Endophytes in Cannabis sativa: Identifying and Characterizing Microbes with Beneficial and Detrimental Effects on Plant Health
Abstract
1. Introduction
2. Isolation and Identification of Endophytes in Cannabis and Hemp Plants
3. Recovery of Microbes from Cannabis and Hemp Plants
3.1. Effect of Geographic Origin and Lineage on Microbial Diversity
3.2. Effect of Growing Substrate on Microbial Diversity
3.3. Influence of Plant Organ Tissues on Microbial Diversity
3.4. Impact of Endophytes in the Root Zone on Plant Development
4. Influence of Bacterial and Fungal Endophytes on Plant Growth
4.1. Beneficial Bacterial Endophytes
4.2. Beneficial Fungal Endophytes
4.3. Detrimental Bacterial Endophytes
4.4. Detrimental Fungal Endophytes
5. Analysis of Cannabis Endophytes Using Whole Genome Sequencing
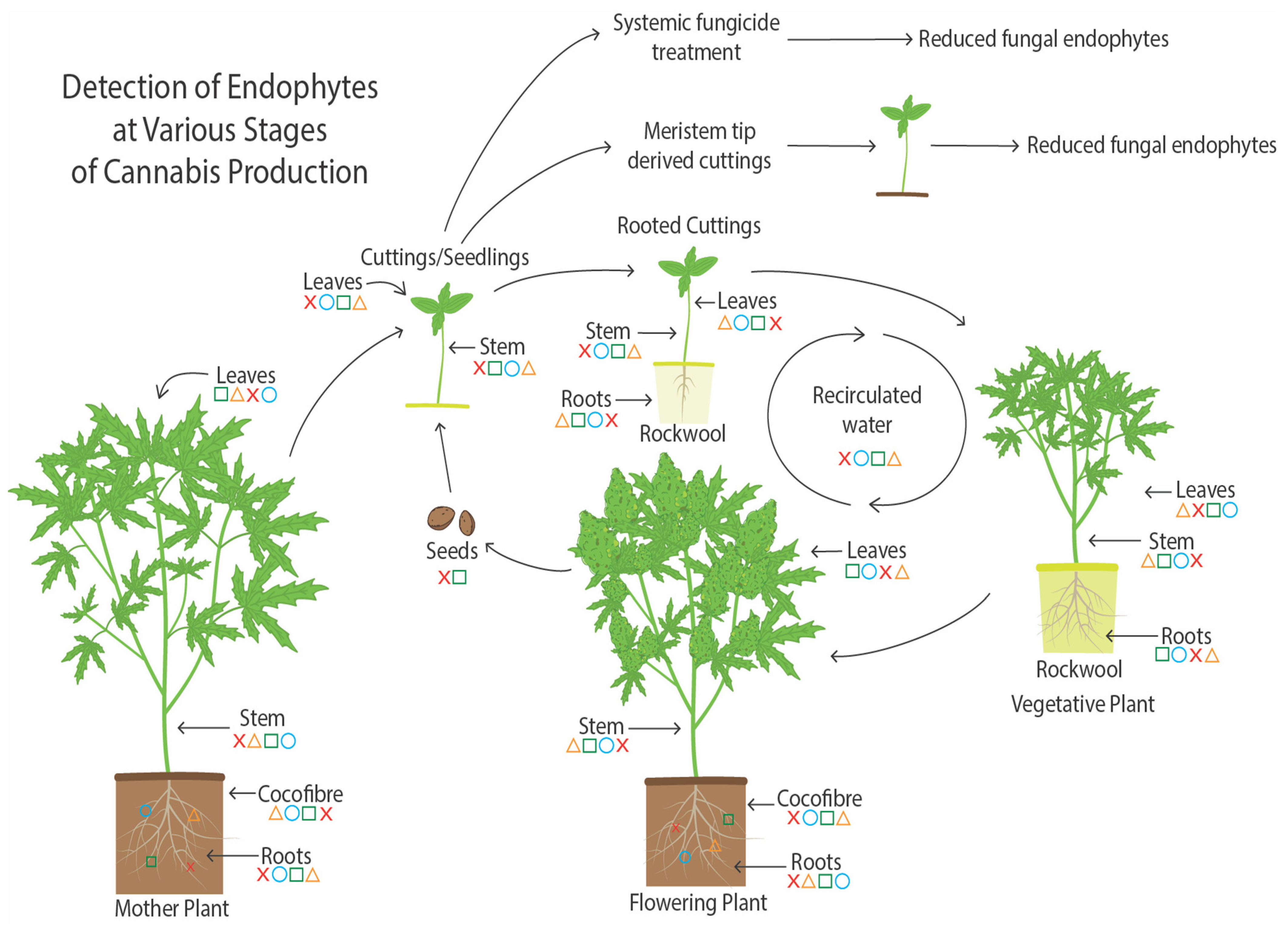
6. The Endophytes in Cannabis Cuttings
7. Determining Endophyte Presence in Cannabis Tissues Using Scanning Electron Microscopy
8. Future Research
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Long, T.; Wagner, M.; Demske, D.; Leipe, C.; Tarasov, P.E. Cannabis in Eurasia: Origin of human use and Bronze Age trans-continental connections. Veg. Hist. Archaeobot. 2017, 26, 245–258. [Google Scholar] [CrossRef]
- Small, E. Classification of Cannabis sativa L. in relation to agricultural, biotechnological, medical and recreational utilization. In Cannabis sativa L.—Botany and Biotechnology; Chandra, S., Lata, H., ElSohly, M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–63. [Google Scholar]
- Li, H.L. An archaeological and historical account of cannabis in China. Econ. Bot. 1973, 28, 437–448. [Google Scholar] [CrossRef]
- Fleming, M.P.; Clarke, R.C. Physical evidence for the antiquity of Cannabis sativa L. (Cannabaceae). J. Int. Hemp Assoc. 1998, 5, 80–92. [Google Scholar]
- Chandra, S.; Lata, H.; ElSohly, M.A.; Walker, L.A.; Potter, D. Cannabis cultivation: Methodological issues for obtaining medical-grade product. Epilepsy Behav. 2017, 70, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Stasiak, M.; Dixon, M. Growing facilities and environmental control. In Handbook of Cannabis Production in Controlled Environments, 1st ed.; Zheng, Y., Ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 13–40. [Google Scholar]
- Fleming, H.; Chamberlain, Z.; Zager, J.J.; Lange, B.M. Controlled environments for cannabis cultivation to support “omics” research studies and production. Methods Enzymol. 2023, 680, 353–380. [Google Scholar]
- Buirs, L.; Punja, Z.K. Integrated management of pathogens and microbes in Cannabis sativa L. under greenhouse conditions. Plants 2024, 13, 786. [Google Scholar] [CrossRef]
- Turner, T.R.; James, E.K.; Poole, P.S. The plant microbiome. Genome Biol. 2013, 14, 209. [Google Scholar] [CrossRef]
- Bacon, C.W.; White, J.F. Functions, mechanisms and regulation of endophytic and epiphytic microbial communities of plants. Symbiosis 2016, 68, 87–98. [Google Scholar] [CrossRef]
- Kumar, J.; Singh, D.; Ghosh, P.; Kumar, A. Endophytic and epiphytic modes of microbial interactions and benefits. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Singh, D., Singh, H., Prabha, R., Eds.; Springer: Singapore, 2017; pp. 227–253. [Google Scholar]
- Barnett, S.E.; Cala, A.R.; Hansen, J.L.; Crawford, J.; Viands, D.R.; Smart, L.B. Evaluating the microbiome of hemp. Phytobiomes J. 2020, 4, 351–363. [Google Scholar] [CrossRef]
- Comeau, D.; Novinscak, A.; Joly, D.L.; Filion, M. Spatio-temporal and cultivar-dependent variations in the cannabis microbiome. Front. Microbiol. 2020, 11, 491. [Google Scholar] [CrossRef]
- Gwinn, K.D.; Leung, M.C.K.; Stephens, A.B.; Punja, Z.K. Fungal and mycotoxin contaminants in cannabis and hemp flowers: Implications for consumer health and directions for further research. Front. Microbiol. 2023, 14, 1278189. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Deepa, N.; Trivedi, P.K.; Singh, B.K.; Srivastava, V.; Singh, A. Plants and endophytes interaction: A “secret wedlock” for sustainable biosynthesis of pharmaceutically important secondary metabolites. Microb. Cell Fact. 2023, 22, 226. [Google Scholar] [CrossRef]
- Kusari, P.; Kusari, S.; Spiteller, M.; Kayser, O. Endophytic fungi harbored in Cannabis sativa L.: Diversity and potential as bio-control agents against host plant-specific phytopathogens. Fungal Divers. 2013, 60, 137–151. [Google Scholar] [CrossRef]
- Punja, Z.K.; Collyer, D.; Scott, C.; Lung, S.; Holmes, J.; Sutton, D. Pathogens and molds affecting production and quality of Cannabis sativa L. Front. Plant Sci. 2019, 10, 1120. [Google Scholar] [CrossRef] [PubMed]
- Punja, Z.K.; Scott, C. Organically grown cannabis (Cannabis sativa L.) plants contain a diverse range of culturable epiphytic and endophytic fungi in inflorescences and stem tissues. Botany 2023, 101, 255–269. [Google Scholar] [CrossRef]
- Gautam, A.K.; Kant, M.; Thakur, Y. Isolation of endophytic fungi from Cannabis sativa and study their antifungal potential. Arch. Phytopathol. Plant Prot. 2013, 46, 627–635. [Google Scholar] [CrossRef]
