High Propagule Pressure and Patchy Biotic Resistance Control the Local Invasion Process of the Tree Ligustrum lucidum in a Subtropical Forest of Uruguay
Abstract
1. Introduction
2. Results
2.1. Regenerating Species Assemblage and L. lucidum Propagule Pressure
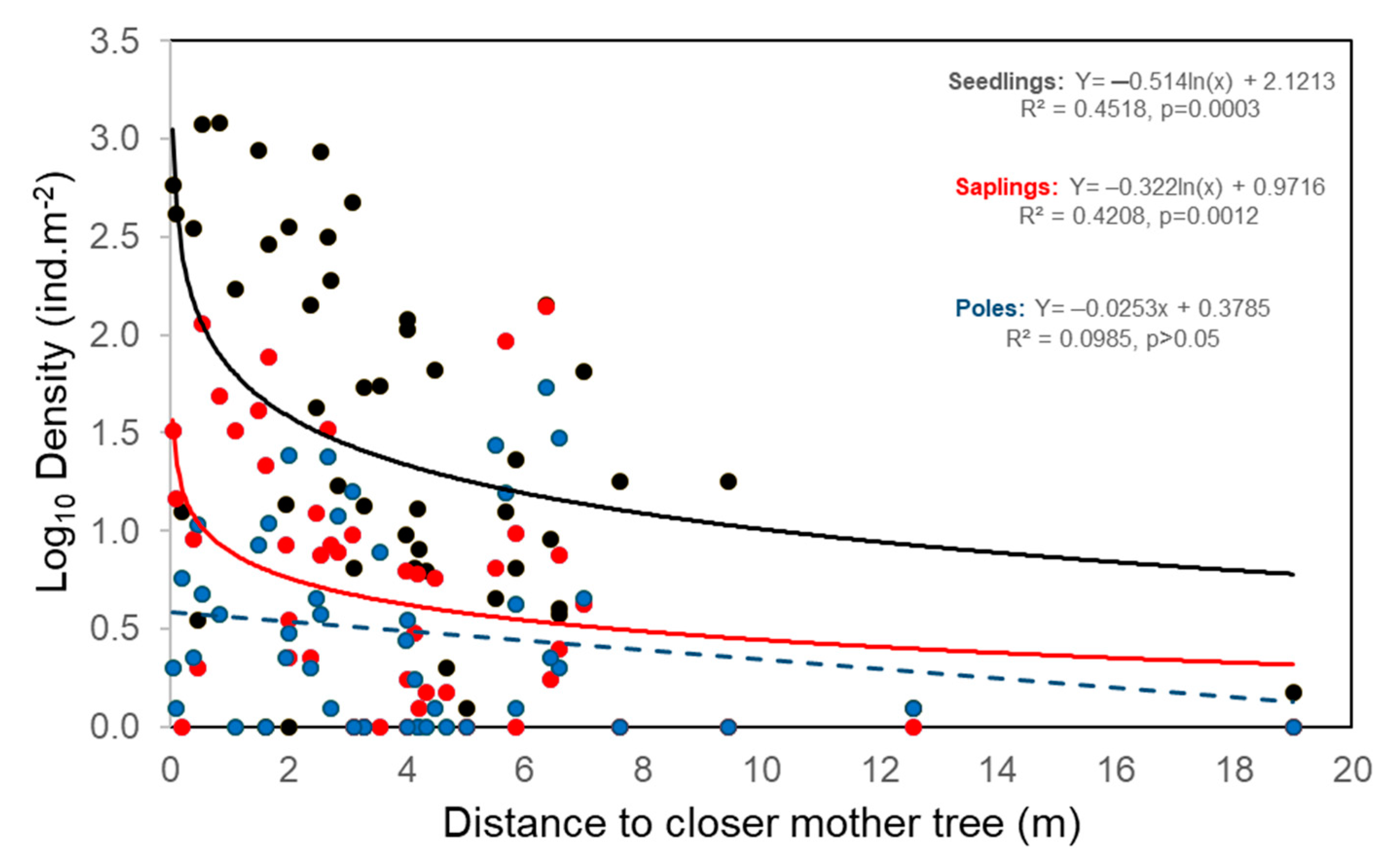
2.2. Exploring Determinant of L. lucidum Recruitment
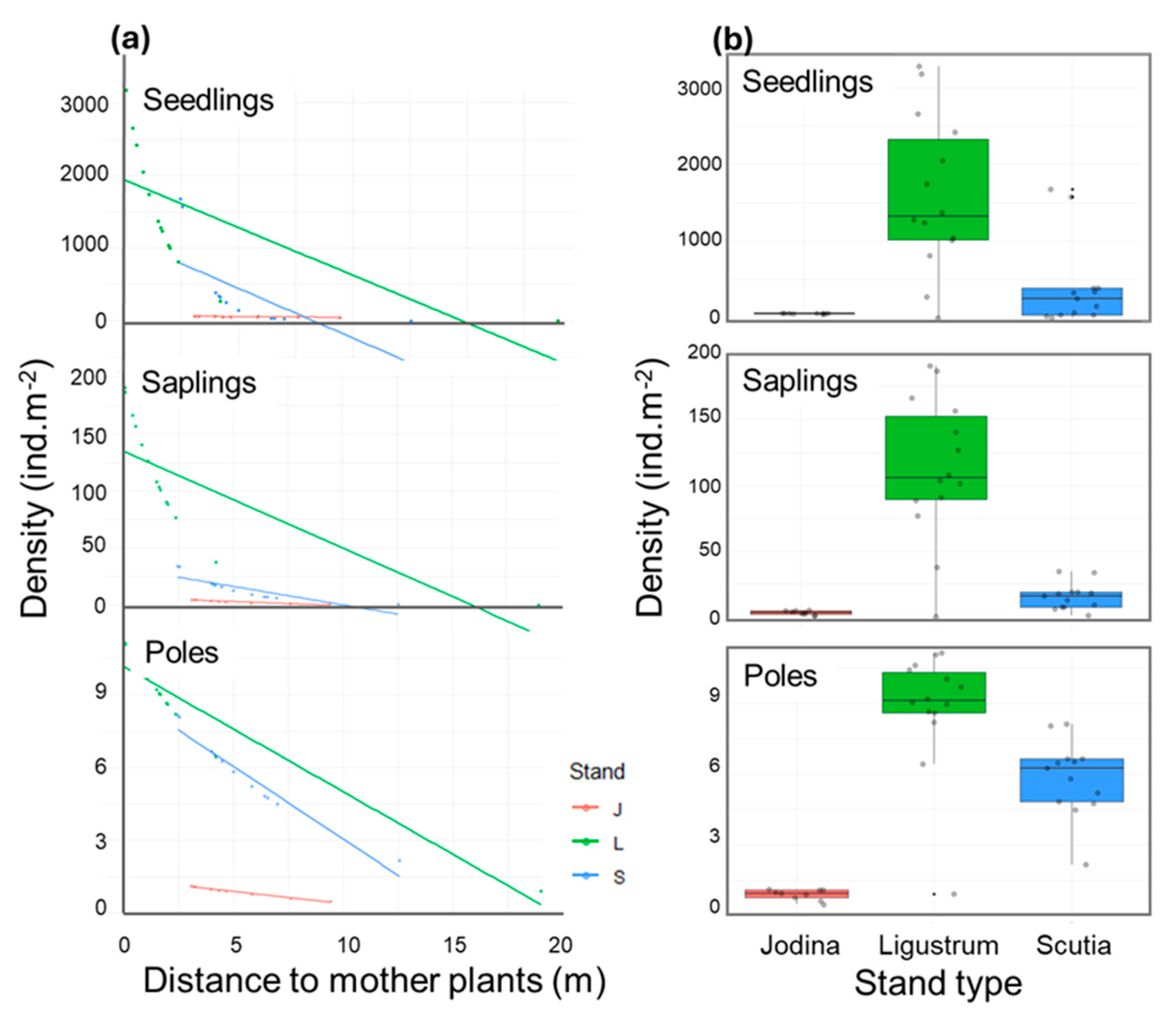
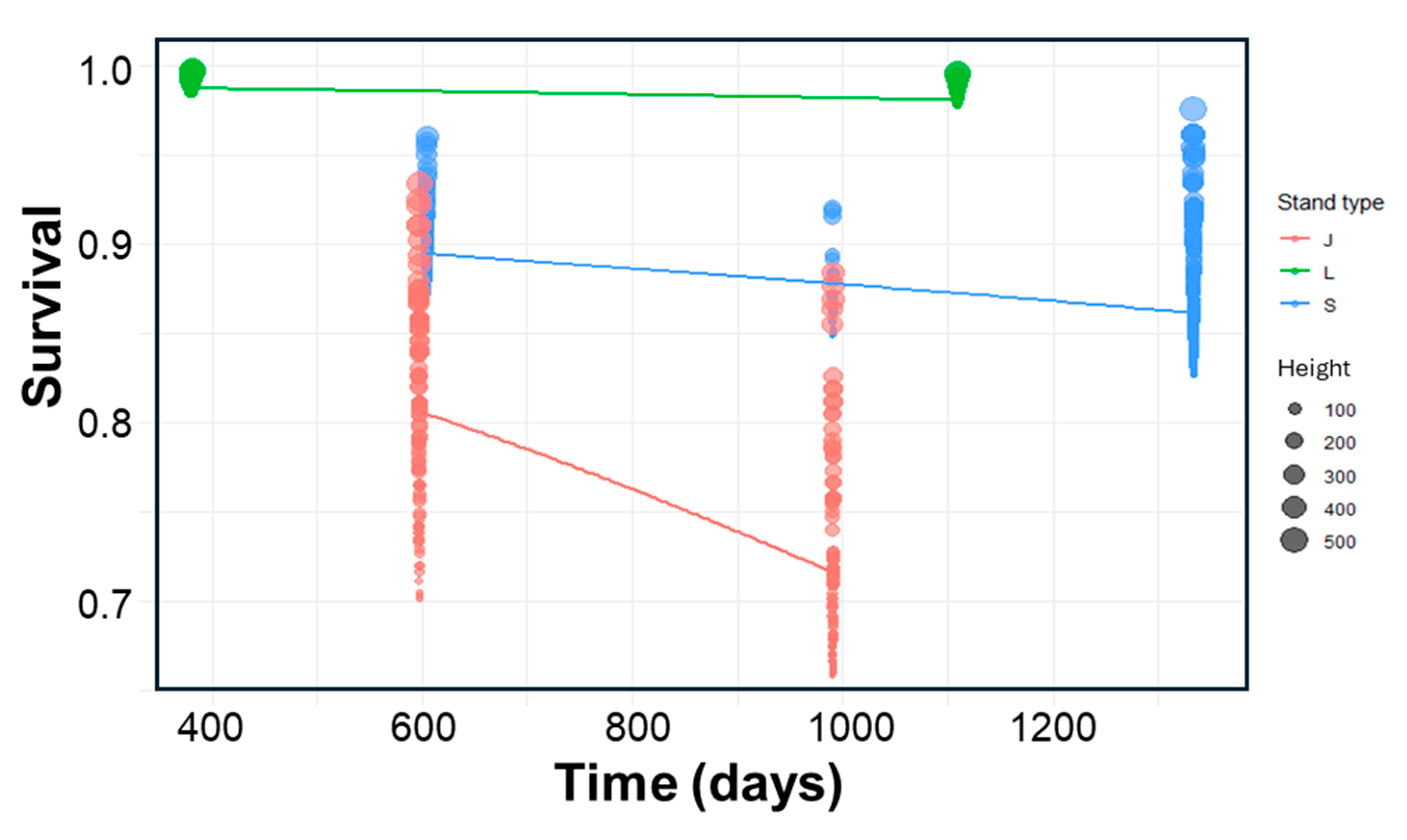
