Preliminary Analysis of the Formation Mechanism of Floret Color in Broccoli (Brassica oleracea L var. italica) Based on Transcriptomics and Targeted Metabolomics
Abstract
1. Introduction
2. Results
2.1. Determination of Color Difference Values and Pigment Contents of Broccoli Florets
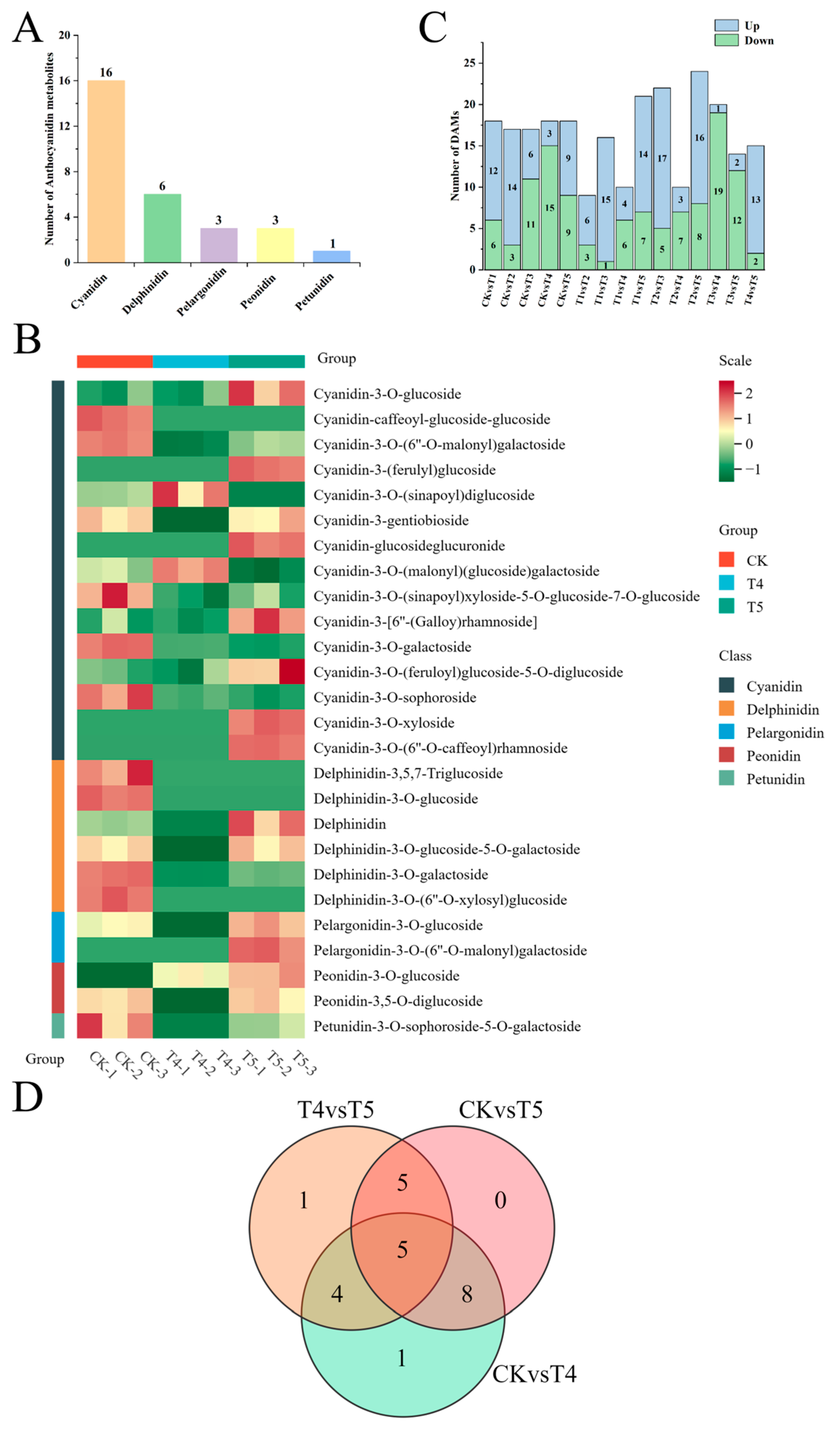
2.2. Metabolic Differences Among Florets of Different Broccoli Varieties
2.3. Overview of the Transcriptome Data of Six Broccoli Varieties
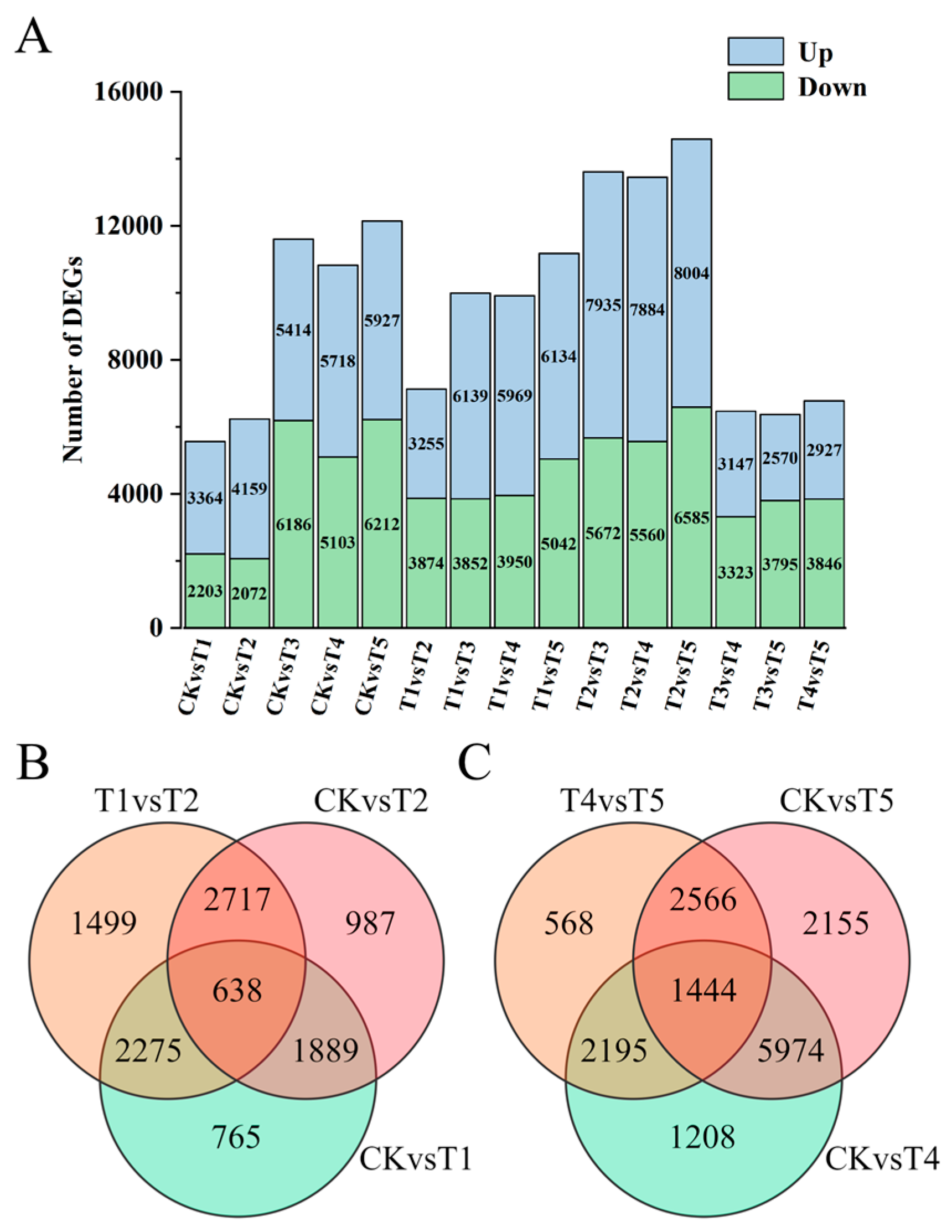
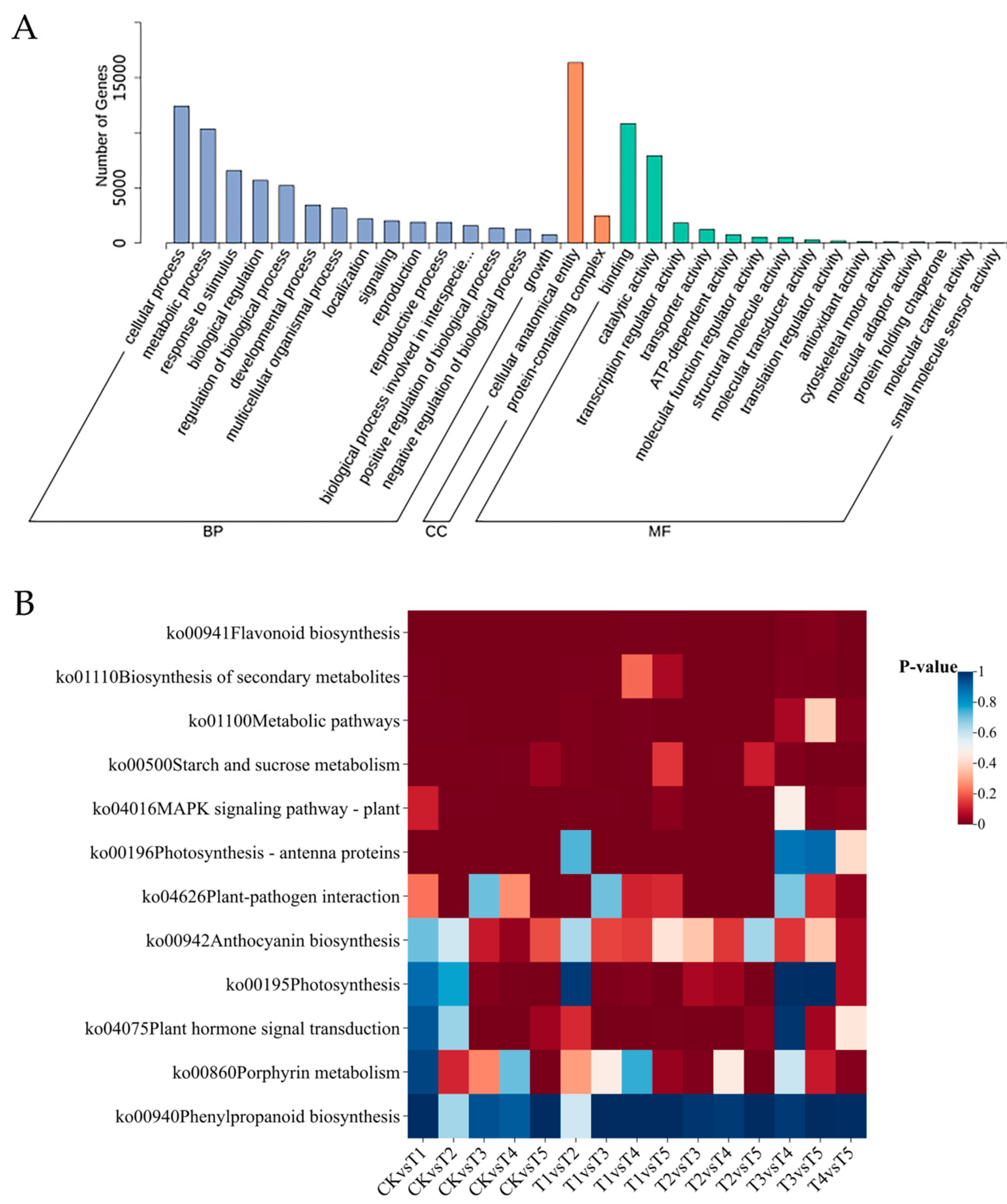
2.4. Screening of Differentially Expressed Genes in Broccoli Florets
2.5. Functional Annotation of Differentially Expressed Genes in Broccoli Florets
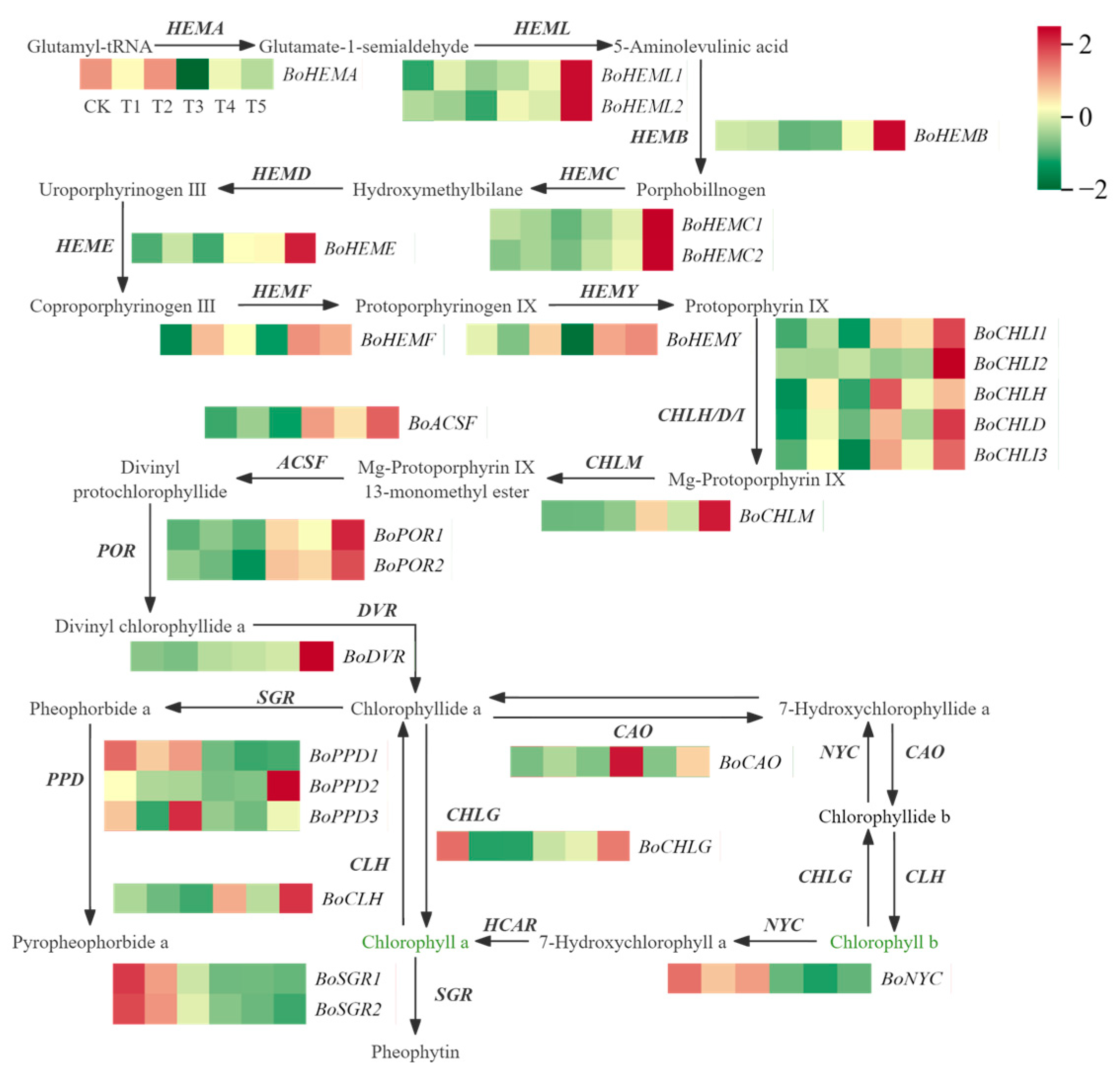
2.6. Identification of Differentially Expressed Genes Related to Chlorophyll Metabolism
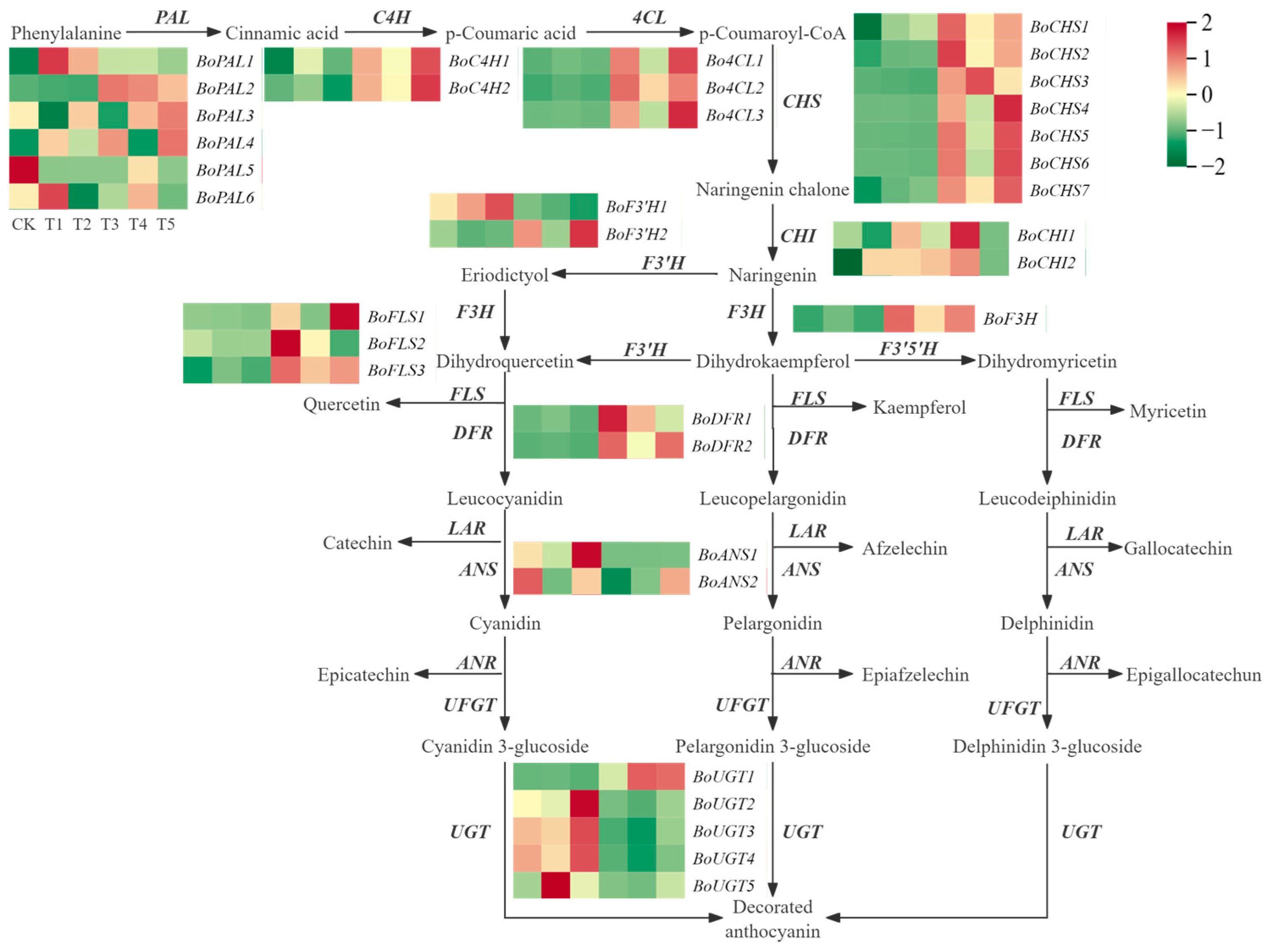
2.7. Identification of Differentially Expressed Genes in Anthocyanin Biosynthesis
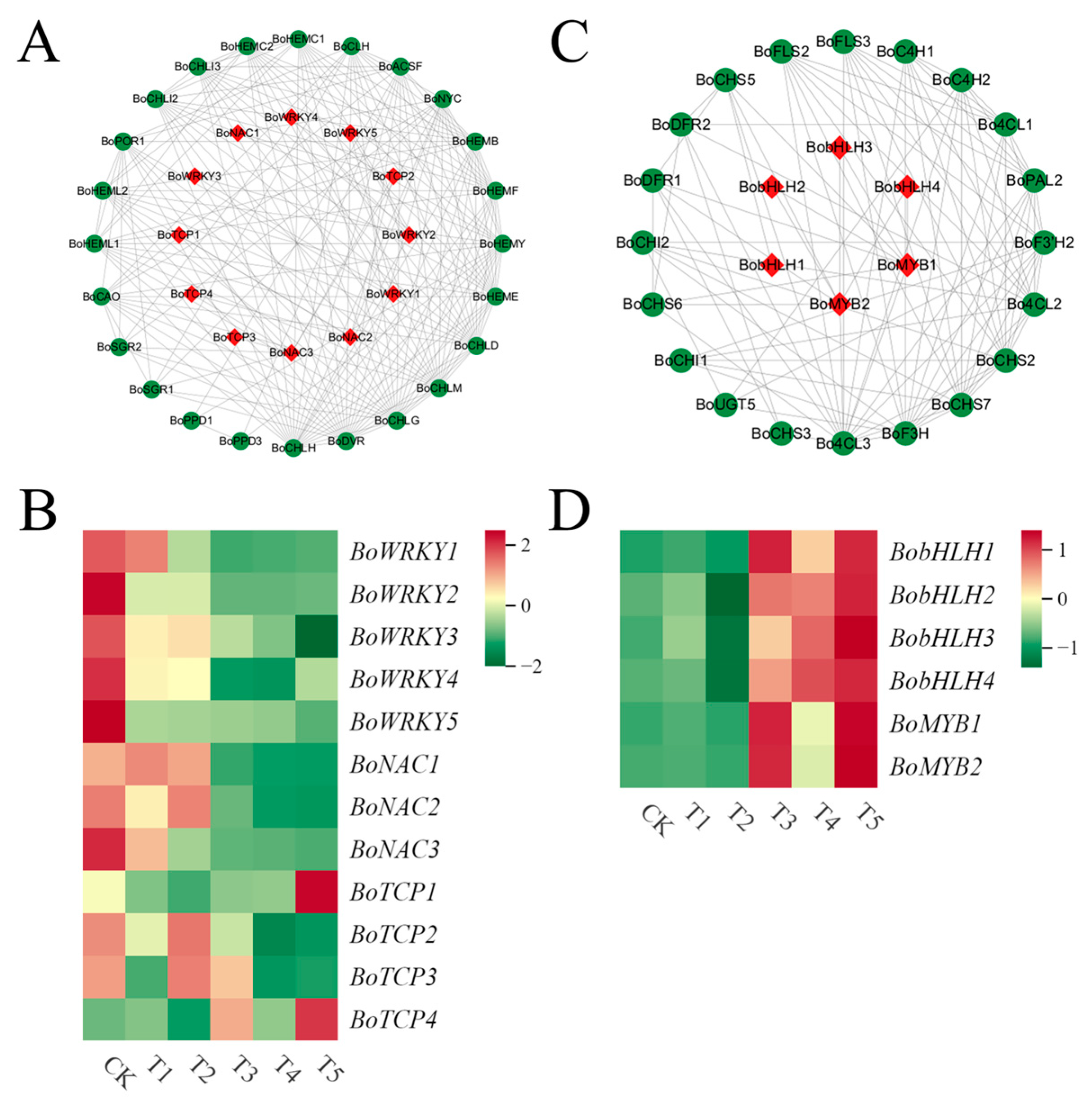
2.8. Identification of Transcription Factors Related to Chlorophyll Metabolism and Anthocyanin Biosynthesis
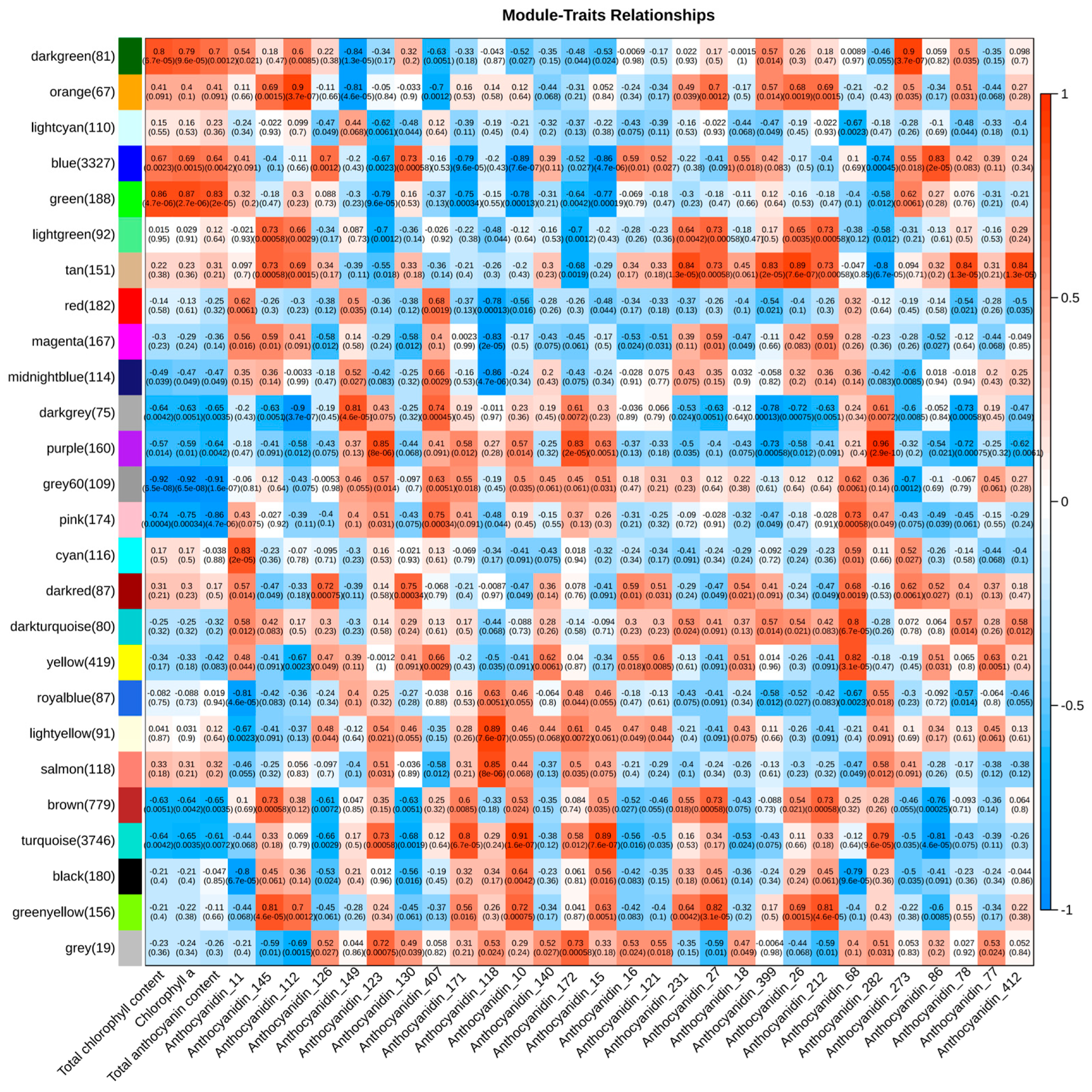
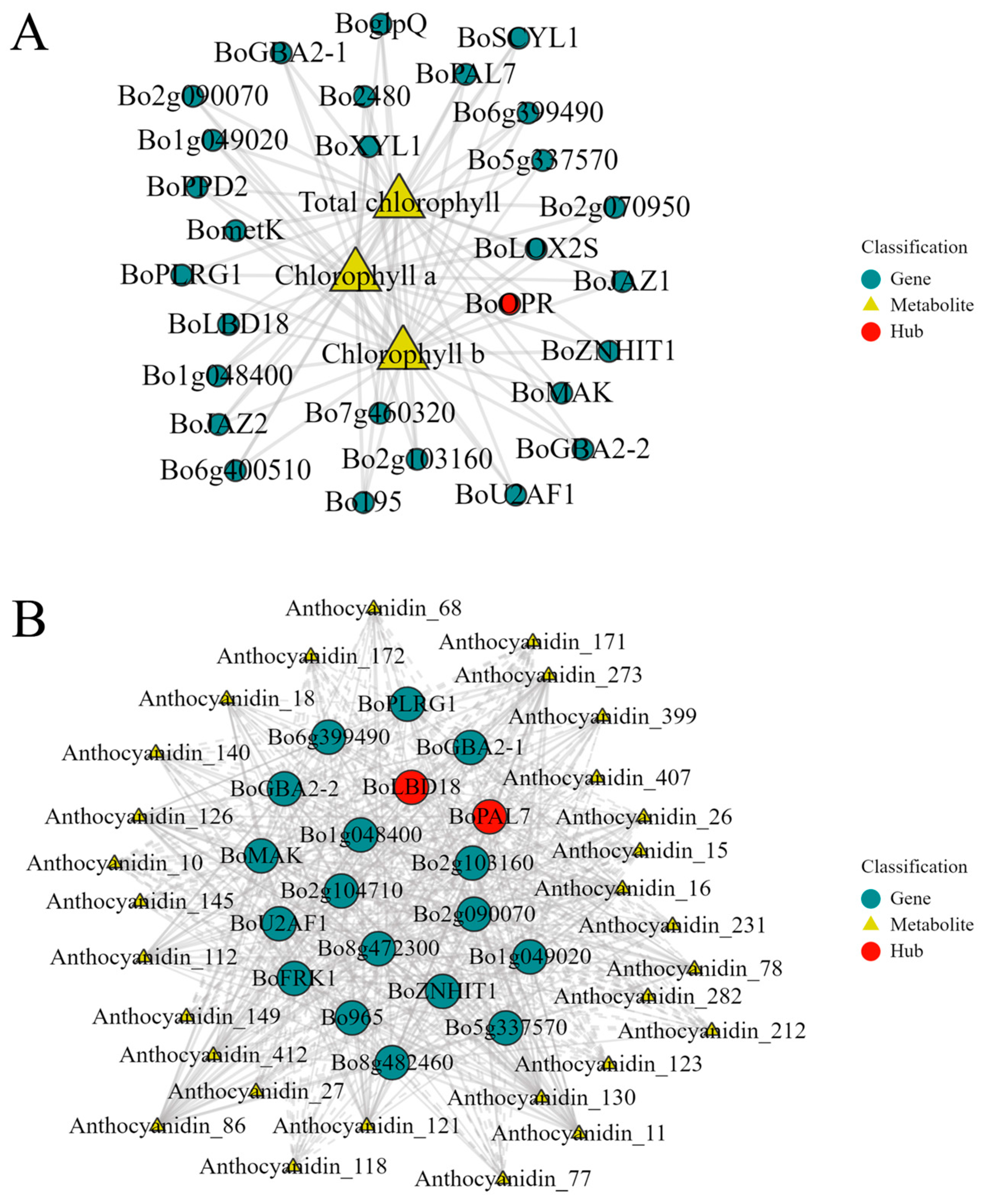
2.9. Construction of the Regulatory Network for Chlorophyll and Anthocyanin Synthesis in Broccoli Florets
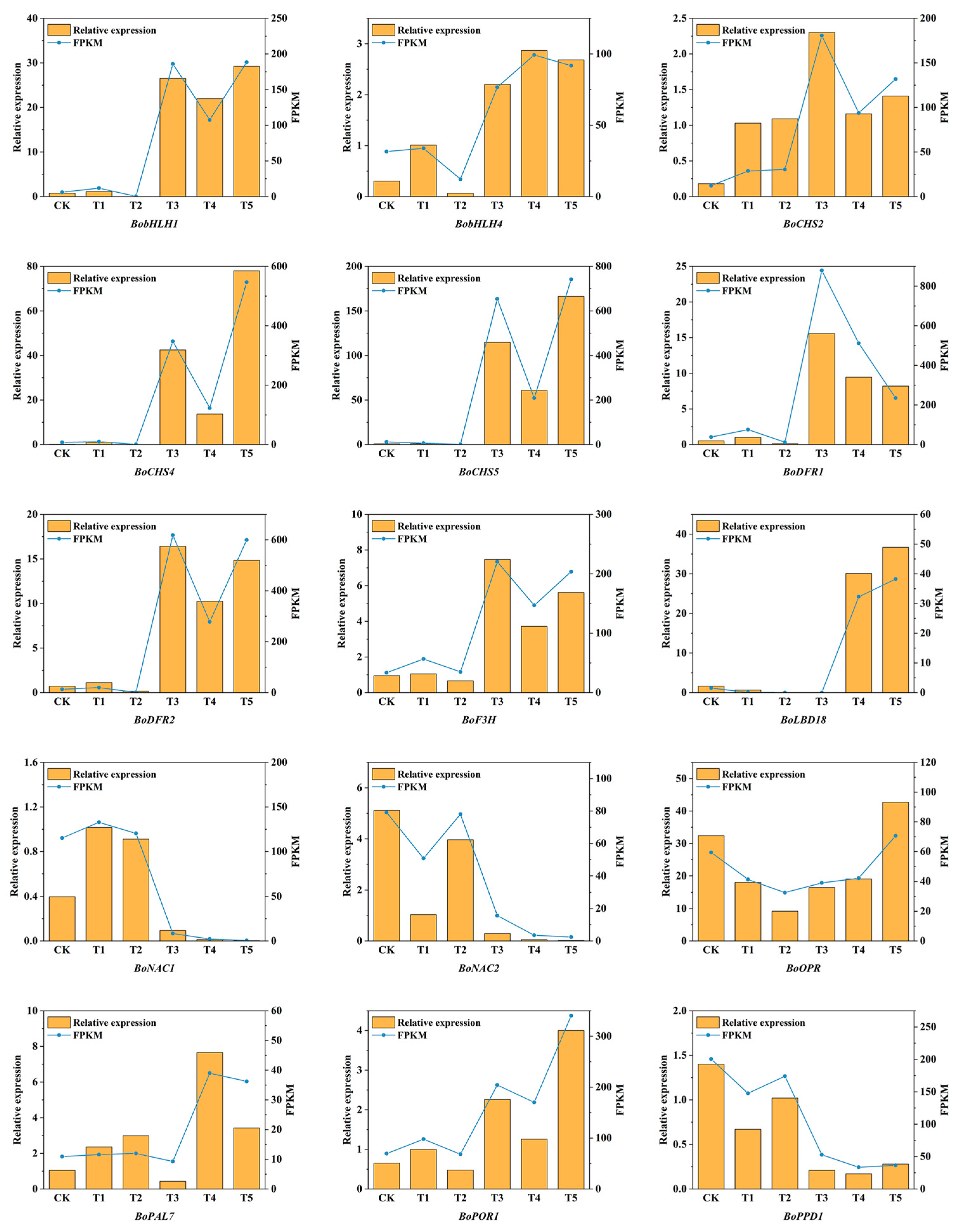
2.10. Confirmation of Differentially Expressed Genes by Quantitative Real-Time PCR Analysis
3. Discussion
3.1. Analysis of Pigment Accumulation in Broccoli Florets with Different Colors
3.2. Metabolic Differences of Anthocyanins in Different Broccoli Varieties
3.3. Analysis of Key Structural Genes in Chlorophyll Metabolism and Anthocyanin Synthesis in Broccoli Florets
3.4. Analysis of Transcription Factors Related to Chlorophyll Metabolism and Anthocyanin Synthesis in Broccoli Florets
3.5. Analysis of Other Key Candidate Genes Related to Chlorophyll Metabolism and Anthocyanin Synthesis in Brassica napus
4. Materials and Methods
4.1. Plant Materials
4.2. Determination of Color Difference Values of Florets
4.3. Determination of the Chlorophyll Content and Flavonoid Content in Florets
4.4. Identification and Quantification of Anthocyanins
4.5. RNA-Seq Analysis
4.6. RNA-seq Data Analysis and Annotation
4.7. Weighted Gene Co-Expression Network Analysis
4.8. Transcription Factor Analysis
4.9. qRT-PCR Analysis
4.10. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.; Xia, Y.; Liu, H.-Y.; Guo, H.; He, X.-Q.; Liu, Y.; Wu, D.-T.; Mai, Y.-H.; Li, H.-B.; Zou, L.; et al. Nutritional Values, Beneficial Effects, and Food Applications of Broccoli (Brassica Oleracea Var. italica Plenck). Trends Food Sci. Technol. 2022, 119, 288–308. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, J.; Feng, B.; Yuan, S.; Yue, X.; Shi, W.; Yan, Z.; Xu, D.; Zuo, J.; Wang, Q. Multi-Omic Analysis of the Extension of Broccoli Quality during Storage by Folic Acid. J. Adv. Res. 2024, 59, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Shi, J.; Yuan, S.; Xu, D.; Zheng, S.; Gao, L.; Wu, C.; Zuo, J.; Wang, Q. Whole-Transcriptome RNA Sequencing Highlights the Molecular Mechanisms Associated with the Maintenance of Postharvest Quality in Broccoli by Red LED Irradiation. Postharvest Biol. Technol. 2022, 188, 111878. [Google Scholar] [CrossRef]
