Optimization of Hydroponic Wheat Sprouts as an Alternative Livestock Feed: Yield and Biochemical Composition Under Different Fertilization Regimes
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydroponic System Setup
2.2. Hydroponic Wheat Sprouts Growth Parameters
2.2.1. Microclimate
2.2.2. Lighting, Photoperiod, and Irrigation Strategy
2.2.3. Preparation of Seeds and Cultivation of Hydroponic Wheat Sprouts
2.2.4. Nutrient Solution
2.2.5. Investigated Quantitative and Qualitative Parameters
2.3. Statistical Analysis
3. Results and Discussion
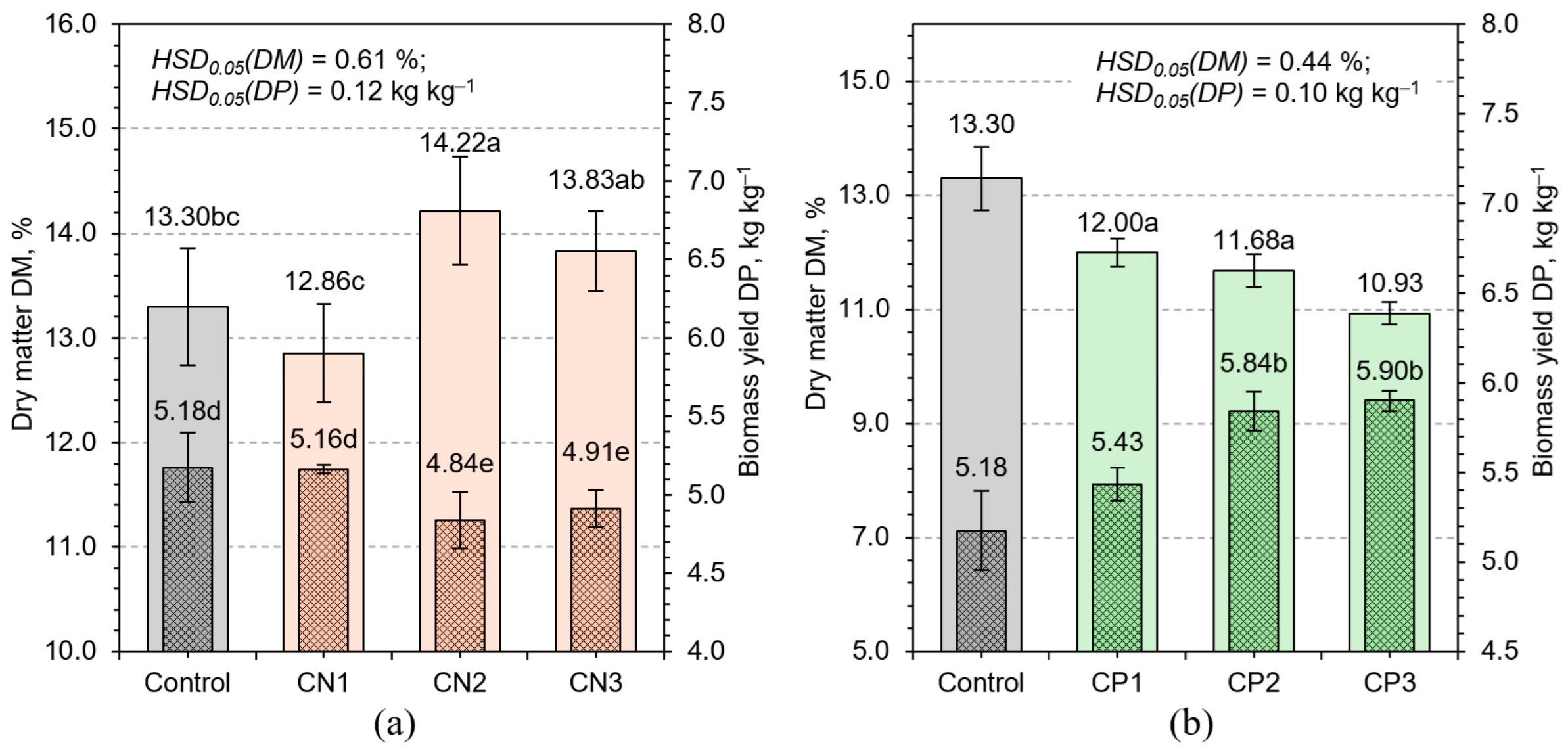
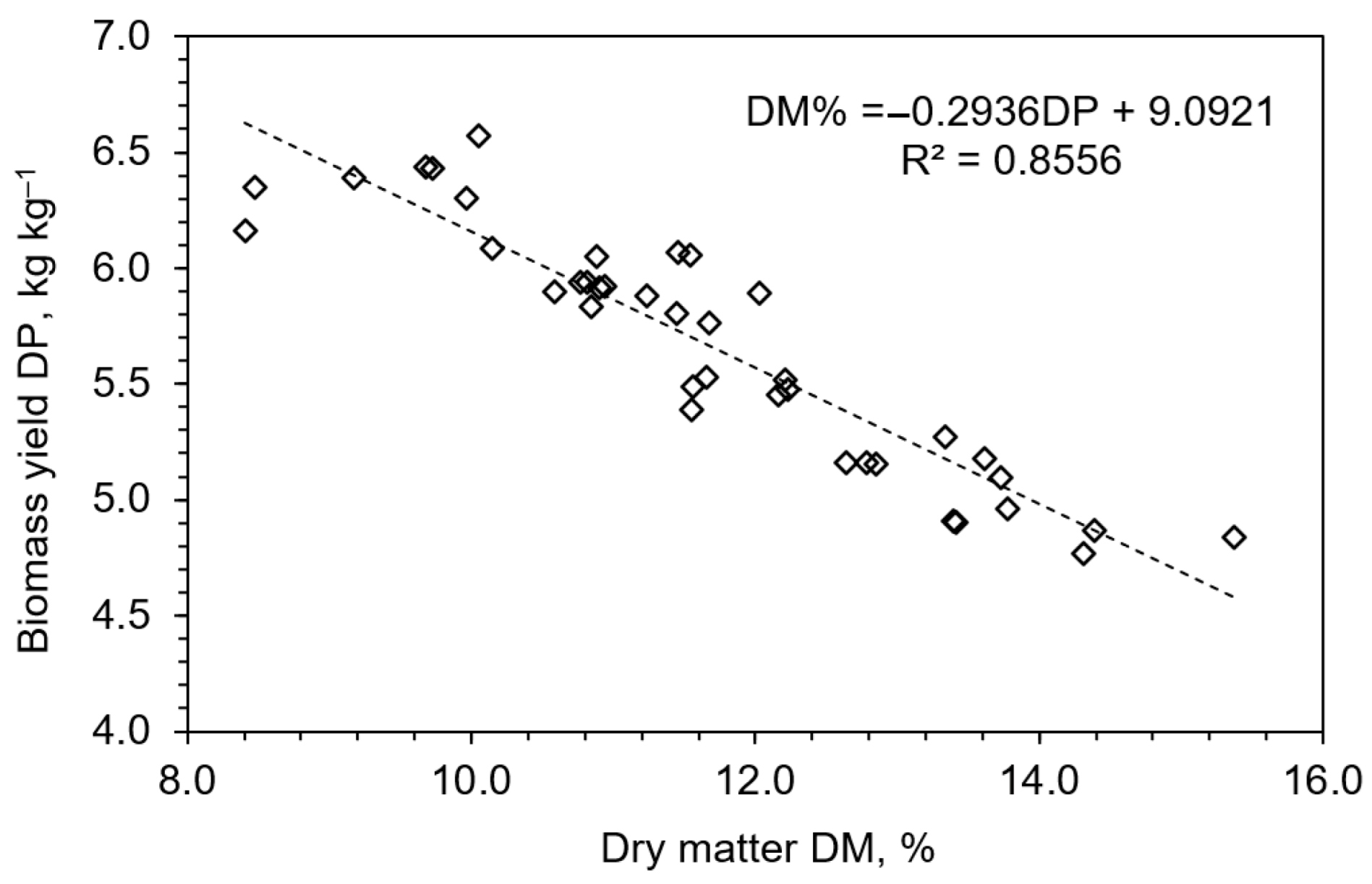
3.1. Biomass Yield and Dry Matter Content Response to Fertilization
3.2. Effect of Fertilization on Feed Quality Parameters
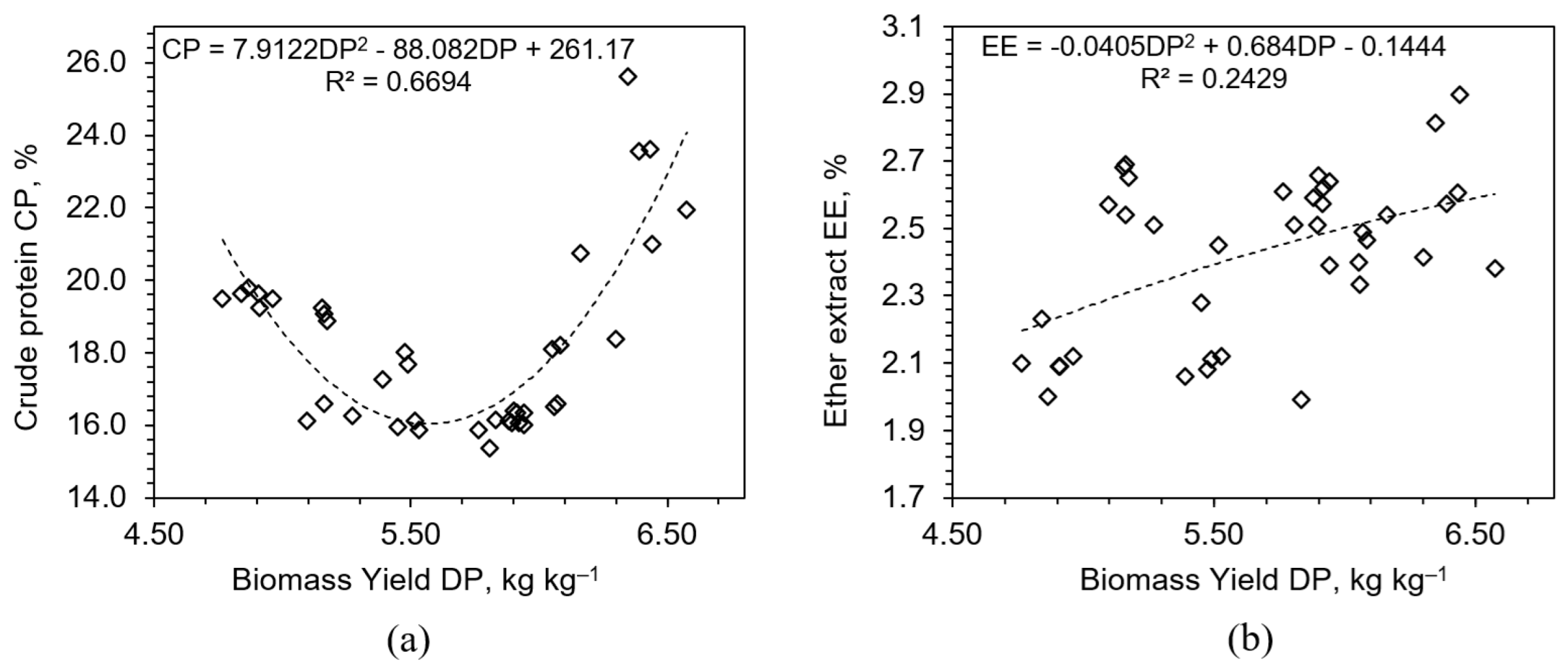
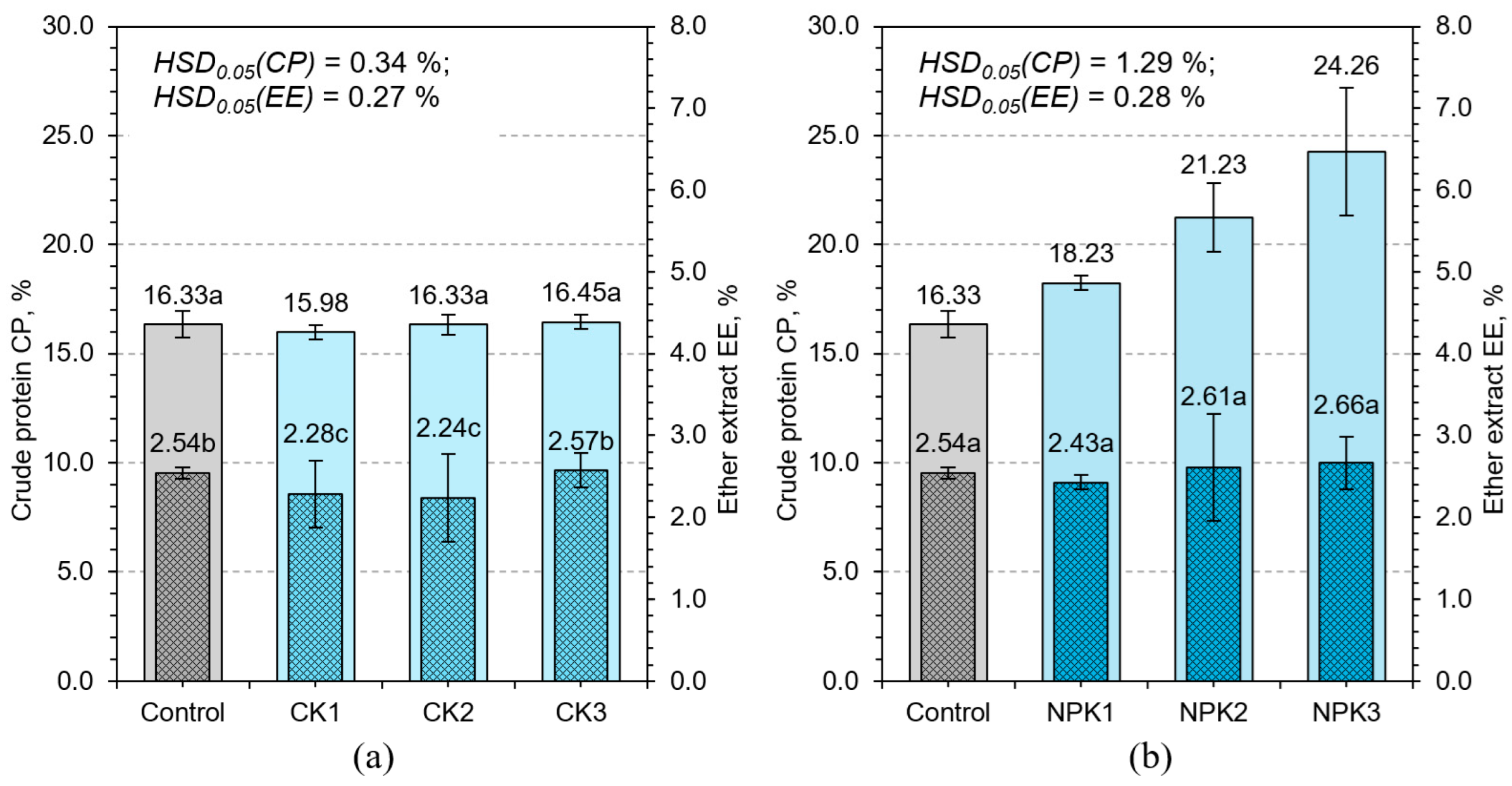
3.2.1. Crude Protein and Ether Extract
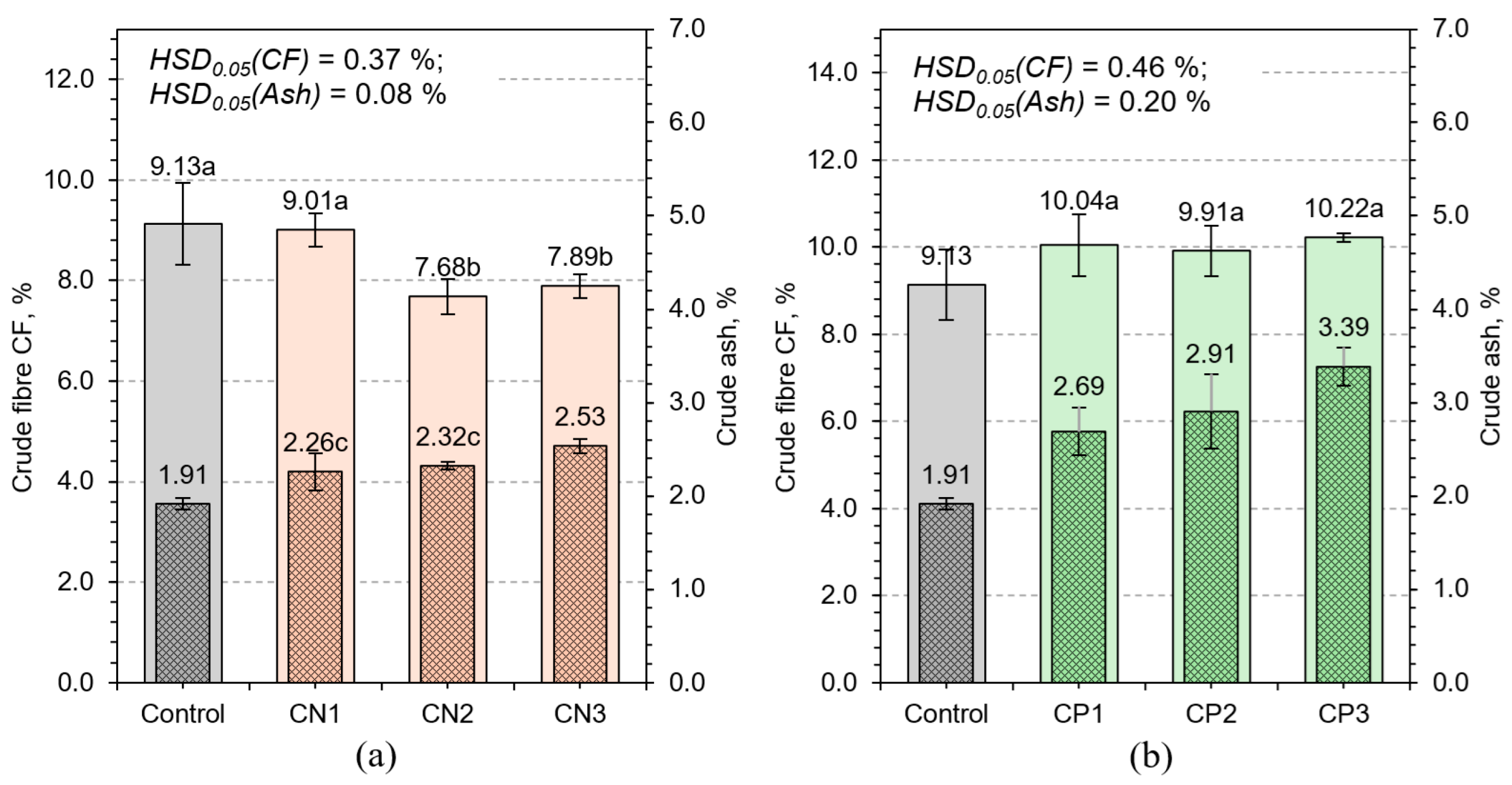
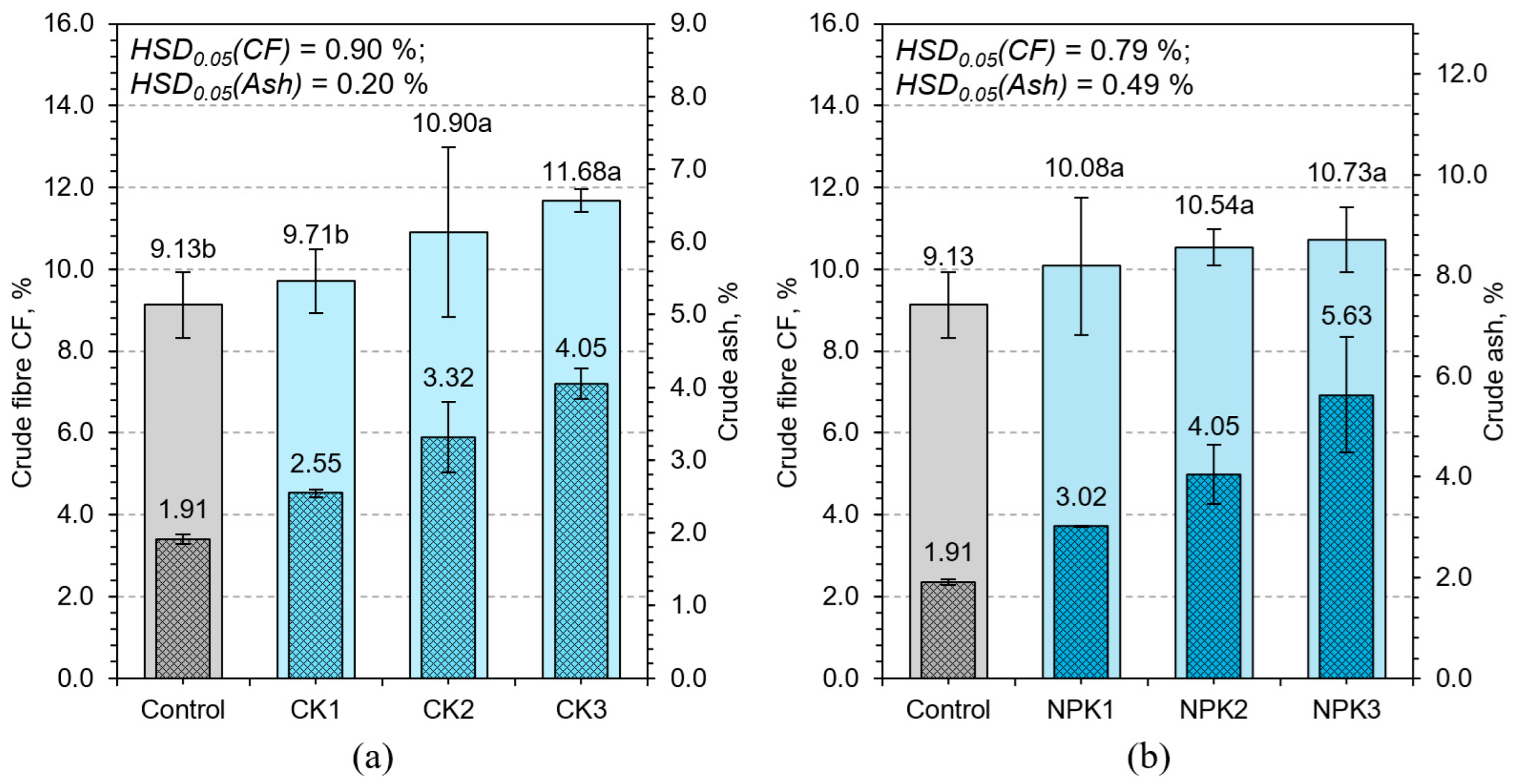
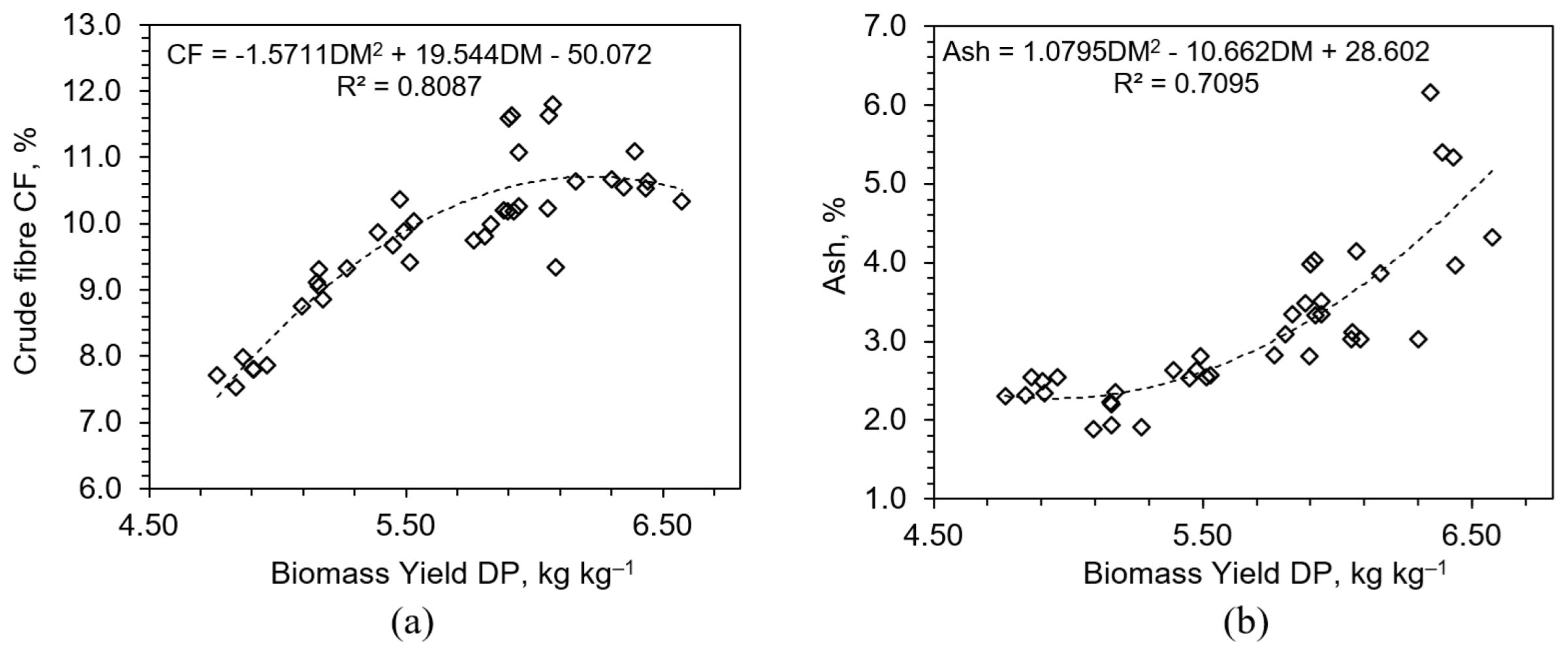
3.2.2. Crude Fiber and Crude Ash
3.3. Practical Implications for Fertilization Strategy
3.4. Limitations of the Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Neto, C.; Catarino, A.; Sobreiro, J.; das Dores, J.; Patanita, M.; Tomaz, A.; Palma, P. Effect of Different Irrigated Crop Successions on Soil Carbon and Nitrogen–Phosphorus–Potassium Budget under Mediterranean Conditions. Agriculture 2024, 14, 1908. [Google Scholar] [CrossRef]
- Rencricca, G.; Froldi, F.; Moschini, M.; Trevisan, M.; Ghnimi, S.; Lamastra, L. The Environmental Impact of Permanent Meadows-Based Farms: A Comparison among Different Dairy Farm Management Systems of an Italian Cheese. Sustain. Prod. Consum. 2023, 37, 53–64. [Google Scholar] [CrossRef]
