Analysis of the Antioxidant and Antimicrobial Activity, Cytotoxic, and Anti-Migratory Properties of the Essential Oils Obtained from Cultivated Medicinal Lamiaceae Species
Abstract
1. Introduction
2. Results
2.1. DPPH Radical-Scavenging Assay of EOs Obtained from Cultivated Medicinal Species Belonging to the Lamiaceae Family
2.2. Antimicrobial Action of the Analysed EOs
2.2.1. Analysis of the Diameters of Inhibition Zones Obtained by the Disc Diffusion Method
2.2.2. Determination of Minimum Inhibitory Concentration and Minimum Concentration
2.3. Antitumoural Effects of the EOs
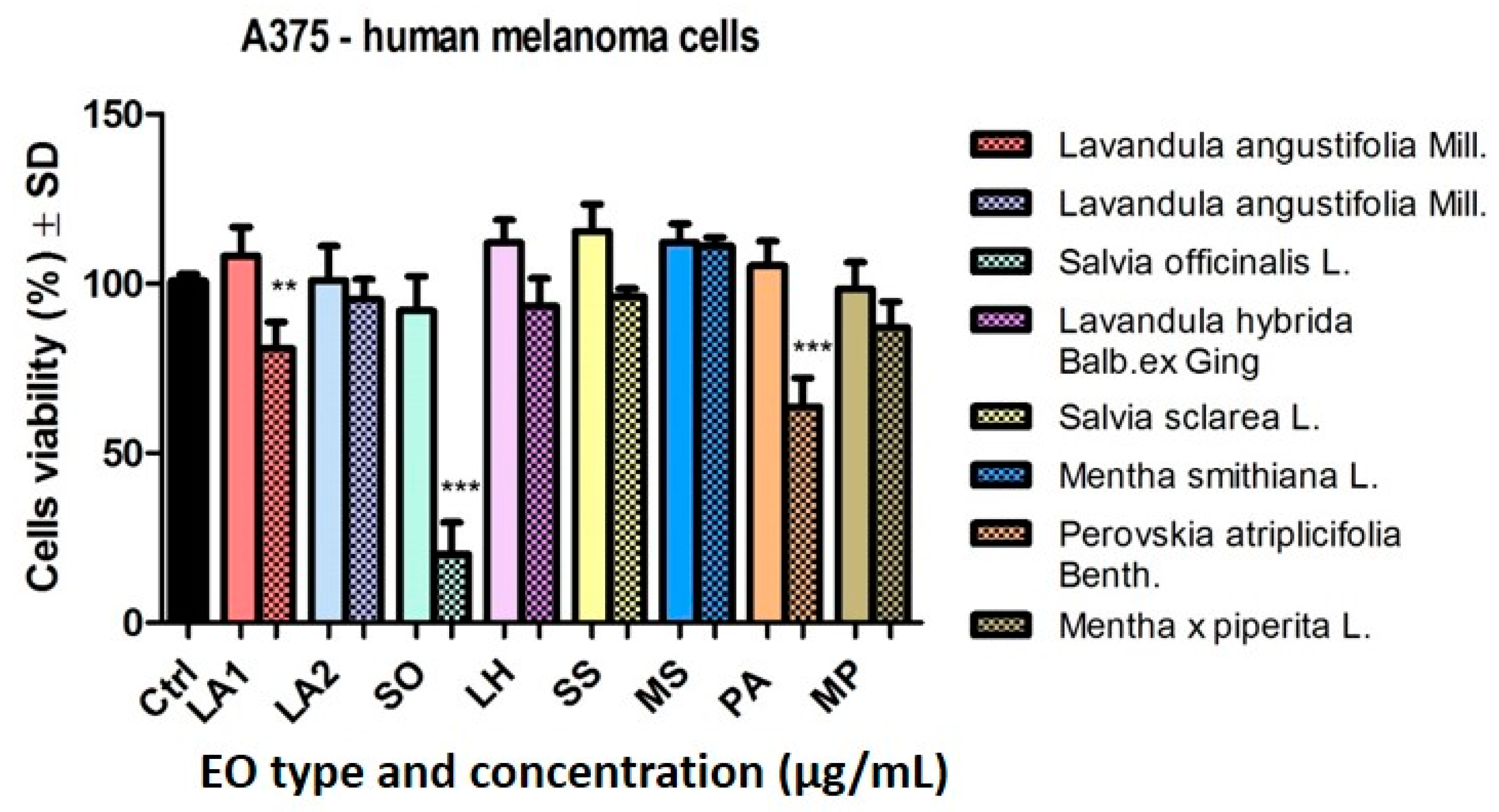
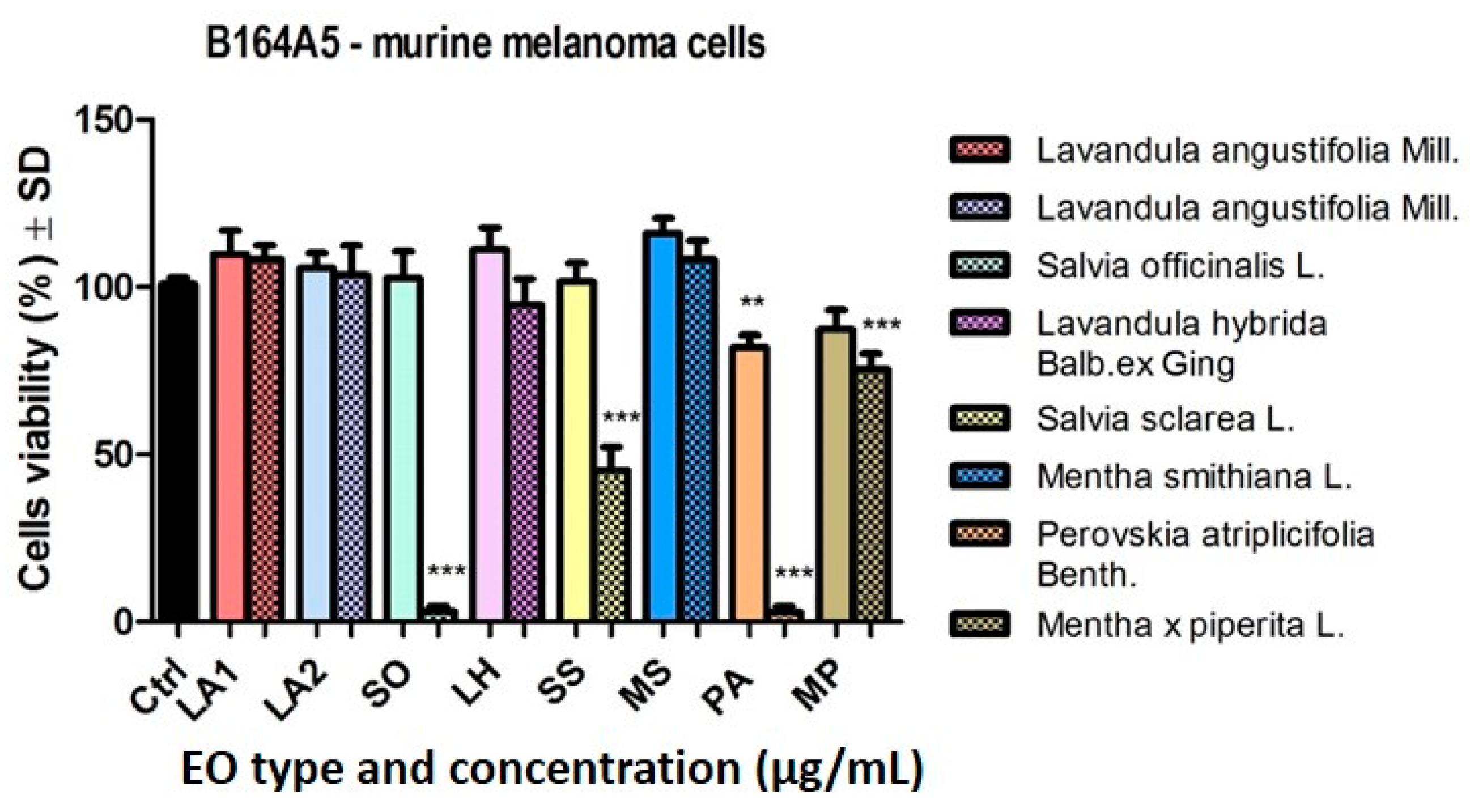
2.3.1. Analysis of the Cytotoxic Effect of the EOs
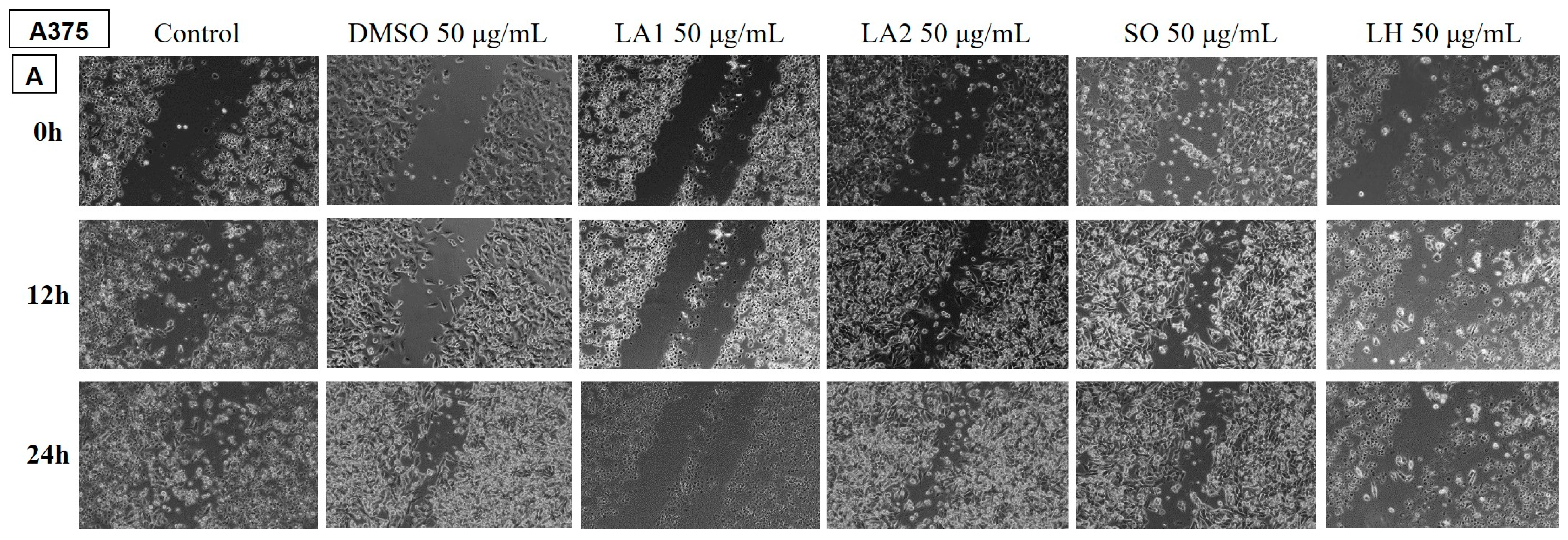
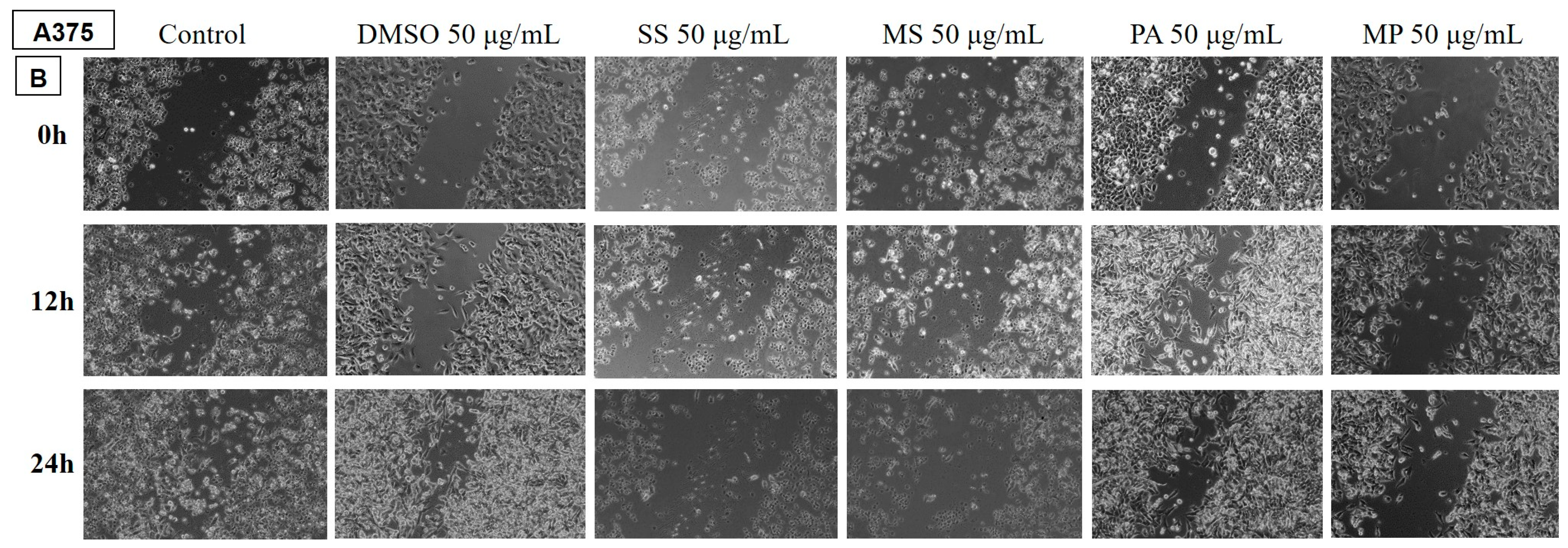
2.3.2. Analysis of the Anti-Migratory Effect of the EOs Using the Scratch Technique
Determination of the Anti-Migratory Effect of the EOs on A375 Human Melanoma Cell Line
Determination of the Anti-Migratory Effect of the EOs on B164A5 Murine Melanoma Cell Line
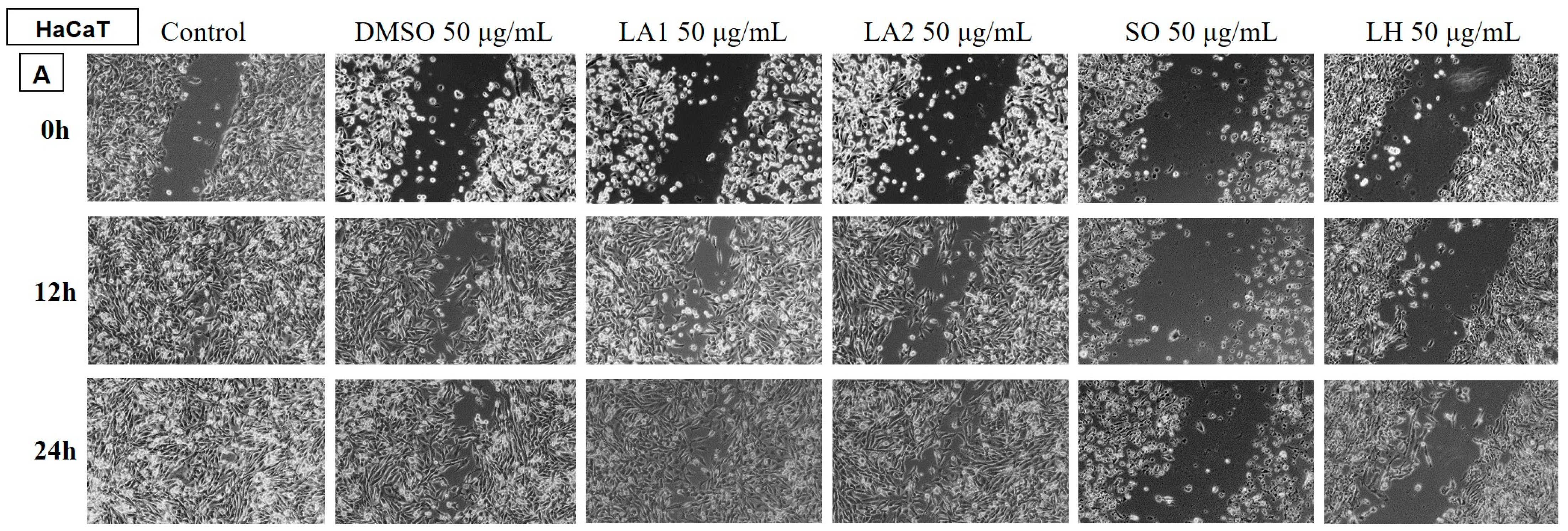
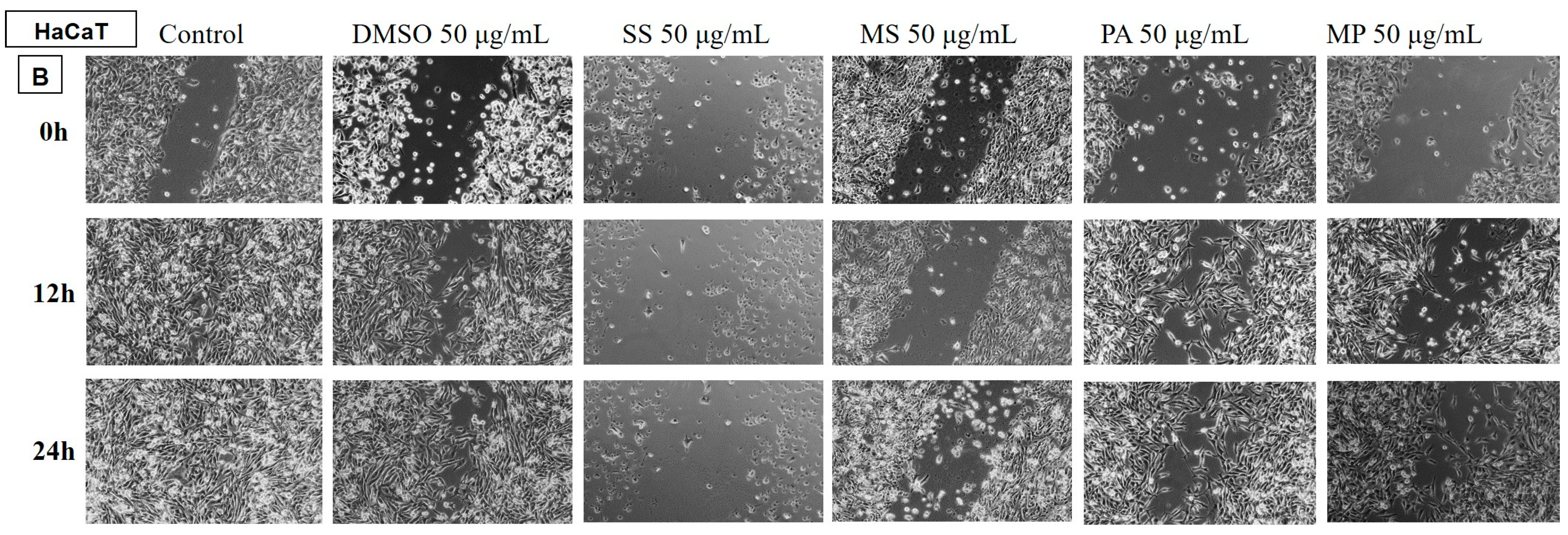
Determination of the Anti-Migratory Effect of the EOs on HaCaT, Human Keratinocyte
3. Discussion
3.1. Antioxidant Capacity of EO
3.2. Antimicrobial Effect of EO
3.3. Cytotoxic and Anti-Migratory Effects of the EOs
4. Materials and Methods
4.1. Materials Used
4.1.1. EO Extraction and Composition Analysis
4.1.2. Reagents
4.1.3. Microbial Strains
4.2. Methods Used
4.2.1. DPPH Radical-Scavenging Assay
4.2.2. Determination of Antimicrobial Activity
- A. Disc-diffusion assay
- B. Macro-dilution method
- C. Determination of CMB/CMF
4.2.3. Determination of the Antitumour Activity
- A. MTT assay
- B. Scratch assay
4.2.4. Statistical Methods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Chemical Compound | % | RT (min) |
|---|---|---|
| Eucalyptol | 2.144 | |
| Trans-Beta-Ocimene | 5.976 | 10.836 |
| cis-beta-Ocimene | 2.477 | 14.876 |
| Linalool | 22.11 | 17.589 |
| endo-Borneol | 2.23 | 18.043 |
| Terpinen-4-ol | 3.811 | 18.59 |
| Alpha-Terpineol | 2.13 | 21.202 |
| Linalyl acetate | 20.384 | 22.22 |
| Lavandulyl acetate | 7.599 | 26.35 |
| Carvacrol | 3.333 | 27.255 |
| Diphenhydramine | 7.31 | 28.742 |
| cis-beta-Farnesene | 3.86 | 31.771 |
| tau-Cadinol | 2.473 |
| Chemical Compound | % | RT (min) |
|---|---|---|
| Alpha-Thujene | 0.12 | 5.656 |
| Alpha-Pinene | 0.38 | 5.893 |
| Camphene | 0.33 | 6.379 |
| Sabinene | 0.6 | 7.487 |
| Beta-Pinene | 0.51 | 8.075 |
| n-Hexyl acetate | 0.2 | 8.908 |
| 3-Carene | 0.26 | 9.139 |
| p-Cymene | 0.13 | 9.643 |
| Benzene, 1-methyl-3-(1-methylethyl)- | 0.84 | 9.861 |
| Eucalyptol | 1.45 | 10.126 |
| D-Limonene | 1.02 | 10.193 |
| trans-beta-Ocimene | 2.18 | 10.819 |
| 1,3,6-Octatriene, 3,7-dimethyl-, (Z)- | 0.85 | 11.577 |
| Sabinene hydrate | 0.49 | 13.177 |
| Linalool | 30.15 | 15.322 |
| (+)-2-Bornanone | 1.57 | 16.321 |
| endo-Borneol | 2.46 | 18.258 |
| (−)-4-Terpineol | 7.19 | 18.47 |
| Alpha-Terpineol | 1.83 | 19.243 |
| Borneol, acetate | 0.24 | 19.983 |
| cis-(−)-1,2-Epoxy-p-menth-8-ene | 0.16 | 20.228 |
| Linalyl acetate | 23.71 | 21.207 |
| (Z)-Geraniol | 0.81 | 21.605 |
| Bicyclo[2.2.1]heptan-2-ol, 1,7,7-trimethyl-, acetate, (1S-endo)- | 0.39 | 22.004 |
| Lavandulol acetate | 6.21 | 22.251 |
| Hexyl tiglate | 0.07 | 23.491 |
| (R)-lavandulyl (R)-2-methylbutanoate | 0.59 | 24.482 |
| cis-Geranyl acetate | 0.9 | 25.03 |
| Hexanoic acid, hexyl ester | 0.23 | 25.15 |
| (−)-beta-Bourbonene | 0.09 | 25.35 |
| Sesquithujene | 0.1 | 25.497 |
| Beta-Curcumene | 0.05 | 25.934 |
| Alpha-Cedrene | 0.1 | 26.183 |
| Bicyclo[5.2.0]nonane, 2-methylene-4,8,8-trimethyl-4-vinyl- | 4.79 | 26.361 |
| cis-alpha-Bergamotene | 0.2 | 26.768 |
| Aromandendrene | 0.12 | 27.005 |
| Bicyclo[7.2.0]undec-4-ene, 4,11,11-trimethyl-8-methylene-,[1R-(1R*,4Z,9S*)]- | 2.54 | 27.26 |
| Hexadecane | 0.21 | 27.59 |
| Germacrene D | 0.19 | 28.001 |
| (R)-lavandulyl (R)-2-methylbutanoate | 0.05 | 28.51 |
| Alpha-Guaiene | 0.12 | 28.596 |
| Naphthalene, 1,2,3,4,4a,5,6,8a-octahydro-7-methyl-4-methylene-1-(1-methylethyl)-, (1.alpha.,4a.beta.,8a.alpha.)- | 1.07 | 28.853 |
| delta-Cadinene | 0.18 | 29.003 |
| Bicyclo[2.2.1]hept-2-ene, 1,7,7-trimethyl- | 0.07 | 29.165 |
| 1-Methyl-6-(3-methylbuta-1,3-dienyl)-7-oxabicyclo[4.1.0]heptane | 0.1 | 29.792 |
| Spiro[tricyclo[3.3.1.1(3,7)]decane-2,2′-oxetan]-4′-one, 3′-methylene- | 0.16 | 30.155 |
| Caryophyllene oxide | 2.63 | 30.589 |
| (1R,3E,7E,11R)-1,5,5,8-Tetramethyl-12-oxabicyclo[9.1.0]dodeca-3,7-diene | 0.1 | 31.239 |
| Epicubenol | 0.12 | 31.488 |
| tau-Cadinol | 1.06 | 32.165 |
| Muurol-5-en-4-one <cis-14-nor-> | 0.08 | 33.115 |
| Chemical Compound | % | RT (min) |
|---|---|---|
| 5-Undecene, 7-ethenyl- | 0.32 | 3.832 |
| 3-Decyne | 0.07 | 4.013 |
| Alpha-Thujene | 0.22 | 5.663 |
| 1R-alpha-Pinene | 2.59 | 5.908 |
| Camphene | 2.17 | 6.385 |
| Sabinen | 9 | 7.488 |
| Beta-Pinene | 0.34 | 8.083 |
| Eucalyptol | 1.04 | 9.913 |
| D-Limonene | 15.04 | 10.209 |
| trans-beta-Ocimene | 0.15 | 10.843 |
| Sabinene hydrate | 0.12 | 13.119 |
| Beta-Thujone | 16.84 | 14.713 |
| Alpha-Thujone | 8.81 | 15.247 |
| (+)-2-Bornanone | 3.83 | 16.317 |
| trans-Sabinol | 0.2 | 16.87 |
| L-Pinocarveol | 0.37 | 17.127 |
| L-Borneol | 6.8 | 18.259 |
| trans-Ocimenol | 0.37 | 19.211 |
| (−)-Myrtenol | 0.19 | 19.523 |
| Pentanoic acid, 4-hexen-1-yl ester | 0.17 | 20.305 |
| Linalyl acetate | 0.05 | 21.075 |
| L-bornyl acetate | 0.73 | 21.995 |
