Abstract
Oaks are characterized by high plasticity and intense interspecific gene flow due to natural hybridization. This generates a wide phenotypic spectrum, which creates taxonomic confusion within the genus. We compared the acorn traits across a temperature gradient in two types of Mediterranean Quercus (Quercus faginea Lam. and Q. pyrenaica Willd.) and their hybrids. Genetic groups were identified using amplified fragment length polymorphism (AFLPs) analysis. Acorns sampled from each of the three genetic groups were used for comparative purposes by means of 15 morphological characteristics. Eight of the traits showed discriminant value among the three groups. The acorn height tended to decrease with decreasing temperatures across the gradient, whereas the acorn width exhibited the opposite response. However, fruit traits allowed discrimination between the three groups, and the differences were consistent in the different zones. Both the number of acorns produced and the individual acorn size were larger for Q. pyrenaica. Hybrids showed intermediate traits between both parent species. Traditionally, the persistence of parental species in the absence of reproductive barriers has been explained by the lower fitness of the hybrids. Our results, however, do not reveal the presence of transgressive characteristics in the hybrids that could justify a lower competitive capacity.
1. Introduction
The leaf morphology has traditionally been one of the main aspects used in plant species delimitation [1,2,3]. However, the use of leaf traits as discriminants poses some problems, particularly for some genera, such as Quercus. Oak species are characterized by their high leaf plasticity in response to environmental changes [4,5,6], which generates strong variability in the leaf morphology among species, among populations of the same species, among individuals of a population, and even within the crown of a single specimen [7,8,9]. Quercus species are also known for their intense gene flow due to frequent phenomena of natural hybridization [10,11,12]. This generates a wide phenotypic spectrum, making identification difficult and producing taxonomic confusion within the genus. Both within-species plasticity and hybridization can lead to uncertainties when the taxonomical identification of oak individuals is based exclusively on the observation of morphological leaf traits [13,14,15]. For this reason, different authors have also proposed the use of reproductive characteristics, specifically the acorn morphology, for the classification of oak species and determination of hybridization, and numerous studies have indeed revealed their taxonomic utility in the study of the genus [16,17]. Since the characteristics of reproductive organs are key ecological traits that play a fundamental role in plant life cycles (affecting the rates of germination; the emergence, development, and survival of the seedlings; predation; and dispersal), their study can also provide key information to understand the patterns of natural regeneration and distribution in different species [18]. Additionally, knowing the pattern of variation in the fruit morphology and its relationship with environmental factors can help us to infer the consequences of current environmental changes for population persistence and the role of genetics and the environment in plant adaptation.
In this study, we set out to characterize the acorn morphology and production in two types of Mediterranean Quercus (Quercus faginea Lam. and Q. pyrenaica Willd.) and their hybrids, preliminarily identified from molecular markers, and to analyze their changes across a climatic gradient. These two species are among the most widespread oaks in the Iberian Peninsula. Both are deciduous and coexist in many places. Previous studies of these two closely related species have addressed some aspects related to their leaf morphology and physiology, as well as their ecological requirements, highlighting significant differences between them [6,19]. However, we do not know of any study that has compared the morphological characteristics of the acorns of both species and their hybrids, although they share numerous areas with high levels of hybridization [20]. Although between-population variability in the acorn morphology has been observed in different Quercus species over a broad range of conditions [21,22,23], we do not know of studies addressing the plasticity in the fruits of Q. faginea and Q. pyrenaica in response to changes across environmental gradients, despite the importance of trait plasticity for the persistence of both species under the new expected climatic conditions. Mediterranean deciduous oaks are known for their extreme interannual variability in fruit production [24]. In the two studied species, acorn production may be almost nil for years, so fruit traits can only be adequately characterized during mast years. During the past year (2024), an extremely large amount of acorn production has been recorded in our study region, with strong synchronization between both species and different populations, giving us the opportunity to compare the acorn characteristics of the different genetic groups both within the same geographical area and across a climatic gradient.
In a previous study of the leaf morphologies of the same species and their hybrids [20], we observed that, although practically all of the morphological traits studied differed significantly between the two species, the hybrids showed, in general, leaf traits that were more similar to those of one of the parental species (Q. faginea), so that the characteristics related to the leaf morphology did not seem to offer much discriminant value in our study complex. Among the three genetic groups (Q. faginea, Q. pyrenaica, and hybrids), Q. faginea exhibited the strongest responses in its leaf traits to temperature changes across a geographic gradient. Since greater phenotypic plasticity has been considered important to allow plants to respond successfully to changing environmental conditions [25,26], the greater plasticity of Q. faginea could confer an advantage to this species compared to Q. pyrenaica and to the hybrids in the face of the changing climatic conditions predicted.
In this work, firstly, we propose to study the acorn characteristics of the two species and their hybrids and to detect the characteristics that allow discrimination among the three genotypes. We intend to verify whether, unlike the leaf morphology, the fruit morphology does offer a criterion for the identification of hybrids. Secondly, we analyze the variability in the fruit traits in response to changes across a climatic gradient and check whether there are differences in plasticity among traits and among the three genetic groups (the two species and their hybrids). Thirdly, we check whether the possible differences among the three genetic groups are similar in different areas distributed across a climatic gradient. Given the crucial influence of the size and other acorn traits in the regeneration of oak forests, analyzing the fruit responses to climatic gradients is especially important to formulate predictions about the future evolution of forest masses in one of the climates (Mediterranean) and one of the areas (the Iberian Peninsula) considered most vulnerable in a climate change scenario [27,28].
2. Results
2.1. Effects of Taxa on Fruit Trait Variability
Visual counts indicated significantly higher acorn production in Q. pyrenaica (around 50% more acorns counted than for Q. faginea) in the three study areas (Figure 1a). Among the other two groups, acorn production was lower in the hybrids in the cold and middle zones, and it was only lower in Q. faginea than in the hybrids in the warm zone (Figure 1a). Q. pyrenaica was also the group with the lowest percentage of acorns predated by insect larvae, regardless of the study area, while Q. faginea showed the highest percentage of acorn predation, with the hybrids exhibiting intermediate values (Figure 1b).
