Organic Fertilization and Biostimulant Application to Improve Yield and Quality of Eggplant While Reducing the Environmental Impact
Abstract
1. Introduction
2. Results
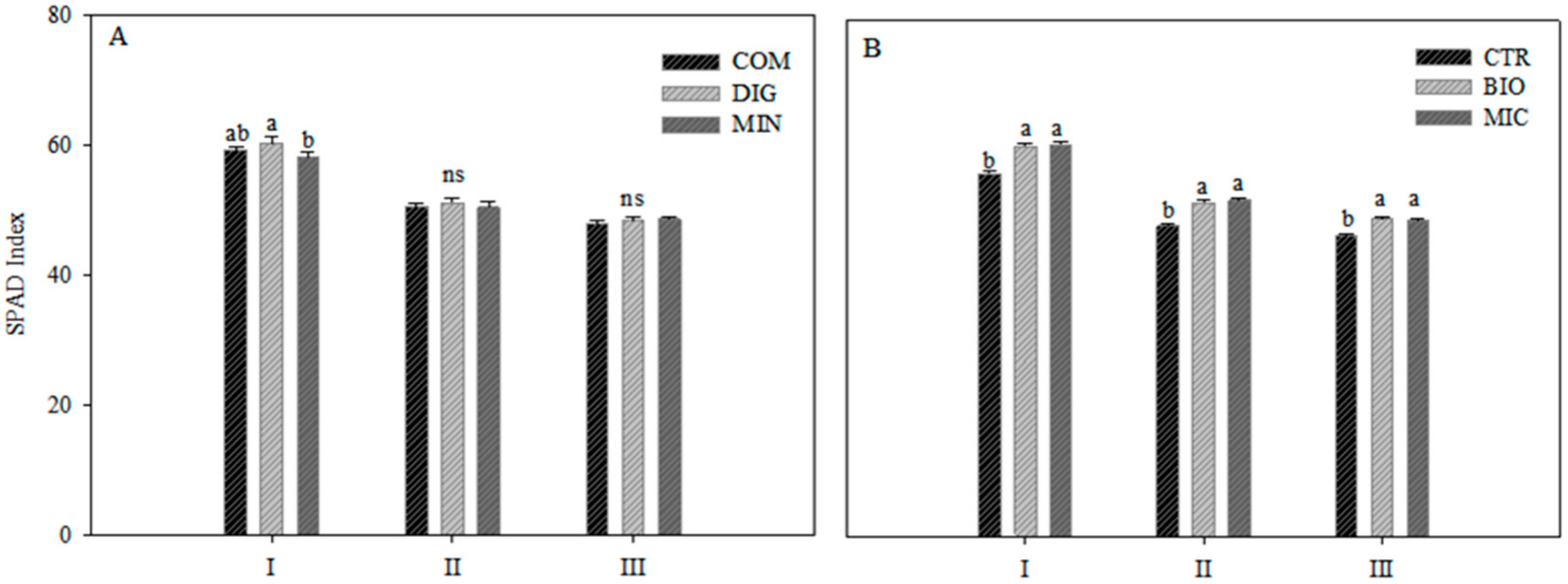
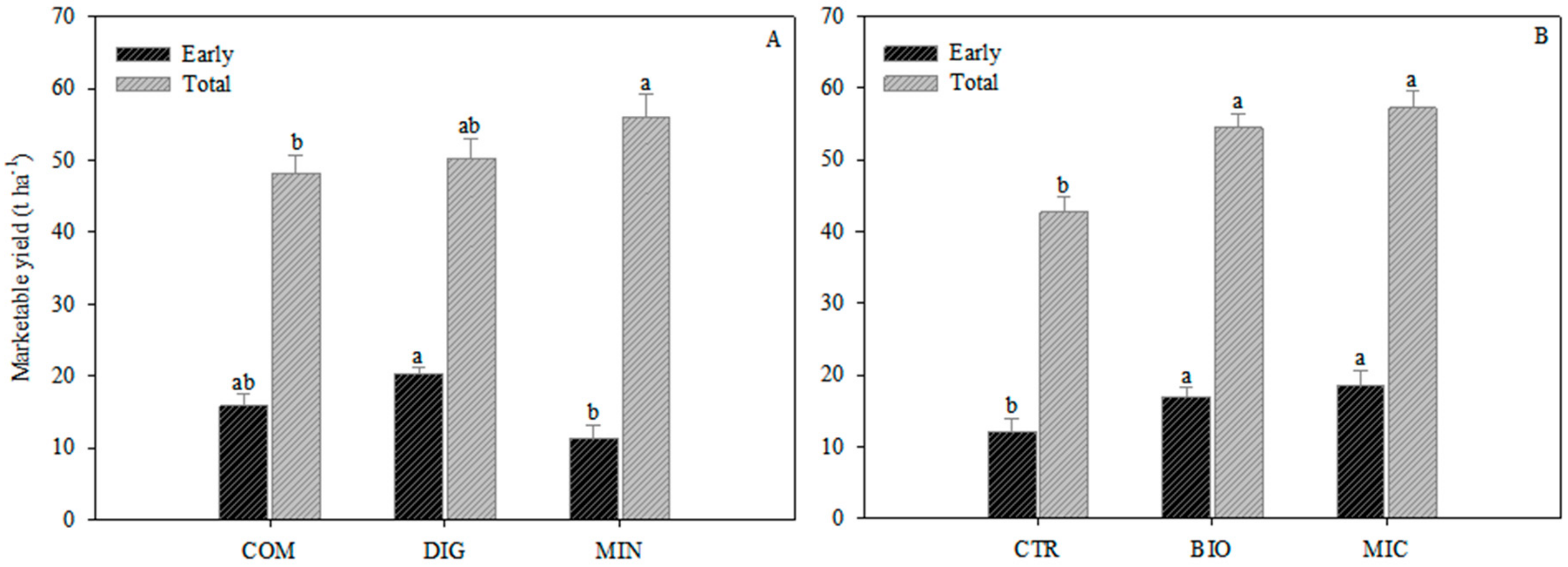
2.1. Plant Growth and Fruit Production
2.2. Eggplant Fruit Quality
2.2.1. Color Parameters
2.2.2. Antioxidant Activity and Main Nutraceutical Compounds
3. Discussion
4. Materials and Methods
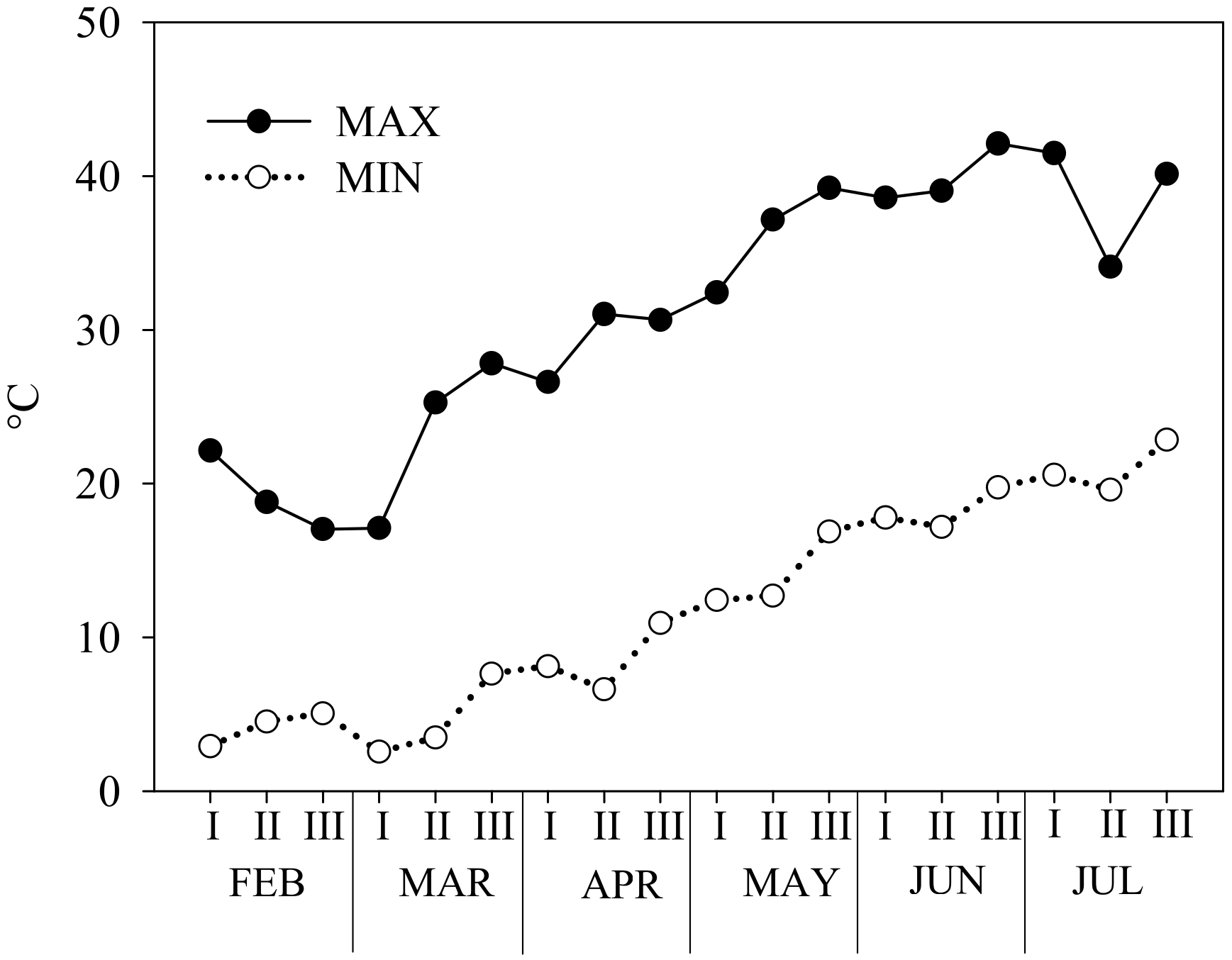
4.1. Plant Material, Growth Condition, and Experimental Treatments
4.2. Biometric Measurements, Leaf Greenness, and Fruit Yield
4.3. Qualitative Traits
4.3.1. Color Parameters and Firmness
4.3.2. Nitrogen and Carotenoid Content
4.3.3. Ascorbic Acid and Total Phenol Content, and Antioxidant Activity
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montemurro, F.; Ciaccia, C.; Leogrande, R.; Ceglie, F.; Diacono, M. Suitability of Different Organic Amendments from Agro-Industrial Wastes in Organic Lettuce Crops. Nutr. Cycl. Agroecosystems 2015, 102, 243–252. [Google Scholar] [CrossRef]
- Amsili, J.P.; van Es, H.M.; Schindelbeck, R.R. Cropping System and Soil Texture Shape Soil Health Outcomes and Scoring Functions. Soil Secur. 2021, 4, 100012. [Google Scholar] [CrossRef]
- Rubio, V.; Núñez, A.; Berger, A.; van Es, H. Biomass Inputs Drive Agronomic Management Impacts on Soil Health. Agric. Ecosyst. Environ. 2025, 378, 109316. [Google Scholar] [CrossRef]
- Clapp, C.E.; Hayes, M.H.B.; Ciavatta, C. Organic Wastes in Soils: Biogeochemical and Environmental Aspects. Soil Biol. Biochem. 2007, 39, 1239–1243. [Google Scholar] [CrossRef]
- Verrillo, M.; Khan, M.R.; Volpe, S.; Spaccini, R.; Torrieri, E. Valorization of Organic Biomass through the Production of Active Biopolymer Film Based on Sodium Caseinate, Guar Gum, and Beeswax. Food Biosci. 2023, 53, 102757. [Google Scholar] [CrossRef]
- Tambone, F.; Scaglia, B.; D’Imporzano, G.; Schievano, A.; Orzi, V.; Salati, S.; Adani, F. Assessing Amendment and Fertilizing Properties of Digestates from Anaerobic Digestion through a Comparative Study with Digested Sludge and Compost. Chemosphere 2010, 81, 577–583. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The Use of Biostimulants for Enhancing Nutrient Uptake. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2015; pp. 141–174. [Google Scholar] [CrossRef]