- Tijskens, L.M.M.; Barringer, S.A.; Biekman, E.S.A. Modelling the Effect of pH on the Colour Degradation of Blanched Broccoli. Innov. Food Sci. Emerg. Technol. 2001, 2, 315–322. [Google Scholar] [CrossRef]
- Tian, M.; Zhou, F.; Jiang, A.; Zhu, P.; Chen, C.; Liu, C.; Yuan, C. Research progress in coloration mechanism of Brassica plants. Acta Hortic. Sin. 2023, 50, 1971–1986. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, L.; Safdar, L.B.; Xie, M.; Cheng, X.; Liu, Y.; Xiang, Y.; Tong, C.; Tu, J.; Huang, J.; et al. Characterization and Fine Mapping of a Yellow-Virescent Gene Regulating Chlorophyll Biosynthesis and Early Stage Chloroplast Development in Brassica napus. G3 GenesGenomesGenetics 2020, 10, 3201–3211. [Google Scholar] [CrossRef]
- Xie, F.; Yuan, J.-L.; Li, Y.-X.; Wang, C.-J.; Tang, H.-Y.; Xia, J.-H.; Yang, Q.-Y.; Wan, Z.-J. Transcriptome Analysis Reveals Candidate Genes Associated with Leaf Etiolation of a Cytoplasmic Male Sterility Line in Chinese Cabbage (Brassica rapa L. ssp. Pekinensis). Int. J. Mol. Sci. 2018, 19, 922. [Google Scholar] [CrossRef]
- Liu, M.; Lu, Y.; Wang, S.; Wu, F.; Li, J.; Wang, Y.; Zhao, J.; Shen, S. Characterization of the Leaf Color Mutant Hy and Identification of the Mutated Gene in Chinese Cabbage. J. Am. Soc. Hortic. Sci. 2018, 143, 363–369. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, Y.; Huang, S.; Liu, Z.; Li, C.; Feng, H. Defect in Brnym1, a Magnesium-Dechelatase Protein, Causes a Stay-Green Phenotype in an EMS-Mutagenized Chinese Cabbage (Brassica campestris L. ssp. Pekinensis) Line. Hortic. Res. 2020, 7, 8. [Google Scholar] [CrossRef]
- Qian, L.; Voss-Fels, K.; Cui, Y.; Jan, H.U.; Samans, B.; Obermeier, C.; Qian, W.; Snowdon, R.J. Deletion of a Stay-Green Gene Associates with Adaptive Selection in Brassica napus. Mol. Plant 2016, 9, 1559–1569. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Z.; Zhang, Y.; Li, C.; Feng, H. Identification and Fine Mapping of a Stay-Green Gene (Brnye1) in Pakchoi (Brassica campestris L. ssp. Chinensis). Theor. Appl. Genet. 2018, 131, 673–684. [Google Scholar] [CrossRef]
- Fan, Z.-Q.; Tan, X.-L.; Shan, W.; Kuang, J.-F.; Lu, W.-J.; Chen, J.-Y. BrWRKY65, a WRKY Transcription Factor, Is Involved in Regulating Three Leaf Senescence-Associated Genes in Chinese Flowering Cabbage. Int. J. Mol. Sci. 2017, 18, 1228. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Hu, Z.; Zhu, M.; Zhang, B.; Deng, L.; Pan, Y.; Chen, G. Biochemical and Molecular Analysis of a Temperature-Sensitive Albino Mutant in Kale Named “White Dove”. Plant Growth Regul. 2013, 71, 281–294. [Google Scholar] [CrossRef]
