Iron and Zinc Foliar Spraying Affected Sideritis cypria Post. Growth, Mineral Content and Antioxidant Properties
Abstract
1. Introduction
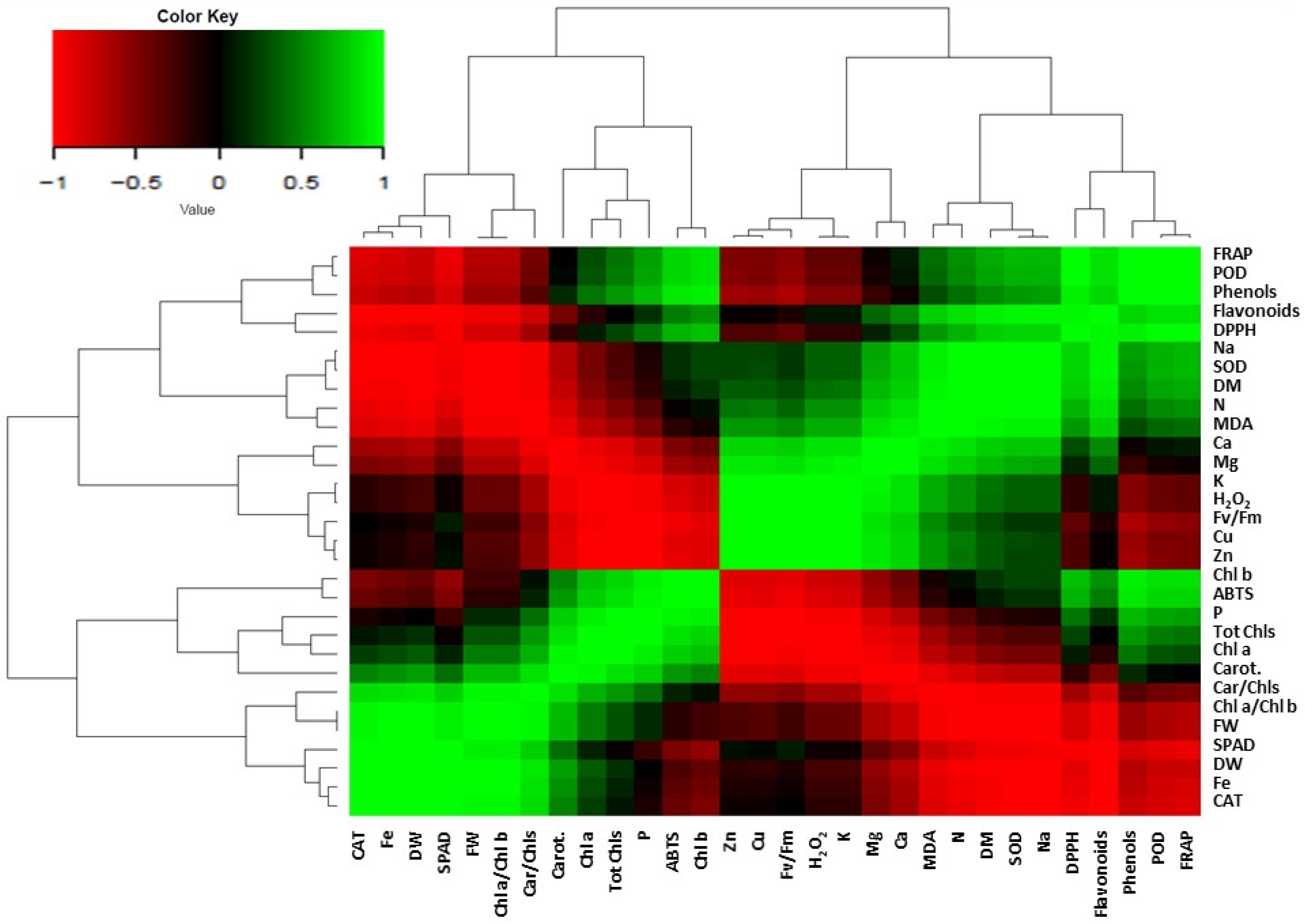
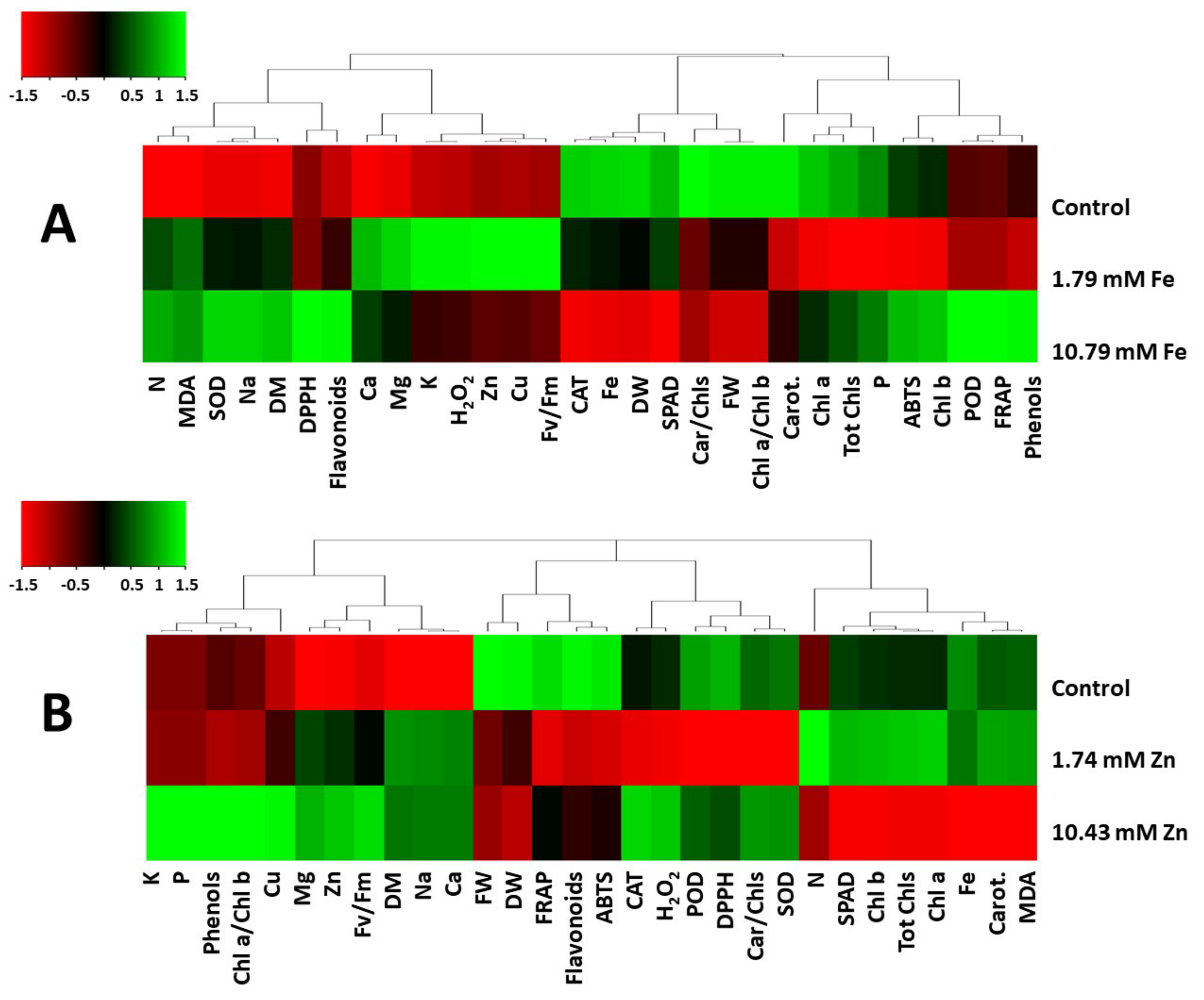
2. Results and Discussion
3. Materials and Methods
3.1. Experimental Setup, Plant Material and Growth Conditions
3.2. Plant Growth Measurements
3.3. Plant Physiology and Photosynthesis-Related Measures
3.4. Plant Tissue and Nutrient Ion Concentration Analysis
3.5. Total Phenols, Total Flavonoids and Antioxidant Activity
3.6. Lipid Peroxidation, Hydrogen Peroxide and Enzyme Antioxidant Activity
3.7. Statistical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nour, V.; Trandafir, I.; Cosmulescu, S. Bioactive compounds, antioxidant activity and nutritional quality of different culinary aromatic herbs. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 179–184. [Google Scholar] [CrossRef]
- Priss, O.; Yevlash, V. Technology of fresh herbs storage using hydrogel and antioxidant composition. Food Environ. Saf. 2017, 16, 256–261. [Google Scholar]
- Chrysargyris, A.; Tomou, E.M.; Goula, K.; Dimakopoulou, K.; Tzortzakis, N.; Skaltsa, H. Sideritis L. essential oils: A systematic review. Phytochemistry 2023, 209, 113607. [Google Scholar] [CrossRef] [PubMed]
- González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. Sideritis spp.: Uses, chemical composition and pharmacological activities—A review. J. Ethnopharmacol. 2011, 135, 209–225. [Google Scholar] [CrossRef]
- Hanoğlu, D.Y.; Hanoğlu, A.; Güvenir, M.; Süer, K.; Demirci, B.; Başer, K.H.C.; Yavuz, D.Ö. Chemical composition and antimicrobial activity of the essential oil of Sideritis cypria Post endemic in Northern Cyprus. J. Essent. Oil Res. 2017, 29, 228–232. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Kloukina, C.; Vassiliou, R.; Tomou, E.-M.E.-M.; Skaltsa, H.; Tzortzakis, N. Cultivation strategy to improve chemical profile and anti-oxidant activity of Sideritis perfoliata L. subsp. perfoliata. Ind. Crops Prod. 2019, 140, 111694. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Evangelides, E.; Tzortzakis, N. Seasonal variation of antioxidant capacity, phenols, minerals and essential oil components of sage, spearmint and sideritis plants grown at different altitudes. Agronomy 2021, 11, 1766. [Google Scholar] [CrossRef]
- Kagde, A.; Kharat, R.; Khomane, S.; Uchale, S. Commercial Cultivation and Collection Aspects of Medicinal and Aromatic Plants. Int. J. Pharm. Sci. 2024, 2, 2235–2247. [Google Scholar]
