Google Scholar] [CrossRef]
- Tang, Z.; Xu, W.; Zhou, G.; Bai, Y.; Li, J.; Tang, X.; Chen, D.; Liu, Q.; Ma, W.; Xiong, G.; et al. Patterns of Plant Carbon, Nitrogen, and Phosphorus Concentration in Relation to Productivity in China’s Terrestrial Ecosystems. Proc. Natl. Acad. Sci. USA 2018, 115, 4033–4038. [Google Scholar] [CrossRef]
- Maity, A.; Marathe, R.A.; Sarkar, A.; Basak, B.B. Phosphorus and Potassium Supplementing Bio-Mineral Fertilizer Augments Soil Fertility and Improves Fruit Yield and Quality of Pomegranate. Sci. Hortic. 2022, 303, 111234. [Google Scholar] [CrossRef]
- Zhang, H.; Gong, L.; Ding, Z.; Wu, X. Fine-Root Soil Stoichiometry of Picea Schrenkiana Fisch. et Mey. and Its Correlation with Soil Environmental Factors under Different Nitrogen Input Levels in the Tianshan Mountains, Xinjiang. Forests 2023, 14, 426. [Google Scholar] [CrossRef]
- Dong, R.; Yang, S.; Wang, X.; Xie, L.; Ma, Y.; Wang, Y.; Zhang, L.; Zhang, M.; Qin, J. C:N:P Stoichiometry in Plant, Soil and Microbe in Sophora Moorcroftiana Shrubs across Three Sandy Dune Types in the Middle Reaches of the Yarlung Zangbo River. Front. Plant Sci. 2023, 13, 1060686. [Google Scholar] [CrossRef]
- Xu, M.; Zhu, Y.; Zhang, S.; Feng, Y.; Zhang, W.; Han, X. Global Scaling the Leaf Nitrogen and Phosphorus Resorption of Woody Species: Revisiting Some Commonly Held Views. Sci. Total Environ. 2021, 788, 147807. [Google Scholar] [CrossRef]
- Hessen, D.O.; Ågren, G.I.; Anderson, T.R.; Elser, J.J.; De Ruiter, P.C. Carbon sequestration in ecosystems: The role of stoichiometry. Ecology 2004, 85, 1179–1192. [Google Scholar] [CrossRef]
- Chen, C.; Hall, S.J.; Coward, E.; Thompson, A. Iron-Mediated Organic Matter Decomposition in Humid Soils Can Counteract Protection. Nat. Commun. 2020, 11, 2255. [Google Scholar] [CrossRef] [PubMed]
- Kirkby, C.A.; Richardson, A.E.; Wade, L.J.; Batten, G.D.; Blanchard, C.; Kirkegaard, J.A. Carbon-Nutrient Stoichiometry to Increase Soil Carbon Sequestration. Soil Biol. 2013, 60, 77–86. [Google Scholar] [CrossRef]
- Tipping, E.; Somerville, C.J.; Luster, J. The C:N:P:S Stoichiometry of Soil Organic Matter. Biogeochemistry 2016, 130, 117–131. [Google Scholar] [CrossRef]
- Tian, H.; Chen, G.; Zhang, C.; Melillo, J.M.; Hall, C.A.S. Pattern and Variation of C:N:P Ratios in China’s Soils: A Synthesis of Observational Data. Biogeochemistry 2010, 98, 139–151. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.; Liu, S.; Shen, J.; Huang, D.; Su, Y.; Wei, W.; Syers, J.K. Is the C:N:P Stoichiometry in Soil and Soil Microbial Biomass Related to the Landscape and Land Use in Southern Subtropical China? Glob. Biogeochem. Cycles 2012, 26, 4399. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Liptzin, D. C:N:P Stoichiometry in Soil: Is There a “Redfield Ratio” for the Microbial Biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Xu, X.; Thornton, P.E.; Post, W.M. A Global Analysis of Soil Microbial Biomass Carbon, Nitrogen and Phosphorus in Terrestrial Ecosystems. Glob. Ecol. Biogeogr. 2013, 22, 737–749. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.; An, X.; Song, Z.; Zhu, Y.; Tan, Y.; Guo, X.; Wang, D. Effects of Nitrogen, Phosphorus and Potassium Formula Fertilization on the Yield and Berry Quality of Blueberry. PLoS ONE 2023, 18, e0283137. [Google Scholar] [CrossRef]
- Chen, K.; Ma, L.; Chen, C.; Liu, N.; Wang, B.; Bao, Y.; Liu, Z.; Zhou, G. Long-Term Impact of N, P, K Fertilizers in Different Rates on Yield and Quality of Anisodus tanguticus (Maxinowicz) Pascher. Plants 2023, 12, 2102. [Google Scholar] [CrossRef]