| Sabinyl isobutanoate | 0.06 | 22.261 |
| Alpha-Cubebene | 0.07 | 24.308 |
| Alpha-ylangene | 0.04 | 24.977 |
| Alpha-Copaene | 0.12 | 25.114 |
| (−)-beta-Bourbonene | 0.09 | 25.347 |
| Bicyclo[5.2.0]nonane, 2-methylene-4,8,8-trimethyl-4-vinyl- | 0.07 | 25.96 |
| Isocaryophyllene | 6.33 | 26.375 |
| Humulene | 5.33 | 27.321 |
| Gamma-Muurolene | 0.24 | 27.859 |
| (+)-Ledene | 0.06 | 28.376 |
| Alpha-Cadinene | 0.06 | 28.51 |
| Naphthalene, 1,2,3,4,4a,5,6,8a-octahydro-7-methyl-4-methylene-1-(1-methylethyl)-, (1alpha,4a.beta.,8a.alpha.)- | 0.11 | 28.853 |
| Delta-Cadinene | 0.18 | 29.033 |
| 1-Methyl-6-(3-methylbuta-1,3-dienyl)-7-oxabicyclo[4.1.0]heptane | 0.27 | 29.796 |
| Caryophyllene oxide | 3.61 | 30.599 |
| Viridiflorol | 7.4 | 31.057 |
| (1R,3E,7E,11R)-1,5,5,8-Tetramethyl-12-oxabicyclo[9.1.0]dodeca-3,7-diene | 2.6 | 31.262 |
| Copalol | 0.2 | 31.751 |
| Caryophylla-4(12),8(13)-dien-5alpha-ol | 0.2 | 32.188 |
| cis-alpha-Bisabolene | 0.06 | 35.543 |
| Cyclopentadecanone | 0.15 | 36.394 |
| Butyl 6,9,12,15-octadecatetraenoate | 0.37 | 36.737 |
| Isopimara-9(11),15-diene | 0.2 | 38.46 |
| n-Hexadecanoic acid | 0.15 | 40.968 |
| Epimanool | 2.46 | 42.17 |
| (+)-Valencene | 0.15 | 42.631 |
| Chemical Compound | % | RT (min) |
|---|---|---|
| Alpha-Pinene | 1.14 | 5.983 |
| Camphene | 0.45 | 6.43 |
| Beta-Ocimene | 1.85 | 7.491 |
| Beta-Pinene | 1.07 | 8.092 |
| Eucalyptol | 17.84 | 10.116 |
| Limonene | 2.01 | 10.225 |
| trans-beta-Ocimene | 5.42 | 10.748 |
| Beta-Ocimene | 1.91 | 11.487 |
| Gamma-Terpinene | 0.47 | 13.847 |
| Linalool | 35.86 | 14.633 |
| (+)-2-Bornanone | 6.06 | 15.902 |
| endo-Borneol | 6.8 | 17.478 |
| Terpinen-4-ol | 3.62 | 17.906 |
| Alpha-Terpineol | 1.98 | 18.473 |
| Linalyl acetate | 5.41 | 20.977 |
| Lavandulol acetate | 0.86 | 22.084 |
| Caryophyllene | 1.04 | 26.247 |
| cis-beta-Farnesene | 4.19 | 27.192 |
| Germacrene D | 0.74 | 27.859 |
| (R)-lavandulyl (R)-2-methylbutanoate | 0.42 | 28.435 |
| Alpha-Bisabolol | 0.86 | 32.678 |
| Chemical Compound | % | RT (min) |
|---|---|---|
| Beta-Pinene | 0.52 | 8.102 |
| Beta-Ocimene | 0.65 | 11.484 |
| Linalool | 10.12 | 14.553 |
| Alpha-Terpineol | 1.15 | 18.483 |
| Linalyl formate | 1.01 | 19.463 |
| Linalyl acetate | 69.41 | 21.099 |
| Neryl acetate | 0.69 | 24.32 |
| Lavandulol acetate | 1.09 | 24.856 |
| Alpha-Cubebene | 0.71 | 25.074 |
| Aromandendrene | 3.23 | 26.252 |
| Germacrene D | 8.61 | 27.87 |
| (1S,2E,6E,10R)-3,7,11,11-Tetramethylbicyclo[8.1.0]undeca-2,6-diene | 1.56 | 28.271 |
| Alpha-Farnesene | 0.43 | 28.503 |
| Alloaromadendrene | 0.82 | 30.819 |
| Chemical Compound | % | RT (min) |
|---|---|---|
| Camphenol, 6- | 0.048 | 9.498 |
| Tricyclenne | 0.006 | 6.942 |
| A-phellandrene | 0.03 | 9.425 |
| A-pinene | 1.009 | 5.983 |
| Camphene | 0.223 | 6.43 |
| Sabinene | 0.912 | 8.200 |
| B-pinene | 1.515 | 7.747 |
| Limonene | 14.18 | 10.225 |
| Eucalyptol | 0.56 | 9.913 |
| 1,3,6 octatriene 3,7 dimethyl (Z) (beta cis ocimene) | 0.071 | 11.577 |
| A-terpinene | 0.029 | 6.726 |
| P-mentha-1,4(8) diene | 0.084 | 10.98 |
| Linalool | 0.336 | 15.322 |
| P-menth-1-en-8-ol | 0.064 | 18.573 |
| Geraniol acetate | 0.256 | 23.70 |
| B-caryophilene | 2.12 | 26.824 |
| B-farnesene | 0.763 | 26.33 |
| Chemical Compound | % | RT (min) |
|---|---|---|
| Phellandrene | 0.14 | 7.85 |
| 2-hexanal | 0.03 | 4.97 |
| Trans-sabinene hydrate | 0.19 | 12.523 |
| borneol | 4.79 | 28.833 |
| camphene | 1.45 | 6.707 |
| sabinene | 1.02 | 6.91 |
| P-cymene | 0.32 | 9.748 |
| Beta-myrcene | 0.53 | 6,98 |
| 1,8-cineole | 5.85 | 10.105 |
| 3-carene | 7,56 | 9.226 |
| Alpha-terpinene | 0.25 | 6.726 |
| o-cymene | 0.38 | 10.30 |
| Limonene | 0.49 | 10.250 |
| Cis-ocimene | 0.75 | 10.82 |
| Beta-ocimeneY | 0.57 | 8.59 |
| y-terpinene | 0.46 | 9.78 |
| Dehydro-p-cymene | 0.03 | 11.09 |
| Alpha-terpinolene | 0.04 | 10.98 |
| Cis--sabinene hydrate | 0.06 | 10.20 |
| linalool | 5.21 | 19.548 |
| Iso-amyl isovalerate | 0.03 | 11.60 |
| camphor | 2.12 | 18.787 |
| 4-terpineol | 0.42 | 17.93 |
| Cumyl alcohol | 0.04 | 2.429 |
| Alfa-terpinol | 0.65 | 18.573 |
| methylcyclohexane | 0.07 | 2.61 |
| calarene | 0.98 | 19.95 |
| valeranone | 2.34 | 35.74 |
| Alpha-thujene | 0.07 | 5,40 |
| Bornyl formate | 0.44 | 17.36 |
| Beta-Pinene | 0.98 | 7.04 |
| Alpha-Pinene | 4.30 | 5.85 |
| Chemical Compound | % | RT (min) |
|---|---|---|
| Alpha-Pinene | 0.93 | 5.994 |
| 3-Octanone | 0.75 | 7.324 |
| Beta-Pinene | 1.38 | 7.51 |
| Beta-Myrcene | 0.7 | 8.114 |
| Eucalyptol | 10.26 | 10.129 |
| D-Limonene | 9.7 | 10.272 |
| Trans-beta-Ocimene | 0.94 | 10.755 |
| Bicyclo[3.1.0]hexan-2-ol, 2-methyl-5-(1-methylethyl)-, (1.alpha.,2.beta.,5.alpha.)- | 4.43 | 12.57 |
| Alpha-Terpineol | 0.31 | 18.508 |
| 8-p-Menthen-2-ol | 5.58 | 18.749 |
| D-Carvone | 42.28 | 20.207 |
| Borane carbonyl | 0.68 | 20.495 |
| 8-p-Menthen-2-yl, acetate, trans | 1.28 | 23.256 |
| 2-Cyclohexen-1-ol, 2-methyl-5-(1-methylethenyl)-, acetate, (1R-cis)- | 0.57 | 24.306 |
| Jasmone | 0.48 | 25.06 |
| Beta-Bourbonene | 1.83 | 25.298 |
| Beta-Elemene | 0.85 | 25.485 |
| Caryophyllene | 4.94 | 26.269 |
| Isogermacrene D | 0.17 | 26.91 |
| Bicyclosesquiphellandrene | 0.34 | 26.962 |
| Beta-copaene | 0.68 | 27.395 |
| Germacrene D | 8.47 | 27.887 |
| Gamma-Elemene | 1.65 | 28.286 |
| Alpha-Selinene | 0.8 | 28.516 |
Appendix B
- A. Disc-diffusion assay