- Scott, M.; Rani, M.; Samsatly, J.; Charron, J.-B.; Jabaji, S. Endophytes of industrial hemp (Cannabis sativa L.) cultivars: Identification of culturable bacteria and fungi in leaves, petioles, and seeds. Can. J. Microbiol. 2018, 64, 664–680. [Google Scholar] [CrossRef]
- Dumigan, C.R.; Deyholos, M.K. Cannabis seedlings inherit seed-borne bioactive and anti-fungal endophytic Bacilli. Plants 2022, 11, 2127. [Google Scholar] [CrossRef]
- Scott, C.; Punja, Z.K. Biological control of Fusarium oxysporum causing damping-off and Pythium myriotylum causing root and crown rot on cannabis (Cannabis sativa L.) plants. Can. J. Plant Pathol. 2023, 45, 238–252. [Google Scholar] [CrossRef]
- Kusari, P.; Spiteller, M.; Kayser, O.; Kusari, S. Recent advances in research on Cannabis sativa L. endophytes and their prospect for the pharmaceutical industry. In Microbial Diversity and Biotechnology in Food Security; Kharwar, R., Upadhyay, R., Dubey, N., Raghuwanshi, R., Eds.; Springer: New Delhi, India, 2014; pp. 3–15. [Google Scholar]
- Steen, A.D.; Carini, A.C.P.; Lloyd, K.G.; Thrash, J.C.; Deangelis, K.M.; Fierer, N. High proportions of bacteria and archaea across most biomes remain uncultured. ISME J. 2019, 13, 3126–3130. [Google Scholar] [CrossRef]
- Lobato, C.; de Freitas, J.M.; Habich, D.; Kögl, I.; Berg, G.; Cernava, T. Wild again: Recovery of a beneficial cannabis seed endophyte from low domestication genotypes. Microbiome 2024, 12, 239. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, M.; Vitali, F.; Chelucci, E.; Chiellini, C. Characterization of the cultivable endophytic bacterial community of seeds and sprouts of Cannabis sativa L. and perspectives for the application as biostimulants. Microorganisms 2022, 10, 1742. [Google Scholar] [CrossRef] [PubMed]
- Afzal, I.; Shinwari, Z.K.; Iqrar, I. Selective isolation and characterization of agriculturally beneficial endophytic bacteria from wild hemp using canola. Pak. J. Bot. 2015, 47, 1999–2008. [Google Scholar]
- Ahmed, B.; Beneš, F.; Hajšlová, J.; Fišarová, L.; Vosátka, M.; Hijri, M. Enhanced production of select phytocannabinoids in medical Cannabis cultivars using microbial consortia. Front. Plant Sci. 2023, 14, 1219836. [Google Scholar] [CrossRef]
- Qadri, M.; Johri, S.; Shah, B.A.; Khajuria, A.; Sidiq, T.; Lattoo, S.K.; Abdin, M.Z.; Riyaz-Ul-Hassan, S. Identification and bioactive potential of endophytic fungi isolated from selected plants of the Western Himalayas. SpringerPlus 2013, 2, 8. [Google Scholar] [CrossRef]
- Kusari, P.; Kusari, S.; Lamshöft, M.; Sezgin, S.; Spiteller, M.; Kayser, O. Quorum quenching is an antivirulence strategy employed by endophytic bacteria. Appl. Microbiol. Biotechnol. 2014, 98, 7173–7183. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.K. Diversity of fungal endophytes in some medicinal plants of Himachal Pradesh, India. Arch. Phytopathol. Plant Prot. 2014, 47, 537–544. [Google Scholar] [CrossRef]
- Punja, Z.K.; Ni, L.; Lung, S.; Buirs, L. Total yeast and mold levels in high THC-containing cannabis (Cannabis sativa L.) inflorescences are influenced by genotype, environment, and pre-and post-harvest handling practices. Front. Microbiol. 2023, 14, 1192035. [Google Scholar] [CrossRef]
- Nelson, E.B. The seed microbiome: Origins, interactions, and impacts. Plant Soil 2018, 422, 7–34. [Google Scholar] [CrossRef]
- Srivastava, A.; Sharbel, T.; Vujanovic, V. Exploring the role of endophytes in Cannabis sativa L. polyploidy and agricultural trait improvement. Int. J. Plant Biol. 2024, 15, 1118–1140. [Google Scholar] [CrossRef]
- Dumigan, C.R.; Deyholos, M.K. Soil and seed both influence bacterial diversity in the microbiome of the Cannabis sativa seedling endosphere. Front. Plant Sci. 2024, 15, 1326294. [Google Scholar] [CrossRef]
- Thomas, B.O.; Lechner, S.L.; Ross, H.C.; Joris, B.R.; Glick, B.R.; Stegelmeier, A.A. Friends and foes: Bacteria of the hydroponic plant microbiome. Plants 2024, 13, 3069. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Ning, K.; Zhang, G.; Yu, H.; Yang, S.; Dai, F.; Dong, L.; Chen, S. Compartment niche shapes the assembly and network of Cannabis sativa-associated microbiome. Front. Microbiol. 2021, 12, 714993. [Google Scholar] [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Babalola, O.O. Exploring the potentialities of beneficial endophytes for improved plant growth. Saudi J. Biol. Sci. 2020, 27, 3622–3633. [Google Scholar] [CrossRef] [PubMed]
- Avis, T.J.; Gravel, V.; Antoun, H.; Tweddell, R.J. Multifaceted beneficial effects of rhizosphere microorganisms on plant health and productivity. Soil Biol. Biochem. 2008, 40, 1733–1740. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Babalola, O.O. The rhizosphere microbial complex in plant health: A review of interaction dynamics. J. Integr. Agric. 2022, 21, 2168–2182. [Google Scholar] [CrossRef]
- El-Shatnawi, M.K.J.; Makhadmeh, I.M. Ecophysiology of the plant-rhizosphere system. J. Agron. Crop Sci. 2001, 187, 1–9. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, M.; Verma, S.; Choudhary, P.; Chakdar, H.; Varma, A.; Prasad, R.; Tripathi, S.; Prasad, R.; Tripathi, S.; et al. Plant microbiome: Trends and prospects for sustainable agriculture. In Plant Microbe Symbiosis; Varma, A., Tripathi, S., Prasad, R., Eds.; Springer International Publishing AG: Cham, Switzerland, 2020; pp. 129–151. [Google Scholar]