- Zhou, F.; Zuo, J.; Xu, D.; Gao, L.; Wang, Q.; Jiang, A. Low Intensity White Light-Emitting Diodes (LED) Application to Delay Senescence and Maintain Quality of Postharvest Pakchoi (Brassica campestris L. ssp. Chinensis (L.) Makino Var. Communis Tsen et Lee). Sci. Hortic. 2020, 262, 109060. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Z.; Jahan, M.S.; Wen, Y.; Yao, X.; Ding, H.; Guo, S.; Xu, Z. RNA-Seq Analysis Reveals the Growth and Photosynthetic Responses of Rapeseed (Brassica napus L.) under Red and Blue LEDs with Supplemental Yellow, Green, or White Light. Hortic. Res. 2020, 7, 206. [Google Scholar] [CrossRef] [PubMed]
- Koeslin-Findeklee, F.; Rizi, V.S.; Becker, M.A.; Parra-Londono, S.; Arif, M.; Balazadeh, S.; Mueller-Roeber, B.; Kunze, R.; Horst, W.J. Transcriptomic Analysis of Nitrogen Starvation- and Cultivar-Specific Leaf Senescence in Winter Oilseed Rape (Brassica napus L.). Plant Sci. 2015, 233, 174–185. [Google Scholar] [CrossRef]
- Yan, K.; Ran, M.; Li, S.; Zhang, J.; Wang, Y.; Wang, Z.; Wei, D.; Tang, Q. The Delayed Senescence of Postharvest Buds in Salt Ions Was Related to Antioxidant Activity, HDA9 and CCX1 in Broccoli (Brassica oleracea L. Var. Italic Planch.). Food Chem. 2020, 324, 126887. [Google Scholar] [CrossRef]
- Zhang, D.; Du, Y.; He, D.; Zhou, D.; Wu, J.; Peng, J.; Liu, L.; Liu, Z.; Yan, M. Use of Comparative Transcriptomics Combined With Physiological Analyses to Identify Key Factors Underlying Cadmium Accumulation in Brassica juncea L. Front. Genet. 2021, 12, 655885. [Google Scholar] [CrossRef]
- Song, B.; Xu, H.; Chen, L.; Fan, X.; Jing, Z.; Chen, S.; Xu, Z. Study of the Relationship between Leaf Color Formation and Anthocyanin Metabolism among Different Purple Pakchoi Lines. Molecules 2020, 25, 4809. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, Y.; Wang, H.; Tian, Z.; Xin, S.; Zhu, P. The Dihydroflavonol 4-Reductase BoDFR1 Drives Anthocyanin Accumulation in Pink-Leaved Ornamental Kale. Theor. Appl. Genet. 2021, 134, 159–169. [Google Scholar] [CrossRef]
- Hichri, I.; Barrieu, F.; Bogs, J.; Kappel, C.; Delrot, S.; Lauvergeat, V. Recent Advances in the Transcriptional Regulation of the Flavonoid Biosynthetic Pathway. J. Exp. Bot. 2011, 62, 2465–2483. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, Z.; Zeng, Q.; Wang, S.; Luo, Y.; Huang, Y.; Xin, Y.; He, N. Abnormal Expression of bHLH3 Disrupts a Flavonoid Homeostasis Network, Causing Differences in Pigment Composition among Mulberry Fruits. Hortic. Res. 2020, 7, 83. [Google Scholar] [CrossRef]
- Wen, S.; Li, N.; Song, S.; Liu, N.; Ding, Y. Comparative Transcriptome and Metabolome Analyses of Broccoli Germplasms with Purple and Green Curds Reveal the Structural Genes and Transitional Regulators Regulating Color Formation. Int. J. Mol. Sci. 2023, 24, 6115. [Google Scholar] [CrossRef] [PubMed]
- An, G.; Chen, J. Frequent Gain- and Loss-of-Function Mutations of the BjMYB113 Gene Accounted for Leaf Color Variation in Brassica juncea. BMC Plant Biol. 2021, 21, 301. [Google Scholar] [CrossRef]