- Harrison-Kirk, T.; Beare, M.H.; Meenken, E.D.; Condron, L.M. Soil Organic Matter and Texture Affect Responses to Dry/Wet Cycles: Effects on Carbon Dioxide and Nitrous Oxide Emissions. Soil Biol. Biochem. 2013, 57, 43–55. [Google Scholar] [CrossRef]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Nocerino, S.; Rouphael, Y.; Colla, G.; El-Nakhel, C.; Mori, M. Nitrogen Use and Uptake Efficiency and Crop Performance of Baby Spinach (Spinacia oleracea L.) and Lamb’s Lettuce (Valerianella locusta L.) Grown under Variable Sub-Optimal N Regimes Combined with Plant-Based Biostimulant Application. Agronomy 2020, 10, 278. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural Uses of Plant Biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Cirillo, V.; Romano, I.; Woo, S.L.; Di Stasio, E.; Lombardi, N.; Comite, E.; Pepe, O.; Ventorino, V.; Maggio, A. Inoculation with a Microbial Consortium Increases Soil Microbial Diversity and Improves Agronomic Traits of Tomato under Water and Nitrogen Deficiency. Front. Plant Sci. 2023, 14, 1304627. [Google Scholar] [CrossRef]
- Telo da Gama, J. The Role of Soils in Sustainability, Climate Change, and Ecosystem Services: Challenges and Opportunities. Ecologies 2023, 4, 552–567. [Google Scholar] [CrossRef]
- Geissdoerfer, M.; Savaget, P.; Bocken, N.M.P.; Hultink, E.J. The Circular Economy—A New Sustainability Paradigm? J. Clean. Prod. 2017, 143, 757–768. [Google Scholar] [CrossRef]
- Taher, D.; Solberg, S.Ø.; Prohens, J.; Chou, Y.; Rakha, M.; Wu, T. World Vegetable Center Eggplant Collection: Origin, Composition, Seed Dissemination and Utilization in Breeding. Front. Plant Sci. 2017, 8, 1484. [Google Scholar] [CrossRef] [PubMed]
- Adamczewska-Sowińska, K.; Krygier, M. Yield Quantity and Quality of Field Cultivated Eggplant in Relation to Its Cultivar and the Degree of Fruit Maturity. Acta Sci. Pol. Hortorum Cultus 2013, 12, 13–23. [Google Scholar]
- Consentino, B.B.; Vultaggio, L.; Allevato, E.; Sabatino, L.; Ntatsi, G.; Ciriello, M.; Rouphael, Y.; Di Miceli, G. Plant Protein Hydrolysate and Arbuscular Mycorrhizal Fungi Synergistically Orchestrate Eggplant Tolerance to Iodine Supply: A Two-Year Study. Sci. Hortic. 2024, 336, 113437. [Google Scholar] [CrossRef]