2.3. Survival Experiment: Assessing Stand Type Effects
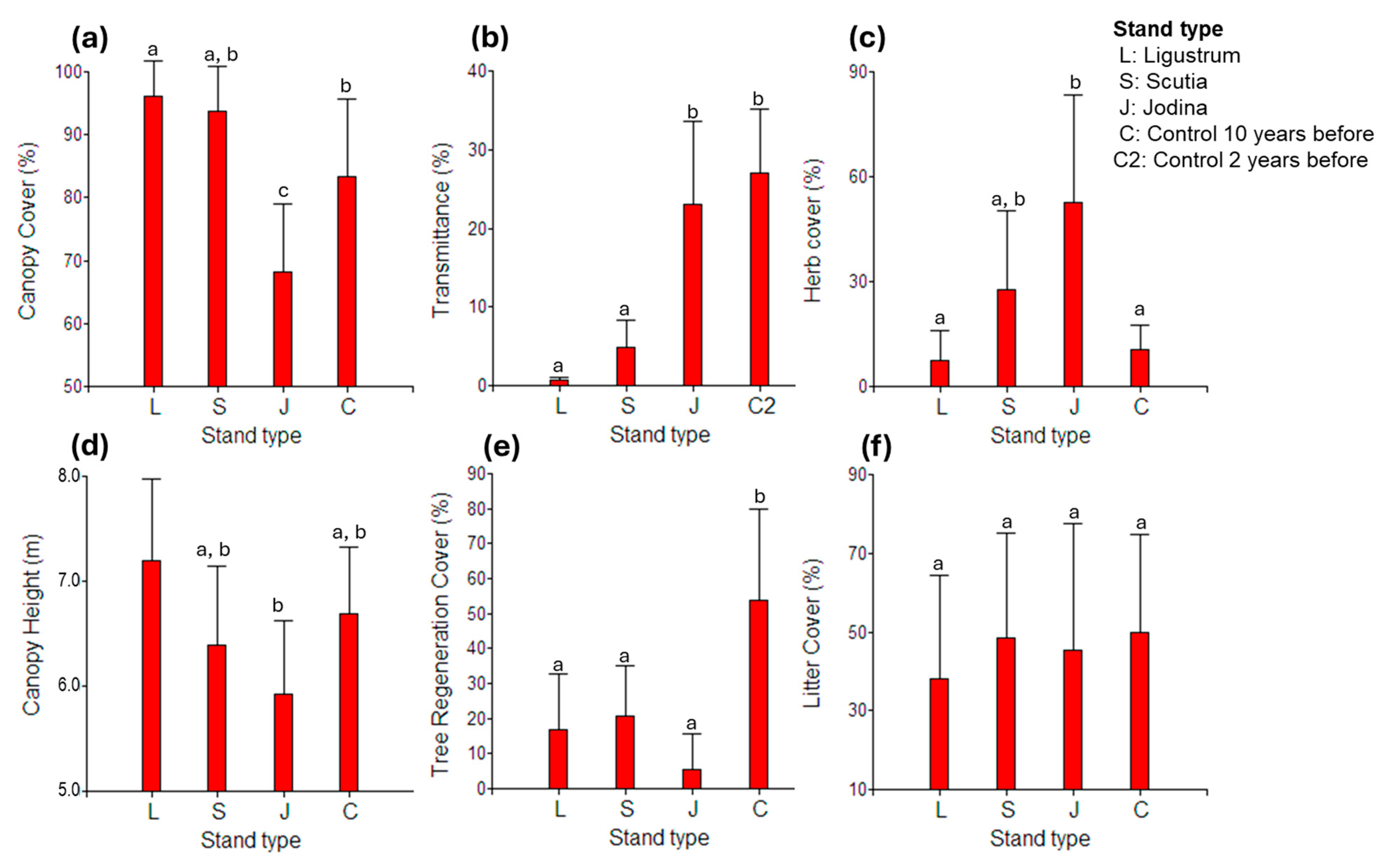
2.4. Possible Underlying Factors Behind Stand Type Effects
2.5. Effects of Previous Control Activities on Current Recruitment of L. lucidum
3. Discussion
3.1. High Propagule Pressure of L. lucidum
3.2. Small-Scale Dispersal from Parental Trees
3.3. Biotic Resistance in Jodina Stands to L. lucidum Invasion
