Stimulatory Effect of Delonix regia Flower Extract in Protecting Syzygium cumini Seedlings from Salinity
Abstract
1. Introduction
2. Materials and Methods
2.1. Planting Methodology
2.2. Poinciana Extract (PFE) Preparation and Treatments
2.3. Saline Water Treatments
2.4. Growth Parameters
2.5. Physiological and Biochemical Analysis
2.5.1. Total Chlorophyll and Carbohydrates
2.5.2. Total Phenols, Proline, and Ferric Reducing Antioxidant Potential (FRAP)
2.5.3. Malondialdehyde (MDA) and Hydrogen Peroxide (H2O2)
2.6. Statistical Design and Data Analysis
3. Results
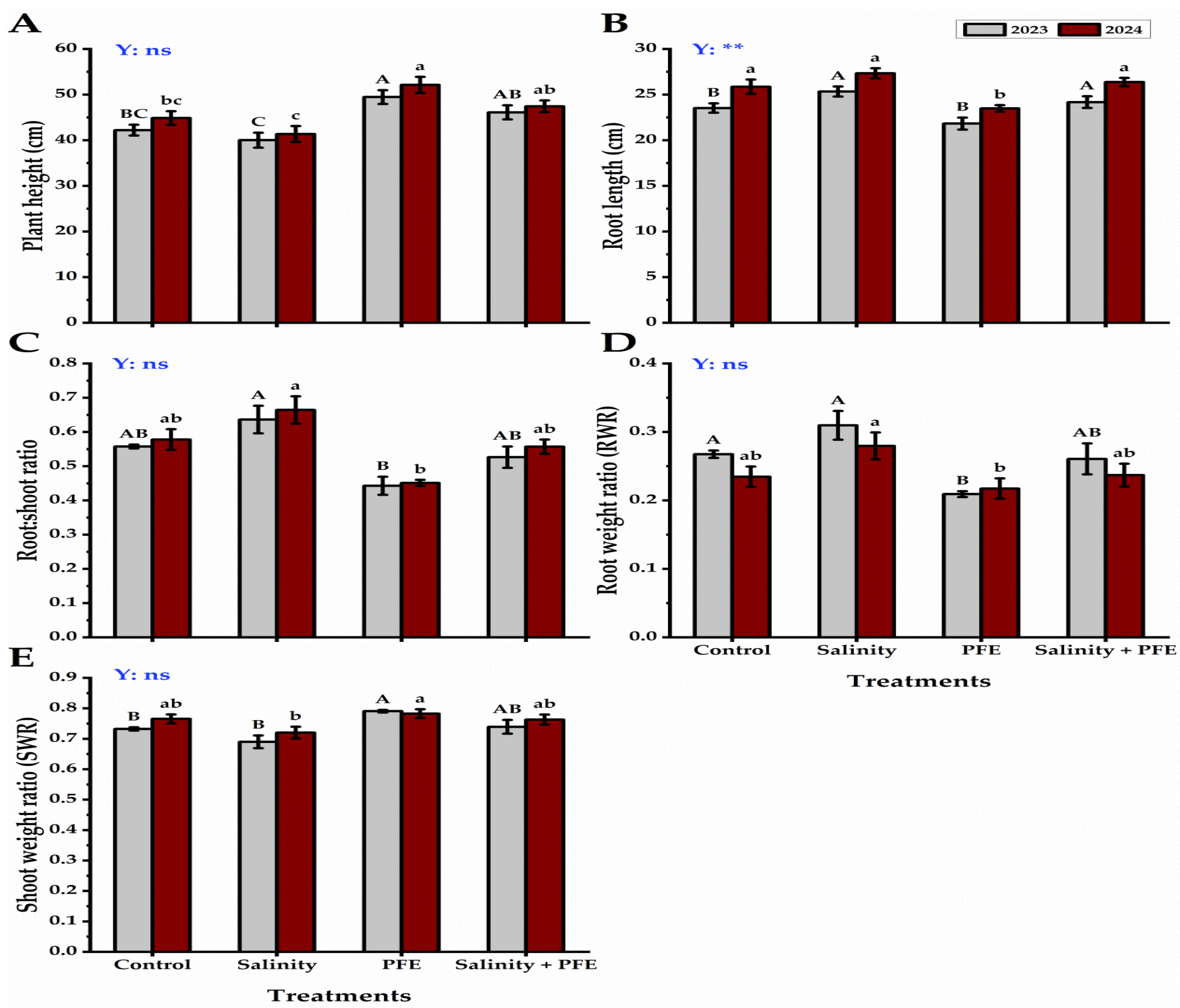
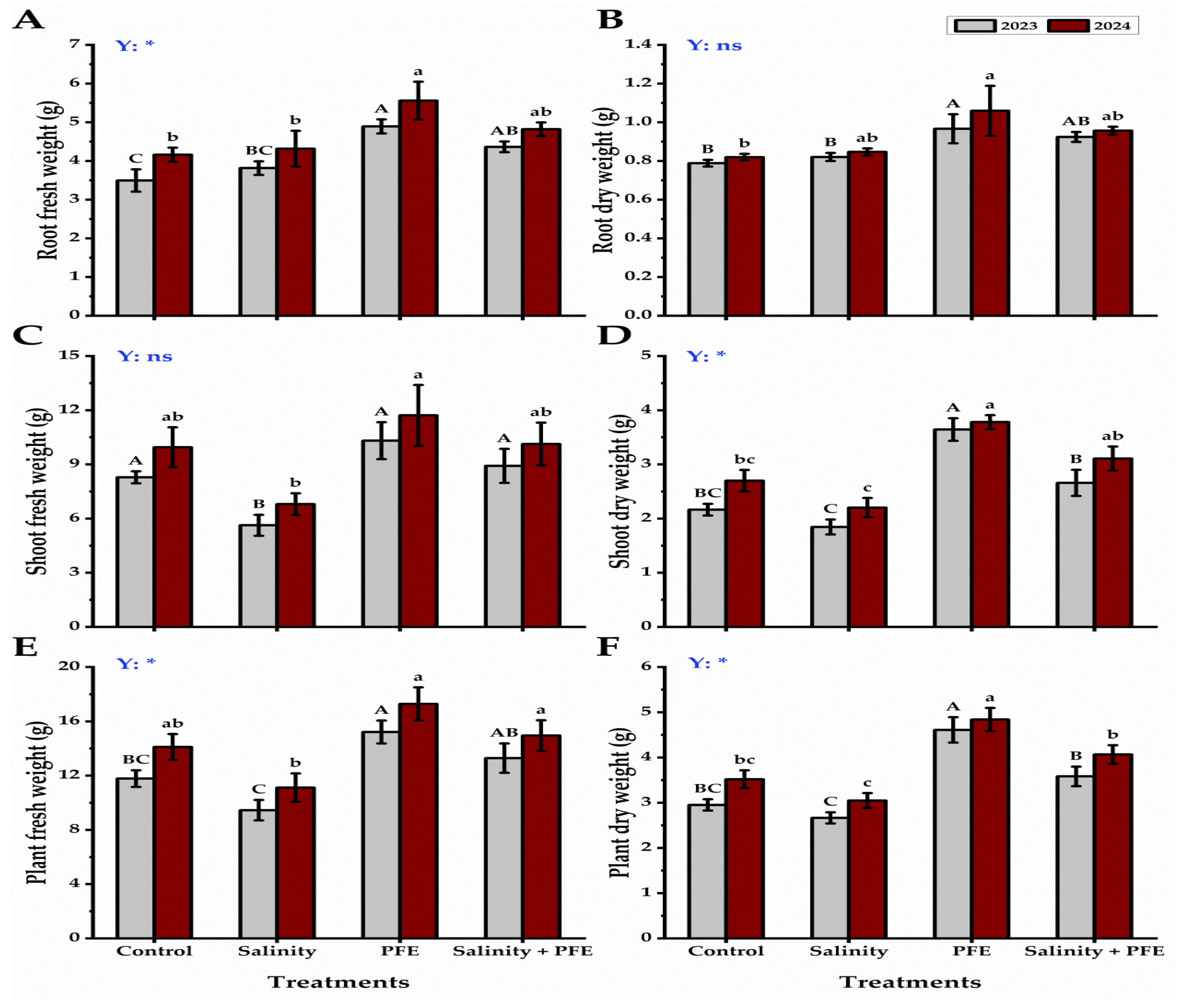
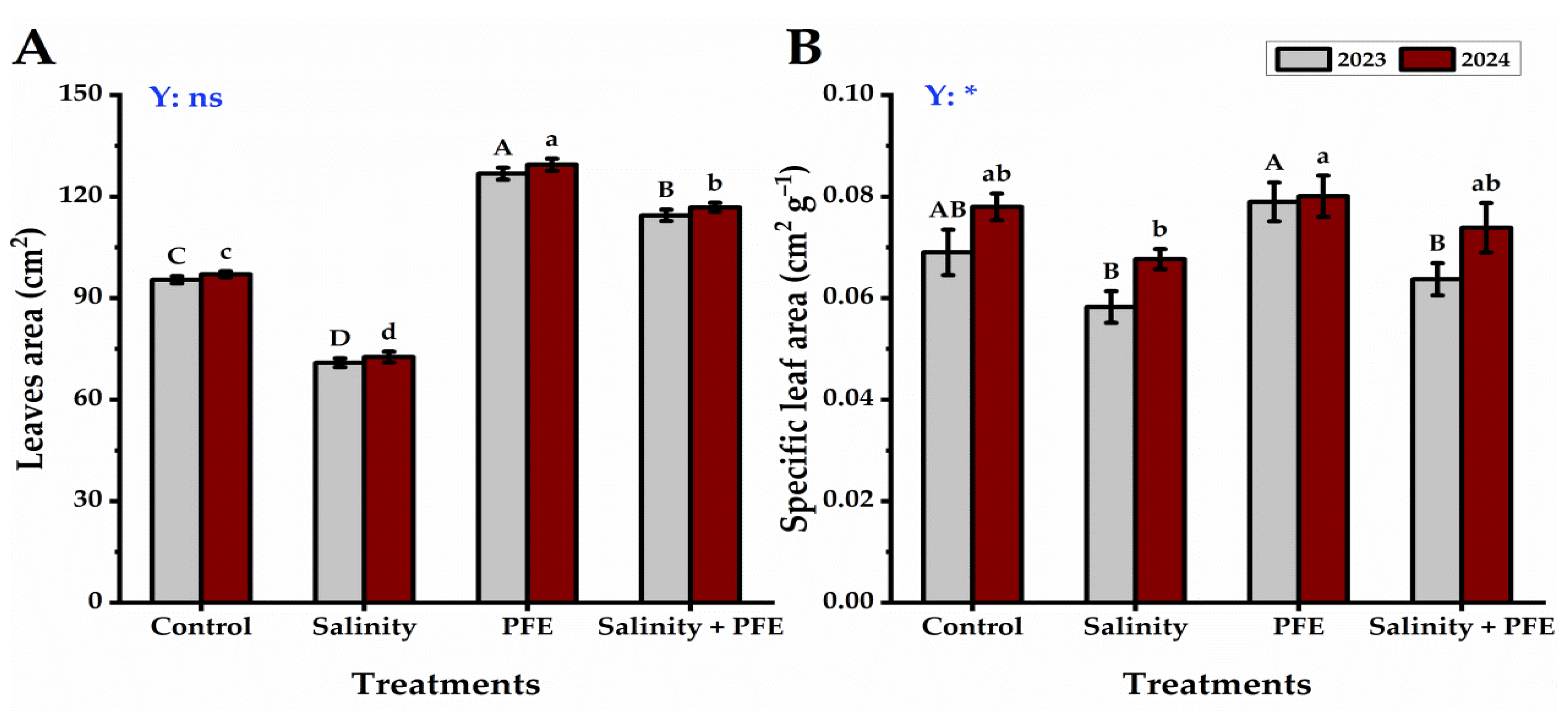
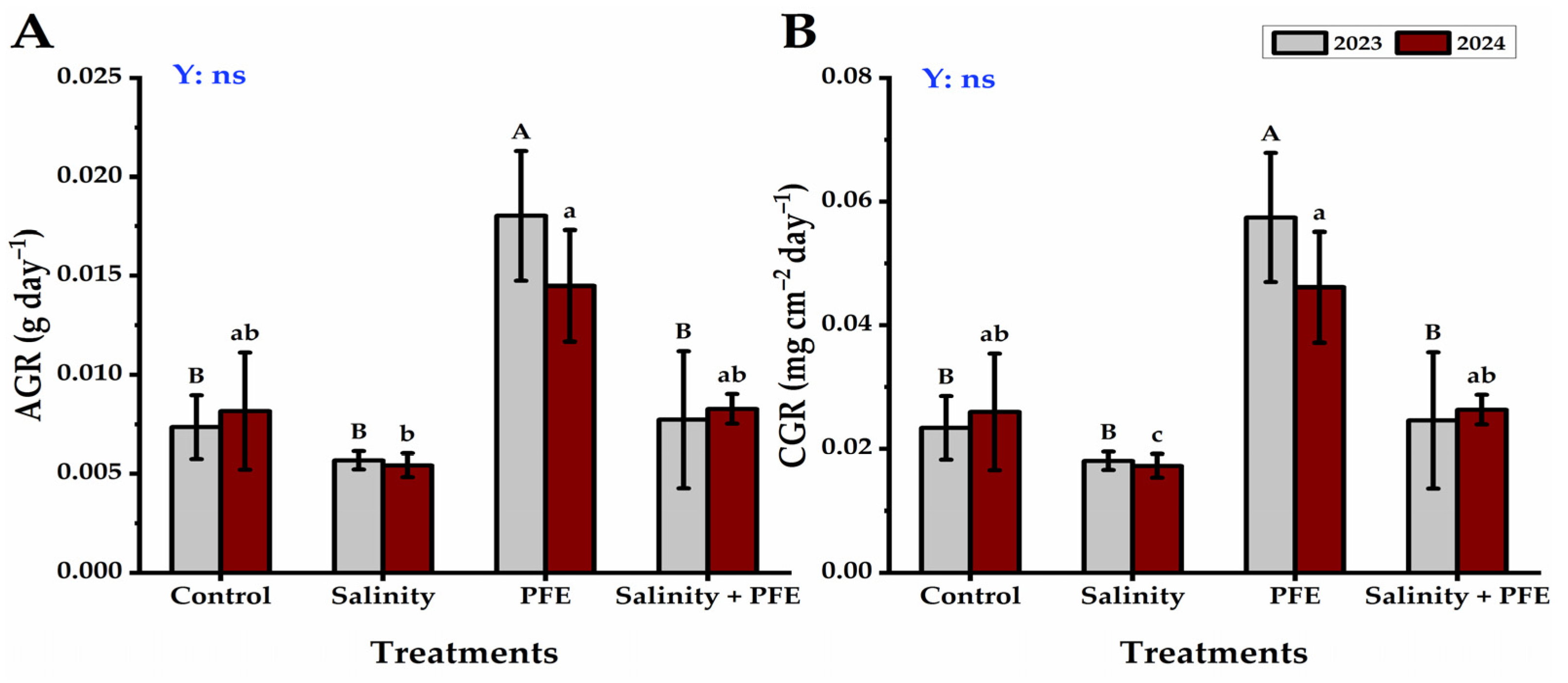
3.1. Growth Parameters
3.2. Physiological and Biochemical Analysis
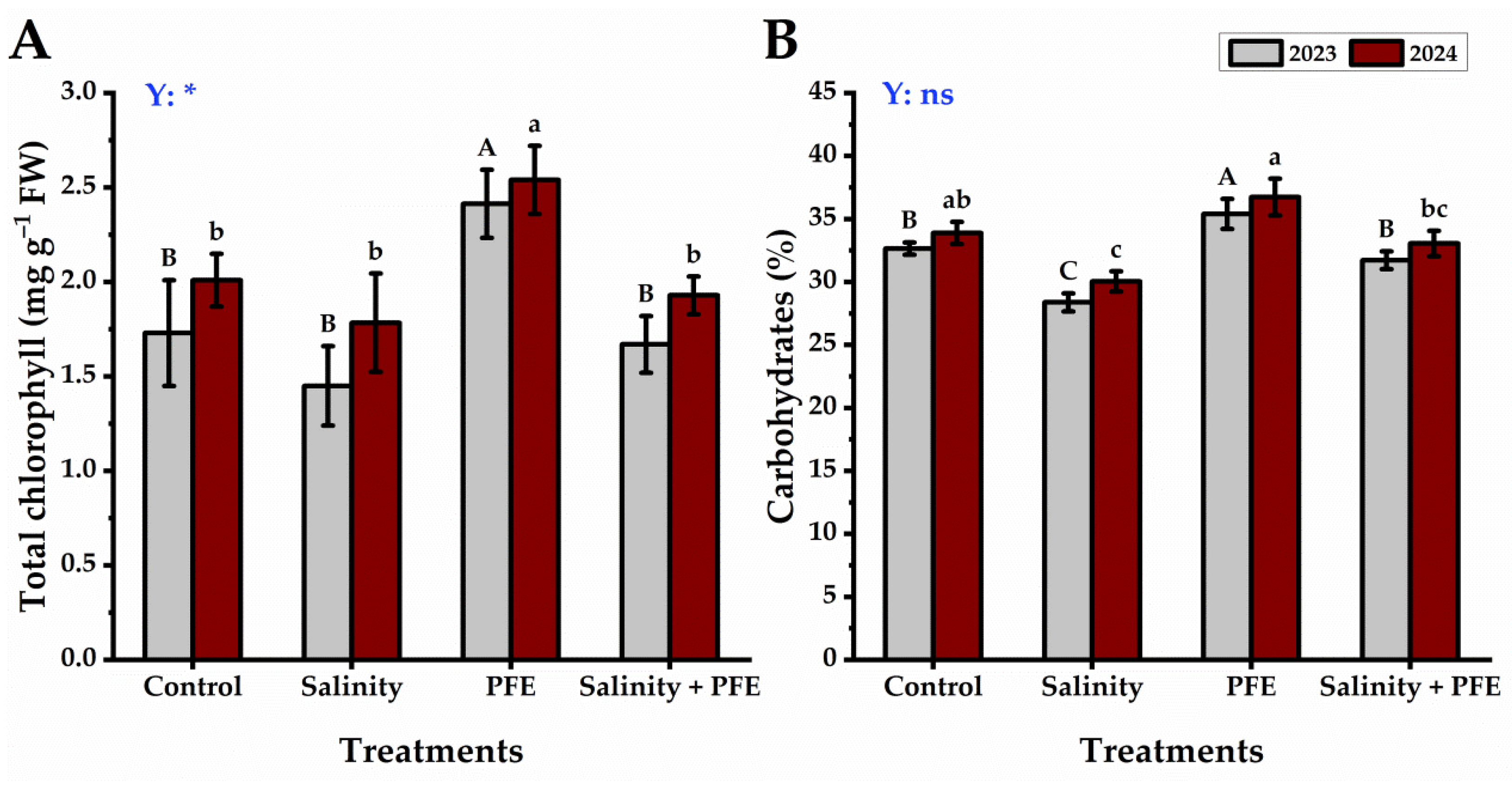
3.2.1. Total Chlorophyll and Carbohydrates Content
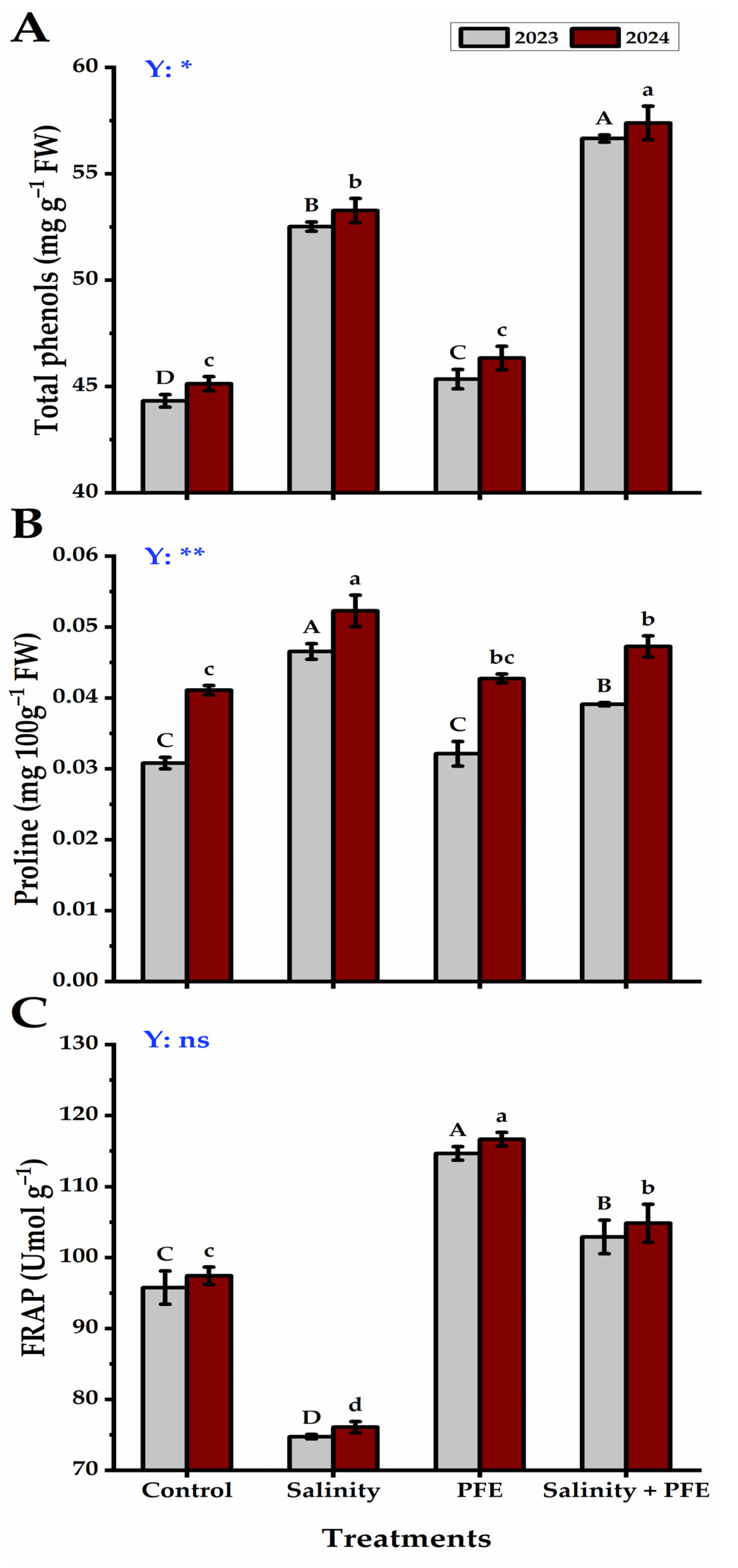
3.2.2. Total Phenols, Proline, and FRAP Content
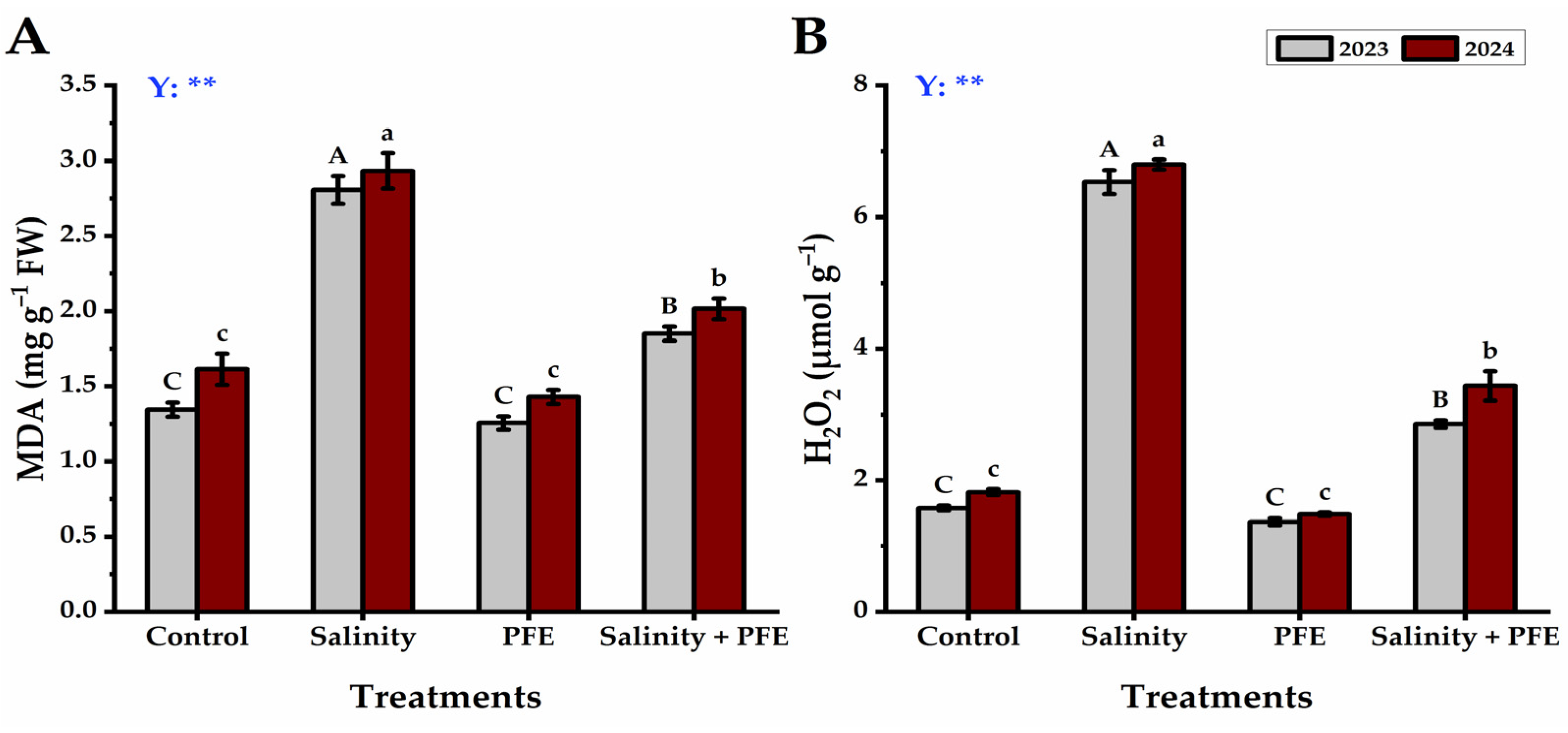
3.2.3. MDA and H2O2
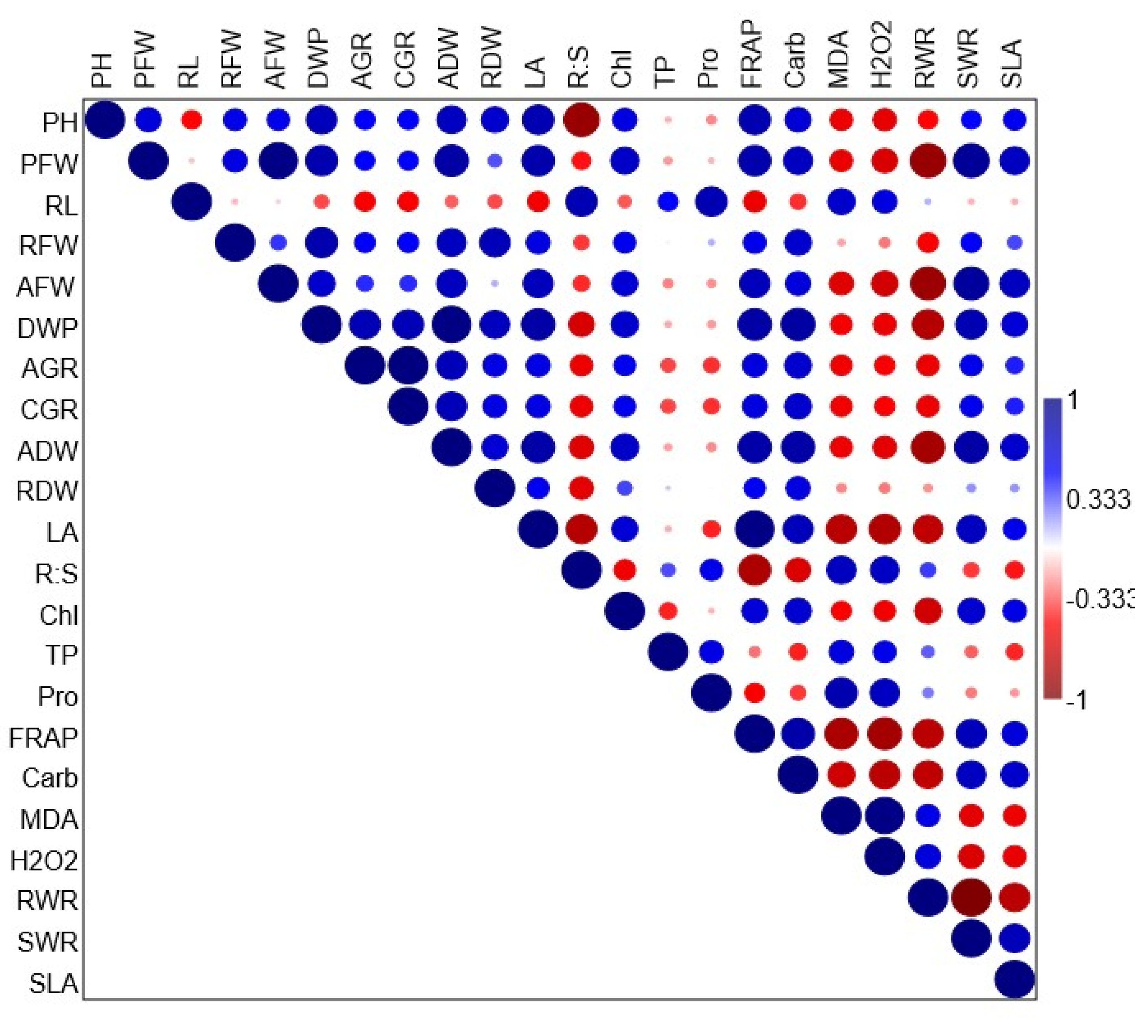
3.3. Correlation Analysis
4. Discussion
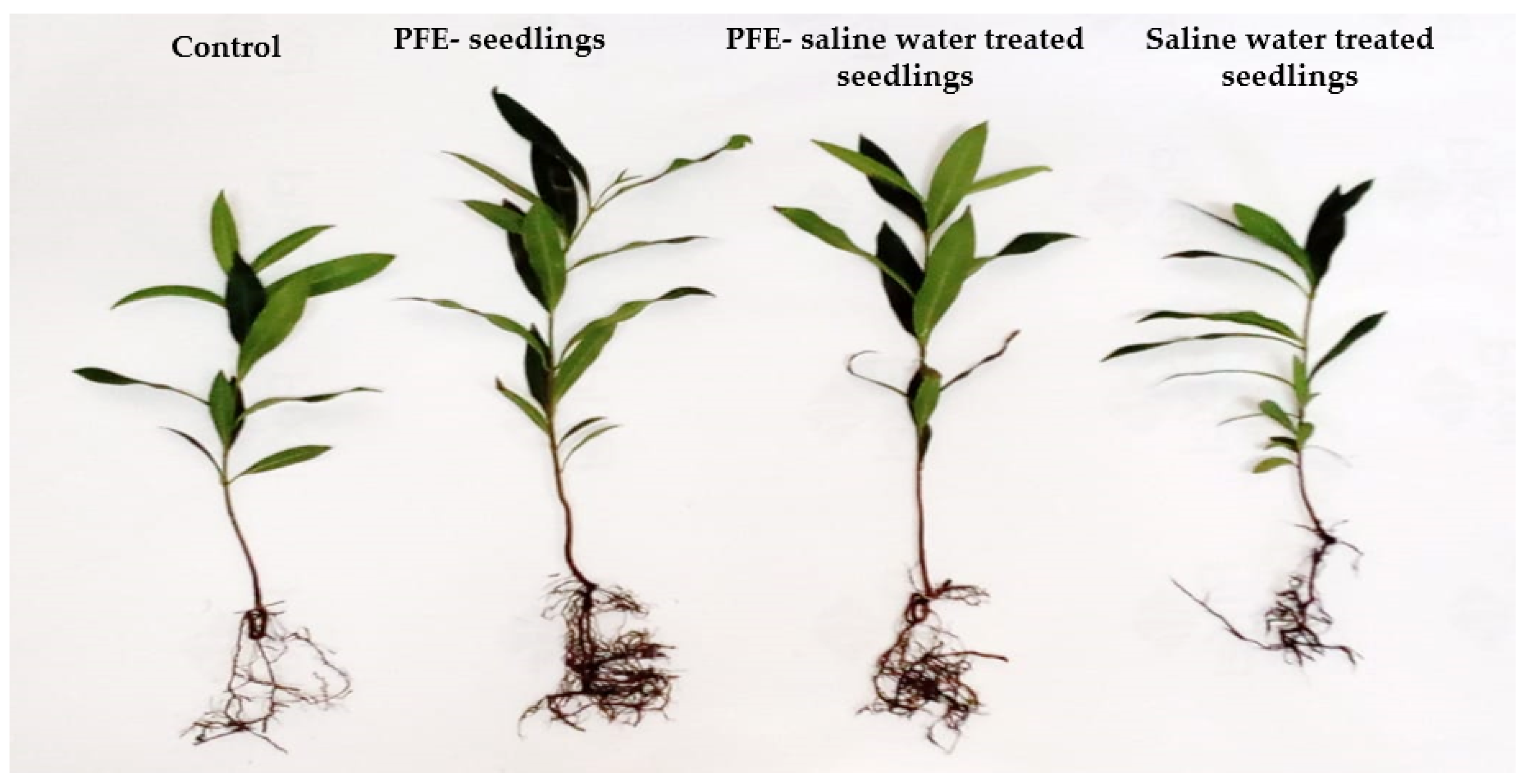
4.1. Vegetative Growth
4.2. Root Growth
4.3. Biochemical Analysis
5. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, A.; Kumar, A.; Kumar, R.; Prakash, J.; Kumar, N.; Verma, A.K. Evaluation of salt tolerance in jamun (Syzygium cumini L. Skeels) using morpho-physiological traits and membership function analysis. Sci. Hortic. 2024, 326, 112742. [Google Scholar] [CrossRef]
- Ayyanar, M.; Subash-Babu, P. Syzygium cumini (L.) Skeels: A review of its phytochemical constituents and traditional uses. Asian Pac. J. Trop. Biomed. 2012, 2, 240–246. [Google Scholar] [CrossRef]