- Yang, S.; Mahmood, M.; Baral, R.; Wu, H.; Almloff, M.; Stanton, L.E.; Min, D.; Smiley, B.K.; Iiams, J.C.; Yu, J. Forage Conservation Is a Neglected Nitrous Oxide Source. PNAS Nexus 2024, 3, 373. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture 2021: Making Agrifood Systems More Resilient to Shocks and Stresses; FAO: Rome, Italy, 2021. [Google Scholar]
- Grigas, A.; Steponavičius, D.; Kemzūraitė, A.; Tarasevičienė, Ž.; Domeika, R. Spatial Heterogeneity in the Properties of Hydroponic Wheat Fodder and Its Sustainability. Sci. Rep. 2024, 14, 19312. [Google Scholar] [CrossRef]
- Bihari, C.; Ahamad, S.; Kumar, M.; Kumar, A.; Kamboj, A.D.; Singh, S.; Srivastava, V.; Gautam, P. Innovative Soilless Culture Techniques for Horticultural Crops: A Comprehensive Review. Int. J. Environ. Clim. Change 2023, 13, 4071–4084. [Google Scholar] [CrossRef]
- Dutta, M.; Gupta, D.; Sahu, S.; Limkar, S.; Singh, P.; Mishra, A.; Kumar, M.; Mutlu, R. Evaluation of Growth Responses of Lettuce and Energy Efficiency of the Substrate and Smart Hydroponics Cropping System. Sensors 2023, 23, 1875. [Google Scholar] [CrossRef]
- Ahamed, M.S.; Sultan, M.; Shamshiri, R.R.; Rahman, M.M.; Aleem, M.; Balasundram, S.K. Present Status and Challenges of Fodder Production in Controlled Environments: A Review. Smart Agric. Technol. 2023, 3, 100080. [Google Scholar] [CrossRef]
- Gwynn-Jones, D.; Dunne, H.; Donnison, I.; Robson, P.; Sanfratello, G.M.; Schlarb-Ridley, B.; Hughes, K.; Convey, P. Can the Optimisation of Pop-up Agriculture in Remote Communities Help. Feed. the World? Glob. Food Sec. 2018, 18, 35–43. [Google Scholar] [CrossRef]
- Czarnek, K.; Tatarczak-Michalewska, M.; Dreher, P.; Rajput, V.D.; Wójcik, G.; Gierut-Kot, A.; Szopa, A.; Blicharska, E. UV-C Seed Surface Sterilization and Fe, Zn, Mg, Cr Biofortification of Wheat Sprouts as an Effective Strategy of Bioelement Supplementation. Int. J. Mol. Sci. 2023, 24, 10367. [Google Scholar] [CrossRef]