- Wang, N.R.; Haney, C.H. Harnessing the genetic potential of the plant microbiome. Biochemist 2020, 42, 20–25. [Google Scholar] [CrossRef]
- Moreira, Z.M.; Haney, C.H. Plant genetic regulation of the microbiome and applications for Canadian agriculture. Can. J. Plant Pathol. 2024, 46, 546–553. [Google Scholar] [CrossRef]
- Parray, J.A.; Jan, S.; Kamili, A.N.; Qadri, R.A.; Egamberdieva, D.; Ahmad, P. Current perspectives on plant growth-promoting rhizobacteria. J. Plant Growth Regul. 2016, 35, 877–902. [Google Scholar] [CrossRef]
- Conant, R.T.; Walsh, R.P.; Walsh, M.; Bell, C.W.; Wallenstein, M.D. Effects of a microbial biostimulant, Mammoth PTM, on Cannabis sativa bud yield. J. Hortic. 2017, 4, 191. [Google Scholar] [CrossRef]
- Pagnani, G.; Pellegrini, M.; Galieni, A.; D’Egidio, S.; Matteucci, F.; Ricci, A.; Stagnari, F.; Sergi, M.; Lo Sterzo, C.; Pisante, M.; et al. Plant growth-promoting rhizobacteria (PGPR) in Cannabis sativa ‘Finola’ cultivation: An alternative fertilization strategy to improve plant growth and quality characteristics. Ind. Crops Prod. 2018, 123, 75–83. [Google Scholar] [CrossRef]
- Comeau, D.; Balthazar, C.; Novinscak, A.; Bouhamdani, N.; Joly, D.L.; Filion, M. Interactions between Bacillus spp., Pseudomonas spp. and Cannabis sativa promote plant growth. Front. Microbiol. 2021, 12, 715758. [Google Scholar] [CrossRef]
- Chitnis, V.R.; Suryanarayanan, T.S.; Nataraja, K.N.; Prasad, S.R.; Oelmüller, R.; Shaanker, R.U. Fungal endophyte-mediated crop improvement: The way ahead. Front. Plant Sci. 2020, 11, 561007. [Google Scholar] [CrossRef] [PubMed]
- Bohra, N.; Singh, R.K.; Jain, R.; Sharma, L.; Sathyanarayana, E.; Quiroz-Figueroa, F.R.; Rajput, V.D.; Singh, A.K.; Kumar, P.; Shukla, A.K.; et al. Endophytic bacteria for plant growth promotion. In Bacterial Endophytes for Sustainable Agriculture and Environmental Management; Springer: Singapore, 2022; pp. 187–199. [Google Scholar]
- Rani, S.; Kumar, P.; Dahiya, P.; Maheshwari, R.; Dang, A.S.; Suneja, P. Endophytism: A multidimensional approach to plant-prokaryotic microbe interaction. Front. Microbiol. 2022, 13, 861235. [Google Scholar] [CrossRef]
- Balthazar, C.; Cantin, G.; Novinscak, A.; Joly, D.L.; Filion, M. Expression of putative defense responses in cannabis primed by Pseudomonas and/or Bacillus strains and infected by Botrytis cinerea. Front. Plant Sci. 2020, 11, 572112. [Google Scholar] [CrossRef]
- Iqrar, I.; Numan, M.; Khan, T.; Shinwari, Z.K.; Ali, G.S. LC–MS/MS-based profiling of bioactive metabolites of endophytic bacteria from Cannabis sativa and their anti-Phytophthora activity. Antonie Van Leeuwenhoek 2021, 114, 1165–1179. [Google Scholar] [CrossRef]
- Pellegrini, M.; Ercole, C.; Gianchino, C.; Bernardi, M.; Pace, L.; Del Gallo, M. Fusarium oxysporum f. sp. cannabis isolated from Cannabis sativa L.: In vitro and in planta biocontrol by a plant growth promoting-bacteria consortium. Plants 2021, 10, 2436. [Google Scholar]
- Balthazar, C.; Novinscak, A.; Cantin, G.; Joly, D.L.; Filion, M. Biocontrol activity of Bacillus spp. and Pseudomonas spp. against Botrytis cinerea and other cannabis fungal pathogens. Phytopathology 2022, 112, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Cavallero, A.; Chelucci, E.; Chiellini, C.; Gabriele, M. Exploring microalgae and endophytes as biostimulants: Antioxidant and anti-inflammatory properties of Cannabis sativa L. sprouts under standard and enrichment conditions. Food Biosci. 2024, 62, 105138. [Google Scholar] [CrossRef]
- Wang, T.; Li, W.; Wang, F.; Li, J.; Qin, J.; Song, Z.; Xu, J.; Qiu, H.; Cheng, Y. Biocontrol potential of Bacillus velezensis SEC-024A against southern blight of industrial hemp. Ind. Crops Prod. 2024, 222, 119767. [Google Scholar] [CrossRef]
- Kakabouki, I.; Mavroeidis, A.; Tataridas, A.; Kousta, A.; Efthimiadou, A.; Karydogianni, S.; Katsenios, N.; Roussis, I.; Papastylianou, P. Effect of Rhizophagus irregularis on growth and quality of Cannabis sativa seedlings. Plants 2021, 10, 1333. [Google Scholar] [CrossRef]
- Seemakram, W.; Paluka, J.; Suebrasri, T.; Lapjit, C.; Kanokmedhakul, S.; Kuyper, T.W.; Ekprasert, J.; Boonlue, S. Enhancement of growth and cannabinoids content of hemp (Cannabis sativa) using arbuscular mycorrhizal fungi. Front. Plant Sci. 2022, 13, 845794. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, S.M.; Injamum-Ul-Hoque, M.; Das, A.K.; Imran, M.; Tavakoli, S.; Kwon, D.B.; Kang, S.-M.; Lee, I.-J.; Choi, H.W. Biological guardians: Unveiling microbial solutions to combat Cannabis sativa fungal pathogens. Stresses 2025, 5, 16. [Google Scholar] [CrossRef]
- Lyu, D.; Backer, R.; Robinson, W.G.; Smith, D.L. Plant growth-promoting rhizobacteria for cannabis production: Yield, cannabinoid profile and disease resistance. Front. Microbiol. 2019, 10, 1761. [Google Scholar] [CrossRef]
- Taghinasab, M.; Jabaji, S. Cannabis microbiome and the role of endophytes in modulating the production of secondary metabolites: An overview. Microorganisms 2020, 8, 355. [Google Scholar] [CrossRef] [PubMed]
- Eljounaidi, K.; Lee, S.K.; Bae, H. Bacterial endophytes as potential biocontrol agents of vascular wilt diseases—Review and future prospects. Biol. Control 2016, 103, 62–68. [Google Scholar] [CrossRef]