- Reference strains were seeded on Columbia agar +5% sheep blood and Sabouraud with fungal chloramphenicol, respectively, with 24 h thermostatting at 37 °C. The inoculum density, i.e., the number of bacteria brought into contact with the tested oil, is an important element and condition for the reproducibility of the results. According to the CLSI [Clinical and Laboratory Standards Institute] standard, a microbial suspension in sterile saline equivalent to 0.5 Mc Farland (108 CFU/mL) is prepared [103].
- For the culture medium, we used Mueller-Hinton agar (bioMerieux, Marcy-l’Étoile, France), which is recommended by CLSI. For the Candida strains, we used Mueller-Hinton medium supplemented with methylene blue. The sterility control of the media consisted of incubating a plate from the batch used for 24 h at 37 °C.
- An unimpregnated microcompressed blank, 6 mm in diameter (BioMaxima, Lublin, Poland), was used.
- Other materials used include sterile saline, cotton wool pads on wooden rods, and tweezers for the deposition of microcompresses.
- B. Macro-dilution method
- C. Determination of CMB/CMF:
References
- Integrating Traditional Medicine in Health Care. Available online: https://www.who.int/southeastasia/news/feature-stories/detail/integrating-traditional-medicine (accessed on 17 October 2024).
- Khan, M.; Kihara, M.; Omoloso, A.D. Antimicrobial Activity of the Alkaloidal Constituents of the Root Bark of Eupomatia Laurina. Pharm. Biol. 2008, 41, 277–280. [Google Scholar] [CrossRef]
- Pavrez, M.; Mahboob, H.K.; Zahuul, I.; Shek, M.H. Antimicrobial Activities of the Petroleum Ether, Methanol and Acetone Extracts of Kaempferia Galangal. Rhizome. J. Life Earth Sci. 2005, 1, 25–29. [Google Scholar]
- Parthasarathy, S.; Bin Azizi, J.; Ramanathan, S.; Ismail, S.; Sasidharan, S.; Said, M.I.M.; Mansor, S.M. Evaluation of Antioxidant and Antibacterial Activities of Aqueous, Methanolic and Alkaloid Extracts from Mitragyna Speciosa (Rubiaceae Family) Leaves. Molecules 2009, 14, 3964–3974. [Google Scholar] [CrossRef]
- Stringaro, A.; Colone, M.; Angiolella, L. Antioxidant, Antifungal, Antibiofilm, and Cytotoxic Activities of Mentha spp. Essential Oils. Medicines 2018, 5, 112. [Google Scholar] [CrossRef] [PubMed]
- Mamadalieva, N.Z.; Akramov, D.K.; Ovidi, E.; Tiezzi, A.; Nahar, L.; Azimova, S.S.; Sarker, S.D. Aromatic Medicinal Plants of the Lamiaceae Family from Uzbekistan: Ethnopharmacology, Essential Oils Composition, and Biological Activities. Medicines 2017, 4, 8. [Google Scholar] [CrossRef]
- Isnaini, N.; Annisa, A.; Prajaputra, V.; Maryam, S.; Idroes, R.; Khairan, K. Chemical Composition and Biological Activities of Essential Oils in the Family Lamiaceae. IOP Conf. Ser. Earth Environ. Sci. 2024, 1356, 012097. [Google Scholar] [CrossRef]
- Galovičová, L.; Borotová, P.; Valková, V.; Vukovic, N.L.; Vukic, M.; Terentjeva, M.; Štefániková, J.; Ďúranová, H.; Kowalczewski, P.Ł.; Kačániová, M. Thymus Serpyllum Essential Oil and Its Biological Activity as a Modern Food Preserver. Plants 2021, 10, 1416. [Google Scholar] [CrossRef] [PubMed]
- Kizi, S.D.S. Pharmacognostic Analysis of Medicinal Plants Containing Essential Oils. Texa. Jour. Medi. Scie. 2023, 25, 99–102. [Google Scholar] [CrossRef]
- Uritu, C.M.; Mihai, C.T.; Stanciu, G.-D.; Dodi, G.; Alexa-Stratulat, T.; Luca, A.; Leon-Constantin, M.-M.; Stefanescu, R.; Bild, V.; Melnic, S.; et al. Medicinal Plants of the Family Lamiaceae in Pain Therapy: A Review. Pain. Res. Manag. 2018, 2018, 7801543. [Google Scholar] [CrossRef]
- Waller, S.B.; Cleff, M.B.; Serra, E.F.; Silva, A.L.; Gomes, A.D.R.; de Mello, J.R.B.; de Faria, R.O.; Meireles, M.C.A. Plants from Lamiaceae Family as Source of Antifungal Molecules in Humane and Veterinary Medicine. Microb. Pathog. 2017, 104, 232–237. [Google Scholar] [CrossRef]
- De Martino, L.; De Feo, V.; Nazzaro, F. Chemical Composition and in Vitro Antimicrobial and Mutagenic Activities of Seven Lamiaceae Essential Oils. Molecules 2009, 14, 4213–4230. [Google Scholar] [CrossRef] [PubMed]
- Ipek, E.; Sivas Zeytinoglu, H.; Okay, S.; Tuylu, B.; Kurkcuoglu, M.; Baser, K.H.C. Genotoxicity and Antigenotoxicity of Origanum Oil and Carvacrol Evaluated by Ames Salmonella/Microsomal Test. Food Chem. 2005, 93, 551–556. [Google Scholar] [CrossRef]
- Evandri, M.G.; Battinelli, L.; Daniele, C.; Mastrangelo, S.; Bolle, P.; Mazzanti, G. The Antimutagenic Activity of Lavandula angustifolia (Lavender) Essential Oil in the Bacterial Reverse Mutation Assay. Food Chem. Toxicol. 2005, 43, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Vizoso Parra, A.; Ramos Ruiz, A.; Decalo Michelena, M.; Betancourt Badell, J. Estudio Genotóxico in Vitro e in Vivo En Tinturas de Melissa officinalis L. (Toronjil) y Mentha piperita L. (Toronjil de Menta). Rev. Cuba. Plantas Med. 1997, 2, 6–11. [Google Scholar]
- Vuković-Gacić, B.; Nikcević, S.; Berić-Bjedov, T.; Knezević-Vukcević, J.; Simić, D. Antimutagenic Effect of Essential Oil of Sage (Salvia officinalis L.) and Its Monoterpenes against UV-Induced Mutations in Escherichia Coli and Saccharomyces Cerevisiae. Food Chem. Toxicol. 2006, 44, 1730–1738. [Google Scholar] [CrossRef]
- Sun, J.; Sun, P.; Kang, C.; Zhang, L.; Guo, L.; Kou, Y. Chemical Composition and Biological Activities of Essential Oils from Six Lamiaceae Folk Medicinal Plants. Front. Plant Sci. 2022, 13, 919294. [Google Scholar] [CrossRef]
- Ramos da Silva, L.R.; Ferreira, O.O.; Cruz, J.N.; de Jesus Pereira Franco, C.; Oliveira Dos Anjos, T.; Cascaes, M.M.; Almeida da Costa, W.; Helena de Aguiar Andrade, E.; Santana de Oliveira, M. Lamiaceae Essential Oils, Phytochemical Profile, Antioxidant, and Biological Activities. Evid. Based Complement. Altern. Med. 2021, 2021, 6748052. [Google Scholar] [CrossRef]