- Yang, J.; Song, H.D.; Chen, Y.; Chen, B.; Kim, M.; Kim, P.; Kawabata, S.; Li, Y.; Wang, Y. A Single Amino Acid Substitution in the R2R3 Conserved Domain of the BrPAP1a Transcription Factor Impairs Anthocyanin Production in Turnip (Brassica rapa Subsp. Rapa). Plant Physiol. Biochem. 2021, 162, 124–136. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, G.; Li, L.; Li, Y.; Zhou, B.; Yan, H. Identification and Expression Analysis of BrTT8 during Anthocyanin Biosynthesis and Exposure to Abiotic Stress in Turnip (Brassica rapa Subsp. Rapa ‘Tsuda’). Sci. Hortic. 2020, 268, 109332. [Google Scholar] [CrossRef]
- Naing, A.H.; Kim, C.K. Abiotic Stress-induced Anthocyanins in Plants: Their Role in Tolerance to Abiotic Stresses. Physiol. Plant. 2021, 172, 1711–1723. [Google Scholar] [CrossRef]
- Maier, A.; Hoecker, U. COP1/SPA Ubiquitin Ligase Complexes Repress Anthocyanin Accumulation under Low Light and High Light Conditions. Plant Signal. Behav. 2015, 10, e970440. [Google Scholar] [CrossRef] [PubMed]
- Cazzonelli, C.I.; Pogson, B.J. Source to Sink: Regulation of Carotenoid Biosynthesis in Plants. Trends Plant Sci. 2010, 15, 266–274. [Google Scholar] [CrossRef]
- Sun, B.; Jiang, M.; Liang, S.; Zheng, H.; Chen, Q.; Wang, Y.; Lin, Y.; Liu, Z.-J.; Wang, X.-R.; Zhang, F.; et al. Functional Differences of BaPDS1 and BaPDS2 Genes in Chinese Kale. R. Soc. Open Sci. 2019, 6, 190260. [Google Scholar] [CrossRef]
- Izadpanah, F.; Frede, K.; Soltani, F.; Baldermann, S. Comparison of Carotenoid, Chlorophyll Concentrations and Their Biosynthetic Transcript Levels in Different Coloured Cauliflower. Hortic. Plant J. 2024, 10, 743–754. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, L.; Ma, S.; Wang, R.; He, Q.; Tian, M.; Zhang, L. Fine Mapping and Candidate Gene Analysis of the White Flower Gene Brwf in Chinese Cabbage (Brassica rapa L.). Sci. Rep. 2020, 10, 6080. [Google Scholar] [CrossRef]
- Solangi, Z.A.; Zhang, Y.; Li, K.; Du, D.; Yao, Y. Fine Mapping and Candidate Gene Analysis of the Orange Petal Colour Gene Bnpc2 in Spring Rapeseed (Brassica napus). Plant Breed. 2021, 140, 294–304. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, C.; Wang, Y.; Yao, X.; Wang, F.; Wu, J.; King, G.J.; Liu, K. Disruption of a CAROTENOID CLEAVAGE DIOXYGENASE 4 Gene Converts Flower Colour from White to Yellow in Brassica Species. New Phytol. 2015, 206, 1513–1526. [Google Scholar] [CrossRef]
- Han, F.; Cui, H.; Zhang, B.; Liu, X.; Yang, L.; Zhuang, M.; Lv, H.; Li, Z.; Wang, Y.; Fang, Z.; et al. Map-Based Cloning and Characterization of BoCCD4, a Gene Responsible for White/Yellow Petal Color in B. Oleracea. BMC Genom. 2019, 20, 242. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.-J.; Manoharan, R.K.; Park, J.-I.; Chung, M.-Y.; Lee, J.; Lim, Y.-P.; Hur, Y.; Nou, I.-S. Identification of Yellow Pigmentation Genes in Brassica rapa ssp. Pekinensis Using Br300 Microarray. Int. J. Genom. 2014, 2014, 204969. [Google Scholar] [CrossRef]
- Alrifai, O.; Hao, X.; Liu, R.; Lu, Z.; Marcone, M.F.; Tsao, R. LED-Induced Carotenoid Synthesis and Related Gene Expression in Brassica Microgreens. J. Agric. Food Chem. 2021, 69, 4674–4685. [Google Scholar] [CrossRef]