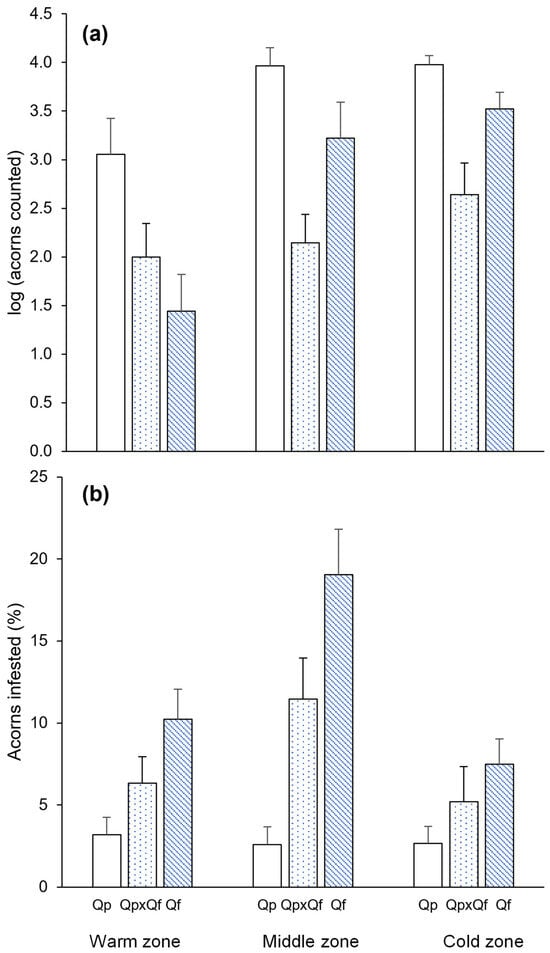
Figure 1.
Mean (+SE) of (a) acorn production (30 s counts in visual surveys, log-transformed) and (b) the percentage of acorns infested by insect larvae for the different genetic groups (Quercus pyrenaica, Qp; hybrids, Qp x Qf; Q. faginea, Qf) in the three climatic zones.
Measurements of 15 characteristics were taken from a total of 900 acorns (100 per site, 10 randomly chosen per tree). These characteristics’ definitions and units are summarized in Table 1 (see Section 4). There were significant differences among the genetic groups in all fruit traits analyzed (Table S1). Eight traits showed discriminant value between the three groups (post hoc Tukey–Kramer HSD test), with Q. pyrenaica presenting larger acorns (length, width, volume, and mass), wider cupules, and shorter but thicker peduncles, while the opposite was observed in Q. faginea, with intermediate values in general for the hybrids (Figure 2 and Figure 3). The mean acorn dry mass in Q. pyrenaica amounted to more than twice that of Q. faginea. For the remaining traits, differences were evident between the two species, with lower AH/AW (Figure 2) and CH/AH but higher CW, CT, CV, and CW/CH (Figure 3) in Q. pyrenaica than in Q. faginea. For most of these traits, the hybrids showed similar values to Q. faginea. The height and width of acorns and cupules, as well as the peduncle characteristics, were the traits that showed the greatest consistency among individuals from the same population, with CVs always lower than 10–15% (Table S2), while the acorn mass and volume and cupule volume were the characteristics with the highest variability in general (more than 20% in some cases). Contrary to our expectations, the CVs were not generally greater for the hybrids than for the parental species.

Table 1.
List of acorn characteristics examined, definitions, and units.
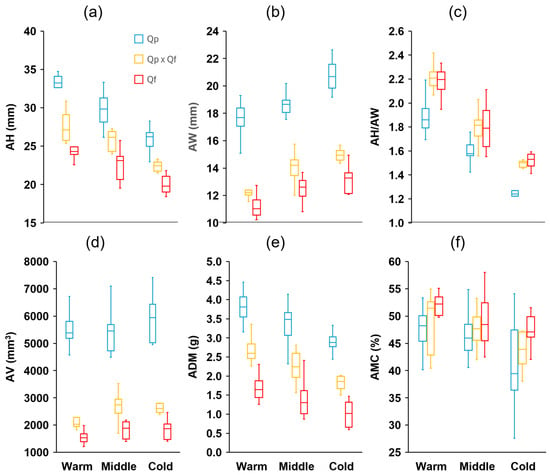
Figure 2.
Boxplot diagrams of (a) height (AH), (b) width (AW), (c) AH/AW ratio, (d) volume (AV), (e) dry mass (ADM), and (f) moisture content (AMC) of acorns from the different genetic groups (Quercus pyrenaica, Qp; hybrids, Qp x Qf; Q. faginea, Qf) in the three climatic zones. The box in each box plot shows the median and the lower and upper quartiles, and the whiskers show the range of variation.
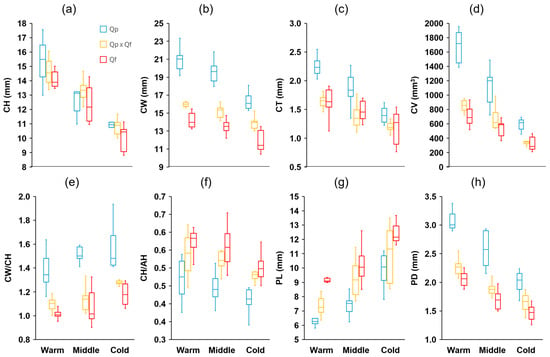
Figure 3.
Boxplot diagrams of (a) height (CH), (b) width (CW), (c) thickness (CT), (d) volume (CV), (e) CW/CH ratio, (f) CH/AH ratio of the cupules, and (g) length (PL) and (h) diameter (PD) of the peduncles of acorns from the different genetic groups (Quercus pyrenaica, Qp; hybrids, Qp x Qf; Q. faginea, Qf) in the three climatic zones. The box in each box plot shows the median and the lower and upper quartiles, and the whiskers show the range of variation.
There were significant correlations between most of the fruit dimensions measured, being especially strong between the width of the acorn and cupule and their respective volumes, the height and mass of the acorns, the mass and moisture content, and the size of the acorn and cupule and the diameter of the peduncle (Table S3). Larger acorns have larger and thicker cupules, but less covering (lower CH/AH ratio) and shorter but thicker peduncles (Table S3). Based on the selected fruit traits, the PCA revealed that the first and second principal components explained 82.2% of the total variance, with the majority, 54.8%, corresponding to PC1 (Table 2). The variables that most contributed to the first principal component were CW, CV, the acorn fresh and dry mass, the acorn height, and PD. The ratio AH/AW and CH for the positive side and CW/CH and AW for the negative side exhibited the strongest correlations with PC2 (Table 2). The first component clearly discriminated Q. pyrenaica (distributed on the right side of the diagram) from Q. faginea (Figure 4). Hybrids tended to occupy the central part of the axis, exhibiting intermediate mean individual scores (Figure 5), although they were somewhat closer to Q. faginea individuals. The second principal component clearly discriminated the three zones across the gradient, with the warmest at the top, the coldest at the bottom, and the intermediate zone occupying positions between the two (Figure 4). The three genetic groups exhibited similar scores with respect to PC2 but they tended to be more negative for Q. pyrenaica in the coldest sites (Figure 5).

Table 2.
Eigenvalues, percentage of the variance explained by the first two principal components, and factor scores of acorn traits (abbreviations and units as in Table 1).