- Benyoucef, F.; Dib, M.E.A.; Arrar, Z.; Costa, J.; Muselli, A. Synergistic Antioxidant Activity and Chemical Composition of Essential Oils From Thymus fontanesii, Artemisia herba-alba and Rosmarinus officinalis. J. Apple Biotechnol. Rep. 2018, 5, 151–156. [Google Scholar] [CrossRef]
- Shanaida, M. Antioxidant Activity of Essential Oils Obtained from Aerial Part of Some Lamiaceae Species. Int. J. Green Pharm. 2018, 12, 200–204. [Google Scholar] [CrossRef]
- Mutlu İngök, A.; Çatalkaya, G.; Çapanoğlu, E.; Karbancıoğlu Güler, F. Antioxidant and Antimicrobial Activities of Fennel, Ginger, Oregano and Thyme Essential Oils. Food Front. 2021, 2, 508–518. [Google Scholar] [CrossRef]
- Jafari, S.; Mori, Y. Chemical Composition and Antioxidant Activity of Essential Oil of Coriandrum sativum L. Seeds Cultivated in Afghanistan. Eur. J. Med. Plants 2021, 32, 82–92. [Google Scholar] [CrossRef]
- Ahmadi-Dastgerdi, A.; Ezzatpanah, H.; Asgary, S.; Dokhani, S.; Rahimi, E.; Gholami-Ahangaran, M. Oxidative Stability of Mayonnaise Supplemented with Essential Oil of Achillea Millefolium Ssp Millefolium during Storage. Food Sci. Technol. 2019, 13, 34–41. [Google Scholar] [CrossRef]
- Ibáñez, M.D.; López-Gresa, M.P.; Lisón, P.; Rodrigo, I.; Bellés, J.M.; González-Mas, M.C.; Blázquez, M.A. Essential Oils as Natural Antimicrobial and Antioxidant Products in the Agrifood Industry. Nereis 2020, 12, 55–69. [Google Scholar] [CrossRef]
- Tsigarida, E.; Skandamis, P.; Nychas, G.J. Behaviour of Listeria Monocytogenes and Autochthonous Flora on Meat Stored under Aerobic, Vacuum and Modified Atmosphere Packaging Conditions with or without the Presence of Oregano Essential Oil at 5 Degrees C. J. Appl. Microbiol. 2000, 89, 901–909. [Google Scholar] [CrossRef]
- Duarte, A.E.; De Menezes, I.R.A.; Bezerra Morais Braga, M.F.; Leite, N.F.; Barros, L.M.; Waczuk, E.P.; Pessoa da Silva, M.A.; Boligon, A.; Teixeira Rocha, J.B.; Souza, D.O.; et al. Antimicrobial Activity and Modulatory Effect of Essential Oil from the Leaf of Rhaphiodon Echinus (Nees & Mart) Schauer on Some Antimicrobial Drugs. Molecules 2016, 21, 743. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M. Essential Oils of Lamiaceae Family Plants as Antifungals. Biomolecules 2020, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, T.; Merecz-Sadowska, A.; Ghorbanpour, M.; Szemraj, J.; Piekarski, J.; Bijak, M.; Śliwiński, T.; Zajdel, R.; Sitarek, P. Enhanced Natural Strength: Lamiaceae Essential Oils and Nanotechnology in In Vitro and In Vivo Medical Research. Int. J. Mol. Sci. 2023, 24, 15279. [Google Scholar] [CrossRef]
- Monzote, L.; Scherbakov, A.; Lizama, R.; Gutiérrez, Y.; Satyal, P.; Cos, P.; Shchekotikhin, A.; Gille, L. Pharmacological Assessment of the Carvacrol Chemotype Essential Oil From Plectranthus Amboinicus Growing in Cuba. Nat. Product. Commun. 2020, 15, 1934578X2096223. [Google Scholar] [CrossRef]
- Kim, S.-W.; Lee, H.-R.; Jang, M.-J.; Jung, C.-S.; Park, I.-K. Fumigant Toxicity of Lamiaceae Plant Essential Oils and Blends of Their Constituents against Adult Rice Weevil Sitophilus oryzae. Molecules 2016, 21, 361. [Google Scholar] [CrossRef]
- Wang, L.; Cao, Z.; Wang, Z.; Guo, J.; Wen, J. Reactive Oxygen Species Associated Immunoregulation Post Influenza Virus Infection. Front. Immunol. 2022, 13, 927593. [Google Scholar] [CrossRef]
- Battaglini, M.; Carmignani, A.; Martinelli, C.; Colica, J.; Marino, A.; Doccini, S.; Mollo, V.; Santoro, F.; Bartolucci, M.; Petretto, A.; et al. In Vitro Study of Polydopamine Nanoparticles as Protective Antioxidant Agents in Fibroblasts Derived from ARSACS Patients. Biomater. Sci. 2022, 10, 3770–3792. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, C.; Pucci, C.; Battaglini, M.; Marino, A.; Ciofani, G. Antioxidants and Nanotechnology: Promises and Limits of Potentially Disruptive Approaches in the Treatment of Central Nervous System Diseases. Adv. Healthc. Mater. 2020, 9, e1901589. [Google Scholar] [CrossRef] [PubMed]
- Klimova, N.; Fearnow, A.; Kristian, T. Role of NAD+-Modulated Mitochondrial Free Radical Generation in Mechanisms of Acute Brain Injury. Brain Sci. 2020, 10, 449. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in Cancer Therapy: The Bright Side of the Moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Iliadis, S.; Papanikolaou, N.A. Reactive Oxygen Species Mechanisms That Regulate Protein–Protein Interactions in Cancer. Int. J. Mol. Sci. 2024, 25, 9255. [Google Scholar] [CrossRef]
- Ju, S.; Singh, M.K.; Han, S.; Ranbhise, J.; Ha, J.; Choe, W.; Yoon, K.-S.; Yeo, S.G.; Kim, S.S.; Kang, I. Oxidative Stress and Cancer Therapy: Controlling Cancer Cells Using Reactive Oxygen Species. Int. J. Mol. Sci. 2024, 25, 12387. [Google Scholar] [CrossRef]
- Wang, L.; Wise, J.T.F.; Zhang, Z.; Shi, X. Progress and Prospects of Reactive Oxygen Species in Metal Carcinogenesis. Curr. Pharmacol. Rep. 2016, 2, 178–186. [Google Scholar] [CrossRef]
- Khokra, S.; Prakash, O.; Jain, S. A Study on Neurological Significance of Vitex Negundo Linn. Int. Res. J. Pharm. 2018, 9, 130–136. [Google Scholar] [CrossRef]
- Rahmi, D.; Yunilawati, R.; Setiawati, I.; Irwinanita, I.; Jati, B.; Riyanto, A.; Yemirta, Y.; Aidha, N. Antioxidant Activity, Skin Irritaion Potential and Chemical Composition of Clove Leaf Oil from West Java Indonesia. J. Sains Mater. Indones. 2021, 23, 24. [Google Scholar] [CrossRef]