- Kim, Y.; Hwang, I.; Jung, H.-J.; Park, J.-I.; Kang, J.-G.; Nou, I.-S. Genome-Wide Classification and Abiotic Stress-Responsive Expression Profiling of Carotenoid Oxygenase Genes in Brassica rapa and Brassica oleracea. J. Plant Growth Regul. 2016, 35, 202–214. [Google Scholar] [CrossRef]
- Liu, C.; Yao, X.; Li, G.; Huang, L.; Xie, Z. Transcriptomic Profiling of Purple Broccoli Reveals Light-Induced Anthocyanin Biosynthetic Signaling and Structural Genes. PeerJ 2020, 8, e8870. [Google Scholar] [CrossRef]
- Yan, J.; Li, Y.; He, H.; Liu, G.; Tang, X.; Wang, Y.; Peng, X. Effects of Electron Beam Irradiation on the Sensory Qualities and Bioactive Compounds of Broccoli Sprout Juice. Food Res. Int. 2025, 199, 115365. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Zhou, L.; You, S.; Deng, H.; Chen, Y.; Alseekh, S.; Yuan, Y.; Fu, R.; Zhang, Z.; et al. MicroTom Metabolic Network: Rewiring Tomato Metabolic Regulatory Network throughout the Growth Cycle. Mol. Plant 2020, 13, 1203–1218. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Y.; He, L.; Yang, J.; Fernie, A.R.; Luo, J. Natural Variance at the Interface of Plant Primary and Specialized Metabolism. Curr. Opin. Plant Biol. 2022, 67, 102201. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, D.; Song, Y.; Zhu, H.; Luo, Y.; Qu, G. Review on Transcriptional Regulation of Chlorophyll Metabolism Network in Tomato Fruits. Acta Hortic. Sin. 2023, 50, 2031–2047. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, X.; Ye, J. Research Progress of Anthocyanin Synthesis and Regulatory Factors in Plants. Life Sci. Res. 2024, 28, 493–502. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A Global Perspective on Carotenoids: Metabolism, Biotechnology, and Benefits for Nutrition and Health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef]
- Yu, H.; Wang, T.; Zhang, P.; Huang, Y.; Yu, T.; Cao, X. Comparison on Morphological and Quality Traits of Different Broccoli Cultivars. Chin. Agric. Sci. Bull. 2021, 37, 49–55. [Google Scholar]
- Mizuno, T.; Sugahara, K.; Tsutsumi, C.; Iino, M.; Koi, S.; Noda, N.; Iwashina, T. Identification of Anthocyanin and Other Flavonoids from the Green–Blue Petals of Puya Alpestris (Bromeliaceae) and a Clarification of Their Coloration Mechanism. Phytochemistry 2021, 181, 112581. [Google Scholar] [CrossRef]
- Wang, J.; Shen, Y.; Yu, H.; Sheng, X.; Song, M.; Gu, H. Research progress of broccoli breeding in China. Acta Agric. Zhejiangensis 2024, 36, 1934–1944. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, G.; Guo, T.; He, G.; Zhao, X.; Liu, S. Recent Advances in Cuticular Was Biosynthesis and Its Molecular Regulation in Plants. J. Agric. Sci. Technol. 2025, 1–14. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, Pharmacology and Health Benefits of Anthocyanins. Phytother. Res. 2016, 30, 1265–1286. [Google Scholar] [CrossRef]