- Whipps, J.M. Microbial interactions and biocontrol in the rhizosphere. J. Exp. Bot. 2001, 52, 487–511. [Google Scholar] [CrossRef]
- Lubna; Asaf, S.; Khan, A.L.; Waqas, M.; Kang, S.-M.; Hamayun, M.; Lee, I.-J.; Hussain, A. Growth-promoting bioactivities of Bipolaris sp. CSL-1 isolated from Cannabis sativa suggest a distinctive role in modifying host plant phenotypic plasticity and functions. Acta Physiol. Plant 2019, 41, 65. [Google Scholar] [CrossRef]
- Kusari, P.; Kusari, S.; Spiteller, M.; Kayser, O. Cannabis endophytes and their application in breeding and physiological fitness. In Cannabis sativa L.—Botany and Biotechnology; Chandra, S., Lata, H., ElSohly, M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 419–437. [Google Scholar]
- Morelli, M.; Bahar, O.; Papadopoulou, K.K.; Hopkins, D.L.; Obradović, A. Editorial: Role of endophytes in plant health and defense against pathogens. Front. Plant Sci. 2020, 11, 1312. [Google Scholar] [CrossRef] [PubMed]
- Busby, P.E.; Ridout, M.; Newcombe, G. Fungal endophytes: Modifiers of plant disease. Plant Mol. Biol. 2016, 90, 645–655. [Google Scholar] [CrossRef] [PubMed]
- De Silva, N.I.; Brooks, S.; Lumyong, S.; Hyde, K. Use of endophytes as biocontrol agents. Fungal Biol. Rev. 2019, 33, 133–148. [Google Scholar] [CrossRef]
- Gupta, R.; Keppanan, R.; Leibman-Markus, M.; Rav-David, D.; Elad, Y.; Ment, D.; Bar, M. The entomopathogenic fungi Metarhizium brunneum and Beauveria bassiana promote systemic immunity and confer resistance to a broad range of pests and pathogens in tomato. Phytopathology 2022, 112, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Jaber, L.R.; Alananbeh, K.M. Fungal entomopathogens as endophytes reduce several species of Fusarium causing crown and root rot in sweet pepper (Capsicum annuum L.). Biol. Control 2018, 126, 117–126. [Google Scholar] [CrossRef]
- Poppeliers, S.W.; Sánchez-Gil, J.J.; de Jonge, R. Microbes to support plant health: Understanding bioinoculant success in complex conditions. Curr. Opin. Microbiol. 2023, 73, 102286. [Google Scholar] [CrossRef]
- Moshe, M.; Frenkel, O.; Sela, N.; Davidovich, C.; Amutuhaire, H.; Banin, E.; Cytryn, E. Persistence and microbiome modification in Rhizoctonia solani-inoculated rhizosphere following amendment with a Bacillus biocontrol agent. Phytobiomes J. 2024, 8, 611–620. [Google Scholar] [CrossRef]
- Holmes, J.E.; Sanghera, H.; Punja, Z.K. Crown gall development on cannabis (Cannabis sativa L., marijuana) plants caused by Agrobacterium tumefaciens species-complex. Can. J. Plant Pathol. 2023, 45, 433–445. [Google Scholar] [CrossRef]
- McPartland, J.M. A review of cannabis diseases. J. Int. Hemp Assoc. 1996, 3, 19–23. [Google Scholar]
- Mahmoud, M.; Benrejeb, I.; Punja, Z.K.; Buirs, L.; Jabaji, S. Understanding bud rot development, caused by Botrytis cinerea, on cannabis (Cannabis sativa L.) plants grown under greenhouse conditions. Botany 2023, 101, 200–231. [Google Scholar] [CrossRef]
- Priyashantha, A.K.H.; Karunarathna, S.C.; Lu, L.; Tibpromma, S. Fungal endophytes: An alternative biocontrol agent against phytopathogenic fungi. Encyclopedia 2023, 3, 759–780. [Google Scholar] [CrossRef]
- Scott, C.; Punja, Z. Management of diseases on cannabis in controlled environment production. In Handbook of Cannabis Production in Controlled Environments, 1st ed.; Zheng, Y., Ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 213–252. [Google Scholar]
- Punja, Z.K. Epidemiology of Fusarium oxysporum causing root and crown rot of cannabis (Cannabis sativa L., marijuana) plants in commercial greenhouse production. Can. J. Plant Pathol. 2021, 43, 216–235. [Google Scholar] [CrossRef]
- McKernan, K.; Spangler, J.; Zhang, L.; Tadigotla, V.; Helbert, Y.; Foss, T.; Smith, D.R. Cannabis microbiome sequencing reveals several mycotoxic fungi native to dispensary grade Cannabis flowers. F1000Research 2016, 2, 1422. [Google Scholar] [CrossRef] [PubMed]
- Hazekamp, A. Evaluating the effects of gamma-irradiation for decontamination of medicinal cannabis. Front. Pharmacol. 2016, 7, 108. [Google Scholar] [CrossRef]
- Jerushalmi, S.; Maymon, M.; Dombrovsky, A.; Freeman, S. Effects of cold plasma, gamma and e-beam irradiations on reduction of fungal colony forming unit levels in medical cannabis inflorescences. J. Cannabis Res. 2020, 2, 12. [Google Scholar] [CrossRef]
- Punja, Z.K. Emerging diseases of Cannabis sativa and sustainable management. Pest. Manag. Sci. 2021, 77, 3857–3870. [Google Scholar] [CrossRef]
- Majumdar, C.G.; ElSohly, M.A.; Ibrahim, E.A.; Elhendawy, M.A.; Stanford, D.; Chandra, S.; Wanas, A.S.; Radwan, M.M. Effect of gamma irradiation on cannabinoid, terpene, and moisture content of cannabis biomass. Molecules 2023, 28, 7710. [Google Scholar] [CrossRef]
- Adhikary, D.; Kulkarni, M.; El-Mezawy, A.; Mobini, S.; Elhiti, M.; Gjuric, R.; Ray, A.; Polowick, P.; Slaski, J.J.; Jones, M.P.; et al. Medical cannabis and industrial hemp tissue culture: Present status and future potential. Front. Plant Sci. 2021, 12, 627240. [Google Scholar] [CrossRef]