- Spiridon, I.; Colceru, S.; Anghel, N.; Teaca, C.A.; Bodirlau, R.; Armatu, A. Antioxidant Capacity and Total Phenolic Contents of Oregano (Origanum vulgare), Lavender (Lavandula angustifolia) and Lemon Balm (Melissa officinalis) from Romania. Nat. Prod. Res. 2011, 25, 1657–1661. [Google Scholar] [CrossRef]
- Robu, S.; Aprotosoaie, A.C.; Miron, A.; Cioanca, O.; Stǎnescu, U.; Hancianu, M. In Vitro Antioxidant Activity of Ethanolic Extracts from Some Lavandula Species Cultivated in Romania. Farmacia 2012, 60, 394–401. [Google Scholar]
- Massoud, R.I.; Bouaziz, M.; Abdallah, H.; Zeiz, A.; Flamini, G.; El-Dakdouki, M.H. Comparative Study on the Chemical Composition and Biological Activities of the Essential Oils of Lavandula angustifolia and Lavandula × intermedia Cultivated in Lebanon. ACS Omega 2024, 9, 30244–30255. [Google Scholar] [CrossRef] [PubMed]
- Truzzi, E.; Chaouch, M.A.; Rossi, G.; Tagliazucchi, L.; Bertelli, D.; Benvenuti, S. Characterization and Valorization of the Agricultural Waste Obtained from Lavandula Steam Distillation for Its Reuse in the Food and Pharmaceutical Fields. Molecules 2022, 27, 1613. [Google Scholar] [CrossRef]
- Hasibi, A.; Abdossi, V.; Ladanmoghadam, A.; Moradi, P. Variation of Some Traits of Lavandula angustifolia to Drought Stress for Optimum Water Usage. Eur. J. Hortic. Sci. 2022, 87, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, M.; Bungau, S.; Tit, D.M.; Copolovici, L.; Behl, T.; Otrisal, P.; Aleya, L.; Cioca, G.; Berescu, D.; Uivarosan, D.; et al. Variations in the Chemical Composition of the Essential Oil of Lavandula angustifolia Mill., Moldoveanca 4 Romanian Variety. Rev. Chim. 2020, 71, 307–315. [Google Scholar] [CrossRef]
- Kamali, H.; Jalilvand, M.R.; Aminimoghadamfarouj, N. Pressurized Fluid Extraction of Essential Oil from Lavandula Hybrida Using a Modified Supercritical Fluid Extractor and a Central Composite Design for Optimization. J. Sep. Sci. 2012, 35, 1479–1485. [Google Scholar] [CrossRef]
- Messaoud, C.; Chograni, H.; Boussaid, M. Chemical Composition and Antioxidant Activities of Essential Oils and Methanol Extracts of Three Wild Lavandula L. Species. Nat. Prod. Res. 2012, 26, 1976–1984. [Google Scholar] [CrossRef]
- Ghanimi, R.; Ouhammou, A.; Atki, Y.E.; Cherkaoui, M. Antioxidant and Antibacterial Activities of Essential Oils from Three Moroccan Species (Lavandula mairei Humbert, Lavandula dentata L. and, Lavandula stoechas L.). J. Pharm. Res. Int. 2021, 33, 64–71. [Google Scholar] [CrossRef]
- Moharam, B.A.; Jantan, I.; Ahmad, F.B.; Jalil, J. Antiplatelet Aggregation and Platelet Activating Factor (PAF) Receptor Antagonistic Activities of the Essential Oils of Five Goniothalamus Species. Molecules 2010, 15, 5124–5138. [Google Scholar] [CrossRef]
- Eldeghedy, H.I.; El-Gendy, A.E.-N.G.; Nassrallah, A.A.; Aboul-Enein, A.M.; Omer, E.A. Chemical Composition of Essential Oils of Lavandula angustifolia and Lavandula Hybrida Cultivated in Egypt and Their Biological Activities. Egypt. J. Chem. 2022, 65, 595–610. [Google Scholar] [CrossRef]
- Afonso, A.F.; Pereira, O.R.; Fernandes, Â.; Calhelha, R.C.; Silva, A.M.S.; Ferreira, I.C.F.R.; Cardoso, S.M. Phytochemical Composition and Bioactive Effects of Salvia Africana, Salvia officinalis ‘Icterina’ and Salvia Mexicana Aqueous Extracts. Molecules 2019, 24, 4327. [Google Scholar] [CrossRef]
- Jažo, Z.; Glumac, M.; Paštar, V.; Bektić, S.; Radan, M.; Carev, I. Chemical Composition and Biological Activity of Salvia officinalis L. Essential Oil. Plants 2023, 12, 1794. [Google Scholar] [CrossRef] [PubMed]
- El Jery, A.; Hasan, M.; Rashid, M.M.; Al Mesfer, M.K.; Danish, M.; Ben Rebah, F. Phytochemical Characterization, and Antioxidant and Antimicrobial Activities of Essential Oil from Leaves of the Common Sage Salvia officinalis L. from Abha, Saudi Arabia. Asian Biomed. (Res. Rev. News) 2020, 14, 261–270. [Google Scholar] [CrossRef]
- Robu, S.; Romila, A.; Buzia, O.D.; Spac, A.F.; Diaconu, C.; Tutunaru, D.; Lisa, E.; Nechita, A. Contribution to the Optimization of a Gas Chromatographic Method by QbD Approach Used for Analysis of Essential Oils from Salvia officinalis. Rev. Chim. 2019, 70, 2015–2020. [Google Scholar] [CrossRef]
- Angelova, V. Heavy Metal Accumulation and Chemical Composition of Essential Oils of Salvia officinalis Cultivated on Heavy Metal Contaminated Soils. 2019; 54. [Google Scholar] [CrossRef]
- Tsai, S.-W.; Hsieh, M.-C.; Li, S.; Lin, S.-C.; Wang, S.-P.; Lehman, C.W.; Lien, C.Z.; Lin, C.-C. Therapeutic Potential of Sclareol in Experimental Models of Rheumatoid Arthritis. Int. J. Mol. Sci. 2018, 19, 1351. [Google Scholar] [CrossRef] [PubMed]
- Gad, H.A.; Mamadalieva, R.Z.; Khalil, N.; Zengin, G.; Najar, B.; Khojimatov, O.K.; Al Musayeib, N.M.; Ashour, M.L.; Mamadalieva, N.Z. GC-MS Chemical Profiling, Biological Investigation of Three Salvia Species Growing in Uzbekistan. Molecules 2022, 27, 5365. [Google Scholar] [CrossRef] [PubMed]
- Kačániová, M.; Vukovic, N.L.; Čmiková, N.; Galovičová, L.; Schwarzová, M.; Šimora, V.; Kowalczewski, P.Ł.; Kluz, M.I.; Puchalski, C.; Bakay, L.; et al. Salvia sclarea Essential Oil Chemical Composition and Biological Activities. Int. J. Mol. Sci. 2023, 24, 5179. [Google Scholar] [CrossRef]
- Ben Akacha, B.; Ben Hsouna, A.; Generalić Mekinić, I.; Ben Belgacem, A.; Ben Saad, R.; Mnif, W.; Kačániová, M.; Garzoli, S. Salvia officinalis L. and Salvia sclarea Essential Oils: Chemical Composition, Biological Activities and Preservative Effects against Listeria Monocytogenes Inoculated into Minced Beef Meat. Plants 2023, 12, 3385. [Google Scholar] [CrossRef]