- Madani, B.; Mirshekari, A.; Yahia, E.M.; Golding, J.B.; Hajivand, S.; Dastjerdy, A.M. Jamun (Syzygium cumini L. Skeels): A promising fruit for the future. Hortic. Rev. 2021, 48, 275–306. [Google Scholar]
- Sarvade, S.; Gautam, D.S.; Bhalawe, S.; Bisen, P.K. An overview of potential multipurpose agroforestry tree species, Syzygium cuminii (L.) Skeels in India. J. Appl. Nat. Sci. 2016, 8, 1714–1719. [Google Scholar] [CrossRef]
- Tewari, S.K.; Singh, D.; Nainwal, R.C. Horticultural management of Syzygium cumini: Syzygium cumini and other underutilized species. In The Genus Syzygium; Nair, K.N., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 215–236. [Google Scholar]
- Singh, G.; Lal, K. Review and case studies on biodrainage: An alternative drainage system to manage waterlogging and salinity. Irrig. Drain. 2018, 67, 51–64. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant growth-promoting bacteria: Biological tools for the mitigation of salinity stress in plants. Front. Microbiol. 2020, 11, 1216. [Google Scholar] [CrossRef] [PubMed]
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.F.; Lutts, S.; Cai, G.; Guerriero, G. Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Ahmad, P.; Nabi, G.; Jeleel, C.A.; Umar, S. Free radical production, oxidative damage and antioxidant defense mechanisms in plants under abiotic stress. In Oxidative Stress: Role of Antioxidants in Plants; Ahmad, P., Umar, S., Eds.; Studium Press: New Delhi, India, 2011; pp. 19–53. [Google Scholar]
- Rasool, S.; Hameed, A.; Azooz, M.M.; Siddiqi, T.O.; Ahmad, P. Salt stress: Causes, types and responses of plants. In Ecophysiology and Responses of Plants Under Salt Stress; Springer: New York, NY, USA, 2013. [Google Scholar]
- Al-Hattab, Z.N.; Al-Ajeel, S.A.; El-Kaaby, E.A. Effect of salinity stress on Capsicum annuum callus growth, regeneration and callus content of capsaicin, phenylalanine, proline and ascorbic acid. J. Life Sci. 2015, 9, 304–310. [Google Scholar]