- Caruso, G.; Pokluda, R.; Sękara, A.; Kalisz, A.; Jezdinský, A.; Kopta, T.; Grabowska, A. Agricultural Practices, Biology and Quality of Eggplant Cultivated in Central Europe. A Review. Hortic. Sci. 2017, 44, 201–212. [Google Scholar] [CrossRef]
- Ji, T.; Guo, X.; Wu, F.; Wei, M.; Li, J.; Ji, P.; Wang, N.; Yang, F. Proper Irrigation Amount for Eggplant Cultivation in a Solar Greenhouse Improved Plant Growth, Fruit Quality and Yield by Influencing the Soil Microbial Community and Rhizosphere Environment. Front. Microbiol. 2022, 13, 981288. [Google Scholar] [CrossRef]
- Morra, L.; Bilotto, M.; Raimo, F.; Pizzolongo, G.; Zaccardelli, M.; Pentangelo, A. Yield Response of an Eggplant Ecotype Fertilized with Compost in Two Localities of Campania (Italy). Italus Hortus 2010, 17, 99–101. [Google Scholar]
- Pane, C.; Francese, G.; Raimo, F.; Mennella, G.; Zaccardelli, M. Activity of Foliar Extracts of Cultivated Eggplants against Sclerotinia Lettuce Drop Disease and Their Phytochemical Profiles. Eur. J. Plant Pathol. 2017, 148, 687–697. [Google Scholar] [CrossRef]
- Yan, L.; Liu, C.; Zhang, Y.; Liu, S.; Zhang, Y. Effects of C/N Ratio Variation in Swine Biogas Slurry on Soil Dissolved Organic Matter: Content and Fluorescence Characteristics. Ecotoxicol. Environ. Saf. 2021, 209, 111804. [Google Scholar] [CrossRef]
- Jin, K.; Ran, Y.; Alengebawy, A.; Yang, G.; Jia, S.; Ai, P. Agro-Environmental Sustainability of Using Digestate Fertilizer for Solanaceous and Leafy Vegetables Cultivation: Insights on Fertilizer Efficiency and Risk Assessment. J. Environ. Manag. 2022, 320, 115895. [Google Scholar] [CrossRef] [PubMed]
- Castro Marín, I.; Loef, I.; Bartetzko, L.; Searle, I.; Coupland, G.; Stitt, M.; Osuna, D. Nitrate Regulates Floral Induction in Arabidopsis, Acting Independently of Light, Gibberellin and Autonomous Pathways. Planta 2011, 233, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Sambo, P.; Sanchez-Cortes, S.; Nardi, S. Capsicum chinensis L. Growth and Nutraceutical Properties Are Enhanced by Biostimulants in a Long-Term Period: Chemical and Metabolomic Approaches. Front. Plant Sci. 2014, 5, 375. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein Hydrolysates as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Dordas, C.A.; Lithourgidis, A.S.; Matsi, T.; Barbayiannis, N. Application of liquid cattle manure and inorganic fertilizers affect dry matter, nitrogen accumulation, and partitioning in Maize. Nutr. Cycl. Agroecosystems 2008, 80, 283–296. [Google Scholar] [CrossRef]