- Holmes, J.E.; Lung, S.; Collyer, D.; Punja, Z.K. Variables affecting shoot growth and plantlet recovery in tissue cultures of drug-type Cannabis sativa L. Front. Plant Sci. 2021, 12, 732344. [Google Scholar] [CrossRef]
- Monthony, A.S.; Page, S.R.; Hesami, M.; Jones, A.M.P. The past, present, and future of Cannabis sativa tissue culture. Plants 2021, 10, 185. [Google Scholar] [CrossRef]
- Jones, M.; Monthony, A.S. Cannabis propagation. In Handbook of Cannabis Production in Controlled Environments; Zheng, Y., Ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 41–90. [Google Scholar]
- Navi, S.S.; Yang, X.B.; Sharma, P.; Sharma, A.K. Use of Trichoderma in the management of diseases in North American row crops. In Trichoderma—Host-Pathogen Interactions and Applications; Sharma, A., Sharma, P., Eds.; Springer: Singapore, 2020; pp. 187–204. [Google Scholar]
- Tõlgo, M.; Hüttner, S.; Rugbjerg, P.; Thuy, N.T.; Thanh, V.N.; Larsbrink, J.; Olsson, L. Genomic and transcriptomic analysis of the thermophilic lignocellulose-degrading fungus Thielavia terrestris LPH172. Biotechnol. Biofuels 2021, 14, 131. [Google Scholar] [CrossRef] [PubMed]
- Kokkoris, V.; Banchini, C.; Paré, L.; Abdellatif, L.; Séguin, S.; Hubbard, K.; Findlay, W.; Dalpé, Y.; Dettman, J.; Corradi, N.; et al. Rhizophagus irregularis, the model fungus in arbuscular mycorrhiza research, forms dimorphic spores. New Phytol. 2024, 242, 1771–1784. [Google Scholar] [CrossRef]
- Houbraken, J.; Frisvad, J.C.; Samson, R.A. Fleming’s penicillin producing strain is not Penicillium chrysogenum but Prubens. IMA Fungus 2011, 2, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.A.; Hadlok, R.; Stolk, A.C. A taxonomic study of the Penicillium chrysogenum series. Antonie Van Leeuwenhoek 1977, 43, 169–175. [Google Scholar] [CrossRef]
- Fierro, F.; Vaca, I.; Castillo, N.I.; García-Rico, R.O.; Chávez, R. Penicillium chrysogenum, a vintage model with a cutting-edge profile in biotechnology. Microorganisms 2022, 10, 573. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-K.; Miao, C.-P.; Chen, H.-H.; Huang, F.-F.; Xia, Y.-M.; Chen, Y.-W.; Zhao, L.-X. Endophytic fungi harbored in Panax notoginseng: Diversity and potential as biological control agents against host plant pathogens of root-rot disease. J. Ginseng Res. 2017, 41, 353–360. [Google Scholar] [CrossRef]
- Li, Y.; Jiao, M.; Li, Y.; Zhong, Y.; Li, X.; Chen, Z.; Chen, S.; Wang, J.; Vissenberg, K. Penicillium chrysogenum polypeptide extract protects tobacco plants from tobacco mosaic virus infection through modulation of ABA biosynthesis and callose priming. J. Exp. Bot. 2021, 72, 3526–3539. [Google Scholar] [CrossRef]
- Pacios-Michelena, S.; Valdés, R.A.; Saldaña-Mendoza, S.A.; Tucuch-Pérez, M.A.; González, C.N.A.; Alvarez-Perez, O.B.; Ilina, A. Encapsulation and application of cell-free extracts produced by Penicillium chrysogenum R1 in tomato plants for the control of Fusarium oxysporum. Environ. Qual. Manag. 2024, 34, 22182. [Google Scholar] [CrossRef]
- Dong, H.; Cohen, Y. Dry mycelium of Penicillium chrysogenum induces resistance against verticillium wilt and enhances growth of cotton plants. Phytoparasitica 2002, 30, 147–157. [Google Scholar] [CrossRef]
- Dawood, M.; Hussein, N.; Ismail, M.; El-Khatib, A.; Ragaey, M. Improvement of germination, phosphate efficiency, antioxidants, metabolic products, and yield of wheat plants by Aspergillus niger and Penicillium chrysogenum. Egypt. J. Bot. 2022, 62, 709–738. [Google Scholar] [CrossRef]
- Galeano, R.M.S.; Silva, S.M.; Yonekawa, M.K.A.; de Alencar Guimarães, N.C.; Giannesi, G.C.; Masui, D.C.; Corrêa, B.O.; da Silva Brasil, M.; Zanoelo, F.F. Penicillium chrysogenum strain 34-P promotes plant growth and improves initial development of maize under saline conditions. Rhizosphere 2023, 26, 100710. [Google Scholar] [CrossRef]
- Khan, S.A.; Hamayun, M.; Yoon, H.; Kim, H.Y.; Suh, S.J.; Hwang, S.K.; Kim, J.-M.; Lee, I.-J.; Choo, Y.-S.; Yoon, U.-H.; et al. Plant growth promotion and Penicillium citrinum. BMC Microbiol. 2008, 8, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Sreevidya, M.; Gopalakrishnan, S.; Melø, T.M.; Simic, N.; Bruheim, P.; Sharma, M.; Srinivas, V.; Alekhya, G. Biological control of Botrytis cinerea and plant growth promotion potential by Penicillium citrinum in chickpea (Cicer arietinum L.). Biocontrol Sci. Technol. 2015, 25, 739–755. [Google Scholar] [CrossRef]
- Waqas, M.; Khan, A.L.; Hamayun, M.; Shahzad, R.; Kang, S.-M.; Kim, J.-G.; Lee, I.-J. Endophytic fungi promote plant growth and mitigate the adverse effects of stem rot: An example of Penicillium citrinum and Aspergillus terreus. J. Plant Interact. 2015, 10, 280–287. [Google Scholar] [CrossRef]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; Pascale, S.D.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. The application of arbuscular mycorrhizal fungi as microbial biostimulant, sustainable approaches in modern agriculture. Plants 2023, 12, 3101. [Google Scholar] [CrossRef]
- Punja, Z.K.; Scott, C.; Tso, H.H.; Munz, J.; Buirs, L. Transmission, spread, longevity and management of hop latent viroid, a widespread and destructive pathogen affecting cannabis (Cannabis sativa L.) plants in North America. Plants 2025, 14, 830. [Google Scholar] [CrossRef]
