Interactive Effects of Climate and Large Herbivore Assemblage Drive Plant Functional Traits and Diversity
Abstract
1. Introduction
2. Results
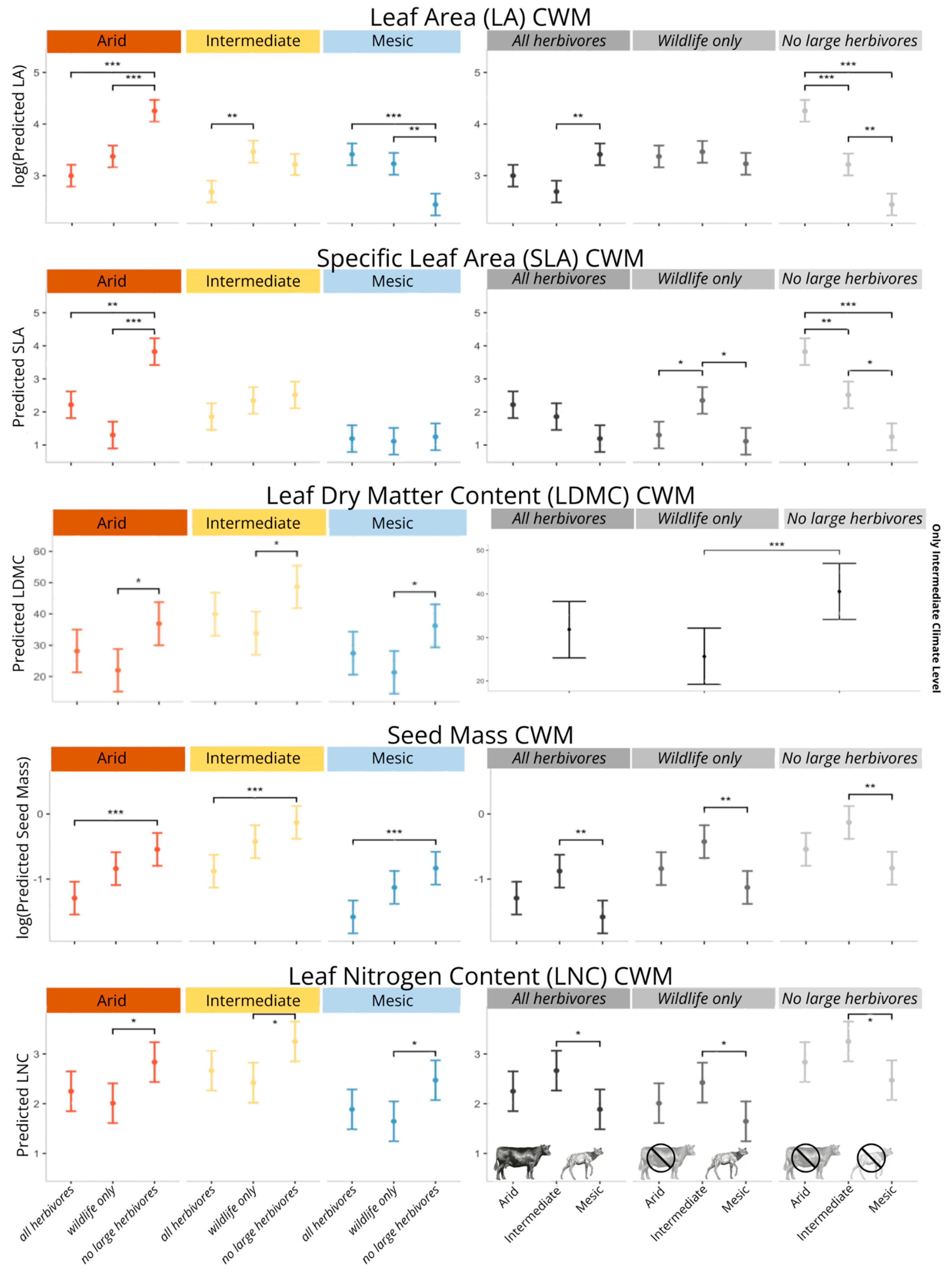
2.1. Community-Level Changes in Functional Traits
2.2. Species Turnover and ITV
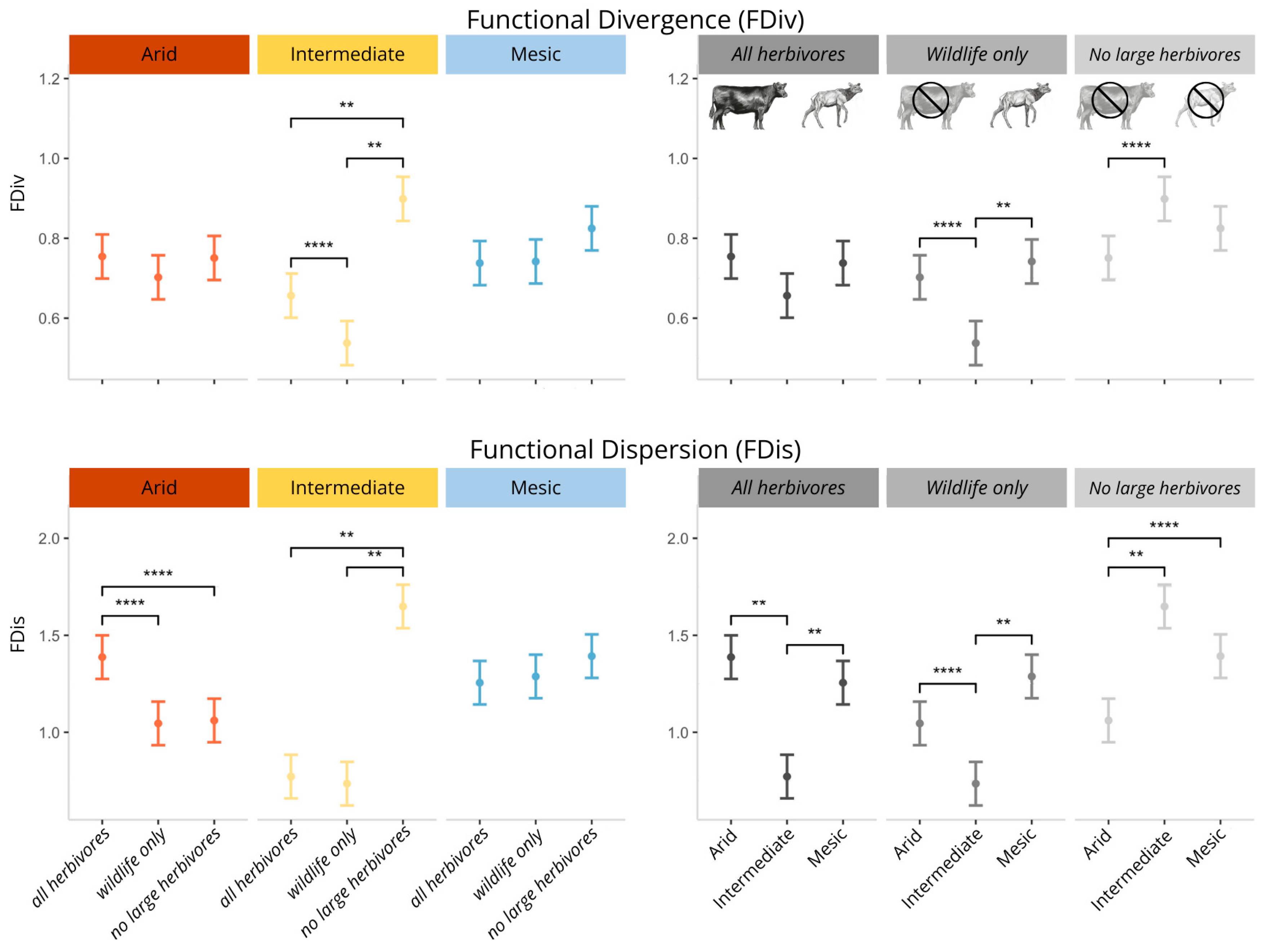
2.3. Functional Diversity
3. Discussion
3.1. The Response of Community-Level Trait Weighted Means to Grazing and Climate
3.2. The Contribution of ITV and Species Turnover to Community-Level Traits
3.3. The Response of Functional Diversity to Climate and Herbivory
3.4. Broader Implications
4. Materials and Methods
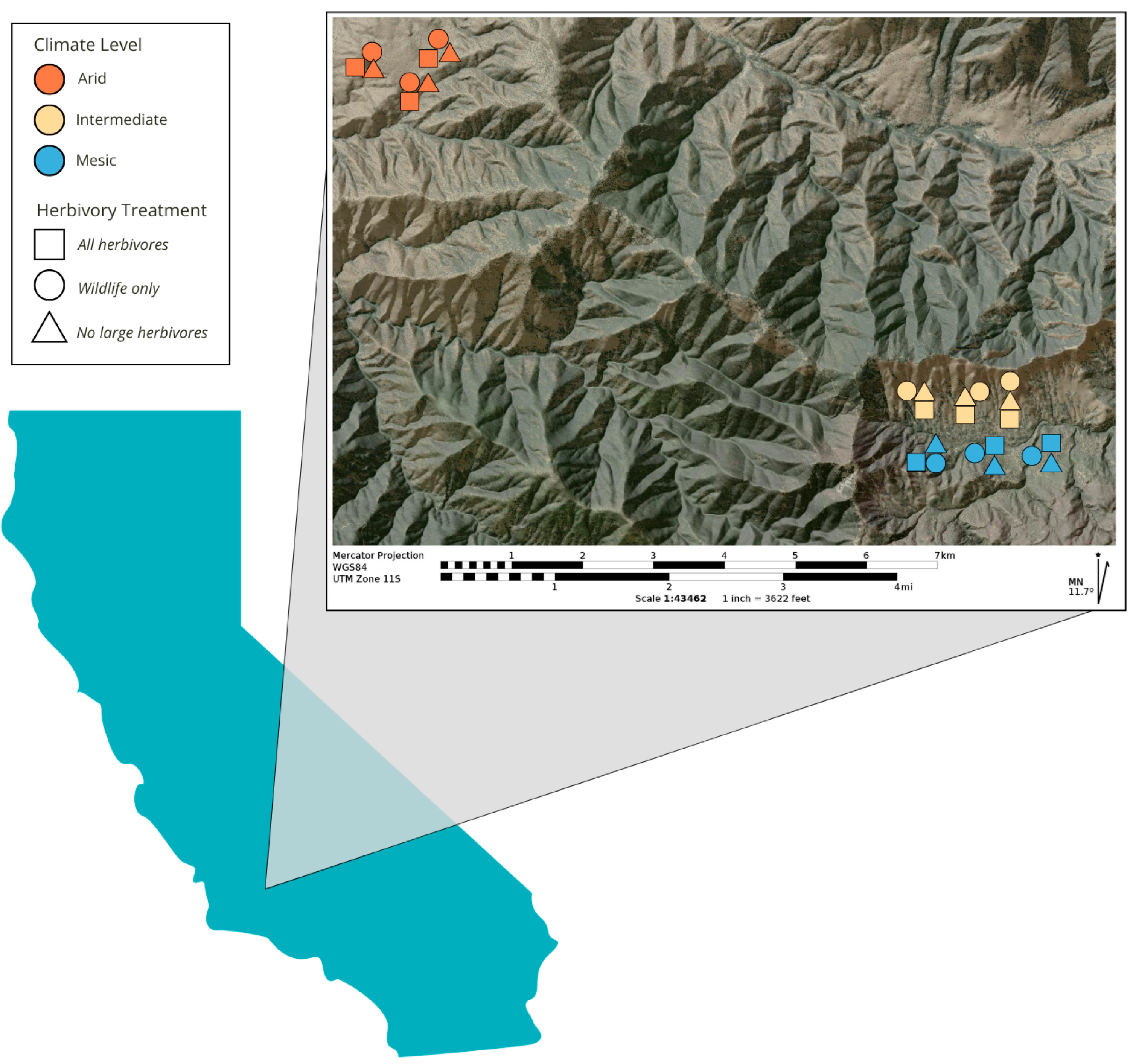
4.1. Study Site
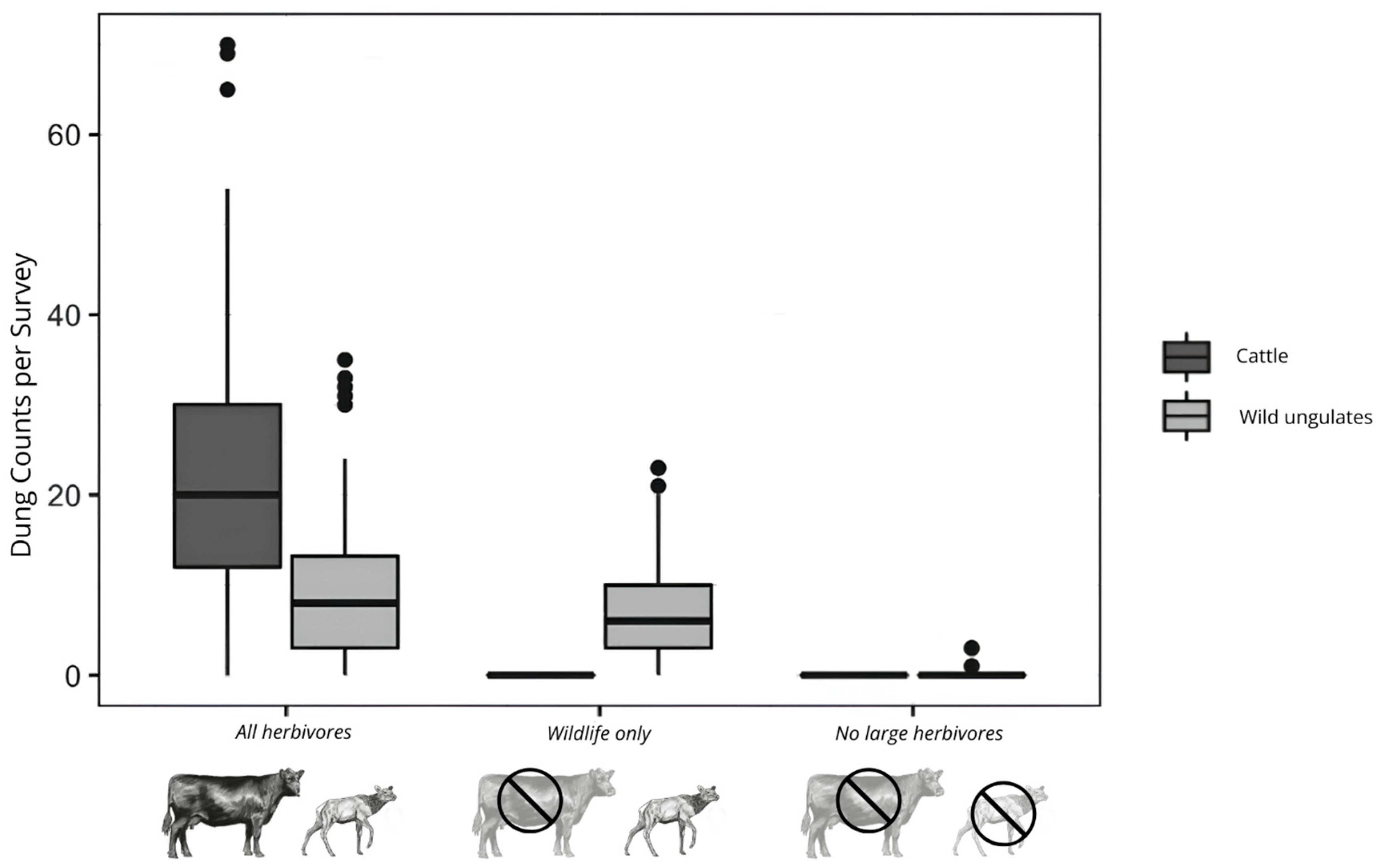
4.2. Herbivore Community
4.3. Plant Functional Trait Collection
4.4. Community-Level Trait Analysis
4.5. Plant Functional Diversity
4.6. Modeling of Plant Functional Traits and Plant Functional Diversity
4.7. Species Turnover and ITV
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Diaz, S.; Lavorel, S.; McIntyre, S.U.E.; Falczuk, V.; Casanoves, F.; Milchunas, D.G.; Skarpe, C.; Rusch, G.; Sternberg, M.; Campbell, B.D. Plant trait responses to grazing—a global synthesis. Glob. Chang. Biol. 2007, 13, 313–341. [Google Scholar] [CrossRef]
- Jia, S.; Wang, X.; Yuan, Z.; Lin, F.; Ye, J.; Hao, Z.; Luskin, M.S. Global signal of top-down control of terrestrial plant communities by herbivores. Proc. Natl. Acad. Sci. USA 2018, 115, 6237–6242. [Google Scholar] [CrossRef] [PubMed]
- Pringle, R.M.; Abraham, J.O.; Anderson, T.M.; Coverdale, T.C.; Davies, A.B.; Dutton, C.L.; Gaylard, A.; Goheen, J.R.; Holdo, R.M.; Hutchinson, M.C.; et al. Impacts of large herbivores on terrestrial ecosystems. Curr. Biol. 2023, 33, R584–R610. [Google Scholar] [CrossRef]
- Koerner, S.E.; Smith, M.D.; Burkepile, D.E.; Hanan, N.P.; Avolio, M.L.; Collins, S.L.; Knapp, A.K.; Lemoine, N.P.; Forrestel, E.J.; Eby, S.; et al. Change in dominance determines herbivore effects on plant biodiversity. Nat. Ecol. Evol. 2018, 2, 1925–1932. [Google Scholar] [CrossRef]
- Ripple, W.J.; Newsome, T.M.; Wolf, C.; Dirzo, R.; Everatt, K.T.; Galetti, M.; Hayward, M.W.; Kerley, G.I.H.; Levi, T.; Lindsey, P.A.; et al. Collapse of the world’s largest herbivores. Sci. Adv. 2015, 1, e1400103. [Google Scholar] [CrossRef]
- Barnosky, A.D. Megafauna biomass tradeoff as a driver of Quaternary and future extinctions. Proc. Natl. Acad. Sci. USA 2008, 105, 11543–11548. [Google Scholar] [CrossRef]
- Forbes, E.S.; Cushman, J.H.; Burkepile, D.E.; Young, T.P.; Klope, M.; Young, H.S. Synthesizing the effects of large, wild herbivore exclusion on ecosystem function. Funct. Ecol. 2019, 33, 1597–1610. [Google Scholar] [CrossRef]
- Malhi, Y.; Doughty, C.E.; Galetti, M.; Smith, F.A.; Svenning, J.-C.; Terborgh, J.W. Megafauna and ecosystem function from the Pleistocene to the Anthropocene. Proc. Natl. Acad. Sci. USA 2016, 113, 838–846. [Google Scholar] [CrossRef]
- Young, H.S.; McCauley, D.J.; Helgen, K.M.; Goheen, J.R.; Otárola-Castillo, E.; Palmer, T.M.; Pringle, R.M.; Young, T.P.; Dirzo, R. Effects of mammalian herbivore declines on plant communities: Observations and experiments in an African savanna. J. Ecol. 2013, 101, 1030–1041. [Google Scholar] [CrossRef]