3.4. Isolated Control of Adult Trees Is Not Sufficient to Restore Forests Invaded by L. lucidum
4. Materials and Methods
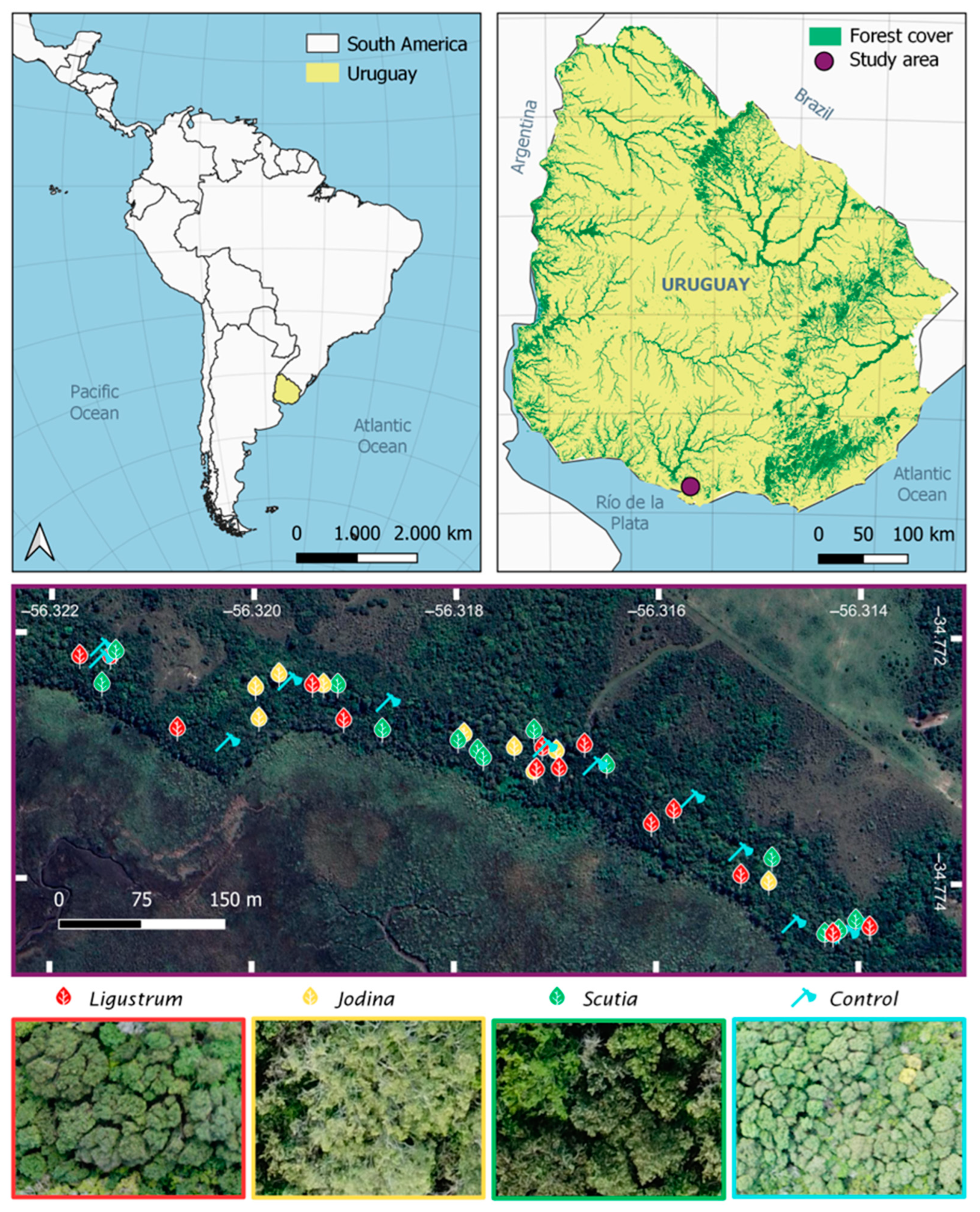
4.1. Study Area
4.2. Field Sampling
4.3. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIC | Akaike Information Criterion |
| DBH | Diameter at Breast Height |
| CV | Coefficient of Variation |
| GLM | General Linear Model |
| INUMET | Instituto Nacional de Meteorología de Uruguay |
| LSD | Least Significant Difference |
| PAR | Photosynthetically Active Range |
References
- Vitousek, P.N.; D’Antonio, C.M.; Loope, L.L.; Westbrook, R. Biological invasions as global environmental change. Am. Sci. 1996, 84, 468–478. [Google Scholar]
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Synthesis; Island Press: Washington, DC, USA, 2005; Available online: https://www.millenniumassessment.org/documents/document.356.aspx.pdf (accessed on 25 September 2023).
