Microfluidic Array Enables Rapid Testing of Natural Compounds Against Xylella fastidiosa
Abstract
1. Introduction
2. Results
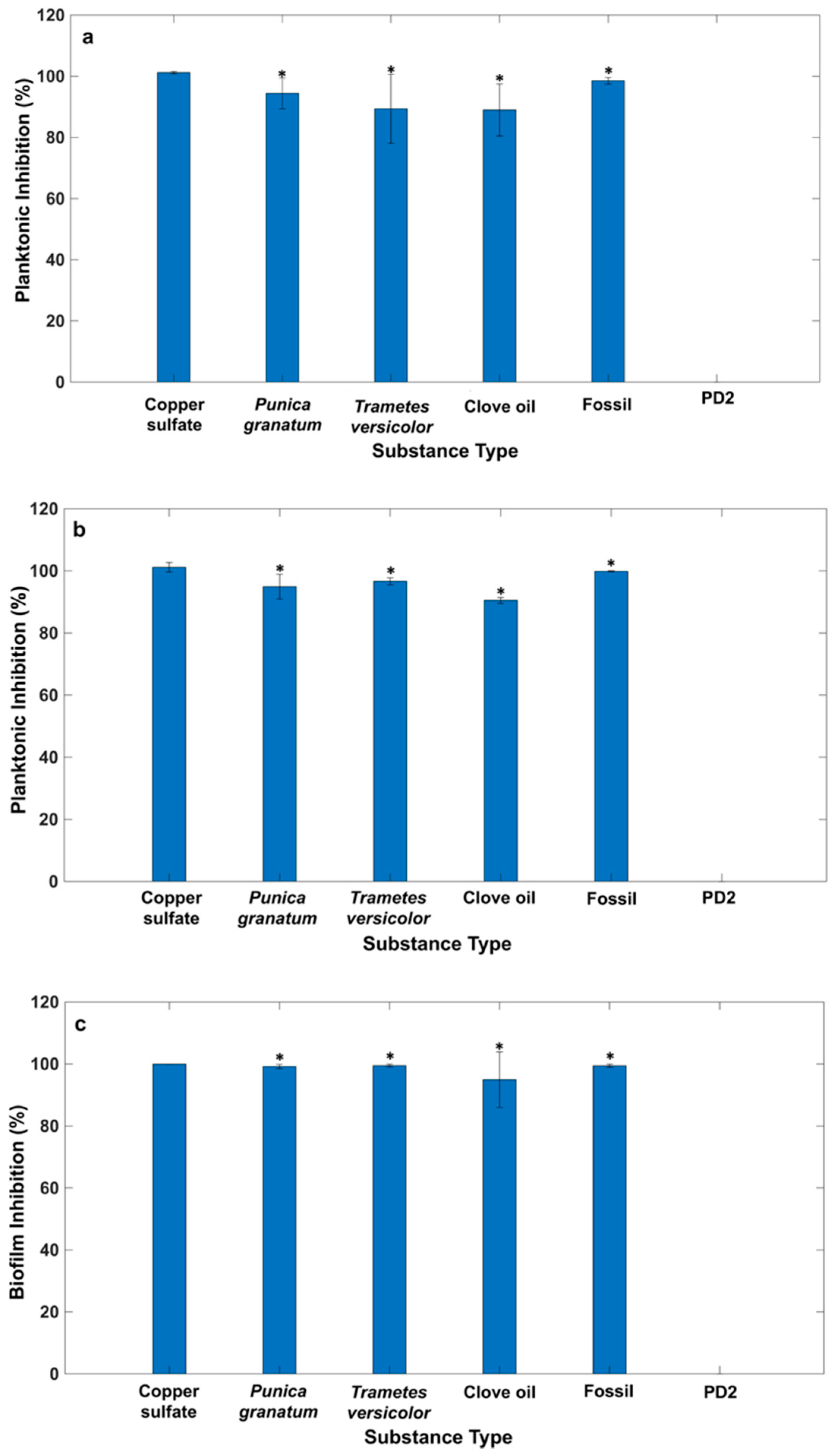
2.1. Macrodilution Broth In Vitro Screening
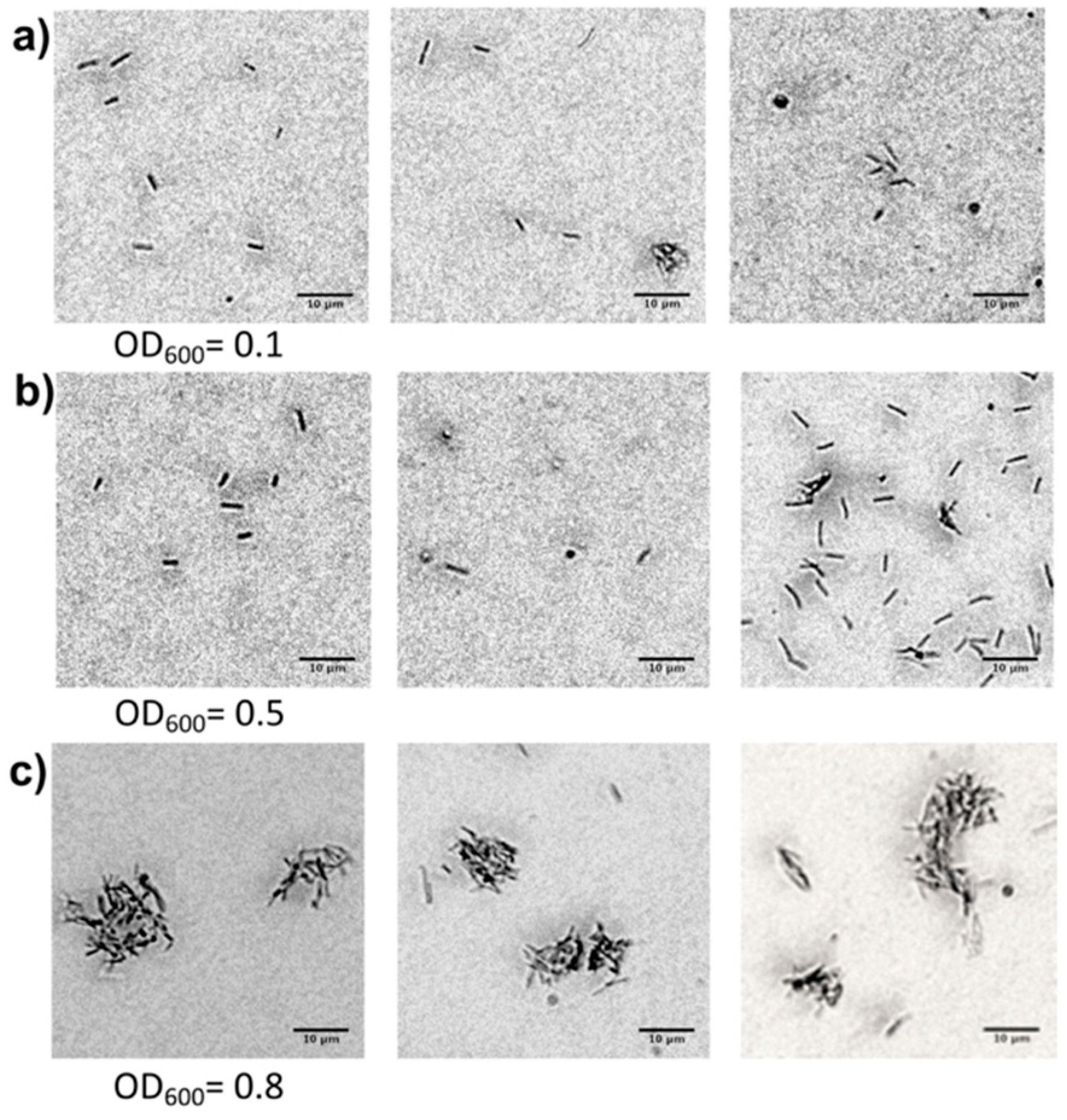
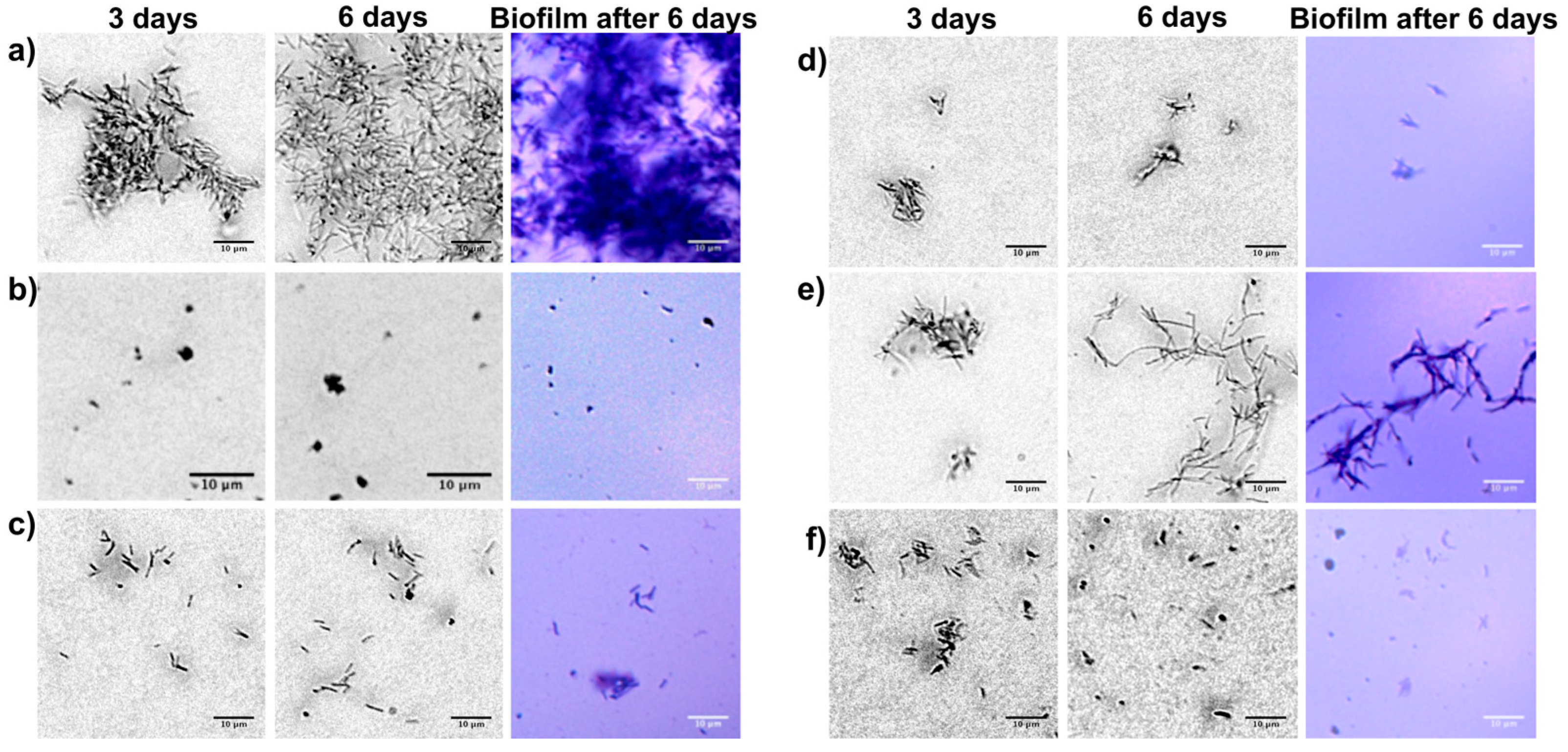
2.2. Microfluidic Channel In Vitro Screening
2.2.1. Cell Attachment on the Inner Channel Glass Surface
2.2.2. Cell Growing
3. Discussion
4. Materials and Methods
4.1. Tested Compounds
4.2. Bacterium
4.3. Minimum Inhibitory Concentration
- For copper sulfate, 0.8 g was dissolved in 10 mL of sterile distilled water.
- For P. granatum extract, 2 g of extract was dissolved prior in 10 mL of sterile distilled water and stored overnight at −80 °C in glass vials; afterward, the solutions were freeze-dried at −40 °C for 2 days and stored at 4° C until use.
- T. versicolor cultural filtrate was obtained following a previously reported procedure [32] and split into aliquots of 10 mL, which were stored overnight at −80 °C in glass vials; afterward, the solutions were freeze-dried at −40°C for 2 days and stored at 4° C until use; the stock solutions were then prepared by dissolving 1 g of the lyophilized powder in sterile distilled water and sterilized using a 0.45 µm sterile filter.
- Clove oil was dissolved prior in dimethyl sulfoxide (DMSO): 20 mg of the oil was added to 250 μL of DMSO and subsequently sterilized using a 0.2 µm sterile filter.