- Díaz, S.; Hodgson, J.G.; Thompson, K.; Cabido, M.; Cornelissen, J.H.; Jalili, A.; Montserrat-Martí, G.; Grime, J.P.; Zarrinkamar, F.; Asri, Y.; et al. The plant traits that drive ecosystems: Evidence from three continents. J. Veg. Sci. 2004, 15, 295–304. [Google Scholar] [CrossRef]
- Lambrecht, S.C. Floral Water Costs and Size Variation in the Highly Selfing Leptosiphon bicolor (Polemoniaceae). Int. J. Plant Sci. 2013, 174, 74–84. [Google Scholar] [CrossRef]
- Lavorel, S.; Garnier, E. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Funct. Ecol. 2002, 16, 545–556. [Google Scholar] [CrossRef]
- Petchey, O.L.; Gaston, K.J. Functional diversity: back to basics and looking forward. Ecol. Lett. 2006, 9, 741–758. [Google Scholar] [CrossRef] [PubMed]
- Borer, E.T.; Seabloom, E.W.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Lind, E.M.; Adler, P.B.; Alberti, J.; Anderson, T.M.; Bakker, J.D.; et al. Herbivores and nutrients control grassland plant diversity via light limitation. Nature 2014, 508, 517–520. [Google Scholar] [CrossRef] [PubMed]
- De Bello, F.; Lavorel, S.; Díaz, S.; Harrington, R.; Cornelissen, J.H.C.; Bardgett, R.D.; Berg, M.P.; Cipriotti, P.; Feld, C.K.; Hering, D.; et al. Towards an assessment of multiple ecosystem processes and services via functional traits. Biodivers. Conserv. 2010, 19, 2873–2893. [Google Scholar] [CrossRef]
- Díaz, S.; Cabido, M. Vive la différence: plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 2001, 16, 646–655. [Google Scholar] [CrossRef]
- Funk, J.L.; Larson, J.E.; Ames, G.M.; Butterfield, B.J.; Cavender-Bares, J.; Firn, J.; Laughlin, D.C.; Sutton-Grier, A.E.; Williams, L.; Wright, J. Revisiting the Holy Grail: using plant functional traits to understand ecological processes. Biol. Rev. 2017, 92, 1156–1173. [Google Scholar] [CrossRef]
- Quétier, F.; Thébault, A.; Lavorel, S. Plant traits in a state and transition framework as markers of ecosystem response to land-use change. Ecol. Monogr. 2007, 77, 33–52. [Google Scholar] [CrossRef]
- Tilman, D.; Knops, J.; Wedin, D.; Reich, P.; Ritchie, M.; Siemann, E. The Influence of Functional Diversity and Composition on Ecosystem Processes. Science 1997, 277, 1300–1302. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, L.; Zhang, Y. Relationships between functional diversity and aboveground biomass production in the Northern Tibetan alpine grasslands. Sci. Rep. 2016, 6, srep34105. [Google Scholar] [CrossRef]
- De Bello, F.; Lepš, J.; Sebastià, M. Predictive value of plant traits to grazing along a climatic gradient in the Mediterranean. J. Appl. Ecol. 2005, 42, 824–833. [Google Scholar] [CrossRef]
- Díaz, S.; Noy-Meir, I.; Cabido, M. Can grazing response of herbaceous plants be predicted from simple vegetative traits? J. Appl. Ecol. 2001, 38, 497–508. [Google Scholar] [CrossRef]
- Del-Val, E.; Crawley, M.J. Are grazing increaser species better tolerators than decreasers? An experimental assessment of defoliation tolerance in eight British grassland species. J. Ecol. 2005, 93, 1005–1016. [Google Scholar] [CrossRef]