- Krishnamurthy, L.; Serraj, R.; Hash, C.T.; Dakheel, A.J.; Reddy, B.V.S. Screening sorghum genotypes for salinity tolerant biomass production. Euphytica 2007, 156, 15–24. [Google Scholar] [CrossRef]
- Veeru, P.; Kishor, M.P.; Meenakshi, M. Screening of medicinal plant extracts for antioxidant activity. J. Med. Plant Res. 2009, 3, 608–612. [Google Scholar]
- Sadat-Hosseini, M.; Naeimi, A.; Boroomand, N.; Aalifar, M.; Farajpour, M. Alleviating the adverse effects of salinity on Roselle plants by green synthesized nanoparticles. Sci. Rep. 2022, 12, 18165. [Google Scholar] [CrossRef] [PubMed]
- Romero-Aranda, R.; Soria, T.; Cuartero, J. Tomato plant-water uptake and plant-water relationships under saline growth conditions. Plant Sci. 2001, 160, 265–272. [Google Scholar] [CrossRef]
- Sharma, I.; Sharma, A.; Bhardwaj, R.; Sirhindi, G. PGPR (Plant Growth Promoting Rhizobacteria) for Plant Stress Management; Nova Science Publishers: Hauppauge, NY, USA, 2023; 311p. [Google Scholar]
- Singh, N.; Maurya, V.; Singh, H.; Sharma, S.; Sharma, I.; Kumar, R.; Sharma, A. Salinity stress in crop plants: Effects and eco-friendly management. Adv. Food Secur. Sustain. 2024, 9, 103–143. [Google Scholar]
- Porcel, R.; Aroca, R.; Ruiz-Lozano, J.M. Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron. Sustain. Dev. 2012, 32, 181–200. [Google Scholar] [CrossRef]
- Paul, D.; Lade, H. Plant-growth-promoting rhizobacteria to improve crop growth in saline soils: A review. Agron. Sustain. Dev. 2014, 34, 737–752. [Google Scholar] [CrossRef]
- Azooz, M.M.; Metwally, A.; Abou-Elhamd, M.F. Jasmonate-induced tolerance of Hassawi okra seedlings to salinity in brackish water. Acta Physiol. Plant. 2015, 37, 77. [Google Scholar] [CrossRef]