- Liu, Y.; Tikunov, Y.; Schouten, R.E.; Marcelis, L.F.M.; Visser, R.G.F.; Bovy, A. Anthocyanin Biosynthesis and Degradation Mechanisms in Solanaceous Vegetables: A Review. Front. Chem. 2018, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Wang, X.; Lin, X.; Mostafa, S.; Zou, H.; Wang, L.; Jin, B. Plant Anthocyanins: Classification, Biosynthesis, Regulation, Bioactivity, and Health Benefits. Plant Physiol. Biochem. 2024, 217, 109268. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Liu, Z.; Niu, R.; Feng, H. Mapping of Re, a Gene Conferring the Red Leaf Trait in Ornamental Kale (Brassica oleracea L. Var. Acephala). Plant Breed. 2015, 134, 494–500. [Google Scholar] [CrossRef]
- He, Q.; Zhang, Z.; Zhang, L. Anthocyanin Accumulation, Antioxidant Ability and Stability, and a Transcriptional Analysis of Anthocyanin Biosynthesis in Purple Heading Chinese Cabbage (Brassica rapa L. ssp. Pekinensis). J. Agric. Food Chem. 2016, 64, 132–145. [Google Scholar] [CrossRef]
- Chen, D.; Yang, Y.; Niu, G.; Shan, X.; Zhang, X.; Jiang, H.; Liu, L.; Wen, Z.; Ge, X.; Zhao, Q.; et al. Metabolic and RNA Sequencing Analysis of Cauliflower Curds with Different Types of Pigmentation. AoB Plants 2022, 14, plac001. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, H.; Alegre, S.; Pascual, J.; Trotta, A.; Yang, W.; Yang, B.; Seyednasrollah, F.; Burow, M.; Kangasjärvi, S. Growth Conditions Trigger Genotype-Specific Metabolic Responses That Affect the Nutritional Quality of Kale Cultivars. J. Exp. Bot. 2024, erae169. [Google Scholar] [CrossRef]
- Li, J.; Yu, X.; Cai, Z.; Wu, F.; Luo, J.; Zheng, L.; Chu, W. An Overview of Chlorophyll Biosynthesis in Higher Plants. Mol. Plant Breed. 2019, 17, 6013–6019. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Z.; Shan, X.; Li, C.; Tang, X.; Chi, M.; Feng, H. Physiological Properties and Chlorophyll Biosynthesis in a Pak-Choi (Brassica rapa L. ssp. Chinensis) Yellow Leaf Mutant, Pylm. Acta Physiol. Plant. 2016, 39, 22. [Google Scholar] [CrossRef]
- Gao, F.; Guo, J.; Shen, Y. Advances from Chlorophyll Biosynthesis to Photosynthetic Adaptation, Evolution and Signaling. Plant Stress 2024, 12, 100470. [Google Scholar] [CrossRef]
- Wang, S. Anthocyanin Biosynthesis in Sweetpotato: Current Status and Future Perspectives. J. Food Compos. Anal. 2024, 132, 106353. [Google Scholar] [CrossRef]
- Gu, C.; Liao, L.; Zhou, H.; Wang, L.; Deng, X.; Han, Y. Constitutive Activation of an Anthocyanin Regulatory Gene PcMYB10.6 Is Related to Red Coloration in Purple-Foliage Plum. PLoS ONE 2015, 10, e0135159. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, S.; Yang, Y.; Liu, J.; Zhuang, Y. Integrated Metabolome and Transcriptome Analysis Reveals a Regulatory Network of Fruit Peel Pigmentation in Eggplant (Solanum melongena L.). Int. J. Mol. Sci. 2022, 23, 13475. [Google Scholar] [CrossRef]
- Li, W.-F.; Mao, J.; Yang, S.-J.; Guo, Z.-G.; Ma, Z.-H.; Dawuda, M.M.; Zuo, C.-W.; Chu, M.-Y.; Chen, B.-H. Anthocyanin Accumulation Correlates with Hormones in the Fruit Skin of ‘Red Delicious’ and Its Four Generation Bud Sport Mutants. BMC Plant Biol. 2018, 18, 363. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Hymus, G.J.; Cai, S.; Kohl, E.A.; Holtan, H.E.; Marion, C.M.; Tiwari, S.; Maszle, D.R.; Lundgren, M.R.; Hong, M.C.; Channa, N.; et al. Application of HB17, an Arabidopsis Class II Homeodomain-Leucine Zipper Transcription Factor, to Regulate Chloroplast Number and Photosynthetic Capacity. J. Exp. Bot. 2013, 64, 4479–4490. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Xiao, X.; Xu, Y.; Fan, Z.; Tan, X.; Shan, W.; Kuang, J.; Lu, W.; Su, X.; Chen, J. BrJUB1, a NAC Family Transcription Factor, Regulates Postharvest Leaf Senescence of Chinese Flowering Cabbage through the Transcriptional Activation of BrCCGs. N. Z. J. Crop Hortic. Sci. 2021, 49, 92–105. [Google Scholar] [CrossRef]