- FossilⓇ stock solution was prepared by dissolving 1 g in 10 mL of sterile distilled water and subsequently sterilized by using a 0.2 µm sterile filter.
4.4. Microfluidic Channel In Vitro Screening
4.5. Optical Image Acquisition and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AST | Antibacterial susceptibly testing |
| MIC | Minimal inhibitory concentration |
| Xf | Xylella fastidiosa |
| Xff | Xylella fastidiosa subsp. fastidiosa |
References
- Coletta-Filho, H.D.; Castillo, A.I.; Laranjeira, F.F.; de Andrade, E.C.; Silva, N.T.; de Souza, A.A.; Bossi, M.E.; Almeida, R.P.P.; Lopes, J.R.S. Citrus Variegated Chlorosis: An Overview of 30 Years of Research and Disease Management. Trop. Plant Pathol. 2020, 45, 175–191. [Google Scholar] [CrossRef]
- Kyrkou, I.; Pusa, T.; Ellegaard-Jensen, L.; Sagot, M.F.; Hansen, L.H. Pierce’s Disease of Grapevines: A Review of Control Strategies and an Outline of an Epidemiological Model. Front. Microbiol. 2018, 9, 2141. [Google Scholar] [CrossRef]
- Martelli, G.P.; Boscia, D.; Porcelli, F.; Saponari, M. The Olive Quick Decline Syndrome in South-East Italy: A Threatening Phytosanitary Emergency. Eur. J. Plant Pathol. 2016, 144, 235–243. [Google Scholar] [CrossRef]
- Krugner, R.; Sisterson, M.S.; Backus, E.A.; Burbank, L.P.; Redak, R.A. Sharpshooters: A Review of What Moves Xylella fastidiosa. Austral Entomol. 2019, 58, 248–267. [Google Scholar] [CrossRef]
- Cornara, D.; Cavalieri, V.; Dongiovanni, C.; Altamura, G.; Palmisano, F.; Bosco, D.; Porcelli, F.; Almeida, R.P.P.; Saponari, M. Transmission of Xylella fastidiosa by Naturally Infected Philaenus spumarius (Hemiptera, Aphrophoridae) to Different Host Plants. J. Appl. Entomol. 2017, 141, 80–87. [Google Scholar] [CrossRef]
- Khursheed, A.; Rather, M.A.; Jain, V.; Wani, A.R.; Rasool, S.; Nazir, R.; Malik, N.A.; Majid, S.A. Plant Based Natural Products as Potential Ecofriendly and Safer Biopesticides: A Comprehensive Overview of Their Advantages over Conventional Pesticides, Limitations and Regulatory Aspects. Microb. Pathog. 2022, 173, 105854. [Google Scholar] [CrossRef]
- Katsoulas, N.; Løes, A.K.; Andrivon, D.; Cirvilleri, G.; de Cara, M.; Kir, A.; Knebl, L.; Malińska, K.; Oudshoorn, F.W.; Willer, H.; et al. Current Use of Copper, Mineral Oils and Sulphur for Plant Protection in Organic Horticultural Crops across 10 European Countries. Org. Agric. 2020, 10, 159–171. [Google Scholar] [CrossRef]
- Bleve, G.; Gallo, A.; Altomare, C.; Vurro, M.; Maiorano, G.; Cardinali, A.; D’Antuono, I.; Marchi, G.; Mita, G. In vitro Activity of Antimicrobial Compounds against Xylella fastidiosa, the Causal Agent of the Olive Quick Decline Syndrome in Apulia (Italy). FEMS Microbiol. Lett. 2018, 365, fnx281. [Google Scholar] [CrossRef]
- Scortichini, M.; Chen, J.; De Caroli, M.; D’Alessandro, G.; Pucci, N.; Modesti, V.; L’Aurora, A.; Petriccione, M. A Zinc, Copper and Citric Acid Biocomplex Shows Promise for Control of Xylella fastidiosa subsp. pauca in Olive Trees in Apulia Region (Southern Italy). Phytopathol. Mediterr. 2018, 57, 48–72. [Google Scholar] [CrossRef]
- Lee, S.A.; Wallis, C.M.; Rogers, E.E.; Burbank, L.P. Grapevine Phenolic Compounds Influence Cell Surface Adhesion of Xylella fastidiosa and Bind to Lipopolysaccharide. PLoS ONE 2020, 15, e0240101. [Google Scholar] [CrossRef]