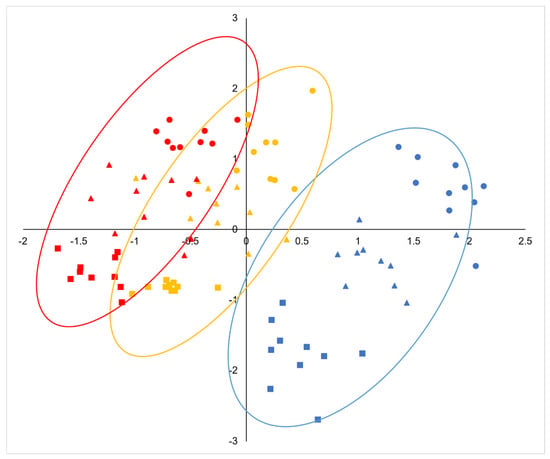
Figure 4.
Principal component analysis (PCA) based on the morphological and anatomical acorn traits (genetic groups are labeled with different colors: Quercus pyrenaica, blue, hybrids, orange, Q. faginea, red; climatic zones are labeled with different symbols: warm, circle, intermediate, triangle, cold, square).
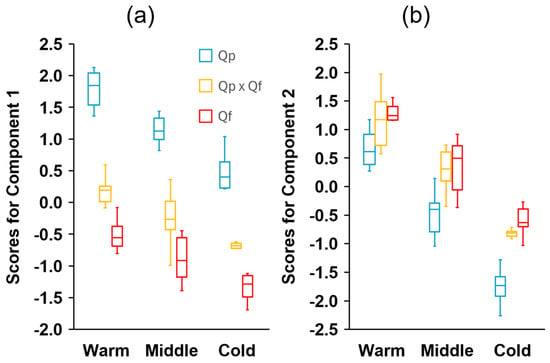
Figure 5.
Boxplot diagrams depicting the distribution of the individual scores for (a) the first and (b) the second principal components of the different genetic groups (Quercus pyrenaica, Qp; hybrids, Qp x Qf; Q. faginea, Qf) in the three climatic zones. The box in each box plot shows the median and the lower and upper quartiles, and the whiskers show the range of variation.
2.2. Effects of Environmental Gradients on Fruit Trait Variability
There were differences between the areas across the climatic gradient in all traits analyzed (Table S1). In 10 of these traits, the differences were significant between the three zones (post hoc Tukey–Kramer HSD test). For the three genetic groups, the acorn height tended to decrease with the decrease in temperature across the gradient, whereas the acorn width exhibited the opposite response (Figure 2). Accordingly, the ratio AH/AW decreased significantly as the mean temperature decreased. The same trend was also apparent for the mean dry mass (ADM, Figure 2), although the mean acorn volume did not change within a single group across the gradient. The three groups tended to produce shorter and narrower cupules, with a smaller thickness (CT) and volume (CV), as well as longer peduncles, but with a smaller diameter (Figure 3) as the temperatures decreased between the zones through the gradient. In the remaining traits, the differences among the zones were less apparent. The ratio CH/AH showed a slight decrease (smaller proportion of the acorn covered by the cupule) across the gradient (Figure 3). Finally, the moisture content (AMC) tended to decrease with decreasing temperatures, although the differences were only evident for the coldest sites (Figure 2).
The combinations of traits present in the different climatic zones showed a consistent change, according to the principal component analysis (Figure 4). For the three genetic groups, the mean individual scores for PC1 and PC2 decreased consistently with decreasing temperatures (Figure 5).
Among the traits that responded to environmental changes, PL, AH/AW, the acorn weight, and CT, and especially CV, were the most variable between the zones within a single group, as indicated by their plasticity indices ranging between 0.20 and 0.40 in most cases (Table 3). Q. pyrenaica tended to exhibit the highest variability for most cupule traits, whereas Q. faginea showed high plasticity in the individual acorn mass (more than 0.40) in response to climatic changes between the sites (Table 3).

Table 3.
Plasticity indices ((maximum value-minimum value)/maximum value) for the different acorn traits (abbreviations as in Table 1) in each genetic group.
3. Discussion
Given the strong variability that characterizes the leaves of the species of the genus Quercus, several authors have proposed including the use of fruit characteristics for the classification of oak species and determination of hybridization [16,17]. Other authors, however, do not consider the fruit morphology to be a reliable discriminant between oak species and their hybrids, either because their studies did not reveal large differences between genetic groups or because of the enormous variation found in these traits within and between populations and individuals [29,30,31]. In the specific case of our two Quercus species, the results revealed that fruits allow the discrimination of the two studied species, showing significant differences in all features considered. In addition, the fruits showed discriminant value for the characterization of hybrids, with 8 of the 15 traits analyzed allowing them to be significantly distinguished from the two parental species and with intermediate values between both. Since these results have been obtained during a single year of sampling, they must be interpreted with caution. The masting behavior in our oak species makes it extremely difficult to obtain comparable data on the fruit morphology for different species under the same conditions. It is known that some fruit traits may be affected by environmental conditions, which implies that different species can only be compared under similar circumstances. The masting episode of 2024 was an opportunity to obtain data for the three genetic groups during the same time period and in the same sites. In fact, some of traits analyzed, such as the acorn volume and mass, did indeed show strong variability (high coefficients of variation) among individuals within the same population, which obviously limits their reliability as discriminant traits among the genetic groups. However, traits such as the acorn height and width, cupule width, and peduncle length and diameter showed greater consistency and relatively low intrapopulation variability (Table S2); in fact, they were smaller than some leaf traits frequently used for species delimitation [20]. Although differences in the leaf morphology also allowed clear discrimination between the two studied species, the hybrids showed leaf traits in most cases that were similar to Q. faginea [20]. The hybrids also shared five of the estimated fruit traits with Q. faginea, but, in most of the remaining fruit traits, the hybrids exhibited intermediate values that were significantly different from both parents, and the differences were consistent in the different climatic zones. This suggests that the discriminating fruit traits are characteristic of the different genetic groups, regardless of the changes that they experience in response to changes in the environmental conditions, and that the genetic differences have a strong influence on the morphology of the acorns [32,33]. Our work, therefore, highlights the usefulness of fruit characteristics to distinguish oak species and their hybrids, at least for our study complex.