- Chrysargyris, A.; Tzortzakis, N. Nitrogen, phosphorus, and potassium requirements to improve Sideritis cypria growth, nutrient and water use efficiency in hydroponic cultivation. Heliyon 2025, 11, e40755. [Google Scholar] [CrossRef]
- Nazari, M.; Zarinkamar, F.; Mohammad Soltani, B.; Niknam, V. Manganese-induced changes in glandular trichomes density and essential oils production of Mentha aquatica L. at different growth stages. J. Trace Elem. Med. Biol. 2018, 50, 57–66. [Google Scholar] [CrossRef]
- Elhindi, K.; Al-Suhaibani, N.A.; Sharaf El-Din, A.F.; Yakout, S.M.; Al-Amri, S.M. Effect of foliar-applied iron and zinc on growth rate and essential oil in sweet basil (Ocimum basilicum L.) under saline conditions. Prog. Nutr. 2016, 18, 288–298. [Google Scholar]
- Jahani, F.; Tohidi-Moghadam, H.R.; Larijani, H.R.; Ghooshchi, F.; Oveysi, M. Influence of zinc and salicylic acid foliar application on total chlorophyll, phenolic components, yield and essential oil composition of peppermint (Mentha piperita L.) under drought stress condition. Arab. J. Geosci. 2021, 14, 691. [Google Scholar] [CrossRef]
- Shoormij, F.; Mirlohi, A.; Saeidi, G.; Shirvani, M. Combined foliar application of Zn and Fe increases grain micronutrient concentrations and alleviates water stress across diverse wheat species and ploidal levels. Sci. Rep. 2022, 12, 20328. [Google Scholar] [CrossRef] [PubMed]
- Oliver, M.A.; Gregory, P.J. Soil, food security and human health: A review. Eur. J. Soil Sci. 2015, 66, 257–276. [Google Scholar] [CrossRef]
- Stein, A.J. Rethinking the measurement of undernutrition in a broader health context: Should we look at possible causes or actual effects? Glob. Food Secur. 2014, 3, 193–199. [Google Scholar] [CrossRef]
- Gregory, P.J.; Wahbi, A.; Adu-Gyamfi, J.; Heiling, M.; Gruber, R.; Joy, E.J.M.; Broadley, M.R. Approaches to reduce zinc and iron deficits in food systems. Glob. Food Secur. 2017, 15, 1–10. [Google Scholar] [CrossRef]
- Alshaal, T.; El-Ramady, H. Foliar application: From plant nutrition to biofortification. Environ. Biodivers. Soil Secur. 2017, 1, 71–83. [Google Scholar] [CrossRef]
- Schlegel, T.K.; Schönherr, J.; Schreiber, L. Rates of foliar penetration of chelated Fe(III): Role of light, stomata, species, and leaf age. J. Agric. Food Chem. 2006, 54, 6809–6813. [Google Scholar] [CrossRef]
- Said-Al Ahl, H.A.H.; Hussein, M.S.; Abd El-Kader, A.A. Effect of nitrogen fertilizer and/or some foliar application on growth, herb yield, essential oil and chemical composition of dragonhead. J. Med. Food Plants 2010, 2, 12–28. [Google Scholar]
