The Combination of Salicylic Acid, Nicotinamide, and Proline Mitigates the Damage Caused by Salt Stress in Nasturtium (Tropaeolum majus)
Abstract
1. Introduction
2. Results
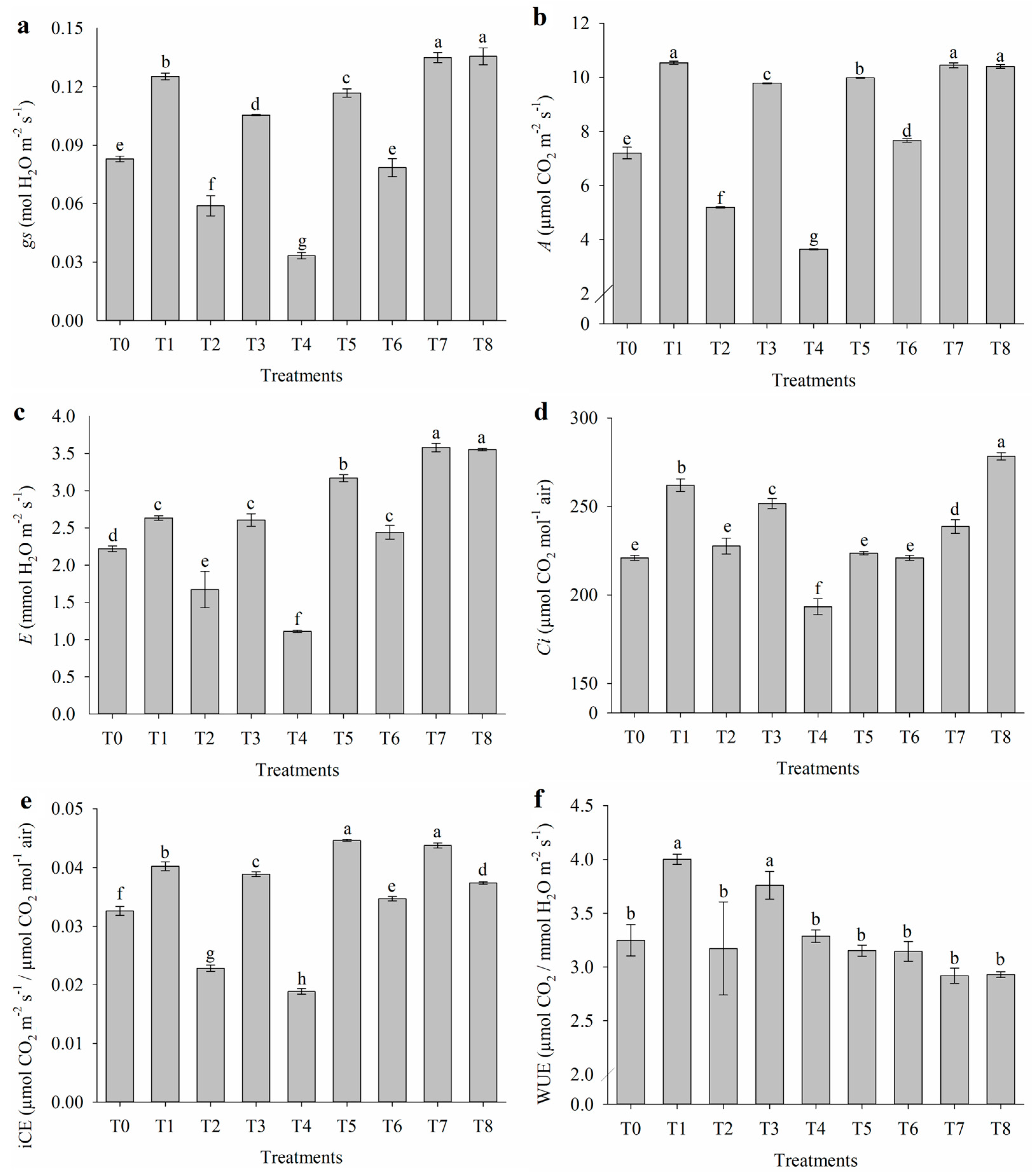
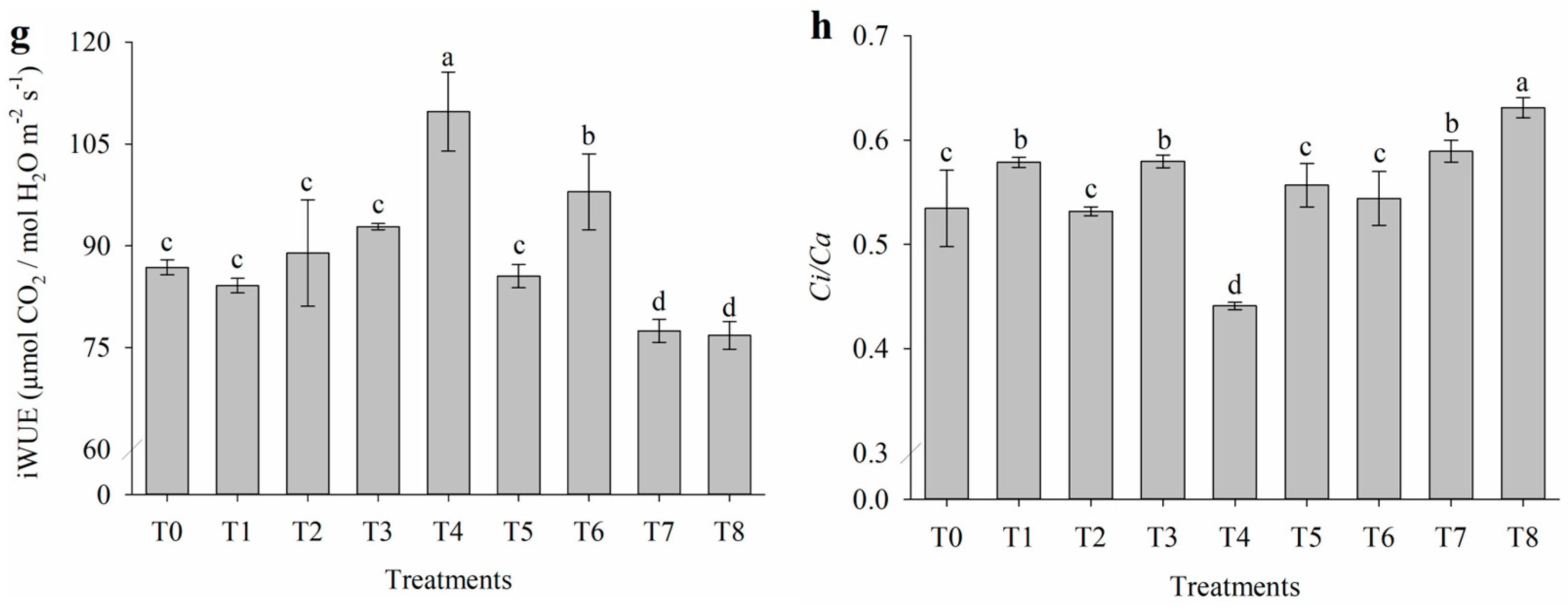
2.1. Effect of Salt Stress on Gas Exchange
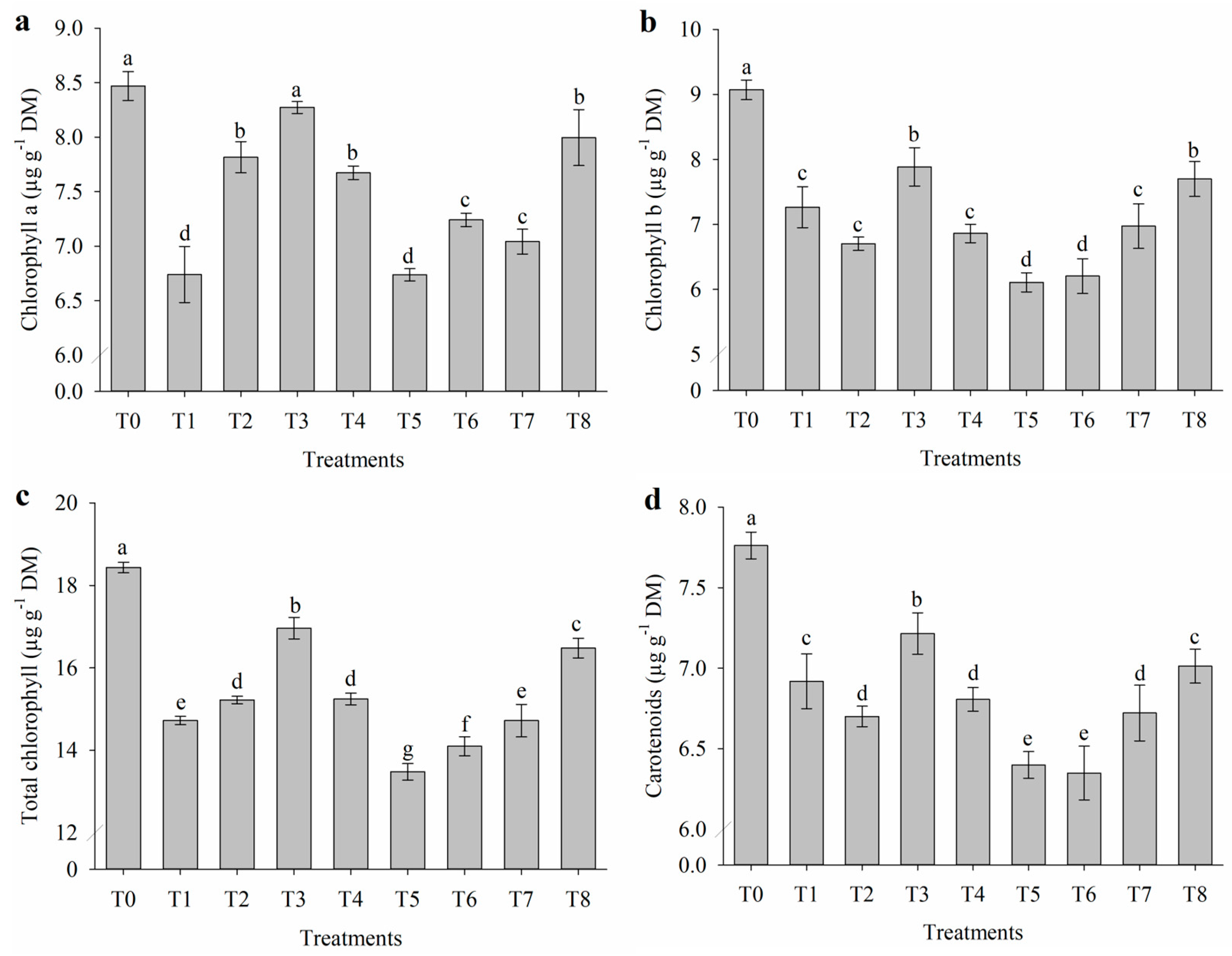
2.2. Impact of Salt Stress on Photosynthetic Pigments
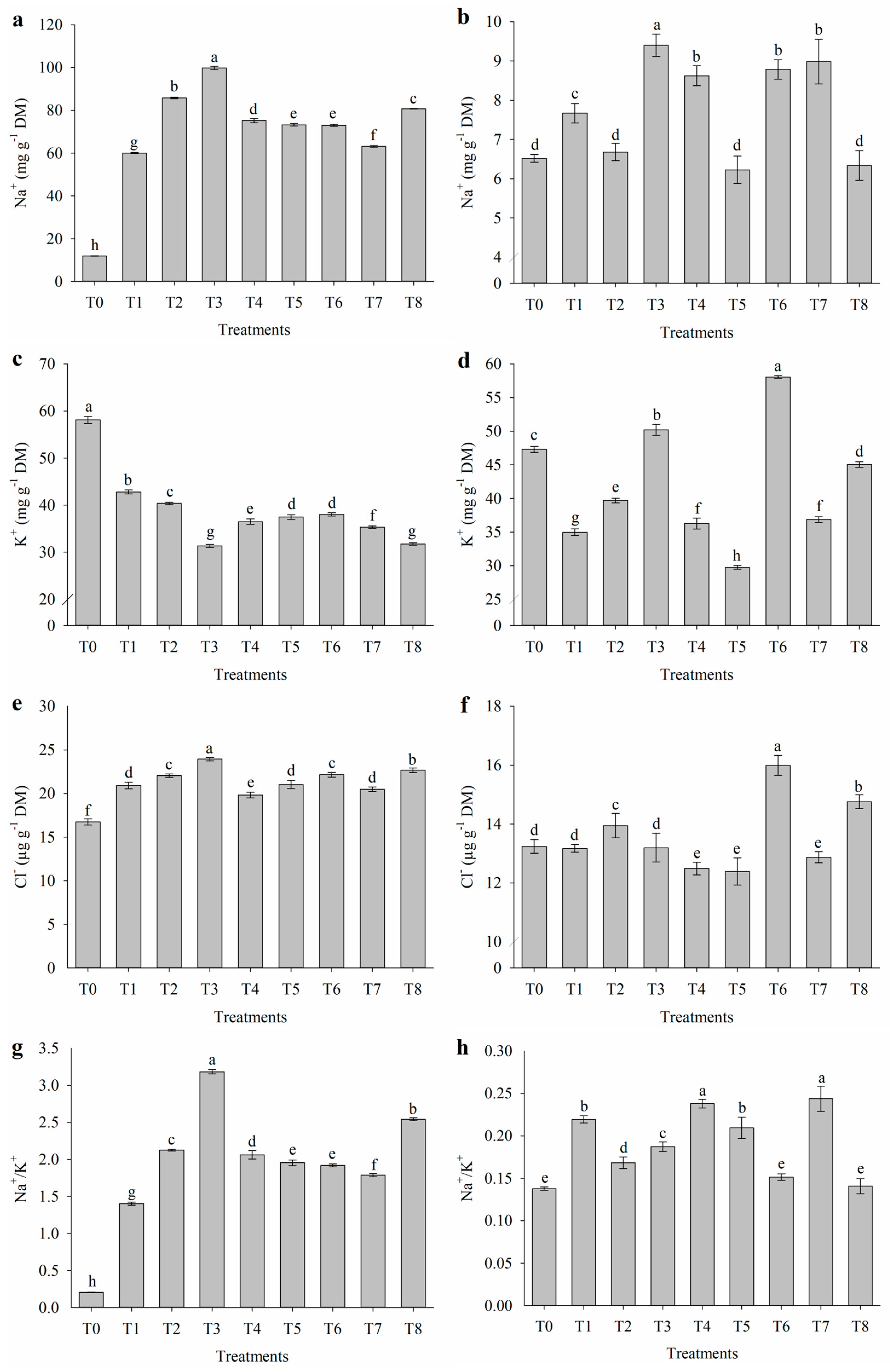
2.3. Inorganic Solutes of Leaves and Flowers
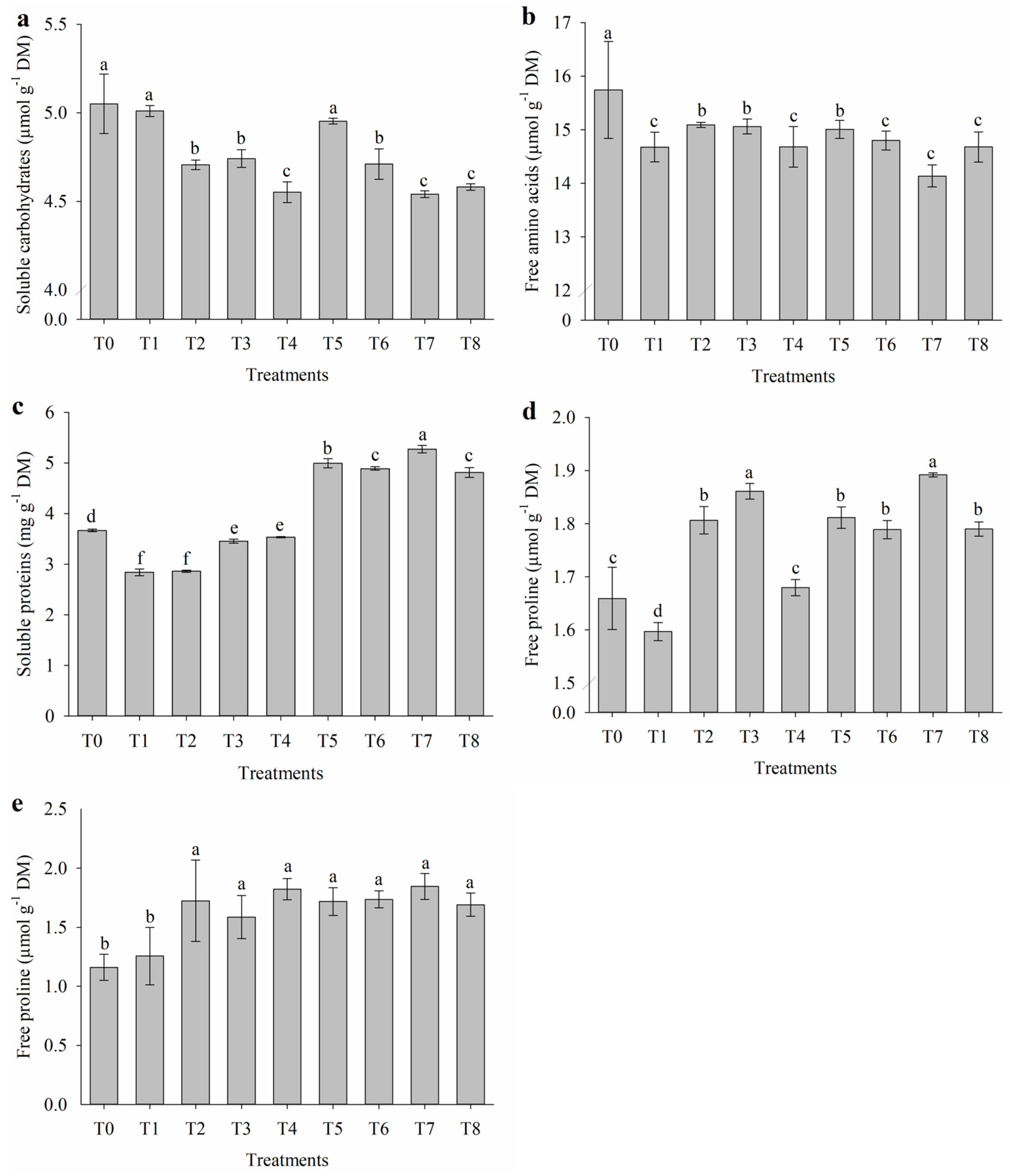
2.4. Organic Solutes of Leaves and Flowers
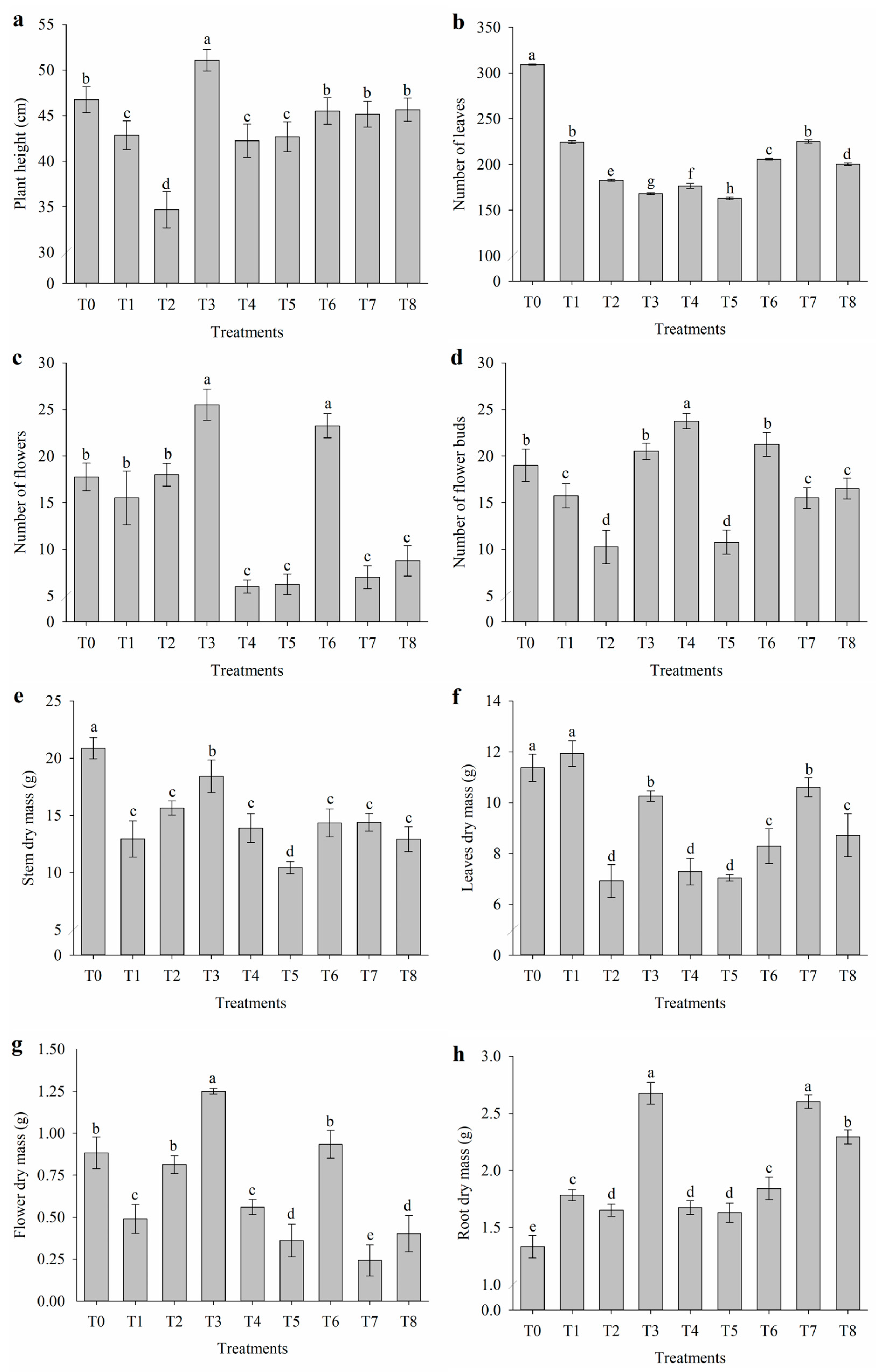
2.5. Growth and Biomass Production
3. Discussion
4. Materials and Methods
4.1. Experimental Place, Experimental Structure, and Design
4.2. Growth Conditions
4.3. Variables Analyzed
4.3.1. Gas Exchange
4.3.2. Photosynthetic Pigments
4.3.3. Inorganic Solutes
4.3.4. Organic Solutes
4.3.5. Growth and Production
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, T.I.; Dias, M.G.; de Araújo, N.O.; Santos, M.N.S.; Ribeiro, W.S.; dos Santos Filho, F.B.; Grossi, J.A.S. Spermine Reduces the Harmful Effects of Drought Stress in Tropaeolum majus. Sci. Hortic. 2022, 304, 111339. [Google Scholar]
- Silva, T.I.D.; Dias, M.G.; Barbosa, L.B.; de Araújo, N.O.; Ferreira, F.D.; Grossi, J.A.S.; Costa, F.B.D.; Marco, C.A.; Ribeiro, D.M. Spermine Decreases Ethylene and Increases Sugars and Phenolic Compounds in Nasturtium Flowers Grown Under Drought and Salt Stress. Bragantia 2023, 82, e20230041. [Google Scholar]
- Xu, W.; Lu, N.; Kikuchi, M.; Takagaki, M. Continuous Lighting and High Daily Light Integral Enhance Yield and Quality of Mass-Produced Nasturtium (Tropaeolum majus L.) in Plant Factories. Plants 2021, 10, 1203. [Google Scholar] [CrossRef]
- Cobus, D.; Nunes, G.; Maior, L.O.; Lacerda, L.G.; Ito, V.C. Unconventional Food Plants (UFPs): An Approach to the Nutritional and Functional Properties of Nasturtium (Tropaeolum majus L.). Food Sci. Today 2023, 1, 1–10. [Google Scholar] [CrossRef]