- Zhu, L.; Luan, L.; Chen, Y.; Wang, X.; Zhou, S.; Zou, W.; Han, X.; Duan, Y.; Zhu, B.; Li, Y.; et al. Community Assembly of Organisms Regulates Soil Microbial Functional Potential through Dual Mechanisms. Glob. Change Biol. 2024, 30, e17160. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.G.; Feng, Y.Z. Research Progresses of Farmland Microorganisms in Fluvo-Aquic Soil of China. Chin. J. Eco-Agric. 2016, 24, 416–434. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Sun, C.; Xi, B.; Li, D.; Chen, Z.; He, Q.; Weng, X.; Jia, L. Global Distribution of Soapberries (Sapindus L.) Habitats under Current and Future Climate Scenarios. Sci. Rep. 2021, 11, 19740. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska-Długosz, A.; Długosz, J.; Frąc, M.; Gryta, A.; Breza-Boruta, B. Enzymatic Activity and Functional Diversity of Soil Microorganisms along the Soil Profile—A Matter of Soil Depth and Soil-Forming Processes. Geoderma 2022, 416, 115779. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, Z.; Zhang, G.; Chen, D.; Zang, L.; Liu, Q.; Guo, Y.; Xie, P.; Chen, H.; He, Y. Variability of Soil Enzyme Activities and Nutrients with Forest Gap Renewal Interacting with Soil Depths in Degraded Karst Forests. Ecol. Indic. 2024, 166, 112332. [Google Scholar] [CrossRef]
- Harish, M.N.; Choudhary, A.K.; Bhupenchandra, I.; Dass, A.; Rajanna, G.A.; Singh, V.K.; Bana, R.S.; Varatharajan, T.; Verma, P.; George, S.; et al. Double Zero-Tillage and Foliar-P Nutrition Coupled with Bio-Inoculants Enhance Physiological Photosynthetic Characteristics and Resilience to Nutritional and Environmental Stresses in Maize–Wheat Rotation. Front. Plant Sci. 2022, 13, 959541. [Google Scholar] [CrossRef]
- Khan, F.; Siddique, A.B.; Shabala, S.; Zhou, M.; Zhao, C. Phosphorus Plays Key Roles in Regulating Plants’ Physiological Responses to Abiotic Stresses. Plants 2023, 12, 2861. [Google Scholar] [CrossRef]
- Van Der Velde, M.; Folberth, C.; Balkovič, J.; Ciais, P.; Fritz, S.; Janssens, I.A.; Obersteiner, M.; See, L.; Skalský, R.; Xiong, W.; et al. African Crop Yield Reductions Due to Increasingly Unbalanced Nitrogen and Phosphorus Consumption. Glob. Change Biol. 2014, 20, 1278–1288. [Google Scholar] [CrossRef]
- Liu, J.; Shu, A.; Song, W.; Shi, W.; Li, M.; Zhang, W.; Li, Z.; Liu, G.; Yuan, F.; Zhang, S.; et al. Long-Term Organic Fertilizer Substitution Increases Rice Yield by Improving Soil Properties and Regulating Soil Bacteria. Geoderma 2021, 404, 115287. [Google Scholar] [CrossRef]
- Li, P.; Yang, Y.; Han, W.; Fang, J. Global Patterns of Soil Microbial Nitrogen and Phosphorus Stoichiometry in Forest Ecosystems. Glob. Ecol. Biogeogr. 2014, 23, 979–987. [Google Scholar] [CrossRef]
- Luo, G.; Xue, C.; Jiang, Q.; Xiao, Y.; Zhang, F.; Guo, S.; Shen, Q.; Ling, N. Soil Carbon, Nitrogen, and Phosphorus Cycling Microbial Populations and Their Resistance to Global Change Depend on Soil C:N:P Stoichiometry. mSystems 2020, 5, e00162-20. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Ma, Q.; Marsden, K.A.; Chadwick, D.R.; Luo, Y.; Kuzyakov, Y.; Wu, L.; Jones, D.L. Microbial Community Succession in Soil Is Mainly Driven by Carbon and Nitrogen Contents Rather than Phosphorus and Sulphur Contents. Soil Biol. Biochem. 2023, 180, 11. [Google Scholar] [CrossRef]
- Liu, K.-L.; Chen, B.-Y.; Zhang, B.; Wang, R.-H.; Wang, C.-S. Understory Vegetation Diversity, Soil Properties and Microbial Community Response to Different Thinning Intensities in Cryptomeria japonica var. sinensis plantations. Front. Microbiol. 2023, 11, 1117384. [Google Scholar] [CrossRef]
- Wang, G.; Xiao, H.; Xin, Z.; Luo, F.; Jin, Y.; Liu, M.; Li, J. Changes in Plant-Soil-Microbe C-N-P Contents and Stoichiometry during Poplar Shelterbelt Degradation. Catena 2024, 243, 108227. [Google Scholar] [CrossRef]