- Zada, T.; Khan, S.; Islam, Z.; Sultan, A.; Khan, K.; Albekairi, T.H.; Khan, A.A.; Aziz, T.; Khan, R.U. Growth Performance, Serum Biochemical Profile and Liver Histopathology of Broiler Chickens Supplemented with Graded Level of Dried Sprouted Wheat Grains. Ital. J. Anim. Sci. 2024, 23, 880–886. [Google Scholar] [CrossRef]
- Mpanza, T.D.E.; Dhlamini, T.C.; Pierneef, R.E.; Mbatha, K.R. Enteric Methane Emission, Rumen Fermentation and Microbial Profiles of Meat-Master Lambs Supplemented with Barley Fodder Sprouts. Fermentation 2022, 8, 434. [Google Scholar] [CrossRef]
- Zang, Y.; Richards, A.T.; Seneviratne, N.; Gutierrez-Oviedo, F.A.; Harding, R.; Ranathunga, S.; McFadden, J.W. Replacing Conventional Concentrates with Sprouted Barley or Wheat: Effects on Lactational Performance, Nutrient Digestibility, and Milk Fatty Acid Profile in Dairy Cows. J. Dairy Sci. 2024, 107, 5529–5541. [Google Scholar] [CrossRef]
- Tripathi, R.; Tewari, R.; Singh, K.P.; Keswani, C.; Minkina, T.; Srivastava, A.K.; De Corato, U.; Sansinenea, E. Plant Mineral Nutrition and Disease Resistance: A Significant Linkage for Sustainable Crop Protection. Front. Plant Sci. 2022, 13, 883970. [Google Scholar] [CrossRef]
- Tariq, A.; Zeng, F.; Graciano, C.; Ullah, A.; Sadia, S.; Ahmed, Z.; Murtaza, G.; Ismoilov, K.; Zhang, Z. Regulation of Metabolites by Nutrients in Plants. In Plant Ionomics: Sensing, Signaling, and Regulation; Wiley: Hoboken, NJ, USA, 2023; pp. 1–18. [Google Scholar]
- Li, T.; Lalk, G.T.; Arthur, J.D.; Johnson, M.H.; Bi, G. Shoot Production and Mineral Nutrients of Five Microgreens as Affected by Hydroponic Substrate Type and Post-Emergent Fertilization. Horticulturae 2021, 7, 129. [Google Scholar] [CrossRef]
- Pastorelli, G.; Serra, V.; Turin, L.; Attard, E. Hydroponic Fodders for Livestock Production—A Review. Ann. Anim. Sci. 2024, 24, 645–656. [Google Scholar] [CrossRef]
- Sriagtula, R.; Martaguri, I.; Sowmen, S. Evaluation of Nutrient Solution Dose and Harvest Time on Forage Sorghum (Sorghum bicolor, L. Moench) in Hydroponic Fodder System. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bandung, Indonesia, 2021; Volume 888, p. 012068. [Google Scholar]