- Aćimović, M.G.; Cvetković, M.T.; Stanković Jeremić, J.M.; Pezo, L.L.; Varga, A.O.; Čabarkapa, I.S.; Kiprovski, B. Biological Activity and Profiling of Salvia sclarea Essential Oil Obtained by Steam and Hydrodistillation Extraction Methods via Chemometrics Tools. Flavour. Fragr. J. 2022, 37, 20–32. [Google Scholar] [CrossRef]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharopov, F.; Antolak, H.; Kręgiel, D.; Sen, S.; Sharifi-Rad, M.; Acharya, K.; Sharifi-Rad, R.; et al. Plants of Genus Mentha: From Farm to Food Factory. Plants 2018, 7, 70. [Google Scholar] [CrossRef]
- Singh, P.; Pandey, A.K. Prospective of Essential Oils of the Genus Mentha as Biopesticides: A Review. Front. Plant Sci. 2018, 9, 1295. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Ouis, N.; Djilali, B.; Benatouche, Z. In Vitro Antioxidant Activity of Essential Oil of Aerial Parts of Mentha pulegium L. Acta Agric. Serbica 2020, 25, 193–197. [Google Scholar] [CrossRef]
- Mondher, B. Comparative Study of the Chemical Properties and Composition of the Mentha pulegium L. of Algerian Origin and That of Tunisian Origin. Int. J. Agric. Sci. Food Technol. 2020, 6, 030–036. [Google Scholar] [CrossRef]
- Gonçalves, R.S.; Battistin, A.; Pauletti, G.; Rota, L.; Serafini, L.A. Antioxidant Properties of Essential Oils from Mentha Species Evidenced by Electrochemical Methods. Rev. Bras. Plantas Med. 2009, 11, 372–382. [Google Scholar] [CrossRef]
- Brahmi, F.; Khodir, M.; Mohamed, C.; Pierre, D.; Brahmi, F.; Khodir, M.; Mohamed, C.; Pierre, D. Chemical Composition and Biological Activities of Mentha Species. In Aromatic and Medicinal Plants—Back to Nature; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef]
- Singh, R.; Shushni, M.; Belkheir, A. Antibacterial and Antioxidant Activities of Mentha piperita L. Arab. J. Chem. 2015, 8, 322–328. [Google Scholar] [CrossRef]
- Mimica-Dukić, N.; Bozin, B.; Soković, M.; Mihajlović, B.; Matavulj, M. Antimicrobial and Antioxidant Activities of Three Mentha Species Essential Oils. Planta Med. 2003, 69, 413–419. [Google Scholar] [CrossRef]
- Kianasab, M.R.; Mohammadhosseini, M.; Nekoei, M.; Mahdavi, B.; Baheri, T. Screening of the Compositions of Essential Oils and Volatiles of Perovskia Abrotanoides Karel. along with Antioxidant, Antibacterial and Cytotoxic Impacts of Its Methanol Extract. Nat. Prod. Res. 2024, 38, 3813–3817. [Google Scholar] [CrossRef]
- Ghaffari, Z.; Rahimmalek, M.; Sabzalian, M.R. Variations in Essential Oil Composition and Antioxidant Activity in Perovskia Abrotanoides Kar. Collected from Different Regions in Iran. Chem. Biodivers. 2018, 15, e1700565. [Google Scholar] [CrossRef]
- Soumahoro, B.; Bohui, G.S.-P.; Kalo, M.; Kanaté, L.; Attioua, B.; Soro, Y. Compound Identification by HPLC-ESI-Q-TOF-MS/ MS Analysis of the Dichloromethane Fraction of Hyptis suaveolens Leaves After Extraction of the Essential Oil. Sci. J. Chem. 2024, 12, 1–14. [Google Scholar] [CrossRef]
- Bajalan, I.; Rouzbahani, R.; Pirbalouti, A.G.; Maggi, F. Chemical Composition and Antibacterial Activity of Iranian Lavandula × hybrida. Chem. Biodivers. 2017, 14, e1700064. [Google Scholar] [CrossRef]
- de Rapper, S.; Viljoen, A.; van Vuuren, S. The In Vitro Antimicrobial Effects of Lavandula angustifolia Essential Oil in Combination with Conventional Antimicrobial Agents. Evid. Based Complement. Altern. Med. 2016, 2016, 2752739. [Google Scholar] [CrossRef] [PubMed]
- Karadağ, A.E.; İpekçi, E.; Yağcılar, A.P.; Demirbolat, İ.; Kartal, M.; Siafaka, P.I.; Okur, N.Ü. Antimicrobial Activities of Mouthwashes Obtained from Various Combinations of Elettaria cardamomum Maton., Lavandula angustifolia Mill. and Salvia triloba L. Essential Oils. Nat. Volatiles Essent. Oils 2020, 7, 9–17. [Google Scholar] [CrossRef][Green Version]
- Adaszyńska-Skwirzyńska, M.; Szczerbińska, D. The Antimicrobial Activity of Lavender Essential Oil (Lavandula angustifolia) and Its Influence on the Production Performance of Broiler Chickens. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1020–1025. [Google Scholar] [CrossRef]
- Biltekin, S.N.; Karadaǧ, A.E.; Demirci, B.; Demirci, F. ACE2 and LOX Enzyme Inhibitions of Different Lavender Essential Oils and Major Components Linalool and Camphor. ACS Omega 2022, 7, 36561–36566. [Google Scholar] [CrossRef]
- Tofah, M.L.; Mseddi, K.; Al-Abbasi, O.K.; Ben Yazid, A.; Khechine, A.; Gdoura, R.; Khannous, L. A New Lavender (Lavandula multifida L.) Ecotype from Arid Tunisia, with Differential Essential Oil Composition and Higher Antimicrobial Potential. Life 2023, 13, 103. [Google Scholar] [CrossRef]
- Álvarez-García, S.; Moumni, M.; Romanazzi, G. Antifungal Activity of Volatile Organic Compounds from Essential Oils against the Postharvest Pathogens Botrytis Cinerea, Monilinia fructicola, Monilinia fructigena, and Monilinia laxa. Front. Plant Sci. 2023, 14, 1274770. [Google Scholar] [CrossRef]
- Bozkurt, İ.A.; Soylu, S.; Kara, M.; Soylu, E.M. Chemical Composition and Antibacterial Activity of Essential Oils Isolated from Medicinal Plants against Gall Forming Plant Pathogenic Bacterial Disease Agents. KSU J. Agric. Nat. 2020, 23, 1474–1482. [Google Scholar] [CrossRef]
- Derradji, L.; Saidi, O.; Hadef, Y. Evaluation of the Antibacterial Activity of Three Essential Oils Extracted from Plants Used in Traditional Medicine in Algeria (Salvia officinalis L, Melissa officinalis L and Origanum vulgare L). GSC Biol. Pharm. Sci. 2020, 12, 181–188. [Google Scholar] [CrossRef]