- Lourenço, E.L.B.; Muller, J.C.; Boareto, A.C.; Gomes, C.; Lourenço, A.C.; Minatovicz, B.; Crestani, S.; Gasparotto Junior, A.; Martino-Andrade, A.; Dalsenter, P.R. Screening for In Vivo (Anti) Estrogenic and (Anti) Androgenic Activities of Tropaeolum majus L. and Its Effect on Uterine Contractility. J. Ethnopharmacol. 2012, 141, 418–423. [Google Scholar]
- Barbosa, L.B.; da Silva, T.I.; Dias, M.G.; Pereira, E.D.; Cruz, R.R.P.; de Souza Silva, J.; Grossi, J.A.S. Application of Phytohormones Reduces Damage Caused by Salt Stress in Tropaeolum majus. S. Afr. J. Bot. 2024, 166, 69–78. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Silva, T.I.; Dias, M.G.; Grossi, J.A.S.; Ribeiro, W.S.; Moraes, P.J.; de Araújo, F.F.; Barbosa, J.G. Application of Phytohormones as Attenuators of Salt Stress in Tropaeolum majus L. (Tropaeolaceae). Acta Bot. Croat. 2022, 81, 51–60. [Google Scholar]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.K.; Shabala, S. Mechanisms of Plant Responses and Adaptation to Soil Salinity. Innovation 2020, 1, 100017. [Google Scholar]
- Ahmadi, M.; Souri, M.K. Growth Characteristics and Fruit Quality of Chili Pepper Under Higher Electrical Conductivity of Nutrient Solution Induced by Various Salts. Agrivita J. Agric. Sci. 2020, 42, 143–152. [Google Scholar] [CrossRef]
- Phour, M.; Sindhu, S.S. Amelioration of Salinity Stress and Growth Stimulation of Mustard (Brassica juncea L.) by Salt-Tolerant Pseudomonas Species. Appl. Soil Ecol. 2020, 149, 103518. [Google Scholar] [CrossRef]
- Xu, W.; Lu, N.; Kikuchi, M.; Takagaki, M. Effects of Node Position and Electric Conductivity of Nutrient Solution on Adventitious Rooting of Nasturtium (Tropaeolum majus L.) Cuttings. Agronomy 2021, 11, 363. [Google Scholar] [CrossRef]
- Bloem, E.; Haneklaus, S.; Kleinwächter, M.; Paulsen, J.; Schnug, E.; Selmar, D. Stress-Induced Changes of Bioactive Compounds in Tropaeolum majus L. Ind. Crops Prod. 2014, 60, 349–359. [Google Scholar] [CrossRef]