- Zörb, C.; Senbayram, M.; Peiter, E. Potassium in Agriculture–Status and Perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef]
- Mohammed, S.B.; Sookoo, R. Nutrient Film Technique for Commercial Production. Agric. Sci. Res. J. 2016, 6, 269–274. [Google Scholar]
- Grigas, A.; Kemzūraitė, A.; Steponavičius, D.; Steponavičienė, A.; Domeika, R. Impact of Slope of Growing Trays on Productivity of Wheat Green Fodder by a Nutrient Film Technique System. Water 2020, 12, 3009. [Google Scholar] [CrossRef]
- Cocetta, G.; Casciani, D.; Bulgari, R.; Musante, F.; Kołton, A.; Rossi, M.; Ferrante, A. Light Use Efficiency for Vegetables Production in Protected and Indoor Environments. Eur. Phys. J. Plus 2017, 132, 43. [Google Scholar] [CrossRef]
- Zeng, Y.J.; Qi, X.N.; Li, Y.Z.; Wang, Y.Q.; Wang, Y.R. Optimising the Preparation Method of Seeds of Agriophyllum Squarrosum Prior to Staining in Tetrazolium Solution. Seed Sci. Technol. 2014, 42, 300–306. [Google Scholar] [CrossRef]
- LST ISO 712:2010; Grains and Their Products, Determination of Moisture Content, Reference Method. Lithuanian Standardization Department: Vilnius, Lithuania, 2010.
- ISO 20483: 2006; Cereals and Pulses-Determination of the Nitrogen Content and Calculation of the Crude Protein Content-Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO 6865: 2000; Animal Feeding Stuffs—Determination of Crude Fiber Content—Method with Intermediate Filtration. ISO (International Organization for Standardization): Geneva, Switzerland, 2000.
- ISO 5984:2022(E); Animal Feeding Stuffs—Determination of Crude Ash. International Organization for Standardization: Geneva, Switzerland, 2022.
- LST ISO 1443:1973; Meat and Meat Products—Determination of Total Fat Content. Lithuanian Standardization Department: Vilnius, Lithuania, 1973.