- Adjé, F.A.; Lozano, Y.F.; Le Gernevé, C.; Lozano, P.R.; Meudec, E.; Adima, A.A.; Gaydou, E.M. Phenolic acid and flavonol water extracts of Delonix regia red flowers. Ind. Crop. Prod. 2012, 37, 303–310. [Google Scholar] [CrossRef]
- Shabir, G.; Anwar, F.; Sultana, B.; Khalid, Z.M.; Afzal, M.; Khan, Q.M.; Ashrafuzzaman, M. Antioxidant and antimicrobial attributes and phenolics of different solvent extracts from leaves, flowers and bark of Gold Mohar [Delonix regia (Bojer ex Hook.) Raf.]. Molecules 2011, 16, 7302–7319. [Google Scholar] [CrossRef]
- Das, A.K.; Kashyap, K.; Bhardwaj, A.K.; Biswas, S.; Roymahapatra, G.; Bhattacharyya, S.; Hait, M. Proximate Analysis, and Mineral Content Determination of Delonix regia Flower. ES Food Agrofor. 2024, 17, 1132. [Google Scholar] [CrossRef]
- Adamu, M.Y.; Peter, P.; Yusuf, M.I. Effect of flamboyant flower (Delonix regia) powder on root knot nematodes (Meloidogyne incognita) infestation on tomato plant (Solanum lycopersicum) in Yola, Adamawa State. Bio-Research 2023, 21, 2121–2130. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis. In Proceedings of the 17th Edition, Association of Official Analytical Chemists, Gaithersburg, MD, USA, 1 January 2000. [Google Scholar]
- Dere, S.; Güne¸s, T.; Sivaci, R. Spectrophotometric determination of chlorophyll-A, B and total carotenoid contents of some algae species using different solvents. Turk. J. Bot. 1998, 22, 13–17. [Google Scholar]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Patterson, B.D.; Macrae, E.A.; Ferguson, I.B. Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal. Biochem. 1984, 139, 487–492. [Google Scholar] [CrossRef]
- Pardo, J.M. Biotechnology of water and salinity stress tolerance. Curr. Opin. Biotechnol. 2010, 21, 185–196. [Google Scholar] [CrossRef]