- Punja, Z.K.; Sutton, D.B.; Kim, T. Glandular trichome development, morphology, and maturation are influenced by plant age and genotype in high THC-containing cannabis (Cannabis sativa L.) inflorescences. J. Cannabis Res. 2023, 5, 12. [Google Scholar] [CrossRef]
- Choat, B.; Cobb, A.R.; Jansen, S. Structure and function of bordered pits: New discoveries and impacts on whole-plant hydraulic function. New Phytol. 2008, 177, 608–626. [Google Scholar] [CrossRef]
- Knox, C.A.P.; Smith, R.H. A scanning electron microscope study of healthy pecan tissues showing the presence or absence of internal fungal contamination. In Vitro 1980, 16, 651–654. [Google Scholar] [CrossRef]
- Blanchette, R.A. Wood decomposition by Phellinus (Fomes) pini: A scanning electron microscopy study. Can. J. Bot. 1980, 58, 1496–1503. [Google Scholar] [CrossRef]
- Khan, M.I.; Pandith, S.A.; Shah, M.A.; Reshi, Z.A. Calcium oxalate crystals, the plant ‘gemstones’: Insights into their synthesis and physiological implications in plants. Plant Cell Physiol. 2023, 64, 1124–1138. [Google Scholar] [CrossRef]
- Konyar, S.T.; Öztürk, N.; Dane, F. Occurrence, types and distribution of calcium oxalate crystals in leaves and stems of some species of poisonous plants. Bot. Stud. 2014, 55, 32. [Google Scholar] [CrossRef] [PubMed]
- De Kreij, C.; Janse, J.; Van Goor, B.J.; Van Doesburg, J.D.J. The incidence of calcium oxalate crystals in fruit walls of tomato (Lycopersicon esculentum Mill.) as affected by humidity, phosphate and calcium supply. J. Hortic. Sci. 1992, 67, 45–50. [Google Scholar] [CrossRef]
- Hudgins, J.W.; Krekling, T.; Franceschi, V.R. Distribution of calcium oxalate crystals in the secondary phloem of conifers: A constitutive defense mechanism? New Phytol. 2003, 159, 677–690. [Google Scholar] [CrossRef]
- Macnish, A.J.; Irving, D.E.; Joyce, D.C.; Vithanage, V.; Wearing, A.H.; Webb, R.I.; Frost, R.L. Identification of intracellular calcium oxalate crystals in Chamelaucium uncinatum (Myrtaceae). Aust. J. Bot. 2003, 51, 565–572. [Google Scholar] [CrossRef]
- Schulz, B.; Boyle, C.S.; Barbara, J.E.; Christine, J.C.; Sieber, T.N. What are endophytes. In Microbial Root Endophytes; Schulz, B., Boyle, C.S., Sieber, T.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 9, pp. 1–13. [Google Scholar]

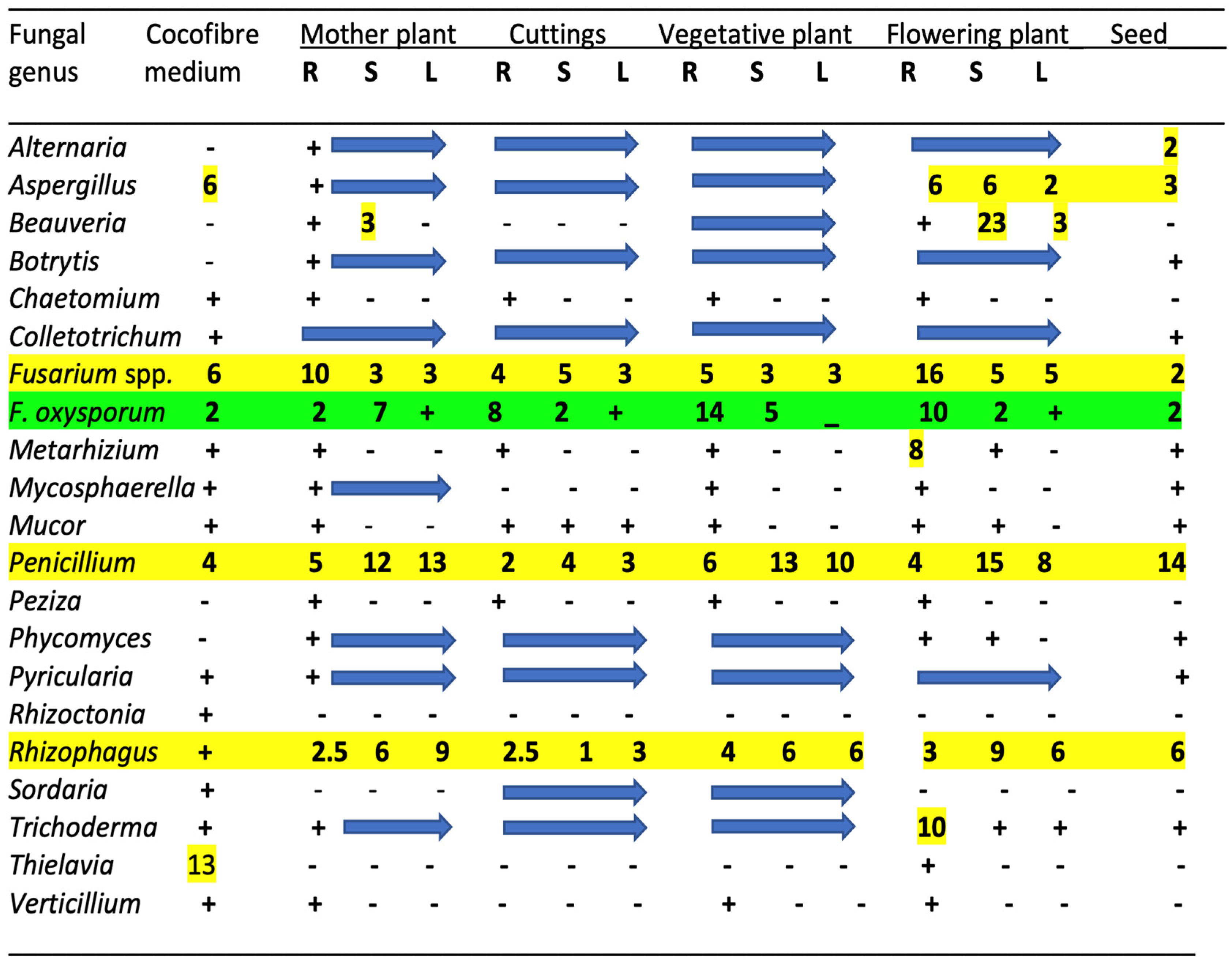
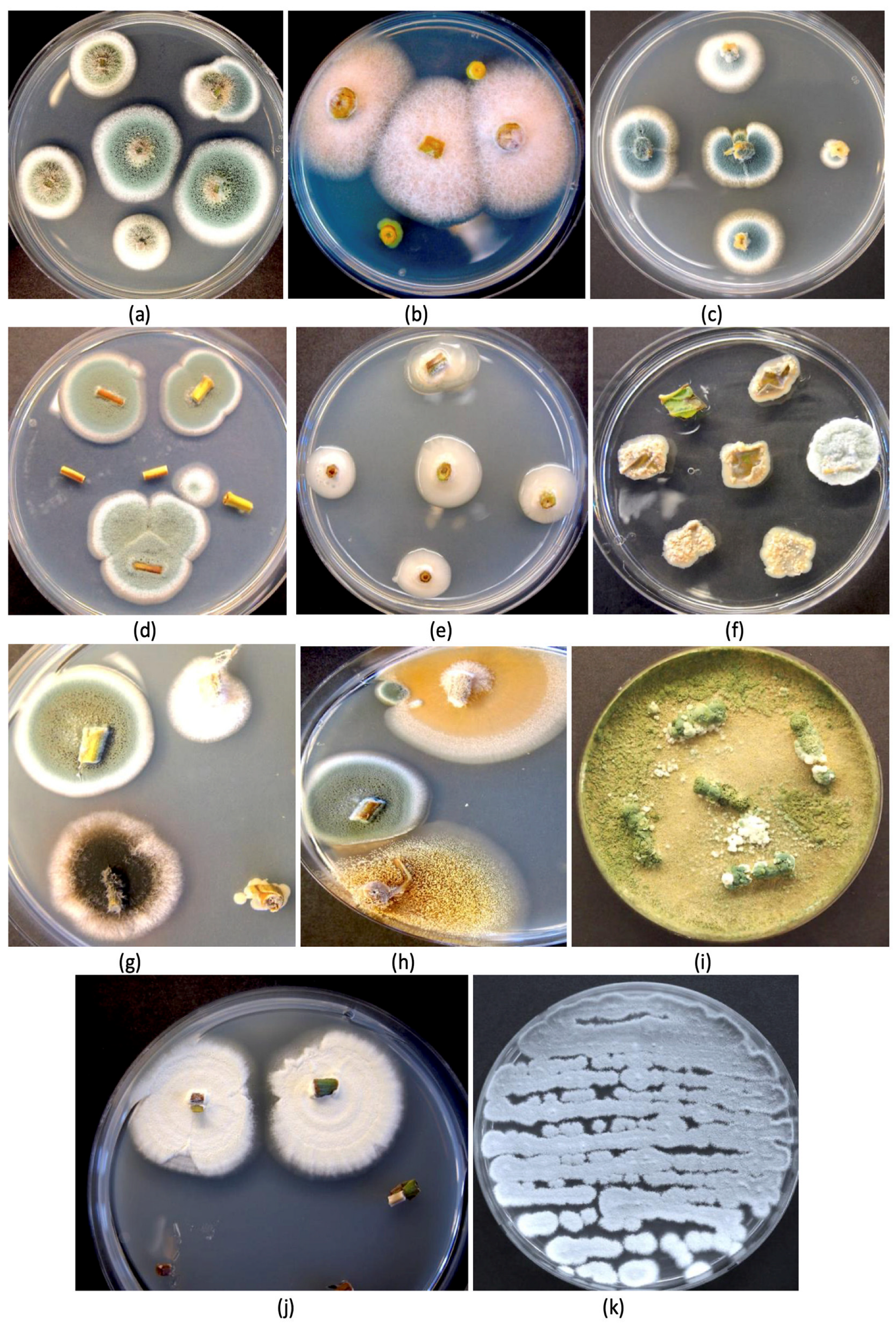
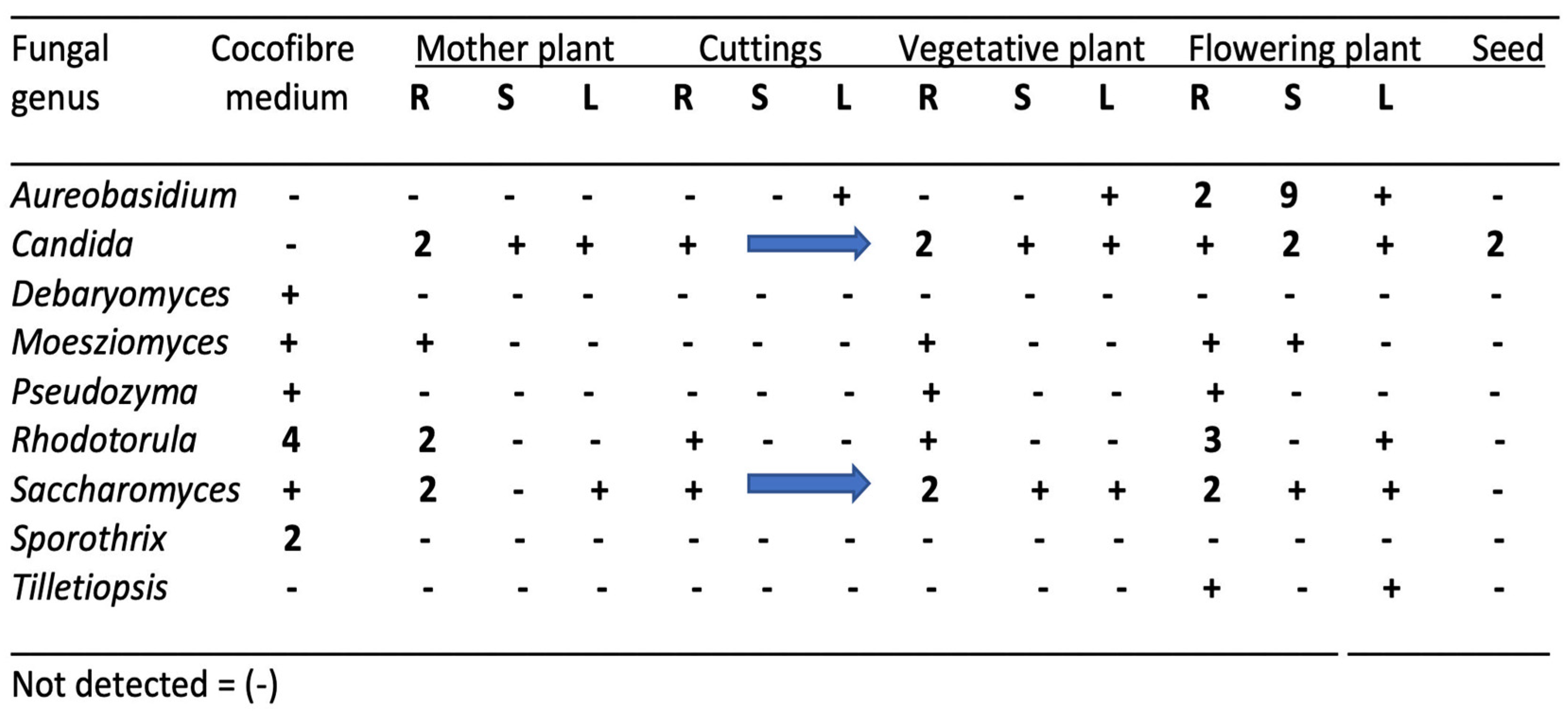
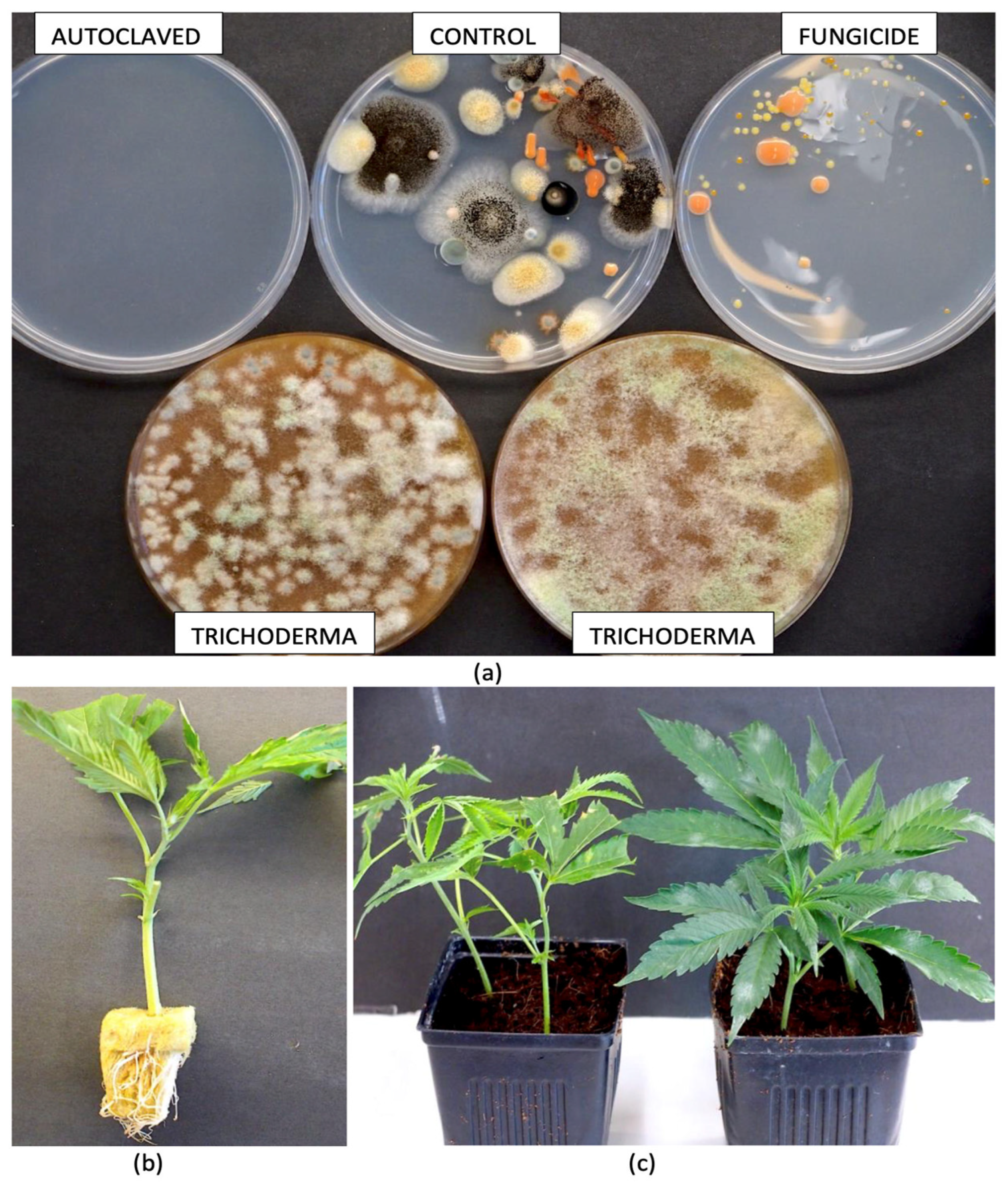


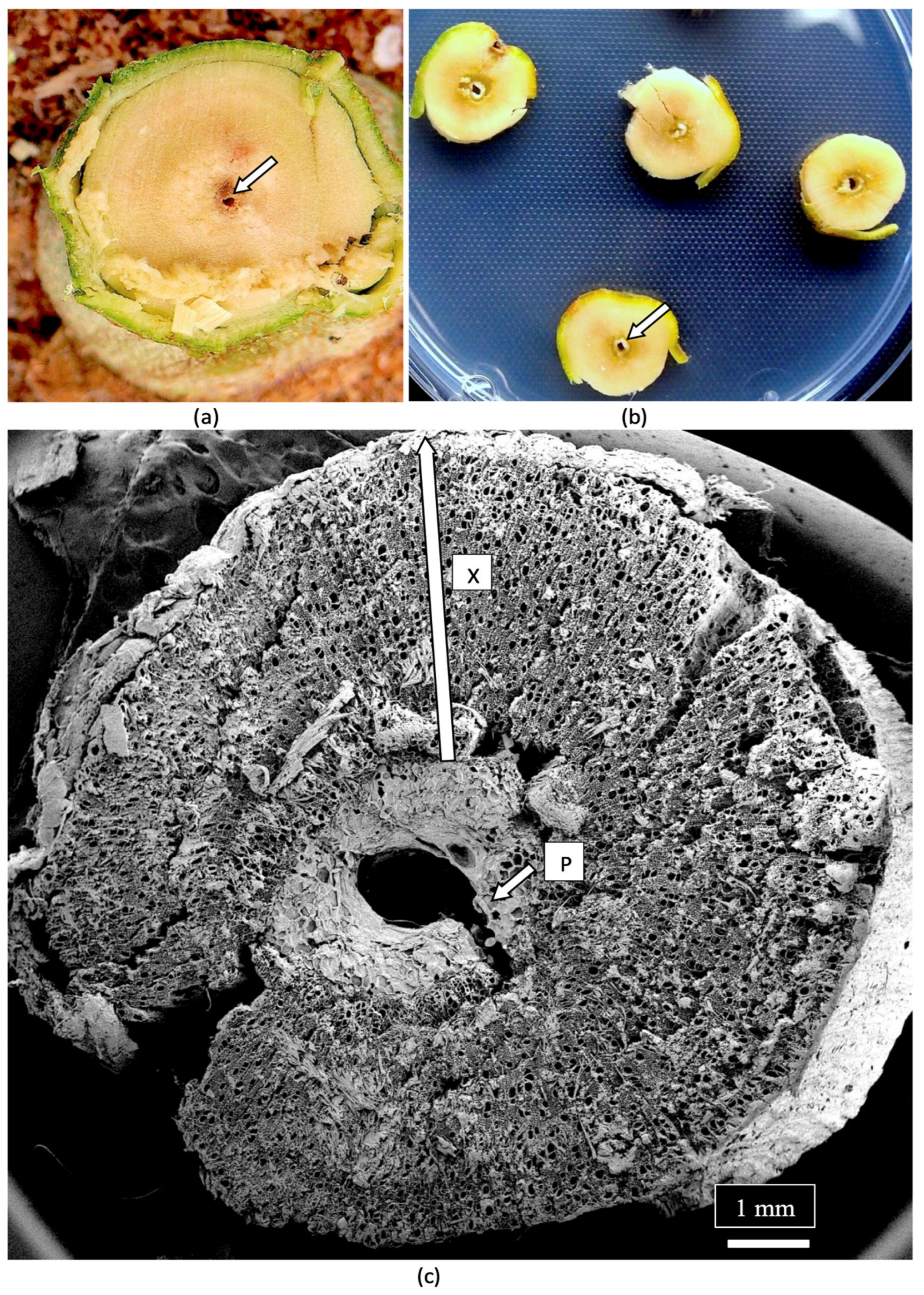
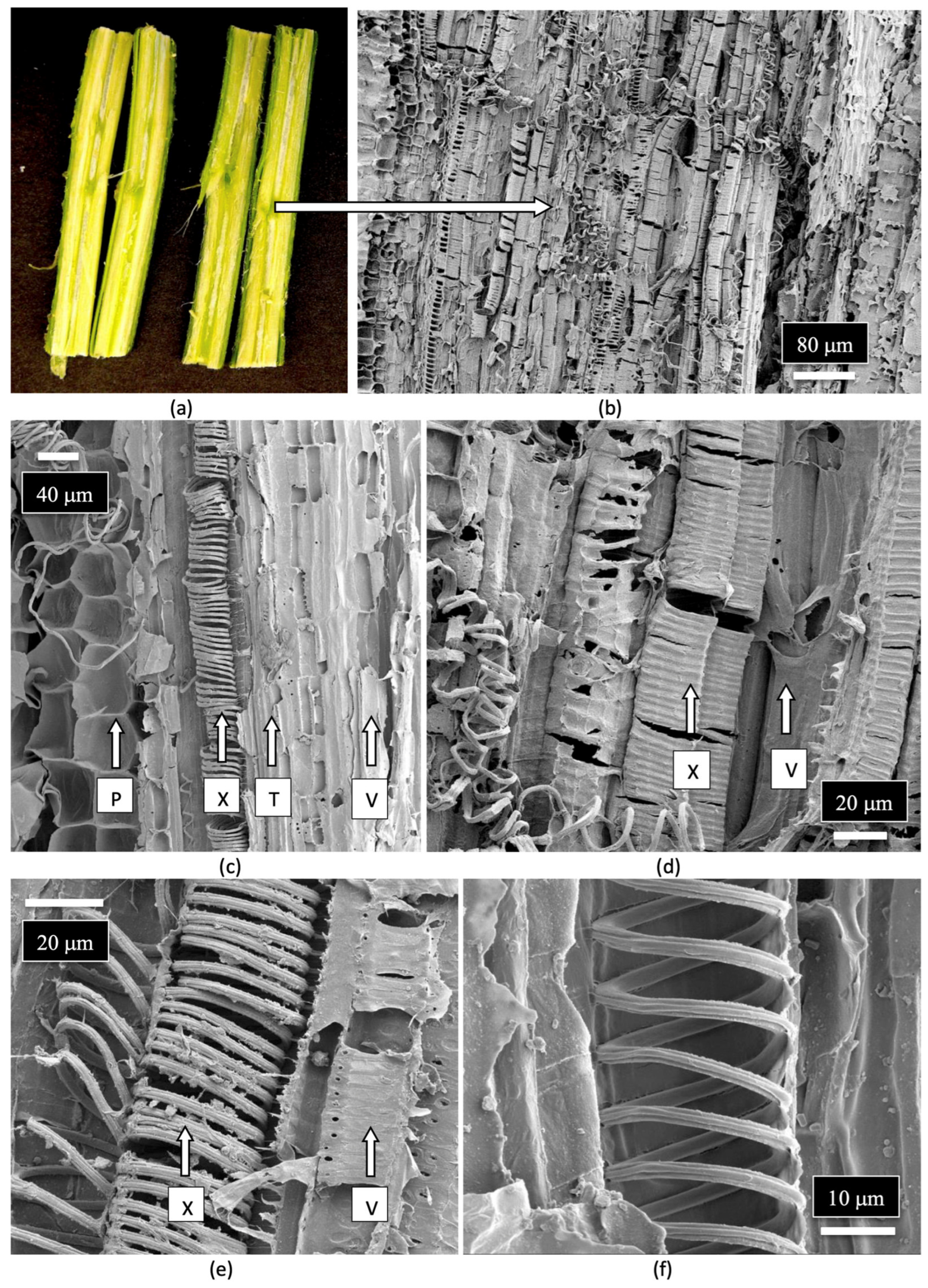
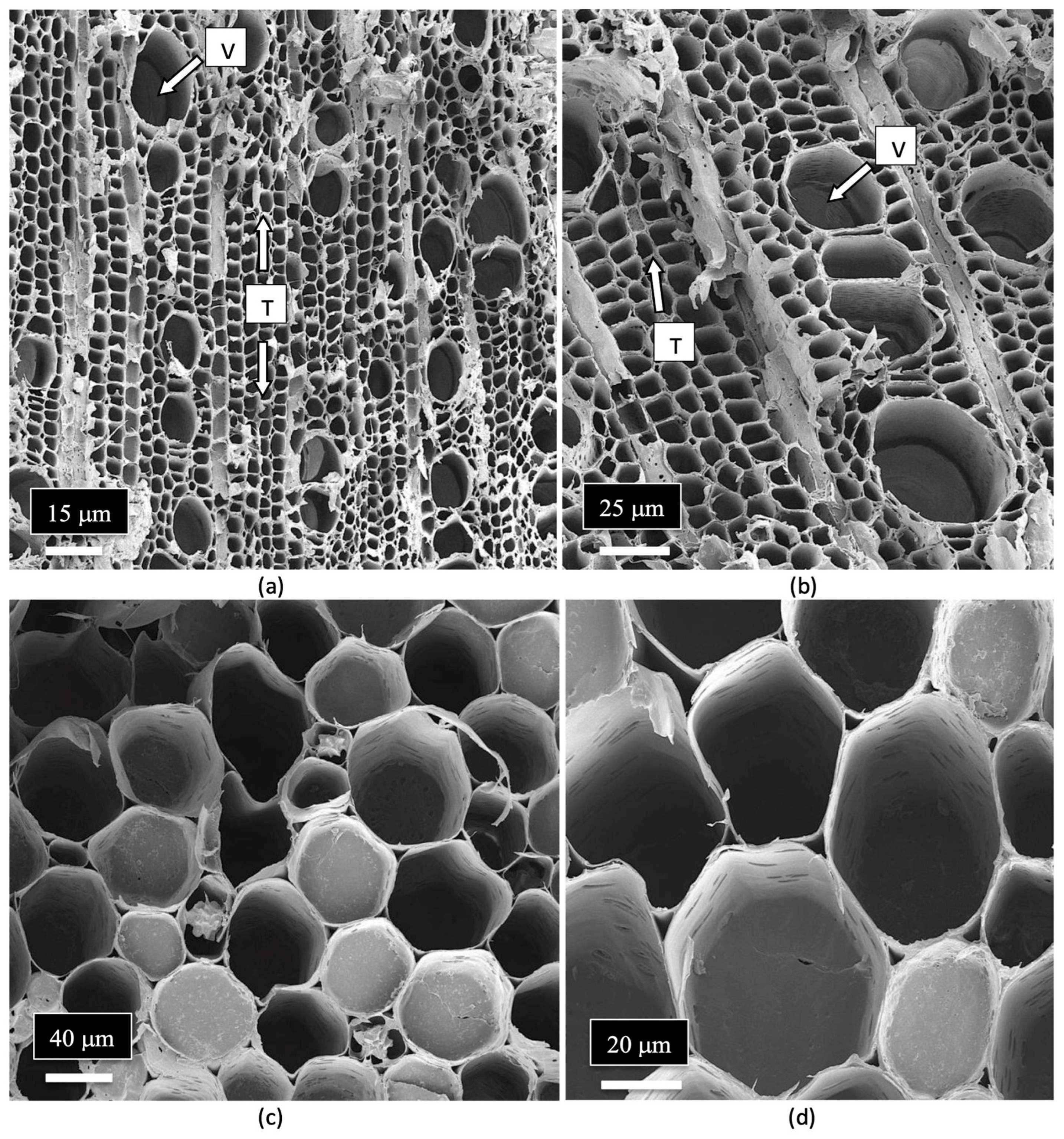

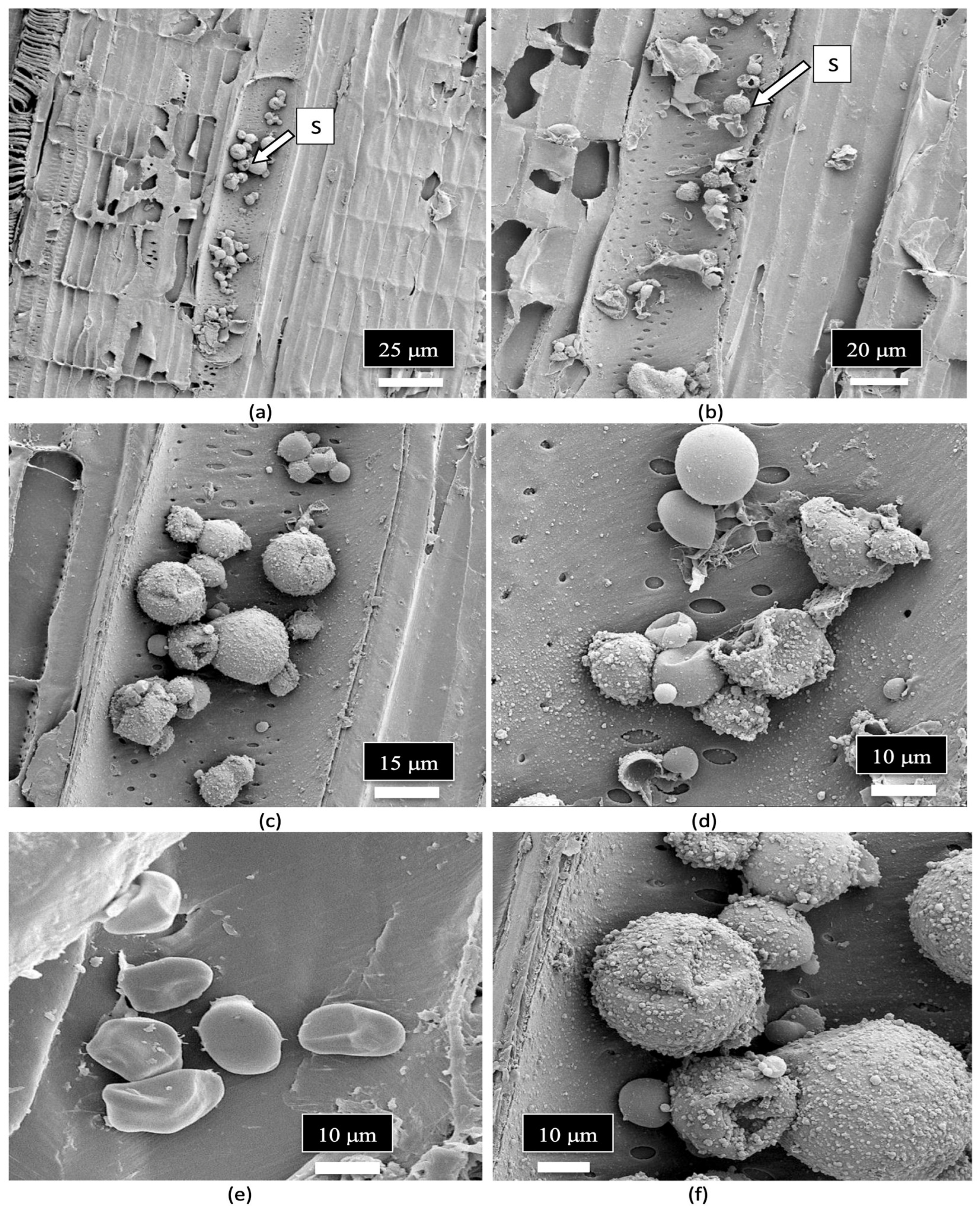

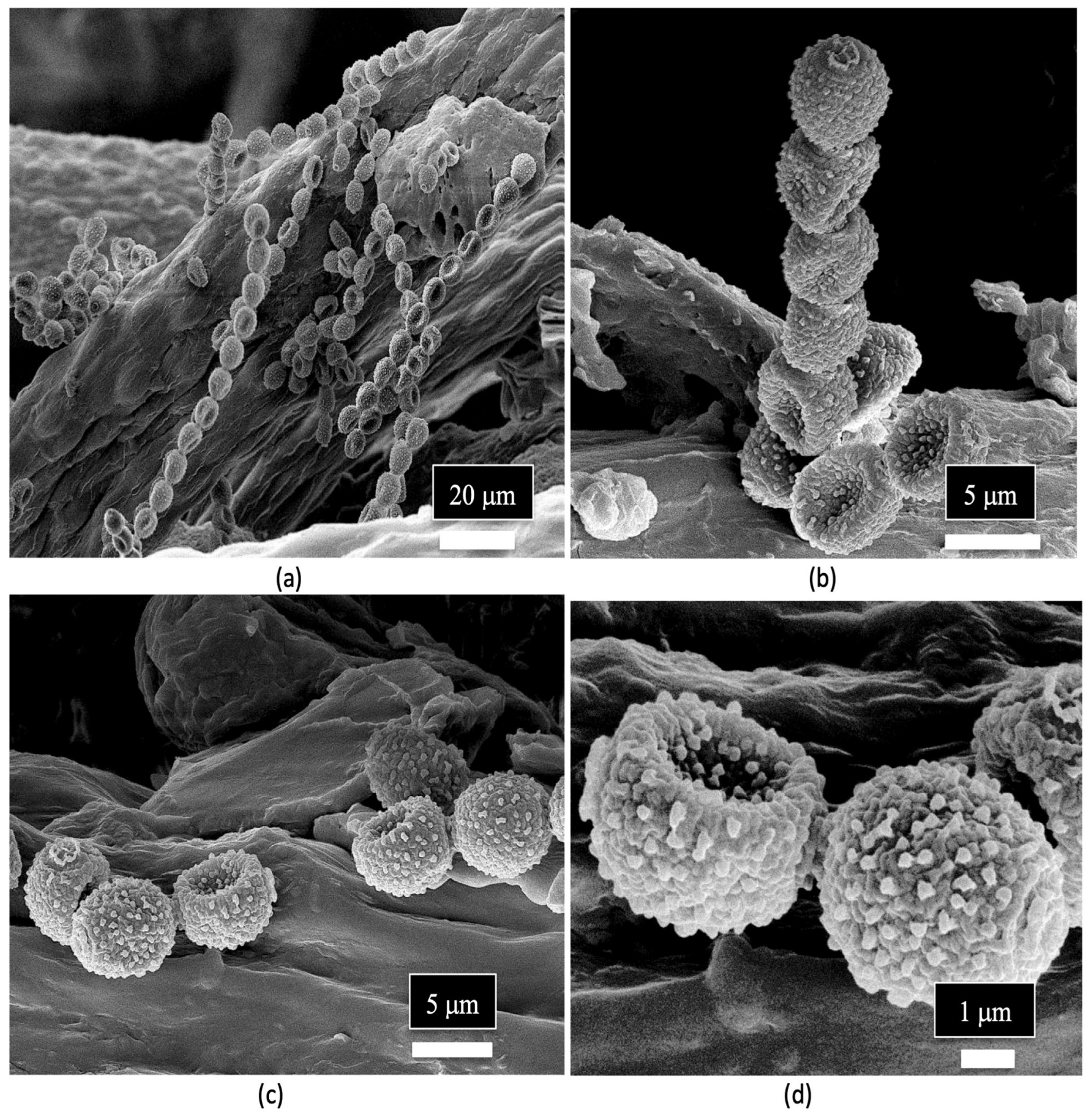
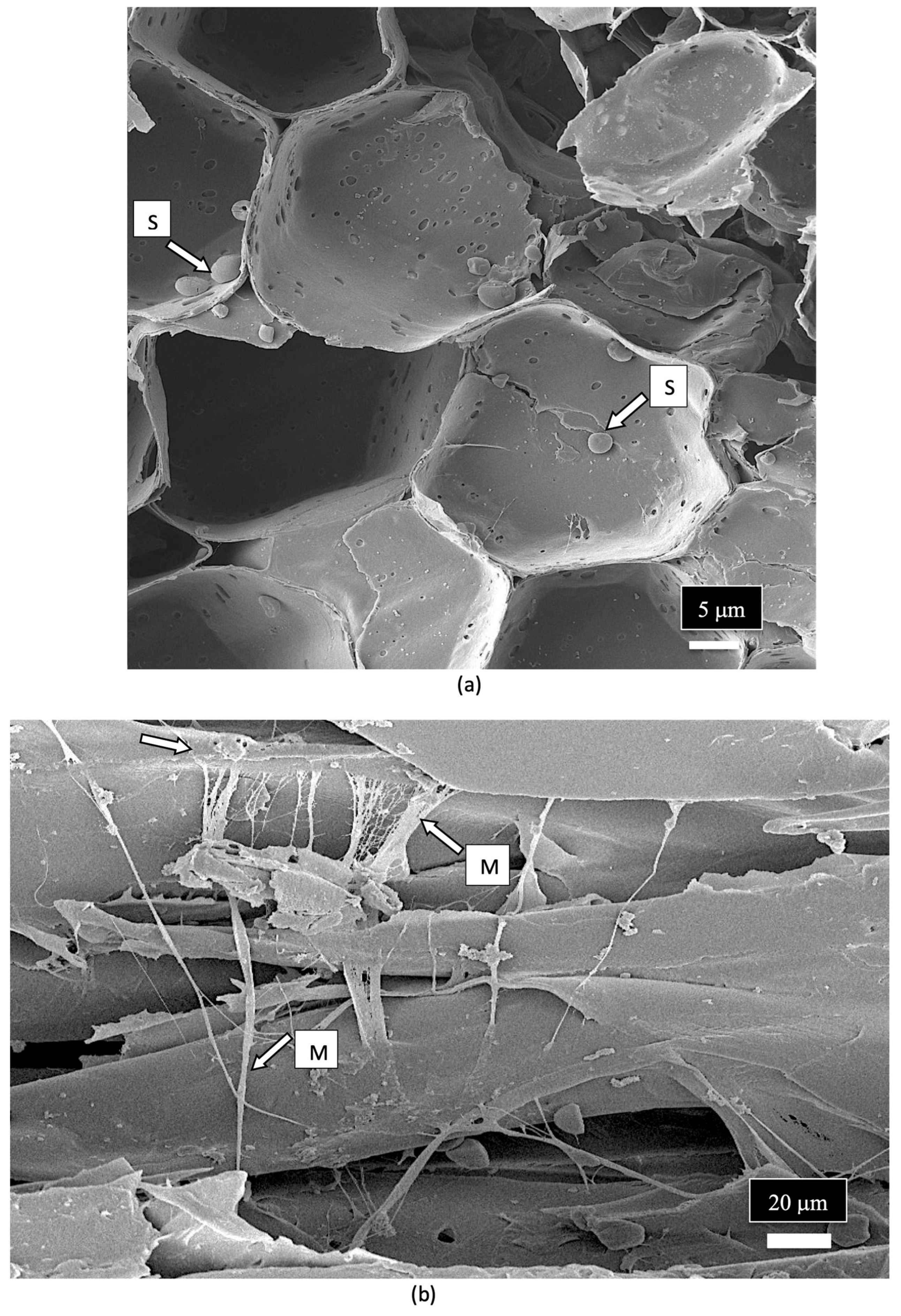
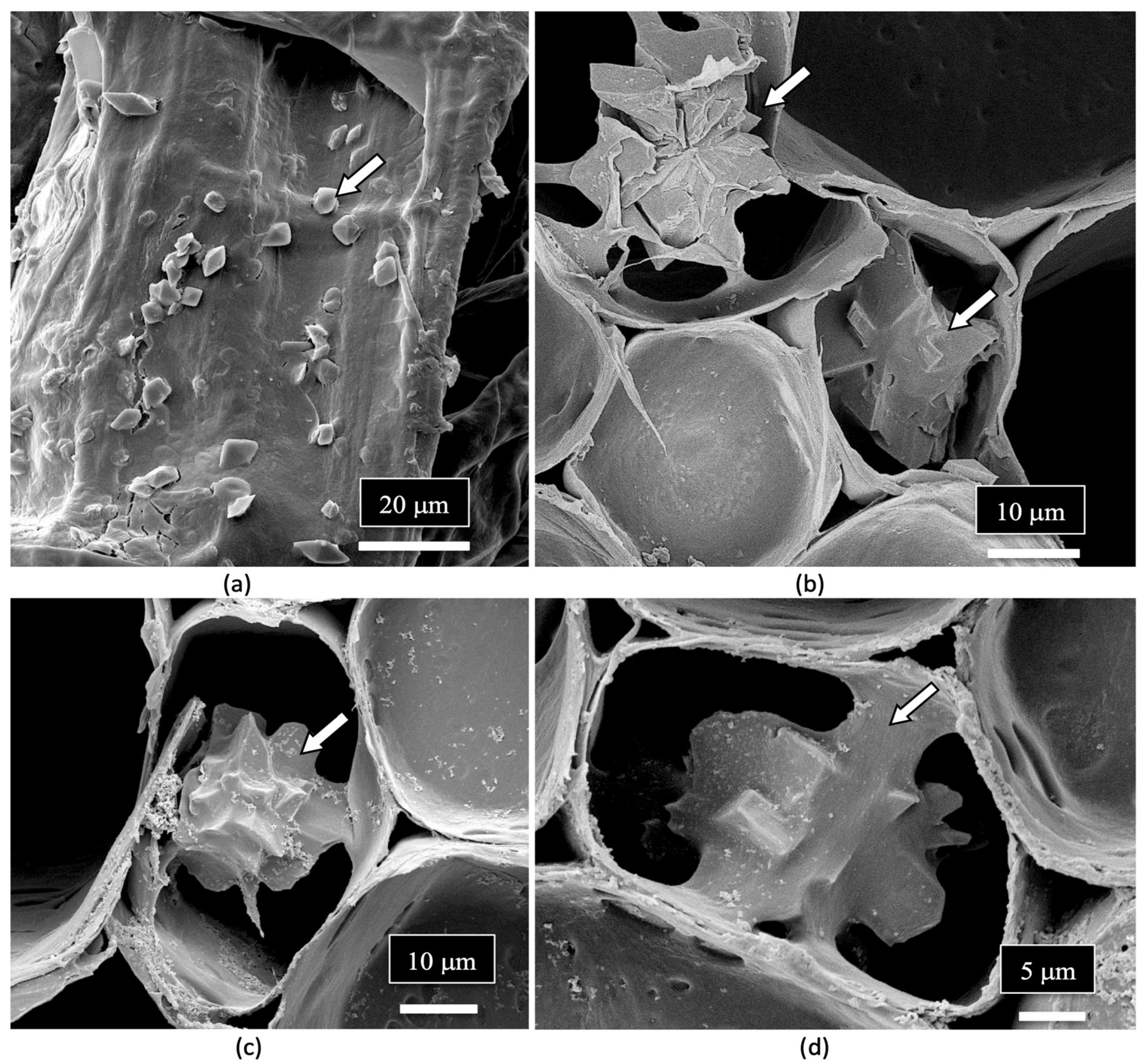
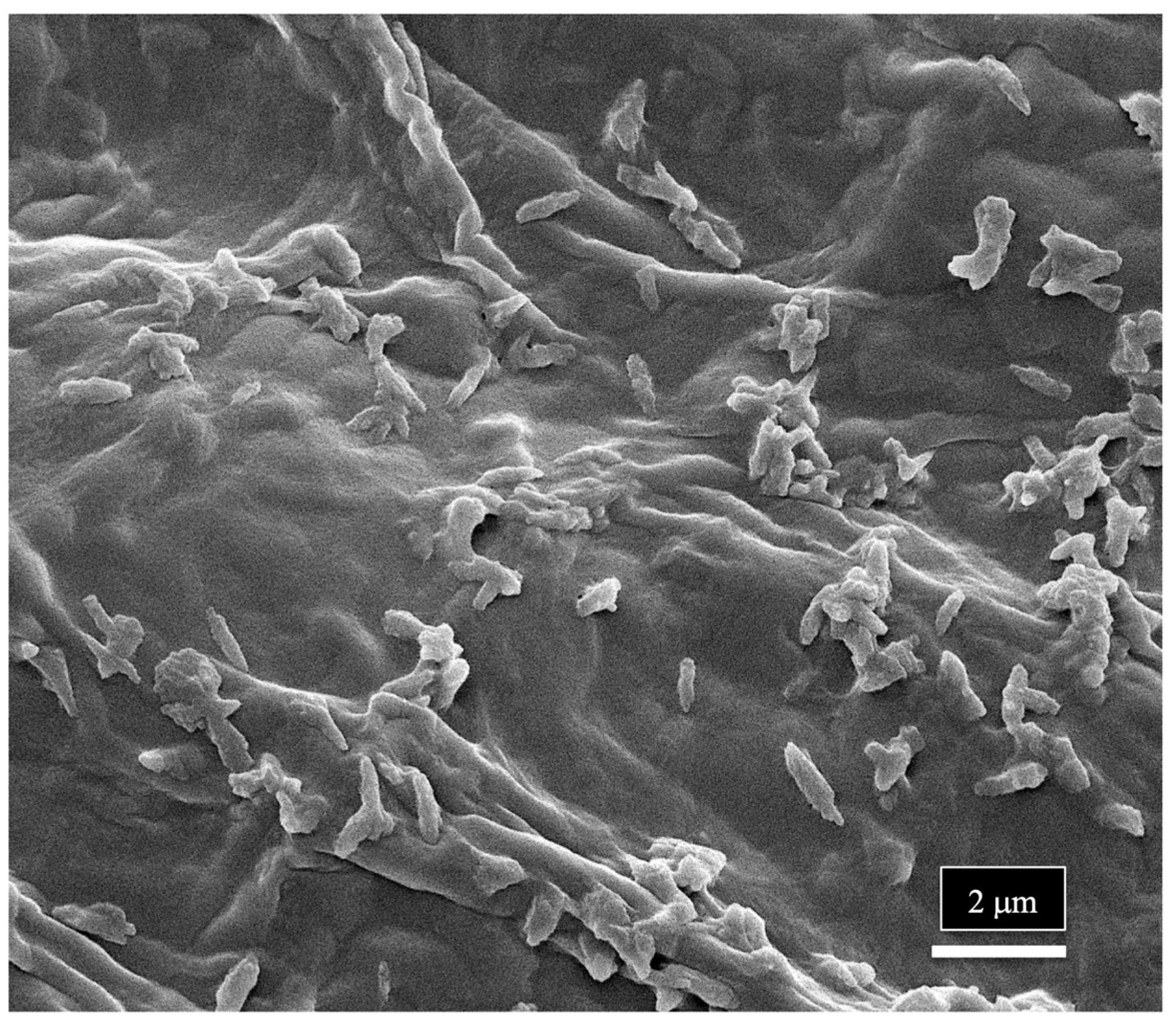
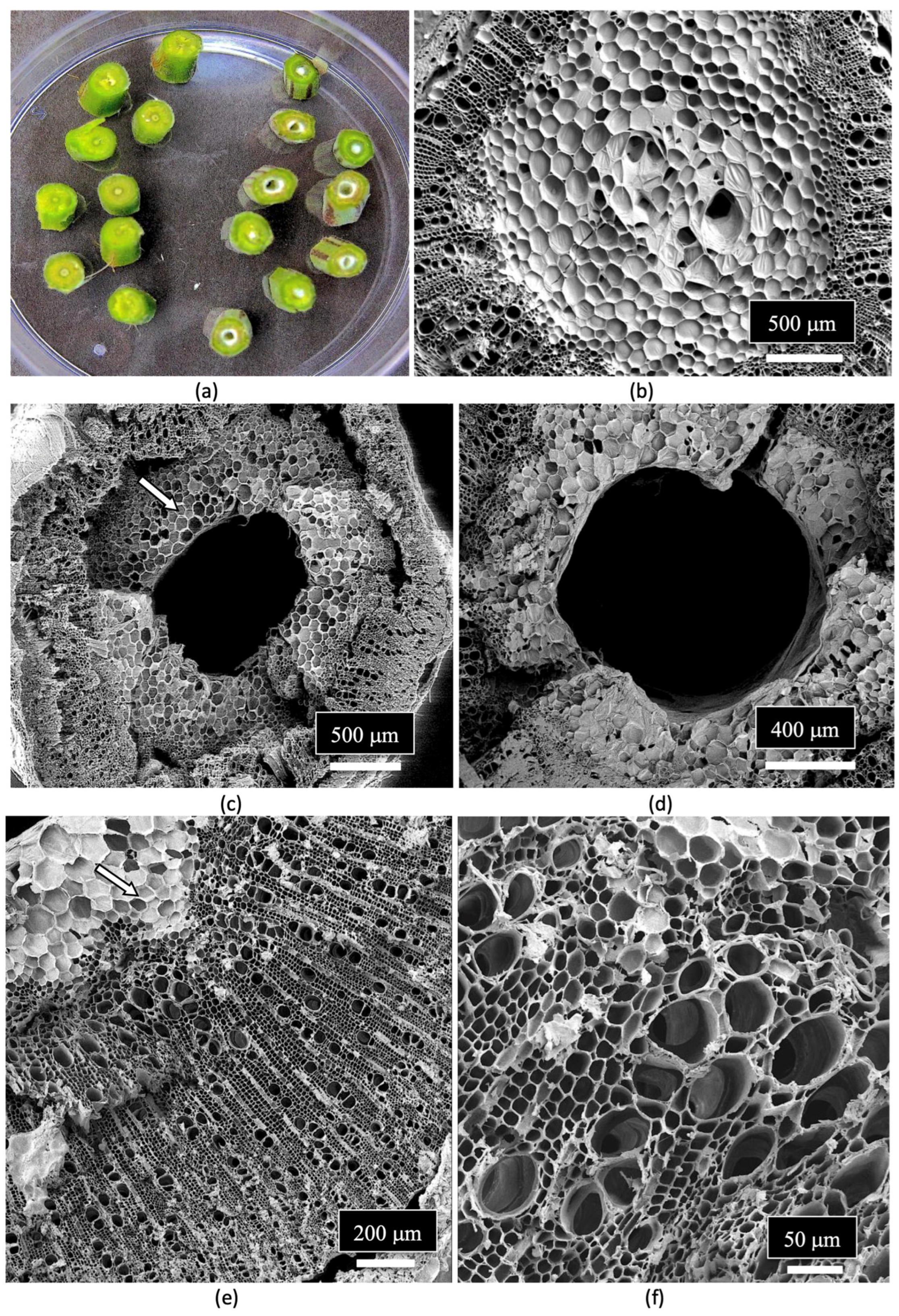
| Reference | Wild Cannabis/Field Hemp | Indoor Cannabis | Reference |
| Seeds | |||
| [21] | Bacillus circulans | Bacillus aerius | [21] |
| Bacillus megaterium | Bacillus inaquosorum | ||
| Bacillus simplex | Bacillus megaterium | ||
| Bacillus subtilis | Bacillus simplex | ||
| Bacillus velezensis | Bacillus stratosphericus | ||
| Bacillus zhangzhouensis | Bacillus subtilis | ||
| Paenibacillus azotifigens | Brevibacillus choshinensis | ||
| Paenibacillus humicus | Paenibacillus illinoisensis | ||
| Paenibacillus illinoisensis | Paenibacillus lautus | ||
| Paenibacillus mobilis | Paenibacillus mobilis | ||
| Paenibacillus pabuli | Paenibacillus pabuli | ||
| Paenibacillus polymyxa | Paenibacillus taohuashanense | ||
| Paenibacillus senegalensis | Psychrobacter pulmonis | ||
| Paenibacillus sinopodophylli | Niallia circulans | [25] | |
| Paenibacillus taohuashanense | Peribacillus frigoritolerans | ||
| Paenibacillus terreus | Pseudomonas congelans | ||
| Paenibacillus vulneris | Pseudomonas punonensis | ||
| Paenibacillus yunnanensis | Rathayibacter festucae | ||
| Pantoea agglomerans | |||
| [26] | Bacillus aryabhattai | ||
| Cellulomonas hominis | |||
| Curtobacterium flaccumfaciens | |||
| Kocuria rhizophila | |||
| Paenibacillus amylolyticus | |||
| Psychrobacillus psychrodurans | |||
| Sphingomonas aerolata | |||
| Staphylococcus epidermidis | |||
| Staphylococcus haemolyticus | |||
| Stenotrophomonas rhizophila | |||
| Reference | Wild Cannabis/Field Hemp | Indoor Cannabis | Reference |
| Roots | |||
| [27] | Acinetobacter gyllenbergii | Fusarium oxysporum | [18] |
| Acinetobacter nosocomialis | Fusarium proliferatum | ||
| Acinetobacter oleivorans strain | Humicola brevis | ||
| Acinetobacter parvus | Humicola fuscoatra | ||
| Acinetobacter pittii | Mucor racemosus | ||
| Agrobacterium tumefaciens | Trichoderma harzianum | ||
| Bacillus anthracis | Afipia felis | [28] | |
| Chryseobacterium kwangjuense | Arthrobotrys conoides | ||
| Chryseobacterium vrystaatense | Asticcacaulis taihuensis | ||
| Enterobacter asburiae | Chaetomium angustispirale | ||
| Enterococcus casseliflavus | Fusarium concentricum | ||
| Nocardioides albus | Fusarium oxysporum | ||
| Nocardioides kongjuensis | Fusarium solani | ||
| Pantoea agglomerans | Mesorhizobium opportunistum | ||
| Pantoea vagans | Penicillium citrinum | ||