Regardless of the study area, Q. pyrenaica showed larger acorns (length, width, and volume) and wider and thicker cupules, covering a smaller proportion of the length of the fruit (shorter CH/AH ratio, Figure 3), as well as shorter and thicker peduncles, while the opposite was observed for Q. faginea. Previous studies have shown that large seeds tend to present increased germination rates in species such as Quercus ilex and Castanea sativa [34,35] and that larger seeds generally produce larger seedlings with higher relative growth rates, presumably because of the greater metabolic energy available for establishment, growth, and development [36,37,38]. Meanwhile, it has traditionally been proposed that larger acorns may suffer higher predation rates [34,39] for several reasons: because they take longer to develop, because optimal foraging theory suggests the predator’s preference for larger seeds over smaller ones, and because larger seeds may host both large and small insects [40,41,42]. However, in our study, Q. pyrenaica was the species with the fewest signs of consumption in all study areas, with the highest values recorded in Q. faginea, the genetic group with the smallest acorns. Therefore, other factors, apart from the acorn size, may also determine individual differences in predation. Quercus species are well known for their extreme interannual variability in fruit production (masting effect) [24]. The “predator satiation hypothesis” states that masting allows a greater proportion of acorns to escape predation by satiating predators during years of high acorn production and by reducing predator populations during years without acorn production [43,44]. Although both of our study species maintained a strong tendency for mast production, Q. pyrenaica is characterized by more marked and frequent interannual variations in acorn production [24]. Q. pyrenaica was the genetic group with the highest acorn production in the three zones across the gradient. In fact, the acorn production in Q. pyrenaica was, in all cases, much higher than the average, according to existing classifications [45]. On the other hand, larger and thicker cupules, such as those shown by Q. pyrenaica, may play an important role in protecting acorns. Besides providing physical protection, the high phenol levels in the cupules in many species of Quercus may prevent animals from feeding on the acorns prematurely [46,47].
Along the climatic gradient, we observed important differences in the three genetic groups, both in acorn production and in their characteristics. Given their influence on pollination, fertilization, ovulation, and flowering, it has traditionally been considered that the climatic conditions decisively contribute to determining acorn production [48,49]. In general, it tends to be considered that higher temperatures and lower water availability would result in lower levels of production, with water deficits being the main driver, rather than the temperature per se [21,50]. In our case, acorn production tended to be lower in the warm zone and, with slight differences, higher in the coldest area. However, drought seems unlikely as an explanation for these differences in production because, in our study, the warm zone registered the highest levels of precipitation, which, in principle, would allow it to compensate for its higher temperatures, resulting in similar levels of water stress between the zones. Furthermore, the differences between the cold and intermediate zones cannot be explained by precipitation, given the similar levels of annual rainfall between both areas. Similarly, several authors have reported no relationship between seed production and precipitation [22], and others have even been unable to find a significant relationship between acorn crops and the weather (precipitation and temperature) in different oak species [51,52]. In some cases, decreases in acorn production have been attributed to biotic factors, with herbivores affecting fecundity in oaks and causing significant losses of acorns due to infestation by bacterial pathogens that enter through holes or cracks caused by these herbivores [53,54]. In our opinion, however, the most probable explanation for the differences in acorn production among our sites is the trade-off between the acorn size and number, as pointed out by other authors [55,56], since the individual acorn mass tended to decrease with decreasing temperatures across the gradient (Figure 2).
The acorn traits showed clear plasticity in response to changes across the environmental gradient, with a greater width but smaller length and mass, as well as longer and thinner peduncles and smaller and generally thinner cupules, as the temperature decreased (Figure 2 and Figure 3). Negative intraspecific correlations between the size and/or mass of acorns and an increase in latitude or altitude, mediated by a decrease in temperature, have been observed in different Quercus species [57,58]. Several authors have proposed that the increase in the size and mass of acorns makes it possible to slow down water loss in warmer environments [59,60,61]. In fact, positive intraspecific correlations between the seed mass and xerothermic indices have been obtained in different Quercus species [62]. In the same sense, it could be interpreted that the larger and thicker cupules that we found in the warmer areas through the gradient play an important role in limiting evaporative losses when the fruit is on the tree [59,63]. However, in our case, differences in water availability were unlikely to be responsible for these trends in the fruit morphology across the gradient because, as mentioned above, the warm zone registered the highest levels of precipitation. Rather, we posit that these trends could be due to the unfavorable effects on the acorn size of lower primary productivity in colder environments at higher latitudes and altitudes, as other authors have pointed out [64]. In the coldest sites examined in our study, low temperatures and late frosts can extend well into the growing season, imposing a delay in leaf emergence [20], which limits the productivity when the water conditions are still favorable during spring, before the appearance of the summer drought typical of Mediterranean environments.In a previous work on the same species as in the present study [20], our results revealed that greater plasticity in leaf traits could confer an advantage to Q. faginea in coping with changing environmental conditions in the future. However, from the point of view of fruit traits, Q. pyrenaica seems to show more favorable traits, thanks to its trend towards mast acorn production, larger acorn sizes, and less predation, with Q. faginea at the opposite extreme. The predation results, however, must be interpreted with caution. Since acorn sampling was performed in autumn, we could not determine the number of acorns lost before sampling. Fruits may fall before maturity due to abortion, abnormal fruit development, or infestation [53]. In some cases, the losses occur at the early stages of fruit development, when the acorns are very small, and are difficult to quantify. There may have been interspecific differences in the number of premature losses that could not be determined with the data obtained in the present study. Q. pyrenaica also tended to exhibit stronger responses to environmental changes between the climatic zones, with the plasticity indices being higher in general than in Q. faginea and the hybrids for some of the fruit traits. Greater phenotypic plasticity has been considered important to allow plants to respond successfully to changing environmental conditions [26]. Therefore, our results suggest that the vegetative and reproductive traits (leaves versus fruits) vary independently between our three study groups (Q. faginea, Q. pyrenaica, and hybrids) and in response to climatic factors, as other authors have also observed in other Quercus species [65]. With respect to the hybrids’ traits, both the results of our previous study regarding the leaves [20] and the results of the present study regarding the fruits seem to corroborate the fact that hybrids tend to show intermediate traits between both parent species. Traditionally, the persistence of parental species in the absence of reproductive barriers has been explained by the lower fitness of the hybrids [66,67]. Our results, however, do not indicate that, with respect to the production and traits of their fruits, hybrids exhibit transgressive characteristics that could justify their lower competitive capacity with respect to their parental species. In any case, the results of the present study highlight the importance of including, along with the leaves, the analysis of the traits of the fruits to differentiate and characterize species and their hybrids and to help predict the distribution of the three groups in a future scenario.
In summary, fruit traits seem to have discriminant value in differentiating among the three genetic groups. At the contact zones, hybrids exhibit intermediate fruit traits, which may explain their persistence under intermediate environmental conditions. More research is needed to verify whether the results of the present study may be extrapolated to other Mediterranean areas and whether the same differences are present in other masting episodes.
4. Materials and Methods
4.1. Selection of Sites and Individuals and Sampling
This study included nine sites located in three areas covering the regions of Castilla-León and Extremadura (Central–Western Spain). The three zones, due to differences in latitude and altitude, could be classified as cold, middle, and warm, mainly in terms of the absolute minimum temperatures and number of frost days per year (Table S4). The warm zone had the highest values of precipitation (which helped to reduce the differences in the intensity of drought stress between the sites), with a negligible difference between the cold and intermediate zones. In each area, three sites were selected (with a distance of less than 50 km from each other and therefore with few climatic differences between them): one with the apparent dominance of Q. faginea (in the absence of Q. pyrenaica), another with Q. pyrenaica (in the absence of Q. faginea), and intermediate sites with the presence of both species. The climate data corresponded to the average of five study years (2020–2024), during which the air temperature was recorded (at 10-min intervals) through sensors with data loggers (Hobo Pendant temperature/light datalogger, Part UA -002-08, Onset Computer Corporation, Pocasset, MA, USA), with a range of −20° to 70 °C and accuracy ± 0.53 °C from 0° to 50 °C, placed for this purpose at each site (two temperature sensors per site). Precipitation data were provided by the Spanish National Meteorological Institute.