- Chi, Y.; Song, S.; Xiong, K.; Albasher, G.; Fang, J. Responses of Soil Microbial Biomass, Microbial Entropy and Soil-Microorganism Stoichiometry Imbalance to Different Utilization Patterns in the Artificial Grassland of Karst Desertification Area. Front. Microbiol. 2023, 14, 129353. [Google Scholar] [CrossRef]
- Hu, L.; Bentler, P.M. Cutoff Criteria for Fit Indexes in Covariance Structure Analysis: Conventional Criteria versus New Alternatives. Struct. Equ. Model. A Multidiscip. J. 1999, 6, 1–55. [Google Scholar] [CrossRef]
| 0–20 cm | 20–40 cm | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment No. | Euclidean Space Distance D+ | Euclidean Space Distance D− | Ci | Ranking | Treatment No. | Euclidean Space Distance D+ | Euclidean Space Distance D− | Ci | Ranking |
| N0P0K0 | 0.32 | 0.03 | 0.09 | 14 | N0P0K0 | 0.32 | 0.04 | 0.10 | 14 |
| N0P2K2 | 0.29 | 0.07 | 0.19 | 13 | N0P2K2 | 0.29 | 0.05 | 0.16 | 13 |
| N1P2K2 | 0.25 | 0.12 | 0.34 | 11 | N1P2K2 | 0.23 | 0.11 | 0.34 | 12 |
| N2P0K2 | 0.24 | 0.11 | 0.33 | 12 | N2P0K2 | 0.24 | 0.13 | 0.36 | 11 |
| N2P1K2 | 0.17 | 0.20 | 0.53 | 5 | N2P1K2 | 0.14 | 0.22 | 0.61 | 2 |
| N2P2K2 | 0.09 | 0.27 | 0.75 | 1 | N2P2K2 | 0.05 | 0.30 | 0.84 | 1 |
| N2P3K2 | 0.15 | 0.21 | 0.58 | 3 | N2P3K2 | 0.17 | 0.23 | 0.57 | 3 |
| N2P2K1 | 0.21 | 0.15 | 0.42 | 10 | N2P2K1 | 0.23 | 0.15 | 0.40 | 9 |
| N2P2K0 | 0.18 | 0.23 | 0.56 | 4 | N2P2K0 | 0.22 | 0.14 | 0.38 | 10 |
| N2P2K3 | 0.21 | 0.16 | 0.42 | 9 | N2P2K3 | 0.16 | 0.20 | 0.56 | 6 |
| N3P2K2 | 0.18 | 0.19 | 0.50 | 7 | N3P2K2 | 0.17 | 0.21 | 0.56 | 5 |
| N1P1K2 | 0.19 | 0.20 | 0.51 | 6 | N1P1K2 | 0.16 | 0.21 | 0.57 | 4 |
| N1P2K1 | 0.18 | 0.25 | 0.58 | 2 | N1P2K1 | 0.21 | 0.22 | 0.50 | 7 |
| N2P1K1 | 0.19 | 0.16 | 0.45 | 8 | N2P1K1 | 0.22 | 0.15 | 0.42 | 8 |
| Processing Number | Fertilization Levels (kg·ha−1) | ||
|---|---|---|---|
| N | P | K | |
| N0P0K0 | 0 | 0 | 0 |
| N0P2K2 | 0 | 500 | 400 |
| N1P2K2 | 300 | 500 | 400 |
| N2P0K2 | 600 | 0 | 400 |
| N2P1K2 | 600 | 250 | 400 |
| N2P2K2 | 600 | 500 | 400 |
| N2P3K2 | 600 | 750 | 400 |
| N2P2K0 | 600 | 500 | 0 |
| N2P2K1 | 600 | 500 | 200 |
| N2P2K3 | 600 | 500 | 600 |
| N3P2K2 | 900 | 500 | 400 |
| N1P1K2 | 300 | 250 | 400 |
| N1P2K1 | 300 | 500 | 200 |
| N2P1K1 | 600 | 250 | 200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Yang, H.; Zhou, L.; Zhang, S.; Chen, J.; Wang, X.; Wu, S.; Gong, Y.; Zhang, G.; Zhang, W.; et al. Fertilization Improves the Yield of Sapindus saponaria by Affecting Leaf–Soil–Microbial C–N–P Content and Stoichiometry. Plants 2025, 14, 1360. https://doi.org/10.3390/plants14091360
Liu J, Yang H, Zhou L, Zhang S, Chen J, Wang X, Wu S, Gong Y, Zhang G, Zhang W, et al. Fertilization Improves the Yield of Sapindus saponaria by Affecting Leaf–Soil–Microbial C–N–P Content and Stoichiometry. Plants. 2025; 14(9):1360. https://doi.org/10.3390/plants14091360
Chicago/Turabian StyleLiu, Juntao, Hongbing Yang, Ling Zhou, Shangpeng Zhang, Jie Chen, Xu Wang, Shixiong Wu, Yingyun Gong, Guoqing Zhang, Weihua Zhang, and et al. 2025. "Fertilization Improves the Yield of Sapindus saponaria by Affecting Leaf–Soil–Microbial C–N–P Content and Stoichiometry" Plants 14, no. 9: 1360. https://doi.org/10.3390/plants14091360
APA StyleLiu, J., Yang, H., Zhou, L., Zhang, S., Chen, J., Wang, X., Wu, S., Gong, Y., Zhang, G., Zhang, W., & Jia, L. (2025). Fertilization Improves the Yield of Sapindus saponaria by Affecting Leaf–Soil–Microbial C–N–P Content and Stoichiometry. Plants, 14(9), 1360. https://doi.org/10.3390/plants14091360