- Roy, S.J.; Negrão, S.; Tester, M. Salt resistant crop plants. Curr. Opin. Biotechnol. 2014, 26, 115–124. [Google Scholar] [CrossRef]
- Meng, Y.; Yin, Q.; Yan, Z.; Wang, Y.; Niu, J.; Zhang, J.; Fan, K. Exogenous silicon enhanced salt resistance by maintaining K+/Na+ homeostasis and antioxidant performance in alfalfa leaves. Front. Plant Sci. 2020, 11, 1183. [Google Scholar] [CrossRef]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Almeida, D.M.; Oliveira, M.M.; Saibo, N.J.M. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 2017, 40, 326–345. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, K.Z.; Hasham, M.M.; El-Sheshtawy, A.N.A.; El-Serafy, R.S.; Sheta, M.H. Biochar stimulated actual evapotranspiration and wheat productivity under water deficit conditions in sandy soil based on non-weighing lysimeter. Plants 2022, 11, 3346. [Google Scholar] [CrossRef] [PubMed]
- El-Serafy, R.S.; El-Sheshtawy, A.N.A.; Atteya, A.K.; Al-Hashimi, A.; Abbasi, A.M.; Al-Ashkar, I. Seed priming with silicon as a potential to increase salt stress tolerance in Lathyrus odoratus. Plants 2021, 10, 2140. [Google Scholar] [CrossRef] [PubMed]
- El-Serafy, R.S.; Dahab, A.A.; Ghanem, K.Z.; Elhakem, A.; Bahgat, A.R.; Venkatesh, J.; El-Sheshtawy, A.A.; Badawy, A. As a natural antioxidant: Sesbania Grandiflora leaf extract enhanced growth and yield performance, active ingredients and tolerance of Hibiscus sabdariffa L. under salt-affected soil. J. Soil Sci. Plant Nutr. 2024, 24, 3406–3420. [Google Scholar] [CrossRef]
- Ahmad, A.; Blasco, B.; Martos, V. Combating salinity through natural plant extracts based biostimulants: A review. Front. Plant Sci. 2022, 13, 862034. [Google Scholar] [CrossRef]