- Kohyani, P.T.; Bossuyt, B.; Bonte, D.; Hoffmann, M. Differential herbivory tolerance of dominant and subordinate plant species along gradients of nutrient availability and competition. Plant Ecol. 2009, 201, 611–619. [Google Scholar] [CrossRef]
- Cingolani, A.M.; Posse, G.; Collantes, M.B. Plant functional traits, herbivore selectivity and response to sheep grazing in Patagonian steppe grasslands. J. Appl. Ecol. 2005, 42, 50–59. [Google Scholar] [CrossRef]
- An, H.; Li, G. Differential Effects of Grazing on Plant Functional Traits in the Desert Grassland Differential Effects of Grazing on Plant Functional Traits. Pol. J. Ecol. 2014, 62, 239–251. [Google Scholar]
- Jonas, C.S.; Geber, M.A. Variation among populations of Clarkia unguiculata (Onagraceae) along altitudinal and latitudinal gradients. Am. J. Bot. 1999, 86, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Augustine, D.J.; McNaughton, S.J. Ungulate Effects on the Functional Species Composition of Plant Communities: Herbivore Selectivity and Plant Tolerance. J. Wildl. Manag. 1998, 62, 1165. [Google Scholar] [CrossRef]
- Liu, J.; Feng, C.; Wang, D.; Wang, L.; Wilsey, B.J.; Zhong, Z. Impacts of grazing by different large herbivores in grassland depend on plant species diversity. J. Appl. Ecol. 2015, 52, 1053–1062. [Google Scholar] [CrossRef]
- Török, P.; Penksza, K.; Tóth, E.; Kelemen, A.; Sonkoly, J.; Tóthmérész, B. Vegetation type and grazing intensity jointly shape grazing effects on grassland biodiversity. Ecol. Evol. 2018, 8, 10326–10335. [Google Scholar] [CrossRef]
- Tóth, E.; Deák, B.; Valkó, O.; Kelemen, A.; Miglécz, T.; Tóthmérész, B.; Török, P. Livestock Type is More Crucial Than Grazing Intensity: Traditional Cattle and Sheep Grazing in Short-Grass Steppes. Land Degrad. Dev. 2018, 29, 231–239. [Google Scholar] [CrossRef]
- Catorci, A.; Cesaretti, S.; Malatesta, L.; Tardella, F.M. Effects of grazing vs mowing on the functional diversity of sub-Mediterranean productive grasslands. Appl. Veg. Sci. 2014, 17, 658–669. [Google Scholar] [CrossRef]
- Komac, B.; Pladevall, C.; Domènech, M.; Fanlo, R. Functional diversity and grazing intensity in sub-alpine and alpine grasslands inAndorra. Appl. Veg. Sci. 2015, 18, 75–85. [Google Scholar] [CrossRef]
- Mandle, L.; Ticktin, T. Moderate land use changes plant functional composition without loss of functional diversity in India’s Western Ghats. Ecol. Appl. 2015, 25, 1711–1724. [Google Scholar] [CrossRef]
- Niu, K.; He, J.; Lechowicz, M.J. Grazing-induced shifts in community functional composition and soil nutrient availability in Tibetan alpine meadows. J. Appl. Ecol. 2016, 53, 1554–1564. [Google Scholar] [CrossRef]
- De Bello, F.; Lepš, J.; Sebastià, M. Variations in species and functional plant diversity along climatic and grazing gradients. Ecography 2006, 29, 801–810. [Google Scholar] [CrossRef]
- Lamanna, C.; Blonder, B.; Violle, C.; Kraft, N.J.B.; Sandel, B.; Šímová, I.; Donoghue, J.C.; Svenning, J.-C.; McGill, B.J.; Boyle, B.; et al. Functional trait space and the latitudinal diversity gradient. Proc. Natl. Acad. Sci. USA 2014, 111, 13745–13750. [Google Scholar] [CrossRef]