- Chrysargyris, A.; Loupasaki, S.; Petropoulos, S.A.; Tzortzakis, N. Salinity and cation foliar application: Implications on essential oil yield and composition of hydroponically grown spearmint plants. Sci. Hortic. 2019, 256, 108581. [Google Scholar] [CrossRef]
- Nasiri, Y.; Zehtab-Salmasi, S.; Nasrullahzadeh, S.; Najafi, N.; Ghassemi-Golezani, K. Effects of foliar application of micronutrients (Fe and Zn) on flower yield and essential oil of chamomile (Matricaria chamomilla L.). J. Med. Plants Res. 2010, 4, 1733–1737. [Google Scholar]
- Moghimipour, Z.; Sourestani, M.M.; Ansari, N.A.; Ramezani, Z. The Effect of Foliar Application of Zinc on Essential Oil Content and Composition of Holy Basil [Ocimum sanctum] at First and Second Harvests. J. Essent. Oil-Bear. Plants 2017, 20, 449–458. [Google Scholar] [CrossRef]
- Said-Al Ahl, H.A.H.; Mahmoud, A.A. Effect of zinc and/or iron foliar application on growth and essential oil of sweet basil (Ocimum basilicum L.) under salt stress. Ozean J. Appl. Sci. 2010, 3, 97–111. [Google Scholar]
- Radmanesh, E.; Naghdi Badi, H.; Hadavi, E.; Mehrafarin, A. Shoot growth, gamma-terpinene and essential oil content of Satureja hortensis L. in response to foliar application of FeSO4 and citric acid. J. Med. Plants 2015, 14, 45–57. [Google Scholar]
- Chrysargyris, A.; Michailidi, E.; Tzortzakis, N. Physiological and biochemical responses of Lavandula angustifolia to salinity under mineral foliar application. Front. Plant Sci. 2018, 9, 489. [Google Scholar] [CrossRef]
- Yadegari, M. Effect of micronutrients foliar application and biofertilizeres on essential oils of lemon balm. J. Soil Sci. Plant Nutr. 2016, 16, 702–715. [Google Scholar] [CrossRef]
- Najafian, S.; Zahedifar, M.; Ghasemi, A.R. Effect of organic and inorganic zinc foliar application on the natural product composition and antioxidant activity of lemon balm (Melissa officinalis) Nanomaterials for Soil Remediation View project Effect of organic and inorganic zinc foliar application. Iran Agric. Res. 2022, 40, 85–92. [Google Scholar]
- Sinta, I.; Vijayakumar, A.; Srimathi, P. Effect of micronutrient application in coriander (Coriandrum sativum L.) cv.CO4. Afr. J. Agric. Res. 2015, 10, 84–88. [Google Scholar] [CrossRef]
- Hassanpouraghdam, M.B.; Mehrabani, L.V.; Tzortzakis, N. Foliar Application of Nano-zinc and Iron Affects Physiological Attributes of Rosmarinus officinalis and Quietens NaCl Salinity Depression. J. Soil Sci. Plant Nutr. 2019, 20, 335–345. [Google Scholar] [CrossRef]