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Roy, H.E.; Pauchard, A.; Stoett, P.; Renard Truong, T.; Bacher, S.; Galil, B.S.; Hulme, P.E.; Ikeda, T.; Sankaran, K.; McGeoch, M.A.; et al. IPBES Invasive Alien Species Assessment: Summary for Policymakers; IPBES: Bonn, Germany, 2024. [Google Scholar] [CrossRef]
- Fuentes, M. Frugivory, seed dispersal and plant community ecology. Trends Ecol. Evol. 2000, 15, 487–488. [Google Scholar] [CrossRef]
- Williamson, M. Biological Invasions; Chapman & Hall: New York, NY, USA, 1996. [Google Scholar]
- Lockwood, J.L.; Hoopes, M.F.; Marchetti, M.P. Invasion Ecology; Blackwell Publishing: Oxford, UK, 2007. [Google Scholar]
- Davis, M.A. Invasion Biology; Oxford University Press: New York, NY, USA, 2009. [Google Scholar]
- Fernandez, R.; Ceballos, C.; Aragón, R.; Malizia, A.; Montti, L.; Whitworth-Hulse, J.; Castro-Diez, P.; Grau, H.R. A global review of the invasion of glossy privet (Ligustrum lucidum, Oleaceae). Bot. Rev. 2020, 86, 93–118. [Google Scholar] [CrossRef] [PubMed]
- Montti, L.; Velazco, S.J.; Travis, J.M.; Grau, H.R. Predicting current and future global distribution of invasive Ligustrum lucidum W.T. Aiton: Assessing emerging risks to biodiversity hotspots. Divers. Distrib. 2021, 27, 1568–1583. [Google Scholar] [CrossRef]
- Dreyer, J.B.B.; Higuchi, P.; Silva, A.C. Ligustrum lucidum W. T. Aiton (broad-leaf privet) demonstrates climatic niche shifts during global-scale invasion. Sci. Rep. 2019, 9, 3813. [Google Scholar] [CrossRef]
- Zamora Nasca, L.; Montti, L.; Grau, R.; Paolini, L. Efectos de la invasión del ligustro, Ligustrum lucidum, en la dinámica hídrica de las Yungas del noroeste argentino. Bosque 2014, 35, 195–205. [Google Scholar] [CrossRef]
- Aguirre-Acosta, N.; Kowaljow, E.; Aguilar, R. Reproductive performance of the invasive tree Ligustrum lucidum in a subtropical dry forest: Does habitat fragmentation boost or limit invasion? Biol. Invasions 2014, 16, 1397–1410. [Google Scholar] [CrossRef]
- Aragón, R.; Groom, M. Invasion by Ligustrum lucidum (Oleaceae) in NW Argentina: Early-stage characteristics in different habitat types. Rev. Biol. Trop. 2003, 51, 59–70. [Google Scholar]
- Montaldo, N. Éxito reproductivo de plantas ornitócoras en un relicto de selva subtropical en Argentina. Rev. Chil. Hist. Nat. 2000, 73, 511–524. [Google Scholar] [CrossRef]
- Grau, H.R.; Aragón, R. (Eds.) Ecología de los árboles invasores de la Sierra de San Javier. In Árboles Exóticos de las Yungas Argentinas; LIEY-UNT: Tucumán, Argentina, 2000; pp. 5–20. [Google Scholar]
- Hoyos, L.; Gavier-Pizarro, G.; Kuemmerle, T.; Bucher, E.; Radeloff, V.; Tecco, T. Invasion of glossy privet (Ligustrum lucidum) and native forest loss in the Sierras Chicas of Córdoba, Argentina. Biol. Invasions 2010, 12, 3261–3275. [Google Scholar] [CrossRef]
- Ceballos, S.J.; Malizia, A.; Chacoff, N. Influencia de la invasión de Ligustrum lucidum (Oleaceae) sobre la comunidad de lianas en la sierra de San Javier (Tucumán–Argentina). Ecol. Austral. 2015, 25, 65–74. [Google Scholar] [CrossRef]
- Malizia, A.; Osinaga-Acosta, O.; Powell, P.; Aragón, R. Invasion of Ligustrum lucidum (Oleaceae) in subtropical secondary forests of NW Argentina: Declining growth rates of abundant native tree species. J. Veg. Sci. 2017, 28, 1097–1269. [Google Scholar] [CrossRef]
- Franco, M.G.; Plaza, M.C.; Medina, M.; Pérez, C.; Mundo, I.A.; Cellini, J.M.; Arturi, M.F. Talares del NE bonaerense con presencia de Ligustrum lucidum: Cambios en la estructura y la dinámica del bosque. Ecol. Austral 2018, 28, 502–512. [Google Scholar] [CrossRef]