- Xu, Y.; Xiao, X.; Zeng, Z.; Tan, X.; Liu, Z.; Chen, J.; Su, X.; Chen, J. BrTCP7 Transcription Factor Is Associated with MeJA-Promoted Leaf Senescence by Activating the Expression of BrOPR3 and BrRCCR. Int. J. Mol. Sci. 2019, 20, 3963. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, F.; Wu, F.; Yin, Y.; Gao, S.; Xu, K.; Liu, Y.; Yao, M.; Li, N. Review of Anthocyanin Synthesis on Pepper (Capsicum annuum L.). Mol. Plant Breed. 2024, 1–7. [Google Scholar]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB Transcription Factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef]
- Mushtaq, M.A.; Pan, Q.; Chen, D.; Zhang, Q.; Ge, X.; Li, Z. Comparative Leaves Transcriptome Analysis Emphasizing on Accumulation of Anthocyanins in Brassica: Molecular Regulation and Potential Interaction with Photosynthesis. Front. Plant Sci. 2016, 7, 311. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, M.; Wen, C.; Xie, X.; Tian, W.; Wen, S.; Lu, R.; Liu, L. Integrated Metabolomic and Transcriptomic Analysis of the Anthocyanin Regulatory Networks in Salvia Miltiorrhiza Bge. Flowers. BMC Plant Biol. 2020, 20, 349. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhu, L.; Zhang, X.; Guan, Q.; Xiao, S.; Min, L.; Zhang, X. GhCPK33 Negatively Regulates Defense against Verticillium dahliae by Phosphorylating GhOPR3. Plant Physiol. 2018, 178, 876–889. [Google Scholar] [CrossRef]
- Fang, H.; Luo, F.; Li, P.; Zhou, Q.; Zhou, X.; Wei, B.; Cheng, S.; Zhou, H.; Ji, S. Potential of Jasmonic Acid (JA) in Accelerating Postharvest Yellowing of Broccoli by Promoting Its Chlorophyll Degradation. Food Chem. 2020, 309, 125737. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Li, J.; Wang, J.; Li, R.; Tian, Q.; Wu, Y.; Yu, J. Research progress of anthocyanin biosynthesis, regulation, bioactivity and detection in higher plants. Acta Agric. Zhejiangensis 2024, 36, 978–996. [Google Scholar] [CrossRef]
- Tian, J.; Xu, R.; Chang, K.; Yuan, S.; Huang, C.; Wang, J.; Li, S.; Liu, F.; Zhong, F. Identification of PAL Gene in Purple Cabbage and Functional Analysis Related to Anthocyanin Synthesis. Horticulturae 2023, 9, 469. [Google Scholar] [CrossRef]
- Chen, X.; Wang, P.; Gu, M.; Hou, B.; Zhang, C.; Zheng, Y.; Sun, Y.; Jin, S.; Ye, N. Identification of PAL Genes Related to Anthocyanin Synthesis in Tea Plants and Its Correlation with Anthocyanin Content. Hortic. Plant J. 2022, 8, 381–394. [Google Scholar] [CrossRef]
- Ahn, J.Y.; Jung, Y.H.; Song, H.; Yi, H.; Hur, Y. Alleles Disrupting LBD37-like Gene by an 136 Bp Insertion Show Different Distributions between Green and Purple Cabbages (Brassica oleracea Var. Capitata). Genes Genom. 2021, 43, 679–688. [Google Scholar] [CrossRef]