- Arduini, I.; Cardelli, R.; Pampana, S. Biosolids Affect the Growth, Nitrogen Accumulation and Nitrogen Leaching of Barley. Plant Soil Environ. 2018, 64, 95–101. [Google Scholar] [CrossRef]
- Dion, P.-P.; Jeanne, T.; Thériault, M.; Hogue, R.; Pepin, S.; Dorais, M. Nitrogen Release from Five Organic Fertilizers Commonly Used in Greenhouse Organic Horticulture with Contrasting Effects on Bacterial Communities. Can. J. Soil Sci. 2020, 100, 120–135. [Google Scholar] [CrossRef]
- Rodrigues, M.; Baptistella, J.L.C.; Horz, D.C.; Bortolato, L.M.; Mazzafera, P. Organic Plant Biostimulants and Fruit Quality—A Review. Agronomy 2020, 10, 988. [Google Scholar] [CrossRef]
- Consentino, B.B.; Sabatino, L.; Vultaggio, L.; Rotino, G.L.; La Placa, G.G.; D’Anna, F.; Leto, C.; Iacuzzi, N.; De Pasquale, C. Grafting Eggplant Onto Underutilized Solanum Species and Biostimulatory Action of Azospirillum Brasilense Modulate Growth, Yield, NUE and Nutritional and Functional Traits. Horticulturae 2022, 8, 722. [Google Scholar] [CrossRef]
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat. Rev. Microbiol. 2023, 21, 312–326. [Google Scholar] [CrossRef]
- Hu, H.; Liu, H.; Zhang, H.; Zhu, J.; Yao, X.; Zhang, X.; Zheng, K. Assessment of Chlorophyll Content Based on Image Color Analysis, Comparison with SPAD-502. In Proceedings of the 2010 2nd International Conference on Information Engineering and Computer Science, IEEE, Hangzhou, China, 4–6 December 2010; pp. 1–3. [Google Scholar] [CrossRef]
- Jiang, C.; Johkan, M.; Hohjo, M.; Tsukagoshi, S.; Maruo, T. A Correlation Analysis on Chlorophyll Content and SPAD Value in Tomato Leaves. HortResearch 2017, 71, 37–42. [Google Scholar] [CrossRef]
- Jain, A.; Singh, A.; Singh, S.; Singh, H.B. Biological Management of Sclerotinia sclerotiorum in Pea Using Plant Growth Promoting Microbial Consortium. J. Basic Microbiol. 2015, 55, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Singh, S.; Singh, H.B.; Sarma, B.K. Compatible Rhizosphere-Competent Microbial Consortium Adds Value to the Nutritional Quality in Edible Parts of Chickpea. J. Agric. Food Chem. 2017, 65, 6122–6130. [Google Scholar] [CrossRef] [PubMed]