- Rahmanian, S.; Hejda, M.; Ejtehadi, H.; Farzam, M.; Memariani, F.; Pyšek, P. Effects of livestock grazing on soil, plant functional diversity, and ecological traits vary between regions with different climates in northeastern Iran. Ecol. Evol. 2019, 9, 8225–8237. [Google Scholar] [CrossRef]
- Jäschke, Y.; Heberling, G.; Wesche, K. Environmental controls override grazing effects on plant functional traits in Tibetan rangelands. Funct. Ecol. 2020, 34, 747–760. [Google Scholar] [CrossRef]
- Eldridge, D.J.; Delgado-Baquerizo, M.; Travers, S.K.; Val, J.; Oliver, I. Do grazing intensity and herbivore type affect soil health? Insights from a semi-arid productivity gradient. J. Appl. Ecol. 2017, 54, 976–985. [Google Scholar] [CrossRef]
- Pérez-Camacho, L.; Rebollo, S.; Hernández-Santana, V.; García-Salgado, G.; Pavón-García, J.; Gómez-Sal, A. Plant functional trait responses to interannual rainfall variability, summer drought and seasonal grazing in Mediterranean herbaceous communities. Funct. Ecol. 2012, 26, 740–749. [Google Scholar] [CrossRef]
- Rook, A.; Dumont, B.; Isselstein, J.; Osoro, K.; WallisDeVries, M.; Parente, G.; Mills, J. Matching type of livestock to desired biodiversity outcomes in pastures—A review. Biol. Conserv. 2004, 119, 137–150. [Google Scholar] [CrossRef]
- van der Plas, F.; Howison, R.A.; Mpanza, N.; Cromsigt, J.P.G.M.; Olff, H. Different-sized grazers have distinctive effects on plant functional composition of an African savannah. J. Ecol. 2016, 104, 864–875. [Google Scholar] [CrossRef]
- Masudi, S.P.; Odadi, W.O.; Kimuyu, D.M.; Gachuiri, C.K.; Sensenig, R.L.; Young, T.P. Wild herbivores and cattle have differing effects on postfire herbaceous vegetation recovery in an African savanna. Ecol. Appl. 2024, 34, e2975. [Google Scholar] [CrossRef] [PubMed]
- Orr, D.A.; Bui, A.; Klope, M.; McCullough, I.M.; Lee, M.; Motta, C.; Mayorga, I.; Konicek, K.; Young, H.S. Context-dependent effects of shifting large herbivore assemblages on plant structure and diversity. J. Ecol. 2022, 110, 1312–1327. [Google Scholar] [CrossRef]
- Asner, G.P.; Knapp, D.E.; Anderson, C.B.; Martin, R.E.; Vaughn, N. Large-scale climatic and geophysical controls on the leaf economics spectrum. Proc. Natl. Acad. Sci. USA 2016, 113, E4043–E4051. [Google Scholar] [CrossRef]
- Moles, A.T.; Perkins, S.E.; Laffan, S.W.; Flores-Moreno, H.; Awasthy, M.; Tindall, M.L.; Sack, L.; Pitman, A.; Kattge, J.; Aarssen, L.W.; et al. Which is a better predictor of plant traits: temperature or precipitation? J. Veg. Sci. 2014, 25, 1167–1180. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Cornelissen, J.H.C.; Falster, D.S.; Groom, P.K.; Hikosaka, K.; Lee, W.; Lusk, C.H.; Niinemets, Ü.; Oleksyn, J.; et al. Modulation of leaf economic traits and trait relationships by climate. Glob. Ecol. Biogeogr. 2005, 14, 411–421. [Google Scholar] [CrossRef]
- Ahrens, C.W.; Andrew, M.E.; Mazanec, R.A.; Ruthrof, K.X.; Challis, A.; Hardy, G.; Byrne, M.; Tissue, D.T.; Rymer, P.D. Plant functional traits differ in adaptability and are predicted to be differentially affected by climate change. Ecol. Evol. 2020, 10, 232–248. [Google Scholar] [CrossRef]