- Maddox, C.E.; Laur, L.M.; Tian, L. Antibacterial Activity of Phenolic Compounds against the Phytopathogen Xylella fastidiosa. Curr. Microbiol. 2010, 60, 53–58. [Google Scholar] [CrossRef]
- Santiago, B.M.; da Silva Moraes, T.; Estétele Massuco, J.; Oliveira Silva, L.; Lucarini, R.; Fernandes da Silva, D.; Manzini Vieira, T.; Miller Crotti, A.E.; Gomes Martins, C.H. In vitro Evaluation of Essential Oils for Potential Antibacterial Effects against Xylella fastidiosa. J. Phytopathol. 2018, 166, 790–798. [Google Scholar] [CrossRef]
- Rongai, D.; Pucci, N.; Cesari, E.; Di Marco, C.; Valentini, F. Potential of Endotherapeutic Treatments with Pomegranate Peel Extract to Control the Olive Quick Decline Syndrome (OQDS) Caused by Xylella fastidiosa subsp. pauca. Eur. J. Plant Pathol. 2023, 170, 805–817. [Google Scholar] [CrossRef]
- Ahern, S.J.; Das, M.; Bhowmick, T.S.; Young, R.; Gonzalez, C.F. Characterization of Novel Virulent Broad-Host-Range Phages of Xylella fastidiosa and Xanthomonas. J. Bacteriol. 2014, 196, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, F.; De Stradis, A.; Altamura, G.; Vergaro, V.; Citti, C.; Cannazza, G.; Capodilupo, A.L.; Dini, L.; Ciccarella, G. Application of Calcium Carbonate Nanocarriers for Controlled Release of Phytodrugs against Xylella fastidiosa Pathogen. Pure Appl. Chem. 2020, 92, 429–444. [Google Scholar] [CrossRef]
- Bruno, G.L.; Cariddi, C.; Botrugno, L. Exploring a Sustainable Solution to Control Xylella fastidiosa subsp. pauca on Olive in the Salento Peninsula, Southern Italy. Crop Prot. 2021, 139, 105288. [Google Scholar] [CrossRef]
- Qin, N.; Zhao, P.; Ho, E.A.; Xin, G.; Ren, C.L. Microfluidic Technology for Antibacterial Resistance Study and Antibiotic Susceptibility Testing: Review and Perspective. ACS Sens. 2021, 6, 3–21. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Gajic, I.; Kabic, J.; Kekic, D.; Jovicevic, M.; Milenkovic, M.; Mitic Culafic, D.; Trudic, A.; Ranin, L.; Opavski, N. Antimicrobial Susceptibility Testing: A Comprehensive Review of Currently Used Methods. Antibiotics 2022, 11, 427. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Pawar, J.S.; Akhter, N.; Lucy, I.B. Conventional Methods and Future Trends in Antimicrobial Susceptibility Testing. Saudi J. Biol. Sci. 2023, 30, 103582. [Google Scholar] [CrossRef]
- Zhou, W.; Le, J.; Chen, Y.; Cai, Y.; Hong, Z.; Chai, Y. Recent Advances in Microfluidic Devices for Bacteria and Fungus Research. TrAC Trends Anal. Chem. 2019, 112, 175–195. [Google Scholar] [CrossRef]
- Millet, L.J.; Aufrecht, J.; Labbé, J.; Uehling, J.; Vilgalys, R.; Estes, M.L.; Miquel Guennoc, C.; Deveau, A.; Olsson, S.; Bonito, G.; et al. Increasing Access to Microfluidics for Studying Fungi and Other Branched Biological Structures. Fungal Biol. Biotechnol. 2019, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; De La Fuente, L. Calcium Transcriptionally Regulates Movement, Recombination and Other Functions of Xylella fastidiosa under Constant Flow inside Microfluidic Chambers. Microb. Biotechnol. 2020, 13, 548–561. [Google Scholar] [CrossRef]
- Kim, J.; Park, H.D.; Chung, S. Microfluidic Approaches to Bacterial Biofilm Formation. Molecules 2012, 17, 9818–9834. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, F.; De La Fuente, L. Response of Xylella fastidiosa to Zinc: Decreased Culturability, Increased Exopolysaccharide Production, and Formation of Resilient Biofilms under Flow Conditions. Appl. Environ. Microbiol. 2014, 80, 1097–1107. [Google Scholar] [CrossRef]