- Nekoukhou, M.; Fallah, S.; Abbasi-Surki, A.; Pokhrel, L.R.; Rostamnejadi, A. Improved efficacy of foliar application of zinc oxide nanoparticles on zinc biofortification, primary productivity and secondary metabolite production in dragonhead. J. Clean. Prod. 2022, 379, 134803. [Google Scholar] [CrossRef]
- Yadegari, M. Foliar application of micronutrients on essential oils of borago, thyme and marigold. J. Soil Sci. Plant Nutr. 2015, 15, 949–964. [Google Scholar] [CrossRef]
- Iziy, E.; Majd, A.; Vaezi-Kakhki, M.R.; Nejadsattari, T.; Noureini, S.K. Effects of zinc oxide nanoparticles on enzymatic and nonenzymatic antioxidant content, germination, and biochemical and ultrastructural cell characteristics of Portulaca oleracea L. Acta Soc. Bot. Pol. 2019, 88, 3639. [Google Scholar] [CrossRef]
- Asle-Mohammadi, Z.; Kharazmi, M.; Sheikhi, H.; Mohammadkhani, N.; Nicola, S. Foliar Application of Fe, Zn, and Mn as a Practical Strategy to Alleviate the Soil Cu Toxicity and Stimulate the Physiological and Biochemical Properties of Peppermint (Mentha piperita L.). J. Soil Sci. Plant Nutr. 2024, 24, 371–388. [Google Scholar] [CrossRef]
- Aghamirzaei, H.; Mumivand, H.; Nia, A.E.; Raji, M.R.; Maroyi, A.; Maggi, F. Effects of Micronutrients on the Growth and Phytochemical Composition of Basil (Ocimum basilicum L.) in the Field and Greenhouse (Hydroponics and Soil Culture). Plants 2024, 13, 2498. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Sharma, V.; Shukla, A.K.; Verma, V.; Sandhu, P.S.; Behera, S.K.; Singh, P.; Kaur, J.; Singh, H.; Abdel-Hafez, S.H.; et al. Interactive effects of foliar application of zinc, iron and nitrogen on productivity and nutritional quality of indian mustard (Brassica juncea L.). Agronomy 2021, 11, 2333. [Google Scholar] [CrossRef]
- Tuiwong, P.; Cho, H.K.; Rouached, H.; Prom-U-Thai, C. Synergistic Effects of Nitrogen and Zinc Foliar Application on Yield and Nutrient Accumulation in Rice at Various Growth Stages. Plants 2024, 13, 3274. [Google Scholar] [CrossRef] [PubMed]
- Ikram, N.A.; Abdalla, M.A.; Mühling, K.H. Developing Iron and Iodine Enrichment in Tomato Fruits to Meet Human Nutritional Needs. Plants 2024, 13, 3438. [Google Scholar] [CrossRef]
- Bagheri, H.; Ladan Moghadam, A.; Danaee, E.; Abdossi, V. Morphophysiological and phytochemical changes of Mentha piperita using calcium, potassium, iron and manganese nano-fertilizers. Eur. J. Hortic. Sci. 2021, 86, 419–430. [Google Scholar] [CrossRef]
- Reshma, Z.; Meenal, K. Foliar application of biosynthesised zinc nanoparticles as a strategy for ferti-fortification by improving yield, zinc content and zinc use efficiency in amaranth. Heliyon 2022, 8, e10912. [Google Scholar] [CrossRef]