- Kaur, T.; Devi, R.; Negi, R.; Kumar, S.; Singh, S.; Rustagi, S.; Shreaz, S.; Rai, A.K.; Kour, D.; Yadav, A.N. Microbial Consortium with Multifunctional Attributes for the Plant Growth of Eggplant (Solanum melongena L.). Folia Microbiol. 2024, 69, 1255–1266. [Google Scholar] [CrossRef]
- Michalojc, Z.; Buczkowska, H. Yield and Eggplant Fruit Quality (Solanum melongena L.) Dependent on Plant Training and Nitrogen Fertlization. Ecol. Chem. Eng. A 2011, 18, 73–81. [Google Scholar]
- Hassan, S.A.; Mijin, S.; Yusoff, U.K.; Ding, P.; Wahab, P.E.M. Nitrate, Ascorbic Acid, Mineral and Antioxidant Activities of Cosmos Caudatus in Response to Organic and Mineral-Based Fertilizer Rates. Molecules 2012, 17, 7843–7853. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The Hydrophilic and Lipophilic Contribution to Total Antioxidant Activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Aboul-Enein, H.Y.; Berczynski, P.; Kruk, I. Phenolic Compounds: The Role of Redox Regulation in Neurodegenerative Disease and Cancer. Mini Rev. Med. Chem. 2013, 13, 385–398. [Google Scholar] [CrossRef]
- Luna-Guevara, M.L.; Luna-Guevara, J.J.; Hernández-Carranza, P.; Ruíz-Espinosa, H.; Ochoa-Velasco, C.E. Phenolic Compounds: A Good Choice Against Chronic Degenerative Diseases. Stud. Nat. Prod. Chem. 2018, 59, 79–108. [Google Scholar] [CrossRef]
- Krinsky, N.I. Antioxidant Functions of Carotenoids. Free Radic. Biol. Med. 1989, 7, 617–635. [Google Scholar] [CrossRef]
- Avio, L.; Sbrana, C.; Giovannetti, M.; Frassinetti, S. Arbuscular Mycorrhizal Fungi Affect Total Phenolics Content and Antioxidant Activity in Leaves of Oak Leaf Lettuce Varieties. Sci. Hortic. 2017, 224, 265–271. [Google Scholar] [CrossRef]
- Bremner, J.M. Inorganic Forms of Nitrogen. In Methods of Soil Analysis, Agronomy Monograph; Black, C.A., Evans, D.D., White, I.L., Ensminger, L.E., Clark, F.E., Eds.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 1179–1237. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Vanmontagu, M.; Inze, D. Extraction and Determination of Ascorbate and Dehydroascorbate from Plant Tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Fogliano, V.; Verde, V.; Randazzo, G.; Ritieni, A. Method for Measuring Antioxidant Activity and Its Application to Monitoring the Antioxidant Capacity of Wines. J. Agric. Food Chem. 1999, 47, 1035–1040. [Google Scholar] [CrossRef]