- Diaz, S.; Cabido, M.; Casanoves, F. Plant functional traits and environmental filters at a regional scale. J. Veg. Sci. 1998, 9, 113–122. [Google Scholar] [CrossRef]
- Wellstein, C.; Poschlod, P.; Gohlke, A.; Chelli, S.; Campetella, G.; Rosbakh, S.; Canullo, R.; Kreyling, J.; Jentsch, A.; Beierkuhnlein, C. Effects of extreme drought on specific leaf area of grassland species: A meta-analysis of experimental studies in temperate and sub-Mediterranean systems. Glob. Chang. Biol. 2017, 23, 2473–2481. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü. Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology 2001, 82, 453–469. [Google Scholar] [CrossRef]
- Zheng, S.; Li, W.; Lan, Z.; Ren, H.; Wang, K. Functional trait responses to grazing are mediated by soil moisture and plant functional group identity. Sci. Rep. 2015, 5, 18163. [Google Scholar] [CrossRef]
- Ackerly, D.; Knight, C.; Weiss, S.; Barton, K.; Starmer, K. Leaf size, specific leaf area and microhabitat distribution of chaparral woody plants: contrasting patterns in species level and community level analyses. Oecologia 2002, 130, 449–457. [Google Scholar] [CrossRef]
- Gross, N.; Maestre, F.T.; Liancourt, P.; Berdugo, M.; Martin, R.; Gozalo, B.; Ochoa, V.; Delgado-Baquerizo, M.; Maire, V.; Saiz, H.; et al. Unforeseen plant phenotypic diversity in a dry and grazed world. Nature 2024, 632, 808–814. [Google Scholar] [CrossRef]
- Griffin-Nolan, R.J.; Blumenthal, D.M.; Collins, S.L.; Farkas, T.E.; Hoffman, A.M.; Mueller, K.E.; Ocheltree, T.W.; Smith, M.D.; Whitney, K.D.; Knapp, A.K. Shifts in plant functional composition following long-term drought in grasslands. J. Ecol. 2019, 107, 2133–2148. [Google Scholar] [CrossRef]
- Gallagher, R.V.; Hughes, L.; Leishman, M.R. Species loss and gain in communities under future climate change: consequences for functional diversity. Ecography 2013, 36, 531–540. [Google Scholar] [CrossRef]
- Lavorel, S.; Díaz, S.; Cornelissen, J.H.C.; Garnier, E.; Harrison, S.P.; McIntyre, S.; Pausas, J.G.; Pérez-Harguindeguy, N.; Roumet, C.; Urcelay, C. Plant functional types: Are we getting any closer to the Holy Grail? In Terrestrial Ecosystems in a Changing World; Canadell, J.G., Pataki, D.E., Pitelka, L.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 149–164. [Google Scholar]
- Reich, P.B. The world-wide ‘fast–slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Adler, P.B.; Milchunas, D.G.; Lauenroth, W.K.; Sala, O.E.; Burke, I.C. Functional traits of graminoids in semi-arid steppes: a test of grazing histories. J. Appl. Ecol. 2004, 41, 653–663. [Google Scholar] [CrossRef]
- Atkinson, J.; Gallagher, R.; Czyzweski, S.; Kerr, M.; Trepel, J.; Buitenwerf, R.; Svenning, J.-C. Integrating functional traits into trophic rewilding scene. J. Ecol. 2024, 112, 936–953. [Google Scholar] [CrossRef]
- Hamann, E.; Blevins, C.; Franks, S.J.; Jameel, M.I.; Anderson, J.T. Climate change alters plant-herbivore interactions. New Phytol. 2020, 229, 1894–1910. [Google Scholar] [CrossRef]