- Li, B.; Qiu, Y.; Glidle, A.; McIlvenna, D.; Luo, Q.; Cooper, J.; Shi, H.C.; Yin, H. Gradient Microfluidics Enables Rapid Bacterial Growth Inhibition Testing. Anal. Chem. 2014, 86, 3131–3137. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Sun, K.; Abulimiti, A.; Xu, P.P.; Li, Z.Y. Microfluidic System for Observation of Bacterial Culture and Effects on Biofilm Formation at Microscale. Micromachines 2019, 10, 606. [Google Scholar] [CrossRef]
- Costantini, F.; Lovecchio, N.; Nandimandalam, M.; Manglli, A.; Faggioli, F.; Biasin, M.; Manetti, C.; Roversi, P.F.; Nascetti, A.; de Cesare, G.; et al. Biomolecular Monitoring Tool Based on Lab-on-Chip for Virus Detection. Biosensors 2023, 13, 544. [Google Scholar] [CrossRef]
- Nandimandalam, M.; Costantini, F.; Lovecchio, N.; Iannascoli, L.; Nascetti, A.; de Cesare, G.; Caputo, D.; Manetti, C. Split Aptamers Immobilized on Polymer Brushes Integrated in a Lab-on-Chip System Based on an Array of Amorphous Silicon Photosensors: A Novel Sensor Assay. Materials 2021, 14, 7210. [Google Scholar] [CrossRef]
- Costantini, F.; Lovecchio, N.; Ruggi, A.; Manetti, C.; Nascetti, A.; Reverberi, M.; De Cesare, G.; Caputo, D. Fluorescent Label-Free Aptasensor Integrated in a Lab-on-Chip System for the Detection of Ochratoxin A in Beer and Wheat. ACS Appl. Bio Mater. 2019, 2, 5880–5887. [Google Scholar] [CrossRef]
- Wu, F.; Dekker, C. Nanofabricated Structures and Microfluidic Devices for Bacteria: From Techniques to Biology. Chem. Soc. Rev. 2016, 45, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Scarpari, M.; Bello, C.; Pietricola, C.; Zaccaria, M.; Bertocchi, L.; Angelucci, A.; Ricciardi, M.R.; Scala, V.; Parroni, A.; Fabbri, A.A.; et al. Aflatoxin Control in Maize by Trametes versicolor. Toxins 2014, 6, 3426–3437. [Google Scholar] [CrossRef]
- Panel, E.; Plh, H. Treatment Solutions to Cure Xylella fastidiosa Diseased Plants. EFSA J. 2016, 14, e04456. [Google Scholar] [CrossRef]
- Aralekallu, S.; Boddula, R.; Singh, V. Development of Glass-Based Microfluidic Devices: A Review on Its Fabrication and Biologic Applications. Mater. Des. 2023, 225, 111517. [Google Scholar] [CrossRef]
- De La Fuente, L.; Montanes, E.; Meng, Y.; Li, Y.; Burr, T.J.; Hoch, H.C.; Wu, M. Assessing Adhesion Forces of Type I and Type IV Pili of Xylella fastidiosa Bacteria by Use of a Microfluidic Flow Chamber. Appl. Environ. Microbiol. 2007, 73, 2690–2696. [Google Scholar] [CrossRef] [PubMed]
- Hasheminejad, N.; Khodaiyan, F.; Safari, M. Improving the Antifungal Activity of Clove Essential Oil Encapsulated by Chitosan Nanoparticles. Food Chem. 2019, 275, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.L.F.; Lakshman, D.K.; Zasada, I.A.; Vinyard, B.T.; Chitwood, D.J. Phytotoxicity of Clove Oil to Vegetable Crop Seedlings and Nematotoxicity to Root-Knot Nematodes. Horttechnology 2008, 18, 631–638. [Google Scholar] [CrossRef]
- Chen, C.H.; Lu, Y.; Sin, M.L.Y.; Mach, K.E.; Zhang, D.D.; Gau, V.; Liao, J.C.; Wong, P.K. Antimicrobial Susceptibility Testing Using High Surface-to-Volume Ratio Microchannels. Anal. Chem. 2010, 82, 1012–1019. [Google Scholar] [CrossRef]
- Kandel, P.P.; Lopez, S.M.; Almeida, R.P.P.; De La Fuente, L. Natural Competence of Xylella fastidiosa Occurs at a High Frequency inside Microfluidic Chambers Mimicking the Bacterium’s Natural Habitats. Appl. Environ. Microbiol. 2016, 82, 5269–5277. [Google Scholar] [CrossRef]