- Arif, Y.; Sami, F.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salicylic acid in relation to other phytohormones in plant: A study towards physiology and signal transduction under challenging environment. Environ. Exp. Bot. 2020, 175, 104040. [Google Scholar] [CrossRef]
- Jyothi, M.V.; Narayan, M.S.; Kotamballi, N.; Bhagyalakshmi, N. Antioxidative efficacies of floral petal extracts of Delonix regia Rafin. Int. J. Biomed. Pharm. Sci. 2007, 1, 73–82. [Google Scholar]
- Hait, M.; Nemu, S.C.; Kashyap, N.K.; Chaturwedi, A. Physicochemical and phytochemical exploration on flower of Delonex regia. J. Med. Plants Stud. 2018, 6, 15–18. [Google Scholar]
- Aulya, N.R.; Supriyatin; Hartanti, E.P.; Nada, W.A.Q.; Achmad Syahputa, A. Effect of aqueous and ethanol extract of Acacia nilotica L. leaves on seed germination of Vigna radiata L. Indones. J. Sci. Educ. 2020, 4, 146–151. [Google Scholar] [CrossRef]
- Gupta, R.; Chakrabarty, S.K. Gibberellic acid in plant: Still a mystery unresolved. Plant Signal Behav. 2013, 8, e25504. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R. Does mineral fertilization modify essential oil content and chemical composition in medicinal plants? Acta Sci. Pol. Hortorum. Cultus 2013, 12, 3–16. [Google Scholar]
- Sakr, W.R.; El-Sayed, A.A.; Hammouda, A.M.; Saad El-Deen, F.S.A. Effect of NPK, aloe gel and moringa extracts on geranium plants. J. Hortic. Sci. Ornam. Plants 2018, 10, 1–16. [Google Scholar]
- Atteya, A.K.G.; El-Serafy, R.S.; El-Zabalawy, K.M.; Elhakem, A.; Genaidy, E.A.E. Brassinolide maximized the fruit and oil yield, induced the secondary metabolites, and stimulated linoleic acid synthesis of Opuntia ficus-indica oil. Horticulturae 2022, 8, 452. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Drew, M.C.; Saker, L.R. Nutrient supply and the growth of the seminal root system in barley. III. Compensatory increases in growth of lateral roots, and in rates of phosphate uptake in response to a localized supply of phosphate. J. Exp. Bot. 1978, 29, 435–451. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 5th ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2010; 782p. [Google Scholar]