At each site, around 30 specimens (located at least 5 m apart) of each of the dominant groups (Q. faginea, Q. pyrenaica, or hybrids) were selected according to their leaf morphologies and duly identified and geolocated. All selected trees were fully sun-exposed, mature specimens with heights between 8 and 10 m and diameters (at 1.3 m) between 40 and 60 cm. Leaf samples were collected from each specimen throughout 2021, and the genetic structure analysis and categorization were performed using AFLPs, following the method implemented in Structure Harvester version 0.6.93 [68], through the specific procedure detailed in [20]. Hybrid existence was determined by analyzing the admixture coefficient (Q) values. Supported by the previous literature [69,70], a threshold of Q ≥ 0.9 was set to distinguish purebreds from hybrids. In accordance with the obtained Q values, finally, of the 272 specimens sampled, the genetic analyses assigned 70 individuals to pure Q. faginea (26%), 76 individuals to pure Q. pyrenaica (28%), and 126 individuals to hybrids (46%) (the final assignment of individuals to genetic clusters within each of the three study areas is shown in Table S5).
Once the individuals had been categorized, at each site, we selected 10 trees from the dominant genetic group and at least 30 mature and apparently undamaged acorns per tree were collected directly from the trees in autumn 2024. The acorns were taken in the middle part of the periphery of the crown and in southern exposure. Groups of acorns from the same tree were packaged together in plastic bags and immediately transported to the laboratory, where they were first immersed in water to visually identify insect-damaged or infected or dead acorns. The proportion of acorns infested by insect larvae was counted for each tree. Abnormal and defective acorns were then discarded before the remaining acorns were stored in hermetically sealed plastic boxes in the dark at 4 °C until morphological characterization. Furthermore, in the field, on every tree, acorn production was evaluated using the visual survey technique developed by Koenig et al. [45], consisting of two observers on opposite sides of the tree, counting all acorns seen in a 15 s period; the index of acorn production was the total number counted per 30 s of observer time. The counting methods used at all sites closely matched the acorn counts obtained using other methods, such as traps and production categories, offering a fast and efficient method of assessing fruit or cone crops [45,52,71].
4.2. Determination of Fruit Morphological Characteristics
Measurements of 15 characteristics, selected among the most used from the literature [17,65,72], were taken from a total of 900 acorns (100 per site, 10 randomly chosen per tree). These characteristics, with their definitions and units, are summarized in Table 1.
The fruits were separated from the cupules and the length and width of the fruit were measured with a digital caliper (Digimatic Micrometer, Mitutoyo, Tokyo, Japan) with accuracy of 0.01 mm. The acorn width (AW) refers to the distance between the widest points on the left and right sides of the fruit, and the acorn height (AH) refers to the distance from the bottom to the top of the fruit. The height-to-width ratio (AH/AW) of each fruit was then calculated. Each fruit was measured three times and the data were averaged. The individual acorn volume (AV) was approximated assuming a prolate spheroid shape, using the formula [65]. The acorn fresh mass (AFM) and acorn dry mass (ADM) (after oven-drying for 17 h at 103 °C) were measured with an analytical balance (Sauter AR70, Sauter, Ebingen, Germany), and the acorn moisture content (AMC) was later estimated as . Moreover, the cupule height (CH), width (CW), and thickness (CT) (difference between the inner and outer diameters of the cupule) were collected with the digital caliper and then the ratios between the cupule height and width (CW/CH) and between the cupule and acorn height (CH/AH) were estimated. Assuming that a cupule is a semi-ellipsoid rotating around its longitudinal axis, we calculated the cupule volume (CV) via the equation [65]. Finally, the peduncle length (PL) and diameter (PD) were measured with the digital caliper.
4.3. Data Analysis
A value for each trait analyzed was obtained for each tree as an average of the 10 acorns measured in each case; finally, a value for each trait, genetic group, and site was estimated as an average of the 10 trees sampled in each case. An analysis of variance (ANOVA) was carried out to search for differences among the genetic groups (two parental species and hybrids) and among the three study areas for the different parameters considered, followed by a post hoc Tukey–Kramer HSD test (at a significance level of p = 0.05). Prior to any analysis, data were tested for normality (using the Kolmogorov–Smirnov statistic test) and variance homogeneity (Levene’s test) to proceed with data transformation when necessary. Acorn counts for each tree were log-transformed [log (x + 1)] for analysis [45]. Similarly, in the analyses involving fractional data (percentage of acorns infested by insect larvae), the data were logit-transformed [ln (p/(1 − p))] [73]. The variability in the acorns characteristics within each species and zone was measured by calculating the coefficient of variation (CV). Furthermore, for the traits in which significant differences were detected between zones, a plasticity index (PI) ranging from 0 to 1 was calculated as (maximum value-minimum value)/maximum value [74,75]. This index was used to verify which genetic group (Q. faginea, Q. pyrenaica, or hybrids) and which traits showed greater responsiveness to environmental changes across the gradient. Principal component analysis (PCA) was used to detect the most differentiating morphological traits for acorns and to assess the contribution of environmental factors to the variability in acorn traits. As a first step, a Pearson correlation matrix was used to determine associations between the variables, with log transformation used when necessary. The SPSS v28 statistical package was used to analyze the data (SPSS Inc., Chicago, IL, USA).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants14050718/s1, Table S1: Results of ANOVA for the effects of genetic group and climatic zone on the different morphological acorn traits analyzed. Abbreviations and units as in Table 1; Table S2: Coefficients of variation for the different acorn traits within each genetic group and site. Abbreviations as in Table 1; Table S3: Pearson’s correlation coefficients for the different acorn traits measured using the average values obtained for each tree (abbreviations and units as in Table 1). p > 0.05 NS; 0.01 < p < 0.05 *; 0.001 < p < 0.01 **; Table S4: Characteristics of the study sites. Climatic data were obtained as an average of five years (2020–2024); Table S5: Individuals sorted by Q value for genetic clusters K1 (Q. faginea) and K2 (Q. pyrenaica).