- Bellis, L.M.; Astudillo, A.; Gavier-Pizarro, G.; Dardanelli, S.; Landi, M.; Hoyos, L. Glossy privet (Ligustrum lucidum) invasion decreases Chaco Serrano Forest bird diversity but favors its seed dispersers. Biol. Invasions 2021, 23, 723–739. [Google Scholar] [CrossRef]
- Whitworth-Hulse, J.I.; Magliano, P.N.; Zeballos, S.R.; Nosetto, M.D.; Gurvich, D.E.; Ferreras, A.; Spalazzi, F.; Kowaljow, E. Ligustrum lucidum invasion alters the soil water dynamic in a seasonally multispecific dry forest. For. Ecol. Manag. 2023, 549, 121493. [Google Scholar] [CrossRef]
- Brazeiro, A.; Olivera, J.; Betancourt, A.; Lado, I.; Romero, D.; Haretche, F.; Cravino, A. Disentangling the invasion process of subtropical native forests of Uruguay by the exotic tree Ligustrum lucidum: Establishment and dominance determinants. Ecol. Proc. 2024, 13, 49. [Google Scholar] [CrossRef]
- Martin, P.H.; Canham, C.D. Dispersal and recruitment limitation in native versus exotic tree species: Life-history strategies and Janzen-Connell effects. Oikos 2010, 119, 807–824. [Google Scholar] [CrossRef]
- Lamarque, L.J.; Delzon, S.; Lortie, C.J. Tree invasions: A comparative test of the dominant hypotheses and functional traits. Biol. Invasions 2011, 13, 1969–1989. [Google Scholar] [CrossRef]
- Colautti, R.I.; Grigorovich, I.A.; MacIsaac, H.J. Propagule pressure: A null model for biological invasions. Biol. Invasions 2006, 8, 1023–1037. [Google Scholar] [CrossRef]
- Diaz Villa, M.V.E.; Madane, N.; Cristiano, P.M.; Goldstein, G. Composición del banco de semillas e invasión de Ligustrum lucidum en bosques costeros de la provincia de Buenos Aires, Argentina. Bosque 2016, 37, 581–590. [Google Scholar] [CrossRef]
- Emer, A.A.; Oliveira, M.de.A.; Althaus-Ottmann, M.M. Biochemical composition and germination capacity of Ligustrum lucidum ait. seeds in the process of biological invasion. Acta Scient. Biol. Sci. 2012, 34, 353–357. [Google Scholar] [CrossRef][Green Version]
- Powell, A.A.; Araóz, E. Biological and environmental effects on fine-scale seed dispersal of an invasive tree in a secondary subtropical forest. Biol. Invasions 2017, 20, 461–473. [Google Scholar] [CrossRef]
- Marco, E.M.; Montemurro, M.A.; Cannas, S.A. Comparing short and long-distance dispersal: Modelling and field case studies. Ecography 2011, 34, 671–682. [Google Scholar] [CrossRef]
- Mazia, C.N.; Chaneton, E.J.; Ghersa, C.M.; León, R.J.C. Limits to tree species invasion in pampean grassland and forest plant communities. Oecologia 2001, 128, 594–602. [Google Scholar] [CrossRef]
- Luna, M.L.; Giudice, G.E. Anatomy of the haustorium of Jodina rhombifolia (Santalaceae). Nordic J. Bot. 2005, 24, 567–573. [Google Scholar] [CrossRef]
- Matthies, D. Interactions between a root hemiparasite and 27 different hosts: Growth, biomass allocation and plant architecture. Persp. Plant Ecol. Evol. Syst. 2017, 24, 118–137. [Google Scholar] [CrossRef]
- Cameron, D.D.; Seel, W.E. Functional anatomy of haustoria formed by Rhinanthus minor: Linking evidence from histology and isotope tracing. New Phytol. 2007, 174, 412–419. [Google Scholar] [CrossRef]
- Těšitelová, T.; Knotková, K.; Knotek, A.; Cempírková, H.; Těšitel, K. Root hemiparasites suppress invasive alien clonal plants: Evidence from a cultivation experiment. NeoBiota 2024, 90, 97–121. [Google Scholar] [CrossRef]
- Instituto Uruguayo de Meteorología (INUMET). Climatología Estacional. Available online: https://www.inumet.gub.uy/clima/climatologia-estacional (accessed on 25 September 2023).