| Planomicrobium chinense | Pseudochaetosphaeronema pandanicola | ||
| Pseudomonas putida | Trichocladium pyriforme | ||
| Pseudomonas taiwanensis | |||
| Rhizobium radiobacter | |||
| Streptomyces eurocidicus | |||
| Streptomyces werraensis | |||
| Xanthomonas arboricola | |||
| Xanthomonas gardneri | |||
| Reference | Wild Cannabis/Field Hemp | Indoor Cannabis | Reference |
| Crowns/Stems | |||
| [29] | Alternaria alternata | Acremonium alternatum | [22] |
| Alternaria brassicae | Alternaria alternata | ||
| Schizophyllum commune | Aspergillus fumigatus | ||
| [19] | Alternaria alternata | Aspergillus puniceus | |
| Aspergillus flavus | Botrytis cinerea | ||
| Aspergillus nidulans | Chaetomium globosum | ||
| Aspergillus niger | Cladosporium cladosporioides | ||
| Penicillium chrysogenum | Cladosporium globosum | ||
| Penicillium citrinum | Lecanicillium aphanocladii | ||
| Rhizopus stolonifer | Metarhizium anisopliae | ||
| Chaetomium globosum | [17] | ||
| Fusarium oxysporum | |||
| Lecanicillium lanosoniveum | |||
| Penicillium chrysogenum | |||
| Penicillium griseofulvum | |||
| Penicillium olsonii | |||
| Trametes versicolor | |||
| Trichoderma harzianum | |||
| Bacillus licheniformis | [30] | ||
| Bacillus megaterium | |||
| Bacillus pumilus | |||
| Bacillus subtilis | |||
| Brevibacillus borstelensis | |||
| Mycobacterium peregrinum | |||
| Penicillium copticola | [16] | ||
| Reference | Wild Cannabis/Field Hemp | Indoor Cannabis | Reference |
| Leaves/Petioles | |||
| [31] | Alternaria alternata | Chaetomium globosum | [16] |
| Aspergillus flavus | Eupenicillium rubidurum | ||
| Aspergillus niger | Penicillium copticola | ||
| Curvularia lunata | |||
| Penicillium chrysogenum | |||
| [19] | Alternaria alternata | ||
| Aspergillus flavus | |||
| Aspergillus niger | |||
| Curvularia lunata | |||
| Penicillium chrysogenum | |||
| Penicillium citrinum | |||
| Reference | Wild Cannabis/Field Hemp | Indoor Cannabis | Reference |
| Inflorescences | |||
| Aspergillus versicolor | [19] | ||
| Paecilomyces lilacinus | |||
| Penicillium copticola | |||
| Penicillium meleagrinum | |||
| Penicillium sumatrense | |||
| Aspergillus ochraceus | [32] | ||
| Penicillium citrinum | |||
| Bacterial Species | Effect on Plant or Pathogen * | Mode of Action | Reference |
|---|---|---|---|
| Bacillus megaterium and Brevibacillus borstelensis | Significantly reduced Chromobacterium violaceum production of violacein in cell-free axenic filtrate assays | Quorum quenching | [30] |
| Bacillus anthracis and Enterobacter asburiae | Inhibition zone (0.5–1 cm) against Aspergillus niger and Fusarium oxysporum in dual cultures | Antagonistic mechanisms | [27] |
| Pantoea vagans | Inhibition (0.5 cm) against Fusarium oxysporum in dual cultures | Antagonistic mechanisms | [27] |
| Pseudomonas taiwanensis and Xanthomonas gardneri | Inhibition (0.5 cm) against Aspergillus niger and Fusarium oxysporum in dual cultures | Antagonistic mechanisms | [27] |
| Pseudomonas putida, Comamonas testosteroni, Citrobacter freundii, and Enterobacter cloacae added as Mammoth P product | Consortium significantly increased the inflorescence yield (16.5%), plant height (8.9%), and basal stem diameter (13.5%) of hemp | Plant growth promotion mechanisms | [48] |
| Serratia plymuthica | Significantly increased the plant height (11.5%), plant stalk diameter (42%), and plant biomass at harvest (~120%) of field cannabis plants | Plant fitness promotion and biological control | [49] |
| Azospirillum brasilense, Gluconacetobacter diazotrophicus, Burkholderia ambifaria, and Herbaspirillum seropedicae | Consortium significantly increased the stem length (17%), stem dry weight (63%), leaf dry weight (49%), THC (9%), CBN (18%), and CBD (9%) of greenhouse hemp plants | Nitrogen fixation, siderophore production, mineral solubilization, and growth hormone production | [49] |
| Pseudomonas fulva and P. orientalis | Significantly inhibited Sclerotinia sclerotiorum by 49–53.8% in dual cultures | Antagonistic mechanisms | [20] |
| Pseudomonas fulva and P. orientalis | Significantly inhibited Botrytis cinerea by 22–18.6% in dual cultures | Antagonistic mechanisms | [20] |
| Pseudomonas orientalis | Significantly inhibited Rhizoctonia solani by 27.6% in dual cultures | Antagonistic mechanisms | [20] |
| Bacillus subtilis | Significantly increased dried vegetative biomass of cannabis plants by ~57.1% | Plant growth promotion mechanisms | [54] |
| Serratia marcescens, Enterobacter cloacae, and Paenibacillus hunanensis | Significantly inhibited Phytophthora parasitica by up to 89.6–93.8% in detached cannabis leaf assays | Antibiosis (antifungal metabolite production) | [55] |
| Gluconacetobacter diazotrophicus, Burkholderia ambifaria, and Herbaspirillum seropedicae | Significantly inhibited Fusarium oxysporum by 64–68% in dual cultures | Antibiosis (antifungal metabolite production) | [56] |
| Azospirillum brasilense, Gluconacetobacter diazotrophicus, Burkholderia ambifaria, and Herbaspirillum seropedicae | Consortium significantly inhibited Fusarium oxysporum by 70.6% in dual cultures | Antibiosis (antifungal metabolite production) | [56] |