- Lang, B.; Ahlborn, J.; Oyunbileg, M.; Geiger, A.; von Wehrden, H.; Wesche, K.; Oyuntsetseg, B.; Römermann, C. Grazing effects on intraspecific trait variability vary with changing precipitation patterns in Mongolian rangelands. Ecol. Evol. 2020, 10, 678–691. [Google Scholar] [CrossRef]
- Niu, K.; Zhang, S.; Lechowicz, M.J. Harsh environmental regimes increase the functional significance of intraspecific variation in plant communities. Funct. Ecol. 2020, 34, 1666–1677. [Google Scholar] [CrossRef]
- Albert, C.H.; Thuiller, W.; Yoccoz, N.G.; Soudant, A.; Boucher, F.; Saccone, P.; Lavorel, S. Intraspecific functional variability: extent, structure and sources of variation. J. Ecol. 2010, 98, 604–613. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; ter Steege, H.; Morgan, H.D.; van der Heijden, M.G.A.; et al. A handbook of protocols for standardized and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- Garnier, E.; Laurent, G.; Bellmann, A.; Debain, S.; Berthelier, P.; Ducout, B.; Roumet, C.; Navas, M. Consistency of species ranking based on functional leaf traits. New Phytol. 2001, 152, 69–83. [Google Scholar] [CrossRef]
- Westerband, A.C.; Funk, J.L.; Barton, K.E. Intraspecific trait variation in plants: a renewed focus on its role in ecological processes. Ann. Bot. 2021, 127, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Jung, V.; Albert, C.H.; Violle, C.; Kunstler, G.; Loucougaray, G.; Spiegelberger, T. Intraspecific trait variability mediates the response of subalpine grassland communities to extreme drought events. J. Ecol. 2013, 102, 45–53. [Google Scholar] [CrossRef]
- Siefert, A.; Ritchie, M.E. Intraspecific trait variation drives functional responses of old-field plant communities to nutrient enrichment. Oecologia 2016, 181, 245–255. [Google Scholar] [CrossRef]
- Paruelo, J.M.; Lauenroth, W.K.; Burke, I.C.; Sala, O.E. Grassland Precipitation-Use Efficiency Varies Across a Resource Gradient. Ecosystems 1999, 2, 64–68. [Google Scholar] [CrossRef]
- Louthan, A.M.; Doak, D.F.; Goheen, J.R.; Palmer, T.M.; Pringle, R.M. Climatic stress mediates the impacts of herbivory on plant population structure and components of individual fitness. J. Ecol. 2013, 101, 1074–1083. [Google Scholar] [CrossRef]
- Oñatibia, G.R.; Amengual, G.; Boyero, L.; Aguiar, M.R. Aridity exacerbates grazing-induced rangeland degradation: A population approach for dominant grasses. J. Appl. Ecol. 2020, 57, 1999–2009. [Google Scholar] [CrossRef]
- Quiroga, R.E.; Golluscio, R.A.; Blanco, L.J.; Fernández, R.J. Aridity and grazing as convergent selective forces: an experiment with an Arid Chaco bunchgrass. Ecol. Appl. 2010, 20, 1876–1889. [Google Scholar] [CrossRef]
- Huber, H.; Kane, N.C.; Heschel, M.S. Herbivory and plasticity in functional traits affect the performance of native and introduced populations of a perennial forb. Ecol. Evol. 2017, 7, 11192–11202. [Google Scholar] [CrossRef]
- Bannar-Martin, K.H.; Hallett, L.M.; Kleinhesselink, A.R.; Spasojevic, M.J.; Suding, K.N. Climate change is driving rapid trait shifts in grassland communities. Nat. Ecol. Evol. 2024, 8, 455–463. [Google Scholar] [CrossRef]