- Olsson, U.; Engstrand, U.; Rupšys, P. Statistical Methods SAS and MINITAB; LŽŪU Press Center: Akademija, Lithuanian, 2000. (In Lithuanian) [Google Scholar]
- Devienne, F.; Mary, B. Influence of Constant Nitrate Concentration on N Uptake and Growth of Wheat. In Structure and Function of Roots, Proceedings of the Fourth International Symposium on Structure and Function of Roots, Stará Lesná, Slovakia, 20–26 June 1993; Springer: Berlin/Heidelberg, Germany, 1995; pp. 141–147. [Google Scholar]
- Zhang, X.; Franzisky, B.L.; Eigner, L.; Geilfus, C.; Zörb, C. Antagonism of Chloride and Nitrate Inhibits Nitrate Reductase Activity in Chloride-Stressed Maize. Plant Growth Regul. 2021, 93, 279–289. [Google Scholar] [CrossRef]
- Chebli, Y.; Geitmann, A. Cellular Growth in Plants Requires Regulation of Cell Wall Biochemistry. Curr. Opin. Cell Biol. 2017, 44, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Hilty, J.; Muller, B.; Pantin, F.; Leuzinger, S. Plant Growth: The What, the How, and the Why. New Phytol. 2021, 232, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Siddique, A.B.; Shabala, S.; Zhou, M.; Zhao, C. Phosphorus Plays Key Roles in Regulating Plants’ Physiological Responses to Abiotic Stresses. Plants 2023, 12, 2861. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Yao, J.G.; Florin, N.; George, A.; Wang, X.; Labeeuw, L.; Jiang, Y.; Davis, R.W.; Abbas, A.; Ralph, P.; et al. Impact of Flue Gas Compounds on Microalgae and Mechanisms for Carbon Assimilation and Utilization. ChemSusChem 2018, 11, 334–355. [Google Scholar] [CrossRef]
- Souza, S.R.; Stark, E.M.L.M.; Fernandes, M.S.; Magalhaes, J.R. Effects of Supplemental Nitrogen on Nitrogen-assimilation Enzymes, Free Amino Nitrogen, Soluble Sugars, and Crude Protein of Rice. Commun. Soil Sci. Plant Anal. 1999, 30, 711–724. [Google Scholar] [CrossRef]