- Costa, L.F.; Soares, T.M.; Silva, M.G.; Modesto, F.J.N.; Queiroz, L.D.A.; Pereira, J.D.S. Cauliflower Growth and Yield in a Hydroponic System with Brackish Water. Rev. Caatinga 2020, 33, 1060–1070. [Google Scholar] [CrossRef]
- Silva, T.I.; Dias, M.G.; Araújo, N.O.; Santos, M.N.S.; Cruz, R.R.P.; Dias, T.J.; Ribeiro, W.S.; Grossi, J.A.S.; Barbosa, J.G. Spermine Reduces the Harmful Effects of Salt Stress in Tropaeolum majus. Physiol. Mol. Biol. Plants 2022, 28, 687–696. [Google Scholar] [CrossRef]
- Santos Filho, F.B.; Silva, T.I.D.; Dias, M.G.; Grossi, J.A.S. Polyamines Mitigate the Harmful Effects of Salt Stress on the Growth and Gas Exchange of Nasturtium. Ciênc. Agrotecnol. 2022, 46, e000722. [Google Scholar] [CrossRef]
- Silva, J.H.D.; Silva, A.J.D.; Silva, T.I.D.; Henschel, J.M.; Lopes, A.S.; Alves, J.C.; Silva, R.F.; Araújo, D.B.; Santos, J.P.O.; Martins, A.H.P.C.; et al. Salicylic Acid Reduces Harmful Effects of Salt Stress in Tropaeolum majus. Rev. Bras. Eng. Agríc. Ambient. 2024, 28, e278566. [Google Scholar]
- Targino, V.A.; Dias, T.J.; Sousa, V.F.D.O.; Silva, M.D.M.; Silva, A.J.; Ribeiro, J.E.D.S.; Rêgo, M.M.D. Growth, Gas Exchange, and Phytochemical Quality of Nasturtium (Tropaeolum majus L.) Subjected to Proline Concentrations and Salinity. Plants 2025, 14, 301. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Kumar, V.; Kohli, S.K.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Bhardwaj, R.; Zheng, B. Phytohormones Regulate Accumulation of Osmolytes Under Abiotic Stress. Biomolecules 2019, 9, 285. [Google Scholar] [CrossRef]
- Faried, H.N.; Ayyub, C.M.; Amjad, M.; Ahmed, R.; Wattoo, F.M.; Butt, M.; Bashir, M.; Shaheen, M.R.; Waqas, M.A. Salicylic Acid Confers Salt Tolerance in Potato Plants by Improving Water Relations, Gaseous Exchange, Antioxidant Activities and Osmoregulation. J. Sci. Food Agric. 2017, 97, 1868–1875. [Google Scholar]
- Mohamed, M.H.; Badr, E.A.; Sadak, M.S.; Khedr, H.H. Effect of Garlic Extract, Ascorbic Acid and Nicotinamide on Growth, Some Biochemical Aspects, Yield and Its Components of Three Faba Bean (Vicia faba L.) Cultivars Under Sandy Soil Conditions. Bull. Natl. Res. Cent. 2020, 44, 100. [Google Scholar] [CrossRef]