- Ali, I.; Khan, A.; Ali, A.; Ullah, Z.; Dai, D.Q.; Khan, N.; Khan, A.; Al-Tawaha, A.R.; Sher, H. Iron and zinc micronutrients and soil inoculation of Trichoderma harzianum enhance wheat grain quality and yield. Front. Plant Sci. 2022, 13, 960948. [Google Scholar] [CrossRef]
- Chatzistathis, T.A.; Papadakis, I.E.; Therios, I.N.; Giannakoula, A.; Dimassi, K. Is chlorophyll fluorescence technique a useful tool to assess manganese deficiency and toxicity stress in olive plants? J. Plant Nutr. 2011, 34, 98–114. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Torres, N.; Flores-Naveda, A.; Barriga-Castro, E.D.; Camposeco-Montejo, N.; Ramírez-Barrón, S.; Borrego-Escalante, F.; Niño-Medina, G.; Hernández-Juárez, A.; Garza-Alonso, C.; Rodríguez-Salinas, P.; et al. Zinc oxide nanoparticles and zinc sulfate impact physiological parameters and boosts lipid peroxidation in soil grown coriander plants (Coriandrum sativum). Molecules 2021, 26, 1998. [Google Scholar] [CrossRef]
- Gao, D.; Ran, C.; Zhang, Y.; Wang, X.; Lu, S.; Geng, Y.; Guo, L.; Shao, X. Effect of different concentrations of foliar iron fertilizer on chlorophyll fluorescence characteristics of iron-deficient rice seedlings under saline sodic conditions. Plant Physiol. Biochem. 2022, 185, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Deng, S.; Zhou, Y.; Long, J.; Ding, D.; Du, H.H.; Lei, M.; Chen, C.; Tie, B.Q. Application of different foliar iron fertilizers for enhancing the growth and antioxidant capacity of rice and minimizing cadmium accumulation. Environ. Sci. Pollut. Res. 2021, 28, 7828–7839. [Google Scholar] [CrossRef] [PubMed]
- Roosta, H.R.; Mohsenian, Y. Effects of foliar spray of different Fe sources on pepper (Capsicum annum L.) plants in aquaponic system. Sci. Hortic. 2012, 146, 182–191. [Google Scholar] [CrossRef]
- He, C.; Sun, J.; Chen, Y.; Wang, L.; Shi, S.; Qiu, F.; Wang, S.; Tagesson, T. A new vegetation index combination for leaf carotenoid-to-chlorophyll ratio: Minimizing the effect of their correlation. Int. J. Digit. Earth 2023, 16, 272–288. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, W.; Zhang, J.; Kong, W.; Casa, R.; Huang, Y. A novel combined spectral index for estimating the ratio of carotenoid to chlorophyll content to monitor crop physiological and phenological status. Int. J. Appl. Earth Obs. Geoinf. 2019, 76, 128–142. [Google Scholar] [CrossRef]
- Ivanov, L.A.; Ronzhina, D.A.; Yudina, P.K.; Zolotareva, N.V.; Kalashnikova, I.V.; Ivanova, L.A. Seasonal Dynamics of the Chlorophyll and Carotenoid Content in the Leaves of Steppe and Forest Plants on Species and Community Level. Russ. J. Plant Physiol. 2020, 67, 453–462. [Google Scholar] [CrossRef]