Author Contributions
Conceptualization, S.G.-C., A.E. and S.M.; methodology, S.G.-C., A.F.-F., M.M.-O. and S.M.; validation, S.G.-C. and S.M.; formal analysis, S.G.-C., M.M.-O. and S.M.; investigation, S.G.-C. and S.M.; data curation, S.G.-C., A.F.-F., M.M.-O. and S.M.; writing—original draft preparation, S.M.; writing—review and editing, A.E. and S.M.; visualization, S.G.-C., A.E. and S.M.; funding acquisition, M.M.-O. and S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Spanish Ministerio de Ciencia e Innovación—EU-FEDER (Project No. PID2020-113442GB-I00).
Data Availability Statement
The authors will make the raw data supporting this article’s conclusions available upon request.
Acknowledgments
We thank Teresa Malvar-Ferreras for the technical support in the lab at the Plant-DNA Biobank (Nucleus, Universidad de Salamanca).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Valcárcel, V.; Vargas, P. Quantitative morphology and species delimitation under the general lineage concept: Optimization for Hedera (Araliaceae). Am. J. Bot. 2010, 97, 1555–1573. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.I.U.; Reboucas, D.A.; Batista Leite, K.R.; de Oliveira, R.P.; Funch, L.S. Can leaf morphology and anatomy contribute to species delimitation? A case in the Campomanesia xanthocarpa complex (Myrtaceae). Flora 2018, 249, 111–123. [Google Scholar] [CrossRef]
- Oso, O.A.; Jayeola, A.A. Digital morphometrics: Application of Morpholeaf in shape visualization and species delimitation, using Cucurbitaceae leaves as a model. Appl. Plant Sci. 2021, 28, e11448. [Google Scholar] [CrossRef] [PubMed]
- Viscosi, V. Geometric morphometrics and leaf phenotypic plasticity: Assessing fluctuating asymmetry and allometry in European white oaks (Quercus). Bot. J. Linn. Soc. 2015, 179, 335–348. [Google Scholar] [CrossRef]
- Kusi, J.; Karsai, I. Plastic leaf morphology in three species of Quercus: The more exposed leaves are smaller, more lobated and denser. Plant Species Biol. 2020, 35, 24–37. [Google Scholar] [CrossRef]
- Martín-Sánchez, R.; Sancho-Knapik, D.; Alonso-Forn, D.; López-Ballesteros, A.; Ferrio, J.P.; Hipp, A.L.; Peguero-Pina, J.J.; Gil-Pelegrín, E. Oak leaf morphology may be more strongly shaped by climate than by phylogeny. Ann. For. Sci. 2024, 81, 14. [Google Scholar] [CrossRef]
- Mediavilla, S.; Martín, I.; Babiano, J.; Escudero, A. Foliar plasticity related to gradients of heat and drought stress across crown orientations in three Mediterranean Quercus species. PLoS ONE 2019, 14, e0224462. [Google Scholar] [CrossRef] [PubMed]
- Solé-Medina, A.; Robledo-Arnuncio, J.J.; Ramírez-Valiente, J.A. Multi-trait genetic variation in resource-use strategies and phenotypic plasticity correlates with local climate across the range of a Mediterranean oak (Quercus faginea). New Phytol. 2022, 234, 462–478. [Google Scholar] [CrossRef] [PubMed]
- Soheili, F.; Heydari, M.; Woodward, S.; Naji, H.R. Adaptive mechanism in Quercus brantii Lindl. leaves under climatic differentiation: Morphological and anatomical traits. Sci. Rep. 2023, 13, 3580. [Google Scholar] [CrossRef] [PubMed]
- Antonecchia, G.; Fortini, P.; Lepais, O.; Gerber, S.; Legér, P.; Scippa, G.S.; Viscosi, V. Genetic structure of a natural oak community in central Italy: Evidence of gene flow between three sympatric white oak species (Quercus, Fagaceae). Ann. For. Res. 2015, 58, 205–216. [Google Scholar] [CrossRef]
- Song, Y.; Deng, M.; Hipp, A.; Li, Q. Leaf morphological evidence of natural hybridization between two oak species (Quercus austrocochinchinensis and Q. kerrii) and its implications for conservation management. Eu. J. For. Res. 2015, 134, 139–151. [Google Scholar] [CrossRef]
- López De Heredia, U.; Sánchez, H.; Soto, A. Molecular evidence of bidirectional introgression between Quercus suber and Quercus ilex. iForest 2018, 11, 338. [Google Scholar] [CrossRef]
- Jawarneh, M.S.; Brake, M.H.; Muhaidat, R.; Migdadi, H.M.; Lahham, J.N.; El-Oqlah, A.A. Characterization of Quercus species distributed in Jordan using morphological and molecular markers. Afr. J. Biotechnol. 2013, 12, 1326–1334. [Google Scholar]
- Hipp, A.L. Should hybridization make us skeptical of the oak phylogeny? Int. Oaks 2015, 26, 9–18. [Google Scholar]
- Musarella, C.M.; Cano-Ortiz, A.; Piñar Fuentes, J.C.; Navas-Ureña, J.; Pinto Gomes, C.J.; Quinto Canas, R.; Cano, E.; Spampinato, G. Similarity analysis between species of the genus Quercus L. (Fagaceae) in southern Italy based on the fractal dimension. PhytoKeys 2018, 113, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Baki, Y.; Bavak, M.T. Morphological variability of acorns and its taxonomic significance in Quercus L. from Turkey. Bangladesh J. Bot. 2014, 43, 293–299. [Google Scholar] [CrossRef]
- Aykut, Y.; Emel, U.; Tekin, B.M. Morphological variability of evergreen oaks (Quercus) in Turkey. Bangladesh J. Plant Taxon. 2017, 24, 39–47. [Google Scholar] [CrossRef]
- Moles, A.T.; Westoby, M. Seed size and plant strategy across the whole life cycle. Oikos 2006, 113, 91–105. [Google Scholar] [CrossRef]
- Mediavilla, S.; Escudero, A. Stomatal responses to drought at a Mediterranean site: A comparative study of co-occurring woody species differing in leaf longevity. Tree Physiol. 2003, 23, 987–996. [Google Scholar] [CrossRef] [PubMed]
- González-Carrera, S.; Fernández-Fuentes, A.; Escudero, A.; García-Estévez, I.; Martínez-Ortega, M.; Mediavilla, S. Leaf traits and insect herbivory levels in two Mediterranean oaks and their hybrids through contrasting environmental gradients. Tree Physiol. 2025, 45, tpae170. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Humanes, B.; Espelta, J.M. Increased drought reduces acorn production in Quercus ilex coppices: Thinning mitigates this effect but only in the short term. Forestry 2011, 84, 73–82. [Google Scholar] [CrossRef]