- Sheha, A.M.; Abou El-Enin, M.M.; El-Hashash, E.F.; Rady, M.M.; El-Serafy, R.S.; Shaaban, A. The productivity and overall benefits of faba bean-sugar beet intercropping systems interacted with foliar applied nutrients. J. Plant Nutr. 2023, 46, 1683–1700. [Google Scholar] [CrossRef]
- Zali, A.G.; Ehsanzadeh, P. Exogenously applied proline as a tool to enhance water use efficiency: Case of fennel. Agric. Water Manag. 2018, 197, 138–146. [Google Scholar] [CrossRef]
- Gémes, K.; Kim, Y.J.; Park, K.Y.; Moschou, P.N.; Andronis, E.; Valassaki, C.; Roussis, A.; Roubelakis-Angelakis, K.A. An NADPH-oxidase/polyamine oxidase feedback loop controls oxidative burst under salinity. Plant Physiol. 2016, 172, 1418–1431. [Google Scholar] [CrossRef]
- Kaur, G.; Sharma, A.; Guruprasad, K.; Pati, P.K. Versatile roles of plant NADPH oxidases and emerging concepts. Biotechnol. Adv. 2014, 32, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Hnilickova, H.; Kraus, K.; Vachova, P.; Hnilicka, F. Salinity stress affects photosynthesis, malondialdehyde formation, and proline content in Portulaca oleracea L. Plants 2021, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Eraslan, F.; Inal, A.; Savasturk, O.; Gunes, A. Changes in antioxidative system and membrane damage of lettuce in response to salinity and boron toxicity. Sci. Hortic. 2007, 114, 5–10. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Shafi, A.; Zahoor, I.; Mushtaq, U. Proline accumulation and oxidative stress: Diverse roles and mechanism of tolerance and adaptation under salinity stress. In Salt Stress, Microbes, and Plant Interactions: Mechanisms and Molecular Approaches; Akhtar, M.S., Ed.; Springer Nature Singapore Pte Ltd.: Singapore, 2019; Volume 2, pp. 269–300. [Google Scholar]