- El-Shawa, G.M.; Rashwan, E.M.; Abdelaal, K.A. Mitigating Salt Stress Effects by Exogenous Application of Proline and Yeast Extract on Morpho-Physiological, Biochemical and Anatomical Characters of Calendula Plants. Sci. J. Flow. Ornam. Plants 2020, 7, 461–482. [Google Scholar] [CrossRef]
- Jia, T.; Hou, J.; Iqbal, M.Z.; Zhang, Y.; Cheng, B.; Feng, H.; Nie, G.; Ma, X.; Liu, W.; Peng, Y. Overexpression of the White Clover TrSAMDC1 Gene Enhanced Salt and Drought Resistance in Arabidopsis thaliana. Plant Physiol. Biochem. 2021, 165, 147–160. [Google Scholar] [CrossRef]
- Pan, T.; Liu, M.; Kreslavski, V.D.; Zharmukhamedov, S.K.; Nie, C.; Yu, M.; Kuznetsov, V.V.; Allakhverdiev, S.I.; Shabala, S. Non-Stomatal Limitation of Photosynthesis by Soil Salinity. Crit. Rev. Environ. Sci. Technol. 2021, 51, 791–825. [Google Scholar] [CrossRef]
- Lemonnier, P.; Lawson, T. Calvin Cycle and Guard Cell Metabolism Impact Stomatal Function. Semin. Cell Dev. Biol. 2024, 155, 59–70. [Google Scholar] [CrossRef]
- Busch, F.A. Opinion: The Red-Light Response of Stomatal Movement Is Sensed by the Redox State of the Photosynthetic Electron Transport Chain. Photosynth. Res. 2014, 119, 131–140. [Google Scholar] [CrossRef]
- Jezek, M.; Silva-Alvim, F.A.; Hills, A.; Donald, N.; Ishka, M.R.; Shadbolt, J.; He, B.; Lawson, T.; Harper, J.F.; Wang, Y.; et al. Guard Cell Endomembrane Ca2+-ATPases Underpin a ‘Carbon Memory’ of Photosynthetic Assimilation That Impacts on Water-Use Efficiency. Nat. Plants 2021, 7, 1301–1313. [Google Scholar] [CrossRef] [PubMed]
- Araújo, T.A.N.; Vendruscolo, E.P.; Binotti, F.F.S.; Costa, E.; Lima, S.F.; Sant’Ana, G.R.; Bortolheiro, F.P.A.P. Nicotinamide Increases the Physiological Performance and Initial Growth of Maize Plants. Int. J. Agron. 2024, 2024, 5567314. [Google Scholar] [CrossRef]