- Alonso-Paz, E. Descripción de un monte natural en la zona de Melilla, Montevideo, Rep. O. del Uruguay. Discusión de algunos aspectos fitogeográficos. In Proceedings of the III Jornadas de Ciencias Naturales, Montevideo, Uruguay, 19–24 September 1983; pp. 60–61. [Google Scholar]
- Medina, S.; Rachid, C. Estudio de una Sucesión Vegetal en las Barrancas de los Humedales del Río Santa Lucía. Bachelor´s Thesis, Agronomic Engineer. Facultad de Agronomía, Universidad de la República, Montevideo, Uruguay, 2004; 101p. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 12 December 2024).
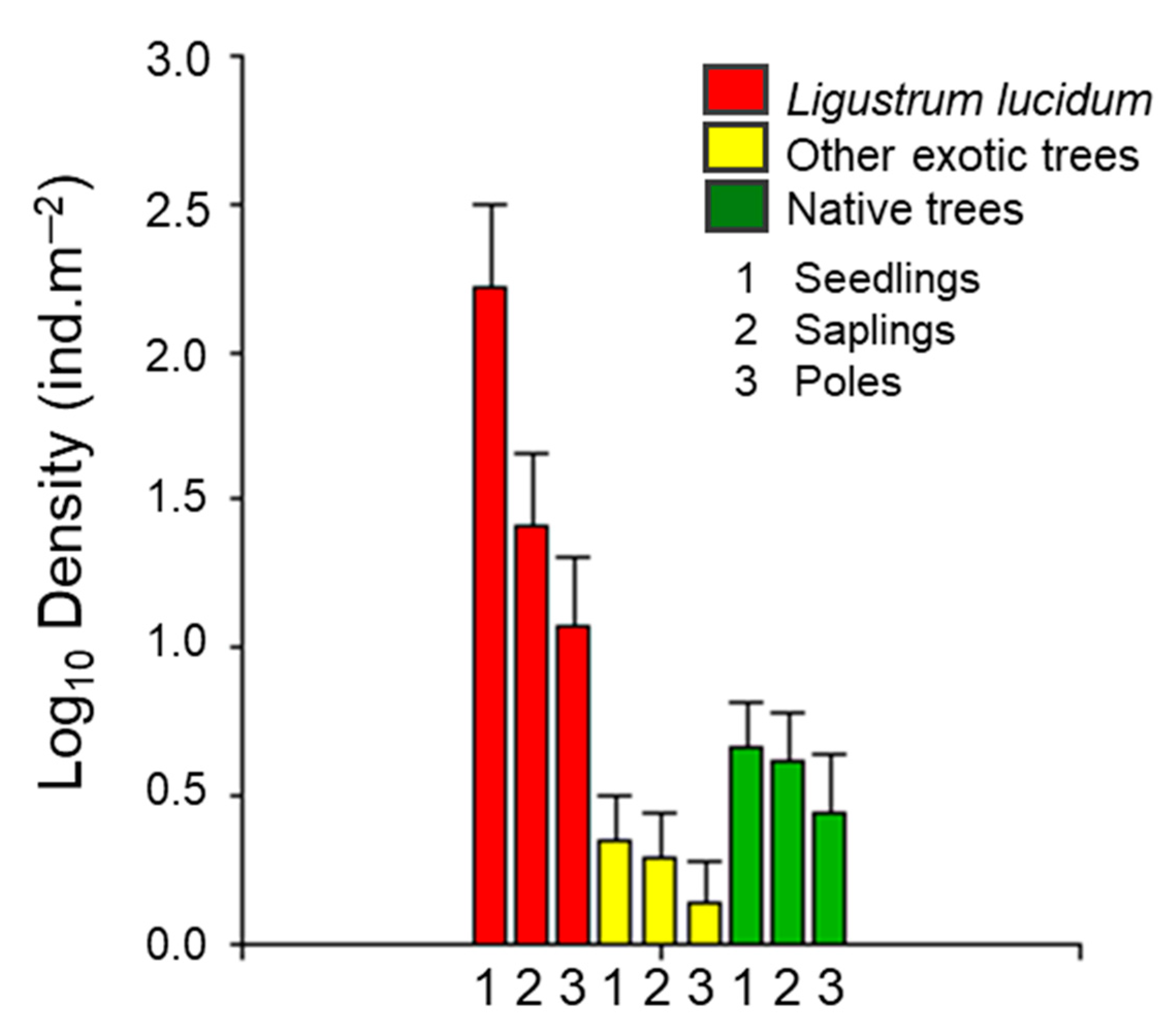

| Density (ind.m−2) (SD) | |||
|---|---|---|---|
| Seedlings | Saplings | Poles | |
| Exotic species | |||
| Ligustrum lucidum W.T. Aiton | 253.7 (621.4) | 15.5 (30.3) | 5.6 (10.2) |
| Ligustrum sinense Lour. | 0.2 (0.3) | 0.05 (0.16) | 0.03 (0.10) |
| Laurus nobilis L. | 0.03 (0.1) | 0.23 (0.5) | 0.06 (0.2) |
| Cotoneaster sp. | 0 | 0.01 (0.1) | 0.02 (0.1) |
| Pyracantha coccinea M. Roem | 0 | 0.01 (0.0) | 0.01 (0.0) |
| Phoenix canariensis H. Wildpret | 0 | 0.01 (0.0) | 0 |
| Morus alba P. | 0 | 0 | 0.01 (0.0) |
| Pittosporum undulatum Vent. | 0 | 0.06 (0.4) | 0.01 (0.0) |
| Native species | |||
| Blepharocalyx salicifolius (Kunth) O. Berg | 1.07 (1.47) | 1.52 (2.10) | 0.44 (1.62) |
| Myrsine laetevirens Mez | 0.08 (0.36) | 0.02 (0.08) | 0.01 (0.04) |
| Jodina rhombifolia (Hook. & Arn.) Reissek | 0.07 (0.26) | 0.02 (0.08) | 0.01 (0.05) |
| Scutia buxifolia Reissek | 0.04 (0.09) | 0.01 (0.05) | 0.04 (0.15) |
| Celtis tala Gillies ex Planch. | 0.04 (0.15) | 0.01 (0.04) | 0.01 (0.05) |
| Acca sellowiana (O. Berg) Burret | 0 | 0.03 (0.18) | 0.01 (0.04) |
| Eugenia uniflora L. | 0 | 0.01 (0.04) | 0.01 (0.04) |
| Schinus longifolia (Lindl.) Speg. | 0 | 0.01 (0.05) | 0 |
| Estimate | Std. Error | z Value | Pr(>|z|) | ||
|---|---|---|---|---|---|
| Seedlings | |||||
| (Intercept) | 4.21835 | 0.11929 | 35.36 | <2 × 10−16 | *** |
| Distance | −0.04003 | 0.02262 | −1.77 | 0.0768 | |
| Stand-L | 3.89434 | 0.11970 | 32.53 | <2 × 10−16 | *** |
| Stand-S | 5.56176 | 0.12813 | 43.41 | <2 × 10−16 | *** |
| Distance-Stand-L | −0.55919 | 0.02410 | −23.21 | <2 × 10−16 | *** |