- Caignard, T.; Kremer, A.; Firmat, C.; Nicolas, M.; Venner, S.; Delzon, S. Increasing Spring temperatures favor oak seed production in temperate areas. Sci. Rep. 2017, 7, 8555. [Google Scholar] [CrossRef]
- Gao, S.; Ren, Y.; Masabnin, J.; Zou, F.; Xiong, H.; Zhu, J. Influence of geographical and climatic factors on Quercus variabilis Blume fruit phenotypic diversity. Diversity 2021, 13, 329. [Google Scholar] [CrossRef]
- Bravo, J.A.; Roig, S.; Serrada, R. Selvicultura en Montes Bajos y Medios de Quercus ilex L., Q. pyrenaica Willd. y Q. faginea Lam; Serrada, R., Montero, G., Reque, J.A., Eds.; Compendio de Selvicultura Aplicada en España, INIA y FUCOVASA: Madrid, Spain, 2008; pp. 657–744. [Google Scholar]
- Hansen, M.M.; Olivieri, I.; Waller, D.M.; Nielsen, E.E. Monitoring adaptive genetic responses to environmental change. Mol. Ecol. 2012, 21, 1311–1329. [Google Scholar] [CrossRef] [PubMed]
- Franks, S.J.; Weber, J.J.; Aitken, S.N. Evolutionary and plastic responses to climate change in terrestrial plant populations. Evol. Appl. 2013, 7, 123–139. [Google Scholar] [CrossRef]
- Ojeda, M.G.-V.; Gámiz-Fortis, S.R.; Romero-Jiménez, E.; Rosa-Cánovas, J.J.; Yeste, P.; Castro-Díez, Y.; Esteban-Parra, M.J. Projected changes in the Iberian Peninsula drought characteristics. Sci. Total Environ. 2021, 757, 143702. [Google Scholar] [CrossRef]
- Hidalgo-Triana, N.; Solakis, A.; Casimiro-Soriguer, F.; Choe, H.; Navarro, T.; Pérez-Latorre, A.V.; Thorne, J.H. The high climate vulnerability of western Mediterranean forests. Sci. Total Environ. 2023, 895, 164983. [Google Scholar] [CrossRef] [PubMed]
- Brookes, P.C.; Wigston, D.L. Variation of morphological and chemical characteristics of acorns from populations of Quercus petraea (Matt.) Liebl., Q. robur L. and their hybrids. Walsonia 1979, 12, 315–324. [Google Scholar]
- Wei, L.; Li, Y.F.; Zhang, H.; Liao, W.J. Variation in morphological traits in a recent hybrid zone between closely related Quercus liaotungensis and Q. mongolica (Fagaceae). J. Plant Ecol. 2015, 8, 224–229. [Google Scholar] [CrossRef][Green Version]
- Proietti, E.; Filesi, L.; Di Marzio, P.; Di Pietro, R.; Masin, R.; Conte, A.L.; Fortini, P. Morphology, geometric morphometrics, and taxonomy in relict deciduous oaks woods in northern Italy. Rend. Lincei. Sci. Fis. Nat. 2021, 32, 549–564. [Google Scholar] [CrossRef]
- Kadomatsu, M.; Funakoshi, S. Annual variations in morphological characters of leaf. acorn and cupule of Quercus. Trans. Jap. For. Soc. 1992, 103, 317–318. [Google Scholar]
- Shi, W.; Villar-Salvador, P.; Li, G.; Jiang, X. Acorn size is more important than nursery fertilization for out planting performance of Quercus variabilis container seedlings. Ann. For. Sci. 2019, 76, 22. [Google Scholar] [CrossRef]
- Gómez, J.M. Bigger is not always better: Conflicting selective pressures on seed size in Quercus ilex. Evolution 2004, 58, 71–80. [Google Scholar] [PubMed]
- Cicek, E.; Tilki, F. Seed size effects on germination, survival and seedling growth of Castanea sativa Mill. J. Biol. Sci. 2007, 7, 438–441. [Google Scholar] [CrossRef]
- Seiwa, K. Effects of seed size and emergence time on tree seedling establishment: Importance of developmental constraints. Oecologia 2000, 123, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.L. Effects of seed mass on seedling success in Artocarpus heterophyllus L., a tropical tree species on north-east India. Acta Oecol. 2004, 25, 103–110. [Google Scholar] [CrossRef]
- Tilki, F. Infuence of acorn size and storage duration on moisture content, germination and survival of Quercus petraea (Mattuschka). J. Environ. Biol. 2010, 31, 325–328. [Google Scholar] [PubMed]
- Muñoz, A.; Bonal, R. Seed choice by rodents: Learning or inheritance? Behav. Ecol. Sociobiol. 2008, 62, 913–922. [Google Scholar] [CrossRef]
- Charnov, E.L. Optimal foraging, the marginal value theorem. Theor. Popul. Biol. 1976, 9, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Moles, A.T.; Westoby, M. Seedling survival and seed size: A synthesis of the literature. J. Ecol. 2004, 92, 372–383. [Google Scholar] [CrossRef]
- Espelta, J.M.; Bonal, R.; Sánchez-Humanes, B. Pre-dispersal acorn predation in mixed oak forests: Interspecific differences are driven by the interplay among seed phenology, seed size and predator size. J. Ecol. 2009, 97, 1416–1423. [Google Scholar] [CrossRef]
- Kelly, D.; Sork, V.L. Mast seeding in perennial plants: Why, how, where? Annu. Rev. Ecol. Syst. 2002, 33, 427–447. [Google Scholar] [CrossRef]
- Greenberg, C.H.; Zarnoch, S.J. A test of the predator satiation hypothesis, acorn predator size, and acorn preference. Can. J. For. Res. 2018, 48, 237–245. [Google Scholar] [CrossRef]
- Koenig, W.D.; Knops, J.M.H.; Carmen, W.J.; Stanback, M.T.; Mumme, R.L. Estimating acorn crops using visual surveys. Can. J. For. Res. 1994, 24, 2105–2112. [Google Scholar] [CrossRef]
- Gezici, S.; Sekeroglu, N. Neuroprotective potential and phytochemical composition of acorn fruits. Ind. Crops Prod. 2018, 128, 13–17. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Q.; Yang, N.; Chang, M.; Ge, Y.; Zhou, H.; Li, G. Traits variation of acorns and cupules during maturation process in Quercus variabilis and Quercus aliena. J. Plant Biochem. Physiol. 2023, 196, 531–541. [Google Scholar] [CrossRef]
- Sherry, R.A.; Zhou, X.; Gu, S.; Arnone, J.A.; Schimel, D.S.; Verburg, P.S.; Wallace, L.L.; Luo, Y. Divergence of reproductive phenology under climate warming. Proc. Natl. Acad. Sci. USA 2007, 104, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Hedhly, A.; Hormaza, J.I.; Herrero, M. Global warming and sexual plant reproduction. Trends Plant Sci. 2009, 14, 30–36. [Google Scholar] [CrossRef]
- Pérez-Ramos, I.M.; Ourcival, J.M.; Limousin, J.M.; Rambal, S. Mast seedling under increasing drought: Results from a long-term data set and from a rainfall exclusion experiment. Ecology 2010, 91, 3057–3068. [Google Scholar] [CrossRef]