- Tsouvaltzis, P.; Kasampalis, D.S.; Aktsoglou, D.-C.; Barbayiannis, N.; Siomos, A.S. Effect of Reduced Nitrogen and Supplemented Amino Acids Nutrient Solution on the Nutritional Quality of Baby Green and Red Lettuce Grown in a Floating System. Agronomy 2020, 10, 922. [Google Scholar] [CrossRef]
- Smith, M.R.; Reis Hodecker, B.E.; Fuentes, D.; Merchant, A. Investigating Nutrient Supply Effects on Plant Growth and Seed Nutrient Content in Common Bean. Plants 2022, 11, 737. [Google Scholar] [CrossRef]
- Nicholls, J.W.F.; Chin, J.P.; Williams, T.A.; Lenton, T.M.; O’Flaherty, V.; McGrath, J.W. On the Potential Roles of Phosphorus in the Early Evolution of Energy Metabolism. Front. Microbiol. 2023, 14, 1239189. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.-H. Regulation of Potassium Transport and Signaling in Plants. Curr. Opin. Plant Biol. 2017, 39, 123–128. [Google Scholar] [CrossRef]
- Barthole, G.; Lepiniec, L.; Rogowsky, P.M.; Baud, S. Controlling Lipid Accumulation in Cereal Grains. Plant Sci. 2012, 185, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.P.S.A.; Yusra, A.F.I.; Bhat, A.H.; Jawaid, M. Cell Wall Ultrastructure, Anatomy, Lignin Distribution, and Chemical Composition of Malaysian Cultivated Kenaf Fiber. Ind. Crops Prod. 2010, 31, 113–121. [Google Scholar] [CrossRef]
- Podgórska, A.; Burian, M.; Gieczewska, K.; Ostaszewska-Bugajska, M.; Zebrowski, J.; Solecka, D.; Szal, B. Altered Cell Wall Plasticity Can Restrict Plant Growth under Ammonium Nutrition. Front. Plant Sci. 2017, 8, 1344. [Google Scholar] [CrossRef]
- Zhang, W.; Qin, W.; Li, H.; Wu, A. Biosynthesis and Transport of Nucleotide Sugars for Plant Hemicellulose. Front. Plant Sci. 2021, 12, 723128. [Google Scholar] [CrossRef]