- Pellegrini, N.; Re, R.; Yang, M.; Rice-Evans, C. Screening of Dietary Carotenoids and Carotenoid-Rich Fruit Extracts for Antioxidant Activities Applying 2,2′-Azinobis (3-Ethylenebenzothiazoline-6-Sulfonic Acid Radical Cation Decolorization Assay). In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; pp. 379–389. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; pp. 152–178. [Google Scholar] [CrossRef]

| Treatments | Early Marketable Yield | Total Marketable Fruits | DM | Firmness | N-Kjeldahl | ||
|---|---|---|---|---|---|---|---|
| n° m−2 | g fruit−1 | n° m−2 | g fruit−1 | % | kg cm−2 | % | |
| Fertilization | |||||||
| COM | 7.8 ± 0.7 b | 199.5 ± 3.9 b | 25.5 ± 1.3 b | 186.2 ± 4.7 b | 6.53 ± 0.1 b | 1.89 ± 0.02 | 1.92 ± 0.04 b |
| DIG | 9.5 ± 0.7 a | 213.5 ± 3.8 a | 25.9 ± 1.2 b | 193.4 ± 2.3 ab | 6.87 ± 0.1 ab | 1.89 ± 0.02 | 2.08 ± 0.02 a |
| MIN | 5.3 ± 0.3 c | 213.5 ± 6.8 a | 27.4 ± 1.1 a | 198.7 ± 2.6 a | 7.14 ± 0.1 a | 1.90 ± 0.02 | 1.98 ± 0.07 ab |
| Biostimulant | |||||||
| CTR | 6.0 ± 0.6 b | 200.3 ± 4.8 b | 22.2 ± 0.7 b | 189.3 ± 1.9 | 6.73 ± 0.15 | 1.84 ± 0.01 b | 1.95 ± 0.05 |
| BIO | 8.1 ± 0.6 a | 208.9 ± 5.9 ab | 27.7 ± 0.6 a | 193.3 ± 2.6 | 6.91 ± 0.12 | 1.90 ± 0.01 ab | 2.01 ± 0.04 |
| MIC | 8.5 ± 0.9 a | 217.3 ± 4.1 a | 28.8 ± 0.8 a | 195.7 ± 2.5 | 6.91 ± 0.11 | 1.94 ± 0.02 a | 2.02 ± 0.05 |
| Significance | |||||||
| Fertilization (F) | ** | * | ** | * | ** | ns | * |
| Biostimulant (B) | ** | * | ** | ns | ns | ** | ns |
| F × B | ns | ns | ns | ns | ns | ns | ns |
| Treatments | L* | a* | b* |
|---|---|---|---|
| Fertilization | |||
| COM | 25.7 ± 0.31 | 7.21 ± 0.58 | 0.96 ± 0.17 ab |
| DIG | 26.3 ± 0.44 | 7.38 ± 0.40 | 1.07 ± 0.32 a |
| MIN | 25.7 ± 0.18 | 7.22 ± 0.45 | 0.60 ± 0.09 b |
| Biostimulant | |||
| CTR | 26.5 ± 0.46 | 8.26 ± 0.36 a | 1.40 ± 0.28 a |
| BIO | 25.5 ± 0.22 | 6.31 ± 0.23 b | 0.51 ± 0.10 b |
| MIC | 25.7 ± 0.17 | 7.24 ± 0.53 ab | 0.71 ± 0.10 b |