| Proximate Analysis | Moisture % | Ash % | Protein % | Fiber % | Fat % | Carbohydrates % | Total Phenols mg g−1 | Total Flavonoids mg g−1 | DPPH (IC50) μg mL−1 | P mg 100 g−1 | K mg 100 g−1 | Ca mg 100 g−1 | Mg mg 100 g−1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 10.35 | 2.11 | 11.32 | 13.82 | 12.76 | 49.64 | 32.50 | 30.1 | 33.72 | 4.11 | 8.25 | 2.34 | 5.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alayafi, A.H.; Dahab, A.A.; El-Sheshtawy, A.-N.A.; Sharma, A.; Elhakem, A.; Youssef, S.M.; El-Serafy, R.S. Stimulatory Effect of Delonix regia Flower Extract in Protecting Syzygium cumini Seedlings from Salinity. Plants 2025, 14, 875. https://doi.org/10.3390/plants14060875
Alayafi AH, Dahab AA, El-Sheshtawy A-NA, Sharma A, Elhakem A, Youssef SM, El-Serafy RS. Stimulatory Effect of Delonix regia Flower Extract in Protecting Syzygium cumini Seedlings from Salinity. Plants. 2025; 14(6):875. https://doi.org/10.3390/plants14060875
Chicago/Turabian StyleAlayafi, Abdullah H., Abeer A. Dahab, Abdel-Nasser A. El-Sheshtawy, Ashutosh Sharma, Abeer Elhakem, Samah M. Youssef, and Rasha S. El-Serafy. 2025. "Stimulatory Effect of Delonix regia Flower Extract in Protecting Syzygium cumini Seedlings from Salinity" Plants 14, no. 6: 875. https://doi.org/10.3390/plants14060875
APA StyleAlayafi, A. H., Dahab, A. A., El-Sheshtawy, A.-N. A., Sharma, A., Elhakem, A., Youssef, S. M., & El-Serafy, R. S. (2025). Stimulatory Effect of Delonix regia Flower Extract in Protecting Syzygium cumini Seedlings from Salinity. Plants, 14(6), 875. https://doi.org/10.3390/plants14060875