- Kavanová, M.; Lattanzi, F.A.; Grimoldi, A.A.; Schnyder, H. Phosphorus Deficiency Decreases Cell Division and Elongation in Grass Leaves. Plant Physiol. 2006, 141, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Hossen, M.S.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A Vital Regulator of Plant Responses and Tolerance to Abiotic Stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Peng, J.-S.; Gong, J.-M. Vacuolar Sequestration Capacity and Long-Distance Metal Transport in Plants. Front. Plant Sci. 2014, 5, 19. [Google Scholar] [CrossRef]
- Sevanto, S. Drought Impacts on Phloem Transport. Curr. Opin. Plant Biol. 2018, 43, 76–81. [Google Scholar] [CrossRef]
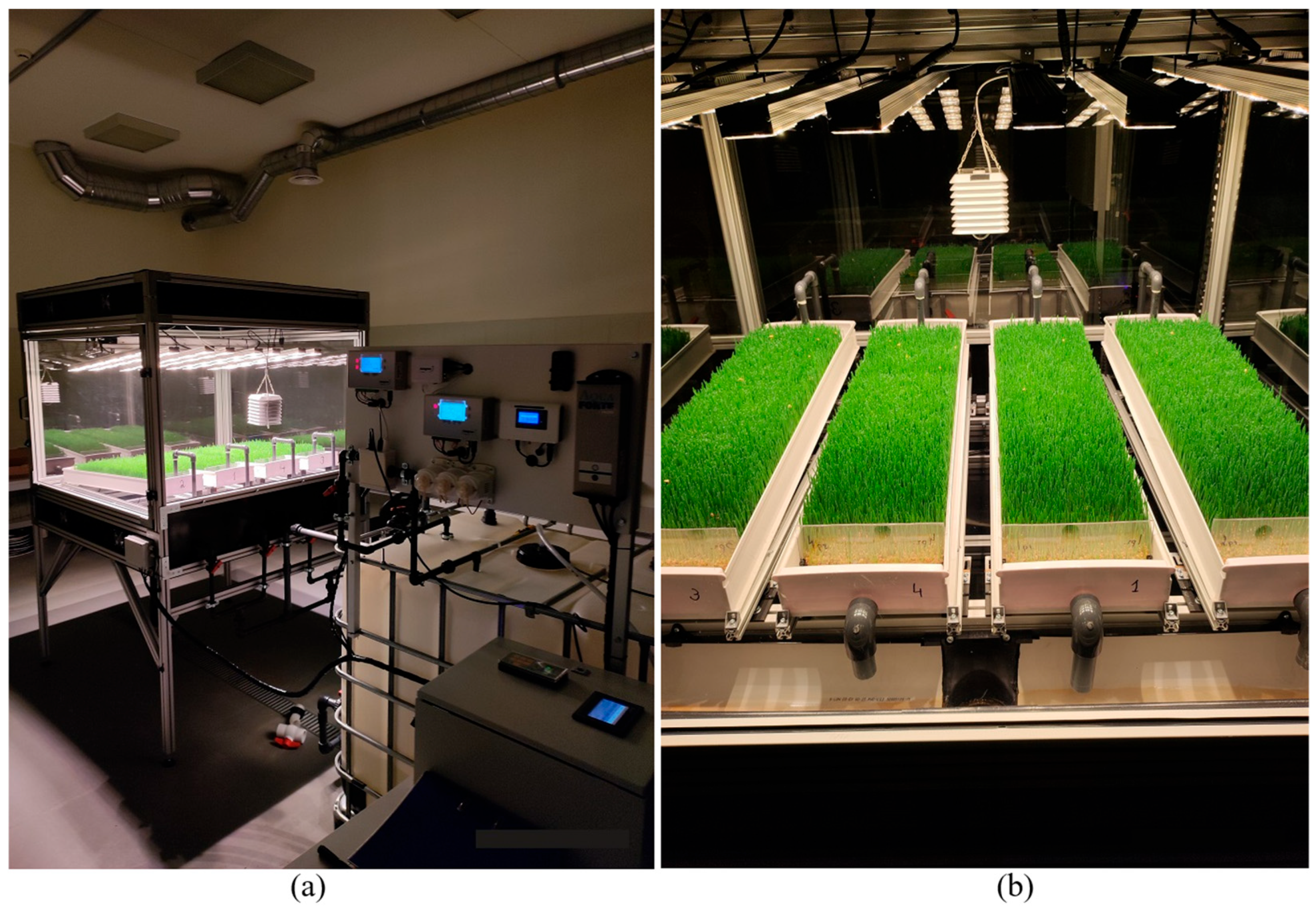



| Temperature, °C | Humidity, % | CO2, ppm | VPD * | |
|---|---|---|---|---|
| Control | 21.87 ± 0.05 | 37.88 ± 0.26 | 591.58 ± 2.26 | 1.616 ± 0.008 |
| CN1 | 21.69 ± 0.05 | 47.56 ± 0.24 | 605.96 ± 2.54 | 1.349 ± 0.008 |
| CN2 | 21.26 ± 0.05 | 44.2 ± 0.21 | 601.91 ± 2.68 | 1.396 ± 0.007 |
| CN3 | 20.95 ± 0.05 | 34.84 ± 0.38 | 616.35 ± 3.02 | 1.602 ± 0.011 |
| CP1 | 20.49 ± 0.05 | 45.73 ± 0.59 | 609.26 ± 2.78 | 1.31 ± 0.005 |
| CP2 | 21.18 ± 0.05 | 48.78 ± 0.52 | 642.97 ± 2.77 | 1.29 ± 0.008 |
| CP3 | 20.67 ± 0.05 | 49.78 ± 0.26 | 636.68 ± 3.78 | 1.23 ± 0.006 |
| CK1 | 21.13 ± 0.05 | 41.82 ± 0.35 | 589.69 ± 3.01 | 1.46 ± 0.006 |
| CK2 | 21.14 ± 0.05 | 47.62 ± 0.62 | 630.42 ± 2.12 | 1.31 ± 0.007 |
| CK3 | 21.47 ± 0.05 | 42.62 ± 0.57 | 633.76 ± 3.59 | 1.47 ± 0.008 |
| NPK1 | 20.57 ± 0.05 | 56.96 ± 0.28 | 624.91 ± 3.16 | 1.04 ± 0.008 |
| NPK2 | 20.46 ± 0.05 | 40.76 ± 0.71 | 648.26 ± 3.43 | 1.43 ± 0.008 |
| NPK3 | 21.18 ± 0.05 | 43.86 ± 0.42 | 591.43 ± 2.23 | 1.41 ± 0.005 |
| Nutrients Concentration in Solution, mg L–1 | EC | pH | ||||
|---|---|---|---|---|---|---|
| Control * | – | – | 0.643 ± 0.015 | 5.8–6.0 | ||
| N (NO3−) | Ca2+ | |||||
| Ca(NO3)2 | CN1 | 100 | 143 | 1.316 ± 0.061 | 5.8–6.0 | |
| CN2 | 200 | 286 | 2.070 ± 0.220 | 5.8–6.0 | ||
| CN3 | 300 | 429 | 2.928 ± 0.086 | 5.8–6.0 | ||
| P (H2PO4−) | K+ | |||||
| KH2PO4 | CP1 | 100 | 126 | 0.782 ± 0.029 | 5.8–6.0 | |