| Azospirillum brasilense, Gluconacetobacter diazotrophicus, Burkholderia ambifaria, and Herbaspirillum seropedicae | Consortium significantly reduced Fusarium oxysporum damage by 65%, increased germination of seeds (79%), and increased the development of the roots (86%), shoots (152%), and leaves (133%) of infected greenhouse hemp plants | Antibiosis (antifungal metabolite production), induced systemic resistance, phytohormone production, nutrient solubilization, and nitrogen fixation | [56] |
| Bacillus velezensis, Bacillus subtilis, Pseudomonas synxantha, and Pseudomonas protegens | Inhibited the following fungal pathogens by ~25–75%: Botrytis cinerea, Sclerotinia sclerotiorum, Fusarium oxysporum, F. culmorum, F. sporotrichioides, Nigrospora oryzae, N. sphaerica, and Alternaria alternata in dual culture assays | Antibiosis (antifungal metabolite production) | [57] |
| Bacillus velezensis, Bacillus subtilis, and Pseudomonas protegens | Significantly inhibited B. cinerea by 25–56% in detached cannabis leaf assays | Antibiosis (antifungal metabolite production) | [57] |
| Bacillus inaquosorum, Paenibacillus polymyxa, Bacillus subtilis, and Bacillus velezensis | Variably inhibited Alternaria destruens, Aspergillus fumigatus, Fusarium fujikuroi, and Penicillium lanosocoeruleum (depending on the species and strain) in dual culture assays | Antagonistic mechanisms | [21] |
| Bacillus amyloliquefaciens added as Stargus | Significantly reduced Fusarium oxysporum disease severity values of cannabis plants | Antagonistic mechanisms | [22] |
| Sphingomonas areolate and Chlorella sp. (algae) | Consortium significantly increased the sprout length (11%), root length (~19%), and shoot length (~5%) of hemp plants in vivo | Phytochemical production stimulation and phytohormone production | [58] |
| Bacillus frigoritolerans | Significantly increased the plant height (43.3%), plant stalk diameter (96%), and plant biomass at harvest (~260%) of field cannabis plants | Phytohormone production, bio-fertilization, immune response stimulation, and competitive exclusion | [25] |
| Bacillus velezensis | Significantly inhibited Agroathelia rolfsii by 80.5% in dual culture assays and 74.1% in greenhouse hemp plants | Antibiosis (antifungal metabolite production, volatile organic compounds) | [59] |
| Fungal Species | Effect on Plant or Pathogen * | Mode of Action | Reference |
|---|---|---|---|
| Aspergillus versicolor, Chaetomium globosum, Eupenicillium rubidurum, Paecilomyces lilacinus, Penicillium copticola, Penicillium meleagrinum, and Penicillium sumatrense | Inhibited Botrytis cinerea by ~15.8–100% depending on the biological control agent species, strain, and media used in dual culture assays | Antagonistic mechanisms | [16] |
| Trichoderma harzianum | Significantly increased the plant height (9.65%), dry biomass weight (12.83%), and root density (13.72%) of greenhouse hemp plants | Plant growth promotion (nutrient capture and solubilization) | [60] |
| Rhizophagus aggregatus | Significantly increased the plant height (27.5%), stem diameter (5.8%), leaf area (50.1%), number of branches (53.1%), number of inflorescences (56.5%), and leaf dry weight (50%), root dry weight (63.8%) of laboratory cannabis plants, compared to fertilized control plants | Nutrient and water capture and induced systemic resistance | [61] |
| Rhizophagus irregularis, Trichoderma harzianum, Dictyosphaerium chlorelloides (algae), and Bacillus subtilis (bacteria) added as Ferticann | Variably affected (cultivar dependent) CBDV, CBG, CBD, CBDA, CBGA, CBN, ∆9-THCA-A, CBNA, CBLA, CBC, CBCA, and flower biomass of laboratory cannabis plants | Production of plant growth compounds and secondary metabolites | [28] |
| Gliocladium catenulatum added as Lalstop, Trichoderma harzianum and Trichoderma virens added as Rootshield Plus, and Trichoderma asperellum added as Asperello | Significantly reduced Fusarium oxysporum disease severity values of cannabis plants | Antibiosis, mycoparasitism, competitive exclusion, and induced systemic resistance | [22] |
| Gliocladium catenulatum added as Lalstop | Significantly reduced Pythium myriotylum disease severity values of cannabis plants | Antibiosis, mycoparasitism, and induced systemic resistance | [22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buirs, L.; Punja, Z.K. Endophytes in Cannabis sativa: Identifying and Characterizing Microbes with Beneficial and Detrimental Effects on Plant Health. Plants 2025, 14, 1247. https://doi.org/10.3390/plants14081247
Buirs L, Punja ZK. Endophytes in Cannabis sativa: Identifying and Characterizing Microbes with Beneficial and Detrimental Effects on Plant Health. Plants. 2025; 14(8):1247. https://doi.org/10.3390/plants14081247
Chicago/Turabian StyleBuirs, Liam, and Zamir K. Punja. 2025. "Endophytes in Cannabis sativa: Identifying and Characterizing Microbes with Beneficial and Detrimental Effects on Plant Health" Plants 14, no. 8: 1247. https://doi.org/10.3390/plants14081247
APA StyleBuirs, L., & Punja, Z. K. (2025). Endophytes in Cannabis sativa: Identifying and Characterizing Microbes with Beneficial and Detrimental Effects on Plant Health. Plants, 14(8), 1247. https://doi.org/10.3390/plants14081247