- Kucukal, E.; Little, J.A.; Gurkan, U.A. Shear Dependent Red Blood Cell Adhesion in Microscale Flow. Integr. Biol. 2018, 10, 194–206. [Google Scholar] [CrossRef]
- Siddique, A.; Meckel, T.; Stark, R.W.; Narayan, S. Improved Cell Adhesion under Shear Stress in PDMS Microfluidic Devices. Colloids Surf. B Biointerfaces 2017, 150, 456–464. [Google Scholar] [CrossRef]
- Wong, I.; Atsumi, S.; Huang, W.C.; Wu, T.Y.; Hanai, T.; Lam, M.L.; Tang, P.; Yang, J.; Liao, J.C.; Ho, C.M. An Agar Gel Membrane-PDMS Hybrid Microfluidic Device for Long Term Single Cell Dynamic Study. Lab Chip 2010, 10, 2710–2719. [Google Scholar] [CrossRef] [PubMed]
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Kahla-Nakbi, A.B.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. The Chemical Composition and Biological Activity of Clove Essential Oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): A Short Review 501 Kamel. Phyther. Res. 2007, 21, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Cesari, E.; Marocchi, F.; L’Aurora, A.; Pucci, N.; Scala, V.; Loreti, S.; Scortichini, M. Occurrence of Copper-Resistant Pseudomonas syringae pv. actinidiae Strains in Kiwifruit Orchards of Central Italy. J. Phytopathol. 2023, 171, 768–774. [Google Scholar] [CrossRef]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef]
- Baldassarre, F.; Tatulli, G.; Vergaro, V.; Mariano, S.; Scala, V.; Nobile, C.; Pucci, N.; Dini, L.; Loreti, S.; Ciccarella, G. Sonication-Assisted Production of Fosetyl-Al Nanocrystals: Investigation of Human Toxicity and in vitro Antibacterial Efficacy against Xylella fastidiosa. Nanomaterials 2020, 10, 1174. [Google Scholar] [CrossRef]
- Park, A.; Jeong, H.H.; Lee, J.; Kim, K.P.; Lee, C.S. Effect of Shear Stress on the Formation of Bacterial Biofilm in a Microfluidic Channel. Biochip J. 2011, 5, 236–241. [Google Scholar] [CrossRef]


| Substance | Concentration 1 (C1) | Concentration 2 (C2) | Concentration 3 (C3) |
|---|---|---|---|
| Copper sulfate | 1 mg/mL | 1.75 mg/mL | 2.5 mg/mL |
| Punica granatum extract * | 0.2 mg/mL | 2 mg/mL | 20 mg/mL |
| Trametes versicolor extract ** | 2.5 mg/mL | 5 mg/mL | 10 mg/mL |
| Clove oil | 0.01 mg/mL | 0.1 mg/m | 1 mg/mL |
| FossilⓇ | 0.01 μg/mL | 0.1 μg/mL | 1 μg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costantini, F.; Cesari, E.; Lovecchio, N.; Scortichini, M.; Scala, V.; Loreti, S.; Pucci, N. Microfluidic Array Enables Rapid Testing of Natural Compounds Against Xylella fastidiosa. Plants 2025, 14, 872. https://doi.org/10.3390/plants14060872
Costantini F, Cesari E, Lovecchio N, Scortichini M, Scala V, Loreti S, Pucci N. Microfluidic Array Enables Rapid Testing of Natural Compounds Against Xylella fastidiosa. Plants. 2025; 14(6):872. https://doi.org/10.3390/plants14060872
Chicago/Turabian StyleCostantini, Francesca, Erica Cesari, Nicola Lovecchio, Marco Scortichini, Valeria Scala, Stefania Loreti, and Nicoletta Pucci. 2025. "Microfluidic Array Enables Rapid Testing of Natural Compounds Against Xylella fastidiosa" Plants 14, no. 6: 872. https://doi.org/10.3390/plants14060872
APA StyleCostantini, F., Cesari, E., Lovecchio, N., Scortichini, M., Scala, V., Loreti, S., & Pucci, N. (2025). Microfluidic Array Enables Rapid Testing of Natural Compounds Against Xylella fastidiosa. Plants, 14(6), 872. https://doi.org/10.3390/plants14060872