| CP2 | 200 | 252 | 0.913 ± 0.059 | 5.8–6.0 | ||
| CP3 | 300 | 378 | 1.045 ± 0.088 | 5.8–6.0 | ||
| K+ | S (SO42−) | |||||
| K2SO4 | CK1 | 100 | 18 | 0.940 ± 0.060 | 5.8–6.0 | |
| CK2 | 200 | 36 | 1.228 ± 0.120 | 5.8–6.0 | ||
| CK3 | 300 | 54 | 1.518 ± 0.175 | 5.8–6.0 | ||
| NPK 20-20-20 | N (NO3−, NH4+, NH2) | P (H2PO4−) | K+ | |||
| NPK1 | 100 | 43.6 | 83 | 1.600 ± 0.124 | 5.8–6.0 | |
| NPK2 | 200 | 87.2 | 166 | 2.551 ± 0.248 | 5.8–6.0 | |
| NPK3 | 300 | 130.8 | 249 | 3.489 ± 0.371 | 5.8–6.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigas, A.; Steponavičius, D.; Bručienė, I.; Krikštolaitis, R.; Krilavičius, T.; Steponavičienė, A.; Savickas, D. Optimization of Hydroponic Wheat Sprouts as an Alternative Livestock Feed: Yield and Biochemical Composition Under Different Fertilization Regimes. Plants 2025, 14, 2166. https://doi.org/10.3390/plants14142166
Grigas A, Steponavičius D, Bručienė I, Krikštolaitis R, Krilavičius T, Steponavičienė A, Savickas D. Optimization of Hydroponic Wheat Sprouts as an Alternative Livestock Feed: Yield and Biochemical Composition Under Different Fertilization Regimes. Plants. 2025; 14(14):2166. https://doi.org/10.3390/plants14142166
Chicago/Turabian StyleGrigas, Andrius, Dainius Steponavičius, Indrė Bručienė, Ričardas Krikštolaitis, Tomas Krilavičius, Aušra Steponavičienė, and Dainius Savickas. 2025. "Optimization of Hydroponic Wheat Sprouts as an Alternative Livestock Feed: Yield and Biochemical Composition Under Different Fertilization Regimes" Plants 14, no. 14: 2166. https://doi.org/10.3390/plants14142166
APA StyleGrigas, A., Steponavičius, D., Bručienė, I., Krikštolaitis, R., Krilavičius, T., Steponavičienė, A., & Savickas, D. (2025). Optimization of Hydroponic Wheat Sprouts as an Alternative Livestock Feed: Yield and Biochemical Composition Under Different Fertilization Regimes. Plants, 14(14), 2166. https://doi.org/10.3390/plants14142166