- Vendruscolo, E.P.; Sant’Ana, G.R.; Lima, S.F.D.; Gaete, F.I.; Bortolheiro, F.P.D.A.; Serafim, G.M. Biostimulant Potential of Azospirillum brasilense and Nicotinamide for Hydroponic Pumpkin Cultivation. Rev. Bras. Eng. Agríc. Ambient. 2024, 28, e278962. [Google Scholar] [CrossRef]
- Shu, S.; Yuan, L.Y.; Guo, S.R.; Sun, J.; Yuan, Y.H. Effects of Exogenous Spermine on Chlorophyll Fluorescence, Antioxidant System and Ultrastructure of Chloroplasts in Cucumis sativus L. Under Salt Stress. Plant Physiol. Biochem. 2013, 63, 209–216. [Google Scholar] [CrossRef]
- Syeed, S.; Sehar, Z.; Masood, A.; Anjum, N.A.; Khan, N.A. Control of Elevated Ion Accumulation, Oxidative Stress, and Lipid Peroxidation with Salicylic Acid-Induced Accumulation of Glycine Betaine in Salinity-Exposed Vigna radiata L. Appl. Biochem. Biotechnol. 2021, 193, 3301–3320. [Google Scholar]
- Zhou, H.; Shi, H.; Yang, Y.; Feng, X.; Chen, X.; Xiao, F.; Lin, H.; Guo, Y. Insights into Plant Salt Stress Signaling and Tolerance. J. Genet. Genomics 2024, 51, 16–34. [Google Scholar] [CrossRef]
- Hussain, S.J.; Khan, N.A.; Anjum, N.A.; Masood, A.; Khan, M.I.R. Mechanistic Elucidation of Salicylic Acid and Sulphur-Induced Defence Systems, Nitrogen Metabolism, Photosynthetic, and Growth Potential of Mungbean (Vigna radiata) Under Salt Stress. J. Plant Growth Regul. 2021, 40, 1000–1016. [Google Scholar]
- Alotaibi, S.; Ali, E.; Darwesh, H.; Ahmed, A.T.; Al-Thubaiti, E. Effect of Proline on Growth and Nutrient Uptake of Simmondsia chinensis (Link) Schneider Under Salinity Stress. Pak. J. Biol. Sci. 2019, 22, 412–418. [Google Scholar]
- Rodríguez-Navarro, A.; Rubio, F. High-Affinity Potassium and Sodium Transport Systems in Plants. J. Exp. Bot. 2006, 57, 1149–1160. [Google Scholar] [PubMed]
- Shelke, D.B.; Nikalje, G.C.; Nikam, T.D.; Maheshwari, P.; Punita, D.L.; Rao, K.R.S.S.; Kishor, K.; Suprasanna, P. Chloride (Cl−) Uptake, Transport, and Regulation in Plant Salt Tolerance. In Molecular Plant Abiotic Stress: Biology and Biotechnology; Wiley: Hoboken, NJ, USA, 2019; pp. 241–268. [Google Scholar]