- Wang, X. Principles and Techniques of Plant Physiology and Biochemistry Experiments, 2nd ed.; Higher Education Press: Beijing, China, 2006. [Google Scholar]
- Zhang, X.; Zhu, S.; Zhong, X.; Luo, Q.; Xiong, A.; Tang, G. Analysis of morphology, anthocyanin content and expression of related genes in different germplasm resources of Oenanthe javanica. J. Guizhou Norm. Univ. Sci. 2025, 43, 105–113. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
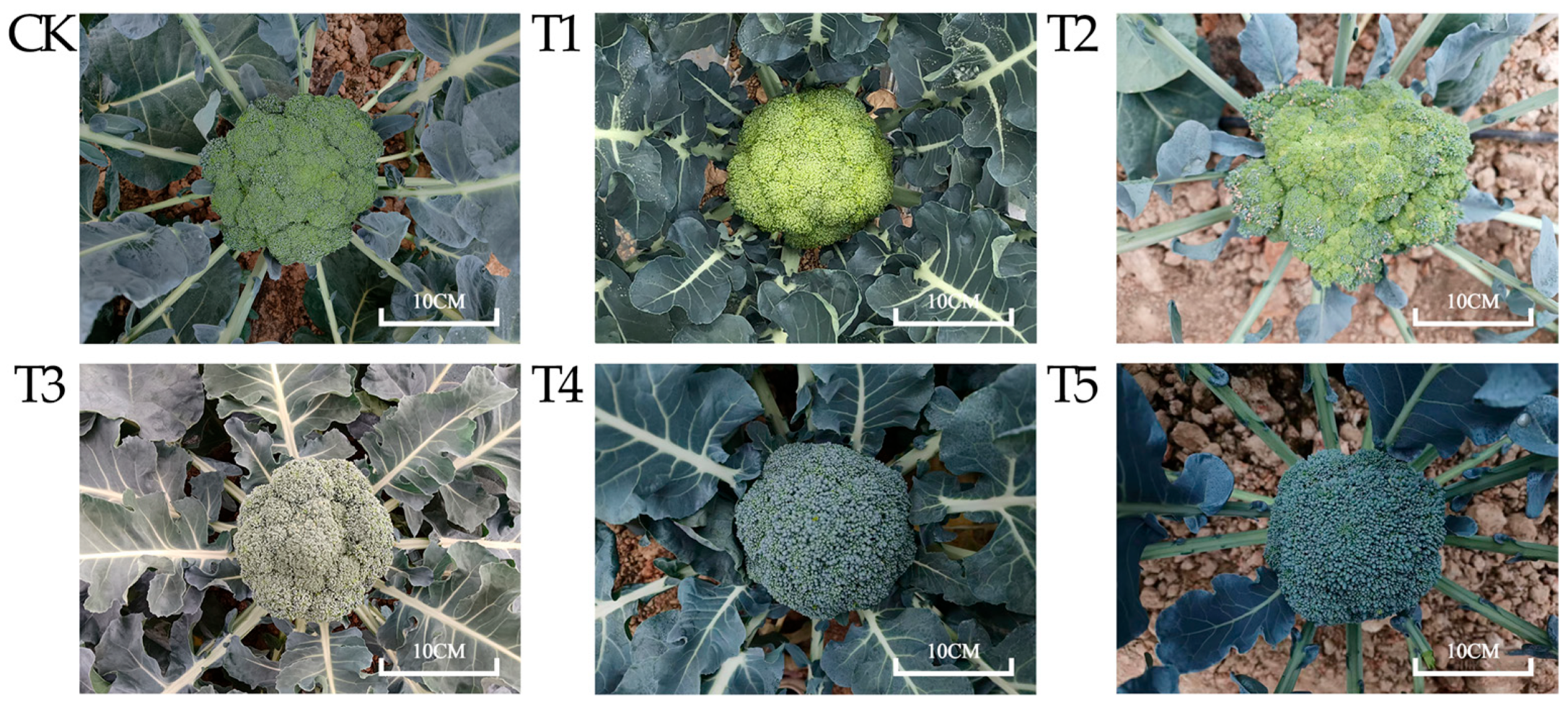
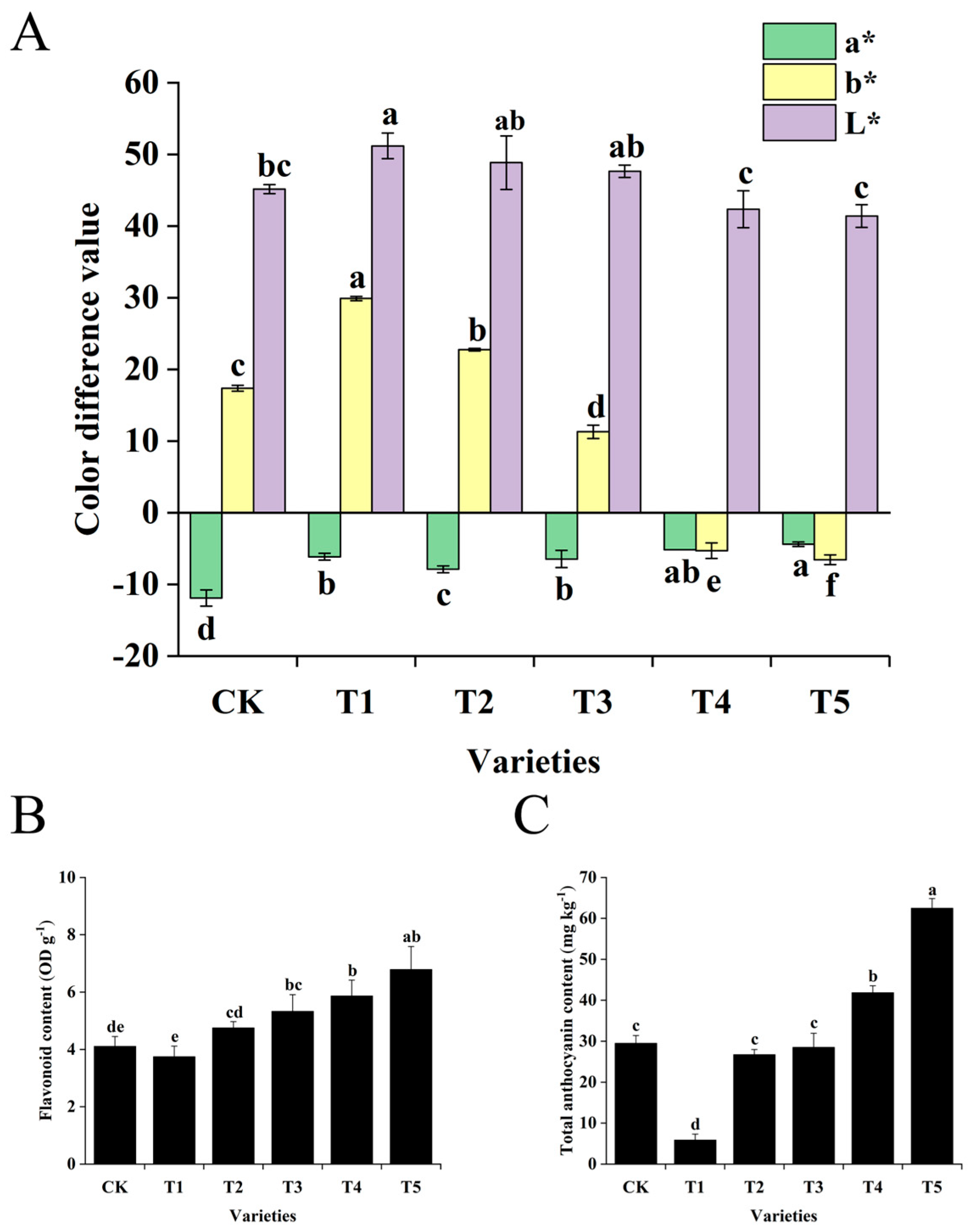

| Varieties | Chlorophyll a mg g−1 FW | Chlorophyll b mg g−1 FW | Total Chlorophyll mg g−1 FW | Carotenoid mg g−1 FW | Chlorophyll a/b | Chlorophyll/Carotenoid |
|---|---|---|---|---|---|---|
| CK | 0.15 ± 0.01 c | 0.07 ± 0.01 c | 0.22 ± 0.02 c | 0.03 ± 0.00 c | 2.36 ± 0.06 a | 0.13 ± 0.01 bc |
| T1 | 0.05 ± 0.01 e | 0.02 ± 0.01 d | 0.07 ± 0.01 d | 0.01 ± 0.00 e | 2.43 ± 0.67 a | 0.17 ± 0.05 a |
| T2 | 0.13 ± 0.01 d | 0.06 ± 0.01 c | 0.19 ± 0.01 c | 0.02 ± 0.00 d | 2.10 ± 0.08 a | 0.11 ± 0.01 c |
| T3 | 0.15 ± 0.01 cd | 0.06 ± 0.01 c | 0.20 ± 0.01 c | 0.03 ± 0.00 c | 2.54 ± 0.14 a | 0.14 ± 0.00 abc |
| T4 | 0.25 ± 0.02 b | 0.10 ± 0.02 b | 0.36 ± 0.03 b | 0.06 ± 0.00 b | 2.47 ± 0.24 a | 0.16 ± 0.02 ab |
| T5 | 0.43 ± 0.00 a | 0.19 ± 0.01 a | 0.62 ± 0.02 a | 0.10 ± 0.00 a | 2.26 ± 0.11 a | 0.16 ± 0.00 ab |
| TF Family | Gene Symbol |
|---|---|
| WRKY | BoWRKY1 |
| BoWRKY2 | |
| BoWRKY3 | |
| BoWRKY4 | |
| BoWRKY5 | |
| NAC | BoNAC1 |
| BoNAC2 | |
| BoNAC3 | |
| TCP | BoTCP1 |
| BoTCP2 | |
| BoTCP3 | |
| BoTCP4 | |
| bHLH | BobHLH1 |
| BobHLH2 | |
| BobHLH3 | |
| BobHLH4 | |
| MYB | BoMYB1 |
| BoMYB2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, Q.; Chen, M.; Cheng, S.; Lin, H.; Lin, B.; Lin, H.; Liu, J.; Zhu, H. Preliminary Analysis of the Formation Mechanism of Floret Color in Broccoli (Brassica oleracea L var. italica) Based on Transcriptomics and Targeted Metabolomics. Plants 2025, 14, 849. https://doi.org/10.3390/plants14060849
Shao Q, Chen M, Cheng S, Lin H, Lin B, Lin H, Liu J, Zhu H. Preliminary Analysis of the Formation Mechanism of Floret Color in Broccoli (Brassica oleracea L var. italica) Based on Transcriptomics and Targeted Metabolomics. Plants. 2025; 14(6):849. https://doi.org/10.3390/plants14060849
Chicago/Turabian StyleShao, Qingqing, Mindong Chen, Saichuan Cheng, Huangfang Lin, Biying Lin, Honghui Lin, Jianting Liu, and Haisheng Zhu. 2025. "Preliminary Analysis of the Formation Mechanism of Floret Color in Broccoli (Brassica oleracea L var. italica) Based on Transcriptomics and Targeted Metabolomics" Plants 14, no. 6: 849. https://doi.org/10.3390/plants14060849
APA StyleShao, Q., Chen, M., Cheng, S., Lin, H., Lin, B., Lin, H., Liu, J., & Zhu, H. (2025). Preliminary Analysis of the Formation Mechanism of Floret Color in Broccoli (Brassica oleracea L var. italica) Based on Transcriptomics and Targeted Metabolomics. Plants, 14(6), 849. https://doi.org/10.3390/plants14060849