- Koenig, W.D.; Knops, J.M.H.; Carmen, W.J.; Stanback, M.T.; Mumme, R.L. Acorn production by oaks in central coastal California: Influence of weather at three levels. Can. J. For. Res. 1996, 26, 1677–1683. [Google Scholar] [CrossRef]
- Garrison, B.A.; Koenig, W.D.; Knops, J.M.H. Spatial synchrony and temporal patterns in acorn production of California black oaks. In Proceedings of the 6th Symposium on Oak Woodlands: Today’s Challenges, Tomorrow’s Opportunities, Rohnert Park, CA, USA, 9–12 October 2006; Merenlender, A., McCreary, D., Purcell, K.L., Eds.; General Technical Report PSW-GTR-217. USDA Forest Service, Pacific SW Forest and Range Experiment Station: Albany, CA, USA, 2008; pp. 343–356. [Google Scholar]
- Pulido, F.J.; Díaz, M. Regeneration of a Mediterranean oak: A whole-cycle approach. Écoscience 2005, 12, 92–102. [Google Scholar] [CrossRef]
- Pulido, F.J.; García, E.; Obrador, J.J.; Moreno, G. Multiple pathways for tree regeneration in anthropogenic savannas: Incorporating biotic and abiotic drivers into management schemes. J. Appl. Ecol. 2010, 47, 1272–1281. [Google Scholar] [CrossRef]
- Wilbur, H.M. Propagule size, number, and dispersion pattern in Ambystoma and Asclepias. Am. Nat. 1977, 111, 43–68. [Google Scholar] [CrossRef]
- Alejano, R.; Vázquez-Piqué, J.; Carevic, F.; Fernández, M. Do ecological and silvicultural factors influence acorn mass in holm oak (southwestern Spain)? Agrofor. Syst. 2011, 83, 25–39. [Google Scholar] [CrossRef]
- Valero-Galván, G.J.; Valledor, L.; Navarro, R.M.; Gil, E.; Jorrín-Novo, J.V. Studies of variability in Holm oak (Quercus ilex subsp. ballota [Desf.] Samp.) through acorn protein profile analysis. J. Proteomics 2011, 74, 1244–1255. [Google Scholar] [CrossRef] [PubMed]
- Koenig, W.D.; Knops, J.M.H. Environmental correlates of acorn production by four species of Minnesota oaks. Popul. Ecol. 2014, 56, 63–71. [Google Scholar] [CrossRef]
- Hill, J.P.; Edwards, W.; Franks, P.J. Size is not everything for desiccation-sensitive seeds. J. Ecol. 2012, 100, 1131–1140. [Google Scholar] [CrossRef]
- Hamilton, K.N.; Offord, C.A.; Cuneo, P.; Deseo, M.A. A comparative study of seed morphology in relation to desiccation tolerance and other physiological responses in 71 Eastern Australian rainforest species. Plant Species Biol. 2013, 28, 51–62. [Google Scholar] [CrossRef]
- Wyse, S.V.; Dickie, J.B. Taxonomic affinity, habitat and seed mass strongly predict seed desiccation response: A boosted regression trees analysis based on 17 539 species. Ann. Bot. 2018, 121, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Ramírez Valiente, J.A.; Valladares, F.; Gilb, L.; Aranda, I. Population differences in juvenile survival under increasing drought are mediated by seed size in cork oak (Quercus suber L.). For. Ecol. Manag. 2009, 257, 1676–1683. [Google Scholar] [CrossRef]
- Xia, K.; Daws, M.I.; Hay, F.R.; Chen, W.Y.; Zhou, Z.K.; Pritchard, H.W. A comparative study of desiccation responses of seeds of Asian evergreen oaks, Quercus subgenus Cyclobalanopsis and Quercus subgenus Quercus. S. Afr. J. Bot. 2012, 78, 47–54. [Google Scholar] [CrossRef]
- Moles, A.T.; Ackerly, D.; Tweddle, J.C.; Dickie, J.B.; Smith, R.; Leishman, M.R.; Mayfield, M.M.; Pitman, A.; Wood, J.T.; Westoby, M. Global patterns in seed size. Glob. Ecol. Biogeogr. 2007, 16, 109–116. [Google Scholar] [CrossRef]
- Chen, X.; Kohyama, T.S. Variation among 91 stone oak species (Fagaceae, Lithocarpus) in fruit and vegetative morphology in relation to climatic factors. Flora 2022, 286, 151984. [Google Scholar] [CrossRef]
- Lepais, O.; Gerber, S. Reproductive patterns shape introgression dynamics and species succession within the European white oak species complex. Evolution 2011, 65, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Leroy, T.; Louvet, J.M.; Lalanne, C.; Le Provost, G.; Labadie, K.; Aury, J.M.; Delzon, S.; Plomion, C.; Kremer, A. Adaptive introgression as a driver of local adaptation to climate in European white oaks. New Phytol. 2020, 226, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Viscosi, V.; Antonecchia, G.; Lepais, O.; Fortini, P.; Gerber, S.; Loy, A. Leaf shape and size differentiation in white oaks: Assessment of allometric relationships among three sympatric species and their hybrids. Int. J. Plant Sci. 2012, 173, 875–884. [Google Scholar] [CrossRef]
- Lyu, J.; Song, J.; Liu, Y.; Wang, Y.; Li, J.; Du, F.K. Species boundaries between three sympatric oak species: Quercus aliena, Q. dentata, and Q. variabilis at the Northern edge of their distribution in China. Front. Plant Sci. 2018, 9, 414. [Google Scholar] [CrossRef] [PubMed]
- Kluge, N.; Sartini, C.; Sedinger, B.; Barringer, B.; Hygnstrom, S. A comparison of visual survey methods to estimate acorn production: A means of standardizing results. For. Ecol. Manag. 2022, 520, 120418. [Google Scholar] [CrossRef]
- Tilki, F.; Alptekin, C.U. Variation in acorn characteristics in three provenances of Quercus aucheri Jaub. et Spach and provenance, temperature and storage effects on acorn germination. Seed Sci. Technol. 2005, 33, 441–447. [Google Scholar] [CrossRef]
- Warton, D.I.; Hui, F.K.C. The arcsine is asinine: The analysis of proportions in ecology. Ecology 2011, 92, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Valladares, F.; Wright, S.J.; Lass, E.; Kitajima, K.; Pearcy, R.W. Plastic phenotypic response to light of 16 rainforests shrubs (Psychotria) differing in shade tolerance. Ecology 2000, 81, 1925–1936. [Google Scholar] [CrossRef]
- López, R.; Climent, J.; Gil, L. Intraspecific variation and plasticity in growth and foliar morphology along a climate gradient in the Canary Island pine. Trees 2010, 24, 343–350. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).