- Rashedy, A.A.; Abd-ElNafea, M.H.; Khedr, E.H. Co-Application of Proline or Calcium and Humic Acid Enhances Productivity of Salt-Stressed Pomegranate by Improving Nutritional Status and Osmoregulation Mechanisms. Sci. Rep. 2022, 12, 14285. [Google Scholar] [CrossRef]
- Ahmad, P.; Hashem, A.; Abd-Allah, E.F.; Alqarawi, A.A.; John, R.; Egamberdieva, D.; Gucel, S. Role of Trichoderma harzianum in Mitigating NaCl Stress in Indian Mustard (Brassica juncea L.) Through Antioxidative Defense System. Front. Plant Sci. 2015, 6, 868. [Google Scholar]
- Zamljen, T.; Medic, A.; Hudina, M.; Veberic, R.; Slatnar, A. Salt Stress Differentially Affects the Primary and Secondary Metabolism of Peppers (Capsicum annuum L.) According to the Genotype, Fruit Part, and Salinity Level. Plants 2022, 11, 853. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants Under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Saddhe, A.A.; Manuka, R.; Penna, S. Plant Sugars: Homeostasis and Transport Under Abiotic Stress in Plants. Physiol. Plant. 2021, 171, 739–755. [Google Scholar]
- Wani, A.B.; Chadar, H.; Wani, A.H.; Singh, S.; Upadhyay, N. Salicylic Acid to Decrease Plant Stress. Environ. Chem. Lett. 2017, 15, 101–123. [Google Scholar]
- Csiszár, J.; Horváth, E.; Váry, Z.; Gallé, Á.; Bela, K.; Brunner, S.; Tari, I. Glutathione Transferase Supergene Family in Tomato: Salt Stress-Regulated Expression of Representative Genes From Distinct GST Classes in Plants Primed With Salicylic Acid. Plant Physiol. Biochem. 2014, 78, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Freitas, P.A.F.; de Carvalho, H.H.; Costa, J.H.; Miranda, R.D.S.; Saraiva, K.D.D.C.; de Oliveira, F.D.B.; Gomes-Filho, E. Salt Acclimation in Sorghum Plants by Exogenous Proline: Physiological and Biochemical Changes and Regulation of Proline Metabolism. Plant Cell Rep. 2019, 38, 403–416. [Google Scholar]
- Naz, M.; Hussain, S.; Ashraf, I.; Farooq, M. Exogenous Application of Proline and Phosphorus Help Improving Maize Performance Under Salt Stress. J. Plant Nutr. 2023, 46, 2342–2350. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar]