- Mottl, O.; Flantua, S.G.A.; Bhatta, K.P.; Felde, V.A.; Giesecke, T.; Goring, S.; Grimm, E.C.; Haberle, S.; Hooghiemstra, H.; Ivory, S.; et al. Global acceleration in rates of vegetation change over the past 18,000 years. Science 2021, 372, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the IPCC; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021; Available online: https://www.ipcc.ch/report/ar6/wg1/ (accessed on 2 April 2025).
- Riginos, C.; Porensky, L.M.; Veblen, K.E.; Young, T.P. Herbivory and drought generate short-term stochasticity and long-term stability in a savanna understory community. Ecol. Appl. 2018, 28, 323–335. [Google Scholar] [CrossRef]
- Kichenin, E.; Wardle, D.A.; Peltzer, D.A.; Morse, C.W.; Freschet, G.T. Contrasting effects of plant inter- and intraspecific variation on community-level trait measures along an environmental gradient. Funct. Ecol. 2013, 27, 1254–1261. [Google Scholar] [CrossRef]
- Siefert, A.; Violle, C.; Chalmandrier, L.; Albert, C.H.; Taudiere, A.; Fajardo, A.; Aarssen, L.W.; Baraloto, C.; Carlucci, M.B.; Cianciaruso, M.V.; et al. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. Ecol. Lett. 2015, 18, 1406–1419. [Google Scholar] [CrossRef]
- Papanikolaou, A.D.; Fyllas, N.M.; Mazaris, A.D.; Dimitrakopoulos, P.G.; Kallimanis, A.S.; Pantis, J.D. Grazing effects on plant functional group diversity in Mediterranean shrublands. Biodivers. Conserv. 2011, 20, 2831–2843. [Google Scholar] [CrossRef]
- Niu, K.; Choler, P.; de Bello, F.; Mirotchnick, N.; Du, G.; Sun, S. Fertilization decreases species diversity but increases functional diversity: A three-year experiment in a Tibetan alpine meadow. Agric. Ecosyst. Environ. 2014, 182, 106–112. [Google Scholar] [CrossRef]
- Mouchet, M.A.; Villéger, S.; Mason, N.W.H.; Mouillot, D. Functional diversity measures: an overview of their redundancy and their ability to discriminate community assembly rules. Funct. Ecol. 2010, 24, 867–876. [Google Scholar] [CrossRef]
- McCullough, I.M.; Davis, F.W.; Dingman, J.R.; Flint, L.E.; Flint, A.L.; Serra-Diaz, J.M.; Syphard, A.D.; Moritz, M.A.; Hannah, L.; Franklin, J. High and dry: high elevations disproportionately exposed to regional climate change in Mediterranean-climate landscapes. Landsc. Ecol. 2016, 31, 1063–1075. [Google Scholar] [CrossRef]
- Soil Survey Staff. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys, 2nd ed.; Natural Resources Conservation Service; U.S. Department of Agriculture Handbook: Washington, DC, USA, 1999; p. 436. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Kattge, J.; Bönisch, G.; Díaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Tautenhahn, S.; Werner, G.D.A.; Aakala, T.; Abedi, M.; et al. TRY plant trait database—Enhanced coverage and open access. Glob. Chang. Biol. 2020, 26, 119–188. [Google Scholar] [CrossRef] [PubMed]
- Villéger, S.; Mason, N.W.H.; Mouillot, D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P.; Shipley, B. FD: Measuring Functional Diversity from Multiple Traits, and Other Tools for Functional ecology (Version 1.0-12.3) [Computer Software]; R package; Université de Sherbrooke: Sherbrooke, QC, Canada, 2014; Available online: https://cran.r-project.org/package=FD (accessed on 2 April 2025).