| Distance-Stand-S | −0.91487 | 0.02656 | −34.45 | <2 × 10−16 | *** |
| Saplings | |||||
| (Intercept) | 2.7507 | 0.222 | 12.381 | <2 × 10−16 | *** |
| Distance | −0.3899 | 0.0242 | −16.109 | <2 × 10−16 | *** |
| Stand-L | 2.5125 | 0.2140 | 11.738 | <2 × 10−16 | *** |
| Stand-S | 1.7401 | 0.2122 | 8.199 | 2.43 × 10−16 | *** |
| Poles | |||||
| (Intercept) | 0.518 | 0.3923 | 1.307 | 0.1912 | |
| Distance | −0.1314 | 0.0372 | −3.532 | 0.0004 | *** |
| Stand-L | 1.9012 | 0.3836 | 4.957 | 7.17 × 10−7 | *** |
| Stand-S | 1.9071 | 0.3717 | 5.131 | 2.89 × 10−7 | *** |
| Estimate | Std. Error | z Value | Pr (>|z|) | ||
|---|---|---|---|---|---|
| (Intercept) | 0.9009358 | 0.4556859 | 1.977 | 0.04803 | * |
| Stand-L | 3.1828581 | 0.4531533 | 7.024 | 2.16 × 10−12 | *** |
| Stand-S | 1.0696586 | 0.2555241 | 4.186 | 2.84 × 10−5 | *** |
| Height | 0.0047637 | 0.0017515 | 2.720 | 0.00653 | ** |
| Time | −0.0004883 | 0.0004007 | −1.219 | 0.22299 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brazeiro, A.; Haretche, F.; Toranza, C.; Cravino, A. High Propagule Pressure and Patchy Biotic Resistance Control the Local Invasion Process of the Tree Ligustrum lucidum in a Subtropical Forest of Uruguay. Plants 2025, 14, 873. https://doi.org/10.3390/plants14060873
Brazeiro A, Haretche F, Toranza C, Cravino A. High Propagule Pressure and Patchy Biotic Resistance Control the Local Invasion Process of the Tree Ligustrum lucidum in a Subtropical Forest of Uruguay. Plants. 2025; 14(6):873. https://doi.org/10.3390/plants14060873
Chicago/Turabian StyleBrazeiro, Alejandro, Federico Haretche, Carolina Toranza, and Alexandra Cravino. 2025. "High Propagule Pressure and Patchy Biotic Resistance Control the Local Invasion Process of the Tree Ligustrum lucidum in a Subtropical Forest of Uruguay" Plants 14, no. 6: 873. https://doi.org/10.3390/plants14060873
APA StyleBrazeiro, A., Haretche, F., Toranza, C., & Cravino, A. (2025). High Propagule Pressure and Patchy Biotic Resistance Control the Local Invasion Process of the Tree Ligustrum lucidum in a Subtropical Forest of Uruguay. Plants, 14(6), 873. https://doi.org/10.3390/plants14060873