- Zhong, Q.; Hu, H.; Fan, B.; Zhu, C.; Chen, Z. Biosynthesis and Roles of Salicylic Acid in Balancing Stress Response and Growth in Plants. Int. J. Mol. Sci. 2021, 22, 11672. [Google Scholar] [CrossRef]
- Dong, W.; Stockwell, V.O.; Goyer, A. Enhancement of Thiamin Content in Arabidopsis thaliana by Metabolic Engineering. Plant Cell Physiol. 2015, 56, 2285–2296. [Google Scholar]
- Bagautdinova, Z.Z.; Omelyanchuk, N.; Tyapkin, A.V.; Kovrizhnykh, V.V.; Lavrekha, V.V.; Zemlyanskaya, E.V. Salicylic Acid in Root Growth and Development. Int. J. Mol. Sci. 2022, 23, 2228. [Google Scholar] [CrossRef]
- El-Bassiouny, H.S.M.; Bakry, B.A.; Attia, A.A.E.M.; Abd Allah, M.M. Physiological Role of Humic Acid and Nicotinamide on Improving Plant Growth, Yield, and Mineral Nutrient of Wheat (Triticum durum) Grown Under Newly Reclaimed Sandy Soil. Agric. Sci. 2014, 5, 687–700. [Google Scholar]
- Furlani, P.R.; Silveira, L.C.P.; Bolonhezi, D.; Faquim, V. Cultivo Hidropônico de Plantas; Instituto Agronômico: Campinas, Brazil, 1999. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Silva, E.N.; Ferreira-Silva, S.L.; Viégas, R.A.; Silveira, J.A.G. The Role of Organic and Inorganic Solutes in the Osmotic Adjustment of Drought-Stressed Jatropha curcas Plants. Environ. Exp. Bot. 2010, 69, 279–285. [Google Scholar] [CrossRef]
- Silva, E.N.D.; Silveira, J.A.G.; Rodrigues, C.R.F.; Lima, C.S.D.; Viégas, R.A. Contribuição de Solutos Orgânicos e Inorgânicos no Ajustamento Osmótico de Pinhão-Manso Submetido à Salinidade. Pesqui. Agropecu. Bras. 2009, 44, 437–445. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, P. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Biochem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Yemm, E.W.; Cocking, E.C.; Ricketts, R. The Determination of Amino Acids With Ninhydrin. Analyst 1955, 80, 209–214. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
| Treatment | Description |
|---|---|
| T0 | 0 mM NaCl (control) |
| T1 | 40 mM NaCl |
| T2 | 40 mM NaCl + Pro |