- Alibi, S.; Asma, F.; Ben Mansour, H.; Navas, J. In Vitro Antibacterial Effects of Salvia sclarea, Eucalyptus Globulus and Eugenia Caryophyllata Essential Oils Against Multidrug Resistant Corynebacterium Spp Clinical Isolates. J. Clin. Res. Rep. 2020, 2, 1–5. [Google Scholar] [CrossRef]
- Ürgeová, E.; Uváčková, Ľ.; Vaneková, M.; Maliar, T. Antibacterial Potential of Microwave-Assisted Extraction Prepared Hydrolates from Different Salvia Species. Plants 2023, 12, 1325. [Google Scholar] [CrossRef]
- Küçük, S.; Soyer, P.; Tunali, Y. Determination of Antimicrobial and Biological Activities of Salvia sclarea L. (Lamiaceae) Extracts. J. Turk. Chem. Soc. Sect. A Chem. 2019, 6, 15–20. [Google Scholar] [CrossRef]
- Akotowanou, O.C.A.; Adjou, E.S.; Sessou, P.; Kougblenou, S.D.; Olubi, A.B.; Michels, F.; Ahoussi, E.D.; Yessoufou, A.; Bankolé, H.; Fauconnier, M.-L.; et al. Antifungal Properties of Pimenta racemosa (Mill.) and Mentha × piperita (L.) Essential Oils against Fusarium Oxysporum Causing Tomato Fruit Rot. J. Adv. Biol. Biotechnol. 2023, 26, 50–59. [Google Scholar] [CrossRef]
- Giridharan, B.; Amutha, C.; Siddhan, N.; Ganeshkumar, A.; Periyasamy, S.; Murali, K. Antibacterial Activity of Mentha piperita L. (Peppermint) from Leaf Extracts—A Medicinal Plant. Acta Agric. Slov. 2007, 89, 73–79. [Google Scholar] [CrossRef]
- Jianu, C.; Golet, I.; Misca, C.; Jianu, A.; Pop, G.; Lukinich-Gruia, A. Antimicrobial Properties and Chemical Composition of Essential Oils Isolated from Six Medicinal Plants Grown in Romania Against Foodborne Pathogens. Rev. Chim.-Buchar.—Orig. Ed. 2016, 67, 1056–1061. [Google Scholar]
- Hamad Al-Mijalli, S.; ELsharkawy, E.R.; Abdallah, E.M.; Hamed, M.; El Omari, N.; Mahmud, S.; Alshahrani, M.M.; Mrabti, H.N.; Bouyahya, A. Determination of Volatile Compounds of Mentha piperita and Lavandula multifida and Investigation of Their Antibacterial, Antioxidant, and Antidiabetic Properties. Evid. Based Complement. Altern. Med. 2022, 2022, 9306251. [Google Scholar] [CrossRef]
- Demir, H. Evaluation of the Chemical Profiling, Total Phenolic Composition, the Antioxidant and Antimicrobial Properties of the Essential Oils of Mentha piperita L., Salvia officinalis L., and Thymus vulgaris L. J. Food Sci. Eng. 2018, 8, 263–270. [Google Scholar] [CrossRef]
- Haydari, M.; Maresca, V.; Rigano, D.; Taleei, A.; Shahnejat-Bushehri, A.A.; Hadian, J.; Sorbo, S.; Guida, M.; Manna, C.; Piscopo, M.; et al. Salicylic Acid and Melatonin Alleviate the Effects of Heat Stress on Essential Oil Composition and Antioxidant Enzyme Activity in Mentha × piperita and Mentha arvensis L. Antioxidants 2019, 8, 547. [Google Scholar] [CrossRef]
- Vakili-Ghartavol, M.; Arouiee, H.; Golmohammadzadeh, S.; Naseri, M. Antifungal Activity of Mentha × piperita L. Essential Oil. Acta Sci. Pol. Hortorum Cultus 2022, 21, 143–152. [Google Scholar] [CrossRef]
- Tullio, V.; Roana, J.; Scalas, D.; Mandras, N. Evaluation of the Antifungal Activity of Mentha × piperita (Lamiaceae) of Pancalieri (Turin, Italy) Essential Oil and Its Synergistic Interaction with Azoles. Molecules 2019, 24, 3148. [Google Scholar] [CrossRef]
- Edris, A.; Jirovetz, L.; Buchbauer, G.; Denkova, Z.; Stoyanova, A.; Slavchev, A. Chemical Composition, Antimicrobial Activities and Olfactive Evaluation of a Salvia officinalis L. (Sage) Essential Oil from Egypt. J. Essent. Oil Res. 2007, 19, 186–189. [Google Scholar] [CrossRef]
- Camargo, K.C.; Batista, L.R.; Alves, E.; Rezende, D.A.d.C.S.; Teixeira, M.L.; Brandão, R.M.; Ferreira, V.R.F.; Nelson, D.L.; Cardoso, M.d.G. Antibacterial Action of the Essential Oil from Cantinoa Carpinifolia Benth. Against Escherichia Coli and Staphylococcus Aureus Strains. Flavour. Fragr. J. 2020, 35, 99–106. [Google Scholar] [CrossRef]
- Nath, S.; Tamuli, K.J.; Saikia, S.; Narzary, B.; Gogoi, B.; Bordoloi, M.; Neipihoi; Dutta, D.; Sahoo, R.K.; Das, A.; et al. Essential Oil from the Leaves of Elsholtzia Communis (Collett & Hemsl.) Diels from North East India: Studies on Chemical Profiling, Antimicrobial, Cytotoxic and ACE Inhibitory Activities. Flavour. Fragr. J. 2021, 36, 626–636. [Google Scholar] [CrossRef]
- Alexa, E.; Danciu, C.; Radulov, I.; Obistioiu, D.; Sumalan, R.M.; Morar, A.; Dehelean, C.A. Phytochemical Screening and Biological Activity of Mentha × piperita L. and Lavandula angustifolia Mill. Extracts. Anal. Cell. Pathol. 2018, 2018, 2678924. [Google Scholar] [CrossRef]
- Cocan, I.; Alexa, E.; Danciu, C.; Radulov, I.; Galuscan, A.; Obistioiu, D.; Morvay, A.A.; Sumalan, R.M.; Poiana, M.-A.; Pop, G.; et al. Phytochemical Screening and Biological Activity of Lamiaceae Family Plant Extracts. Exp. Ther. Med. 2017, 15, 1863. [Google Scholar] [CrossRef] [PubMed]
- Alexa, E.; Sumalan, R.M.; Danciu, C.; Obistioiu, D.; Negrea, M.; Poiana, M.-A.; Rus, C.; Radulov, I.; Pop, G.; Dehelean, C. Synergistic Antifungal, Allelopatic and Anti-Proliferative Potential of Salvia officinalis L., and Thymus vulgaris L. Essential Oils. Molecules 2018, 23, 185. [Google Scholar] [CrossRef]
- NIST 14 Mass Spec Library and Search Programs—User Manual. Available online: https://www.sisweb.com/manuals/nist.htm (accessed on 24 February 2025).
- Wiley GCMS Libraries. MS Wil. Available online: https://www.mswil.com/software/spectral-libraries-and-databases/wiley-spectral-libraries/wiley-gcms-libraries/ (accessed on 24 February 2025).