| Significance | |||
| Fertilization (F) | ns | ns | * |
| Biostimulant (B) | ns | * | ** |
| F × B | ns | ns | ns |
| Treatments | Carotenoids | AsA | Phenols | HAA | ABTS |
|---|---|---|---|---|---|
| µg g−1 fw | mg g−1 fw | mg Gallic Acid g−1 dw | mmol AA 100 g−1 dw | mmol Trolox 100 g−1 dw | |
| Fertilization | |||||
| COM | 0.017 ± 0.001 | 45.42 ± 5.45 a | 2.80 ± 0.09 | 5.26 ± 0.35 ab | 12.87 ± 0.51 |
| DIG | 0.019 ± 0.001 | 32.82 ± 5.06 ab | 2.44 ± 0.16 | 5.71 ± 0.28 a | 13.11 ± 0.65 |
| MIN | 0.020 ± 0.002 | 29.15 ± 3.61 b | 2.73 ± 0.17 | 4.88 ± 0.14 b | 12.47 ± 0.80 |
| Biostimulant | |||||
| CTR | 0.016 ± 0.001 b | 36.87 ± 4.77 | 2.62 ± 0.08 ab | 5.38 ± 0.32 | 12.81 ± 0.69 ab |
| BIO | 0.018 ± 0.001 ab | 37.44 ± 5.46 | 2.46 ± 0.17 b | 5.25 ± 0.26 | 12.11 ± 0.58 b |
| MIC | 0.022 ± 0.002 a | 33.08 ± 5.72 | 2.89 ± 0.16 a | 5.22 ± 0.30 | 13.53 ± 0.65 a |
| Significance | |||||
| Fertilization (F) | ns | * | ns | * | ns |
| Biostimulant (B) | * | ns | * | ns | * |
| F × B | ns | ns | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duri, L.G.; Paradiso, R.; Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Marra, R.; Mori, M. Organic Fertilization and Biostimulant Application to Improve Yield and Quality of Eggplant While Reducing the Environmental Impact. Plants 2025, 14, 962. https://doi.org/10.3390/plants14060962
Duri LG, Paradiso R, Di Mola I, Cozzolino E, Ottaiano L, Marra R, Mori M. Organic Fertilization and Biostimulant Application to Improve Yield and Quality of Eggplant While Reducing the Environmental Impact. Plants. 2025; 14(6):962. https://doi.org/10.3390/plants14060962
Chicago/Turabian StyleDuri, Luigi Giuseppe, Roberta Paradiso, Ida Di Mola, Eugenio Cozzolino, Lucia Ottaiano, Roberta Marra, and Mauro Mori. 2025. "Organic Fertilization and Biostimulant Application to Improve Yield and Quality of Eggplant While Reducing the Environmental Impact" Plants 14, no. 6: 962. https://doi.org/10.3390/plants14060962
APA StyleDuri, L. G., Paradiso, R., Di Mola, I., Cozzolino, E., Ottaiano, L., Marra, R., & Mori, M. (2025). Organic Fertilization and Biostimulant Application to Improve Yield and Quality of Eggplant While Reducing the Environmental Impact. Plants, 14(6), 962. https://doi.org/10.3390/plants14060962