| T3 | 40 mM NaCl + SA |
| T4 | 40 mM NaCl + NAM |
| T5 | 40 mM NaCl + Pro + SA |
| T6 | 40 mM NaCl + Pro + NAM |
| T7 | 40 mM NaCl + NAM + SA |
| T8 | 40 mM NaCl + NAM + SA + Pro |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, T.S.d.; Correia, M.R.S.; Sena, L.S.; Santana, L.P.d.S.; Silva, G.B.G.d.; Lima, K.S.; Dutra, E.V.d.S.; Adas, M.E.; Ribeiro, M.C.B.d.O.; Ribeiro, J.E.d.S.; et al. The Combination of Salicylic Acid, Nicotinamide, and Proline Mitigates the Damage Caused by Salt Stress in Nasturtium (Tropaeolum majus). Plants 2025, 14, 1156. https://doi.org/10.3390/plants14081156
Santos TSd, Correia MRS, Sena LS, Santana LPdS, Silva GBGd, Lima KS, Dutra EVdS, Adas ME, Ribeiro MCBdO, Ribeiro JEdS, et al. The Combination of Salicylic Acid, Nicotinamide, and Proline Mitigates the Damage Caused by Salt Stress in Nasturtium (Tropaeolum majus). Plants. 2025; 14(8):1156. https://doi.org/10.3390/plants14081156
Chicago/Turabian StyleSantos, Thainan Sipriano dos, Marcos Roberto Santos Correia, Luma Santos Sena, Laura Pereira dos Santos Santana, Geovanna Buique Gualberto da Silva, Keilane Silva Lima, Elienay Vinícius da Silva Dutra, Myriam El Adas, Maria Carolina Borges de Oliveira Ribeiro, João Everthon da Silva Ribeiro, and et al. 2025. "The Combination of Salicylic Acid, Nicotinamide, and Proline Mitigates the Damage Caused by Salt Stress in Nasturtium (Tropaeolum majus)" Plants 14, no. 8: 1156. https://doi.org/10.3390/plants14081156
APA StyleSantos, T. S. d., Correia, M. R. S., Sena, L. S., Santana, L. P. d. S., Silva, G. B. G. d., Lima, K. S., Dutra, E. V. d. S., Adas, M. E., Ribeiro, M. C. B. d. O., Ribeiro, J. E. d. S., Ribas, R. F., Silva, E. F. d., Rubio-Casal, A. E., Barros Júnior, A. P., Tang, X., Silva, T. G. F. d., Jardim, A. M. d. R. F., & Silva, T. I. d. (2025). The Combination of Salicylic Acid, Nicotinamide, and Proline Mitigates the Damage Caused by Salt Stress in Nasturtium (Tropaeolum majus). Plants, 14(8), 1156. https://doi.org/10.3390/plants14081156