- Kedare, S.B.; Singh, R.P. Genesis and Development of DPPH Method of Antioxidant Assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Moacă, E.-A.; Pavel, I.Z.; Danciu, C.; Crăiniceanu, Z.; Minda, D.; Ardelean, F.; Antal, D.S.; Ghiulai, R.; Cioca, A.; Derban, M.; et al. Romanian Wormwood (Artemisia absinthium L.): Physicochemical and Nutraceutical Screening. Molecules 2019, 24, 3087. [Google Scholar] [CrossRef]
- Niu, Y.; Yang, C.; Zhou, J.; Huang, S.; Liu, J. Two New Compounds with Antimicrobial Activities from the Seeds of Voacanga africana. Phytochem. Lett. 2016, 18, 208–212. [Google Scholar] [CrossRef]
- Clinical & Laboratory Standards Institute: CLSI Guidelines. Clinical & Laboratory Standards Institute. Available online: https://clsi.org/ (accessed on 19 October 2024).
- M26 AE Bactericidal Activity of Antimicrobial Agents. Clinical & Laboratory Standards Institute. Available online: https://clsi.org/standards/products/microbiology/documents/m26/ (accessed on 9 December 2024).
- Bobadilla, A.V.P.; Arévalo, J.; Sarró, E.; Byrne, H.M.; Maini, P.K.; Carraro, T.; Balocco, S.; Meseguer, A.; Alarcón, T. In Vitro Cell Migration Quantification Method for Scratch Assays. J. R. Soc. Interface 2019, 16, 20180709. [Google Scholar] [CrossRef]
- Pusnik, M.; Imeri, M.; Deppierraz, G.; Bruinink, A.; Zinn, M. The Agar Diffusion Scratch Assay—A Novel Method to Assess the Bioactive and Cytotoxic Potential of New Materials and Compounds. Sci. Rep. 2016, 6, 20854. [Google Scholar] [CrossRef]
- MTT Assay Protocol|Abcam. Available online: https://www.abcam.com/en-us/technical-resources/protocols/mtt-assay?srsltid=AfmBOorh9g61ejVubQ9usSO9HBZVEuIeQBu5txXHKARmWO02oMVNeccU (accessed on 9 December 2024).
- Coricovac, D.-E.; Moacă, E.-A.; Pinzaru, I.; Cîtu, C.; Soica, C.; Mihali, C.-V.; Păcurariu, C.; Tutelyan, V.A.; Tsatsakis, A.; Dehelean, C.-A. Biocompatible Colloidal Suspensions Based on Magnetic Iron Oxide Nanoparticles: Synthesis, Characterization and Toxicological Profile. Front. Pharmacol. 2017, 8, 154. [Google Scholar] [CrossRef] [PubMed]
- PAST. LO4D.com. Available online: https://past.en.lo4d.com/windows (accessed on 9 December 2024).
- SAS OnDemand for Academics|SAS. Available online: https://www.sas.com/en_us/software/on-demand-for-academics.html (accessed on 9 December 2024).
- Prism 5 Updates—GraphPad. Available online: https://www.graphpad.com/support/prism-5-updates/ (accessed on 9 December 2024).





| Sample No. | Sample Code | Antioxidant Capacity [%] | IC50 ± SD [μg/mL] |
|---|---|---|---|
| 1 | LA1 | 88.85 ± 0.024 | 15.84 ± 0.92 |
| 2 | LA2 | 90.90 ± 0.002 | 13.28 ± 0.67 |
| 3 | SO | 55.56 ± 0.187 | 94.73 ± 1.18 |
| 4 | LH | 83.81 ± 0.004 | 19.39 ± 0.98 |
| 5 | SS | 52.05 ± 0.079 | 109.28 ± 1.34 |
| 6 | MS | 70.02 ± 0.117 | 65.78 ± 1.21 |
| 7 | PA | 38.81 ± 0.041 | 186.84 ± 1.56 |
| 8 | MP | 89.18 ± 0.003 | 45.75 ± 1.06 |
| 9 | Ascorbic acid | 95.92 ± 0.026 | 0.7 ± 0.05 |
| EO | K. pneumoniae | S. flexneri | S. enterica | E. coli | P. aeruginosa | S. aureus | E. faecalis | C. albicans | C. parapsilosis |
|---|---|---|---|---|---|---|---|---|---|
| MS | 21 | 20 | 22 | 22 | 15 | 21 | 22 | 33 | 33 |
| SO | 6 | 9 | 9 | 9 | 6 | 10 | 9 | 10 | 10 |
| LA1 | 13 | 14 | 10 | 14 | 6 | 20 | 19 | 30 | 30 |
| PA | 6 | 6 | 6 | 10 | 6 | 20 | 16 | 20 | 19 |
| LH | 6 | 6 | 9 | 10 | 6 | 21 | 20 | 20 | 21 |
| MP | 25 | 20 | 20 | 26 | 21 | 26 | 24 | 20 | 20 |
| LA2 | 10 | 11 | 9 | 9 | 6 | 16 | 15 | 19 | 18 |
| SS | 6 | 6 | 6 | 6 | 6 | 22 | 20 | 20 | 20 |
| EO | MS | SO | LA1 | PA | LH | MP | LA2 | SS |
|---|---|---|---|---|---|---|---|---|
| MS | 0.00 | |||||||
| SO | 45.97 | 0.00 | ||||||
| LA1 | 20.42 | 33.17 | 0.00 | |||||
| PA | 36.11 | 18.68 | 19.36 | 0.00 | ||||
| LH | 33.65 | 21.75 | 17.69 | 5.48 | 0.00 | |||
| MP | 20.95 | 42.40 | 30.17 | 36.54 | 34.66 | 0.00 | ||
| LA2 | 33.41 | 15.39 | 18.47 | 8.37 | 10.10 | 33.57 | 0.00 | |
| SS | 36.84 | 22.18 | 19.95 | 6.08 | 5.20 | 37.55 | 11.18 | 0.00 |
| EO | K. pneumoniae | S. flexneri | S. enterica | E. coli | P. aeruginosa | S. aureus | E. faecalis | C. albicans | C. parapsilosis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| MS | 10 | 20 | 10 | 20 | 10 | 20 | 10 | 20 | 20 | 40 | 10 | 10 | 10 | 10 | 5 | 5 | 5 | 5 |
| LA1 | 10 | 20 | 10 | 20 | 5 | 5 | 5 | 5 | ||||||||||
| PA | 10 | 20 | 10 | 20 | 10 | 10 | 10 | 10 | ||||||||||
| LH | 10 | 20 | 10 | 20 | 10 | 10 | 10 | 10 | ||||||||||
| MP | 10 | 20 | 10 | 20 | 10 | 20 | 10 | 20 | 20 | 40 | 5 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| LA2 | 10 | 20 | 20 | 20 | 10 | 10 | 10 | 10 | ||||||||||
| SS | 20 | 20 | 20 | 20 | 10 | 10 | 10 | 10 | ||||||||||
| Sample Code | Fresh herba (g) | Dry herba (g) | Drying Efficiency (%) | EO from Sample (mL) | EO ml/100 g Dry herba |
|---|---|---|---|---|---|
| LA1 | 3450 | 1100 | 31.88 | 15 | 1.36 |
| LA2 | 2968 | 1012 | 29.33 | 18 | 1.78 |
| SO | 2843 | 987 | 33.25 | 4.6 | 0.47 |
| LH | 3526 | 1188 | 33.69 | 20.1 | 1.69 |
| SS | 2487 | 795 | 31.96 | 10.1 | 1.27 |
| MS | 2507 | 890 | 35.5 | 5.5 | 0.61 |
| PA | 2717 | 918 | 33.78 | 8.3 | 0.9 |
| MP | 11700 | 3630 | 31.02 | 40.5 | 1.11 |
| Microbial Species | ATCC | Manufacturer |
|---|---|---|
| Salmonella enterica serotype typhimurium | 14028 | Thermo Scientific (Waltham, MA, USA) |
| Shigella flexneri serotype 2b | 12022 | |
| Enterococcus faecalis | 51299 | |
| Escherichia coli | 25922 | |
| Klebsiella pneumoniae | 700603 | |
| Pseudomonas aeruginosa | 27853 | |
| Staphylococcus aureus | 25923 | |
| Candida albicans | 10231 | |
| Candida parapsilosis | 22019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciobotaru, G.V.; Goje, I.-D.; Dehelean, C.A.; Danciu, C.; Magyari-Pavel, I.Z.; Moacă, E.-A.; Muntean, D.; Imbrea, I.M.; Sărățeanu, V.; Pop, G. Analysis of the Antioxidant and Antimicrobial Activity, Cytotoxic, and Anti-Migratory Properties of the Essential Oils Obtained from Cultivated Medicinal Lamiaceae Species. Plants 2025, 14, 846. https://doi.org/10.3390/plants14060846
Ciobotaru GV, Goje I-D, Dehelean CA, Danciu C, Magyari-Pavel IZ, Moacă E-A, Muntean D, Imbrea IM, Sărățeanu V, Pop G. Analysis of the Antioxidant and Antimicrobial Activity, Cytotoxic, and Anti-Migratory Properties of the Essential Oils Obtained from Cultivated Medicinal Lamiaceae Species. Plants. 2025; 14(6):846. https://doi.org/10.3390/plants14060846
Chicago/Turabian StyleCiobotaru, Gabriela Valentina, Iacob-Daniel Goje, Cristina Adriana Dehelean, Corina Danciu, Ioana Zinuca Magyari-Pavel, Elena-Alina Moacă, Delia Muntean, Ilinca Merima Imbrea, Veronica Sărățeanu, and Georgeta Pop. 2025. "Analysis of the Antioxidant and Antimicrobial Activity, Cytotoxic, and Anti-Migratory Properties of the Essential Oils Obtained from Cultivated Medicinal Lamiaceae Species" Plants 14, no. 6: 846. https://doi.org/10.3390/plants14060846
APA StyleCiobotaru, G. V., Goje, I.-D., Dehelean, C. A., Danciu, C., Magyari-Pavel, I. Z., Moacă, E.-A., Muntean, D., Imbrea, I. M., Sărățeanu, V., & Pop, G. (2025). Analysis of the Antioxidant and Antimicrobial Activity, Cytotoxic, and Anti-Migratory Properties of the Essential Oils Obtained from Cultivated Medicinal Lamiaceae Species. Plants, 14(6), 846. https://doi.org/10.3390/plants14060846