- Brooks, M.; Kristensen, K.; van Benthem, K.; Magnusson, A.; Berg, C.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. glmmTMB Balances Speed and Flexibility Among Packages for Zero-inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Barkton, K. Mu-MIn: Multi-Model Inference. 2009. Available online: https://cran.r-project.org/web/packages/MuMIn/MuMIn.pdf (accessed on 27 August 2021).
- Burnham, K.P.; Anderson, D.R. (Eds.) . Model Selection and Multimodel Inference; Springer: New York, NY, USA, 2002. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. 2021. Available online: https://cran.r-project.org/package=DHARMa (accessed on 27 August 2021).
- Lenth, R. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. 2021. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 27 August 2021).
- Lepš, J.; de Bello, F.; Šmilauer, P.; Doležal, J. Community trait response to environment: disentangling species turnover vs intraspecific trait variability effects. Ecography 2011, 34, 856–863. [Google Scholar] [CrossRef]
- Davis, F. Downscaled Climate Grids at 30m for a Variety of Bioclimatic Variables over the Tejon Ranch, CA: 2001-2099 ver 1. 2018. Available online: https://portal.edirepository.org/nis/mapbrowse?packageid=edi.170.1 (accessed on 27 August 2021).
| Term | Abbreviation | Definition |
|---|---|---|
| Intraspecific Trait Variation | ITV | Variation in trait values between member of the same plant species |
| Community-Weighted Mean | CWM | Community means of each species’ trait value weighted by their plot-specific abundance |
| Leaf Area | LA | One-sided leaf area (mm2) |
| Specific Leaf Area | SLA | Leaf area/dry mass (mm2 × (mg−1)) |
| Leaf Dry Matter Content | LDMC | Leaf dray mass/fresh leaf mass (mg × (g−1)) |
| Seed Mass | Weight of dry seed (mg) | |
| Leaf Nitrogen Content | LNC | Total leaf nitrogen content per dry mass of leaf matter (mg × (g−1)) |
| Functional Richness | FRic | The convex hull, or volume, of a plant community’s functional trait space |
| Functional Divergence | FDiv | Distance of abundance-weighted trait values from the center of the communities’ functional space |
| Functional Evenness | FEve | The regularity of abundances of each species within the functional space |
| Functional Dispersion | FDis | The average distance of species to centroid weighted by their abundance |
| CWM | Terms | Species Turnover | ITV | Covariation | Total |
|---|---|---|---|---|---|
| Leaf Area (LA) | Climate | 6.11 | 18.29 | −4.75 | 19.65 |
| Treatment | 3.74 | 1.96 | 5.09 | 10.79 | |
| Climate:Treatment | 18.33 | 15.58 | 19.18 | 53.09 | |
| Residuals | 14.87 | 2.97 | −1.36 | 16.47 | |
| Total | 43.05 | 38.79 | 18.15 | 100.00 | |
| Specific Leaf Area (SLA) | Climate | 33.08 | 2.17 | 1.91 | 37.16 |
| Treatment | 15.86 | 0.57 | 4.01 | 20.44 | |
| Climate:Treatment | 16.29 | 2.98 | 7.61 | 26.88 | |
| Residuals | 14.13 | 0.26 | 1.13 | 15.52 | |
| Total | 79.37 | 5.98 | 14.65 | 100.00 | |
| Leaf Dry Matter Content (LDMC) | Climate | 17.01 | 0.79 | 6.70 | 24.51 |
| Treatment | 16.10 | 1.94 | 9.67 | 27.71 | |
| Climate:Treatment | 16.23 | 0.35 | −0.48 | 16.11 | |
| Residuals | 27.02 | 0.59 | 4.06 | 31.67 | |
| Total | 76.37 | 3.68 | 19.96 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klope, M.; Harris-Gavin, R.; Copeland, S.; Orr, D.; Young, H.S. Interactive Effects of Climate and Large Herbivore Assemblage Drive Plant Functional Traits and Diversity. Plants 2025, 14, 1249. https://doi.org/10.3390/plants14081249
Klope M, Harris-Gavin R, Copeland S, Orr D, Young HS. Interactive Effects of Climate and Large Herbivore Assemblage Drive Plant Functional Traits and Diversity. Plants. 2025; 14(8):1249. https://doi.org/10.3390/plants14081249
Chicago/Turabian StyleKlope, Maggie, Ruby Harris-Gavin, Stephanie Copeland, Devyn Orr, and Hillary S. Young. 2025. "Interactive Effects of Climate and Large Herbivore Assemblage Drive Plant Functional Traits and Diversity" Plants 14, no. 8: 1249. https://doi.org/10.3390/plants14081249
APA StyleKlope, M., Harris-Gavin, R., Copeland, S., Orr, D., & Young, H. S. (2025). Interactive Effects of Climate and Large Herbivore Assemblage Drive Plant Functional Traits and Diversity. Plants, 14(8), 1249. https://doi.org/10.3390/plants14081249