- Yadegari, M. Effects of Zn, Fe, Mn and Cu Foliar Application on Essential Oils and Morpho-Physiological Traits of Lemon Balm (Melissa officinalis L.). J. Essent. Oil-Bear. Plants 2017, 20, 485–495. [Google Scholar] [CrossRef]
- Kiani, M.H.; Mokhtari, A.; Zeinali, H.; Abbasnejad, A.; Khoraskani, L.A. Rosmarinic acid and anthocyanin content improvement by foliar application of Fe and Zn fertilizer in Lemon balm (Melissa officinalis L.). Int. J. Adv. Biol. Biomed. Res. 2014, 2, 1525–1530. [Google Scholar]
- Zhao, X.; Zhang, X.; Wang, L.; Huang, Q.; Dai, H.; Liu, L.; Zhu, Y.; El-Sappah, A.H.; Wu, H. Foliar application of iron impacts flavonoid glycosylation and promotes flavonoid metabolism in coloured rice. Food Chem. 2024, 444, 138454. [Google Scholar] [CrossRef] [PubMed]
- Chrysargyris, A.; Solomou, M.; Petropoulos, S.A.S.A.; Tzortzakis, N. Physiological and biochemical attributes of Mentha spicata when subjected to saline conditions and cation foliar application. J. Plant Physiol. 2019, 232, 27–38. [Google Scholar] [CrossRef]
- García-Gómez, C.; Obrador, A.; González, D.; Babín, M.; Fernández, M.D. Comparative effect of ZnO NPs, ZnO bulk and ZnSO4 in the antioxidant defences of two plant species growing in two agricultural soils under greenhouse conditions. Sci. Total Environ. 2017, 589, 11–24. [Google Scholar] [CrossRef]
- Ali, E.F.; Hassan, F.; Abdel-Rahman, S.S.A.; Siddique, K.H.M. Foliar application of potassium and zinc enhances the productivity and volatile oil content of damask rose (Rosa damascena miller var. trigintipetala dieck). Acta Sci. Pol. Hortorum Cultus 2021, 20, 101–114. [Google Scholar] [CrossRef]
- Cakmak, I.; Kalayci, M.; Kaya, Y.; Torun, A.A.; Aydin, N.; Wang, Y.; Arisoy, Z.; Erdem, H.; Yazici, A.; Gokmen, O.; et al. Biofortification and localization of zinc in wheat grain. J. Agric. Food Chem. 2010, 58, 9092–9102. [Google Scholar] [CrossRef]
- Rugeles-Reyes, S.M.; Cecílio Filho, A.B.; López Aguilar, M.A.; Silva, P.H.S. Foliar application of zinc in the agronomic biofortification of arugula. Food Sci. Technol. 2019, 39, 1011–1017. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Chai, T.; Zhang, Y.; Tan, J.; Ma, S. The effects of copper, manganese and zinc on plant growth and elemental accumulation in the manganese-hyperaccumulator Phytolacca americana. J. Plant Physiol. 2012, 169, 1243–1252. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Tzortzakis, N. Optimizing nitrogen, phosphorus, and potassium requirements to improve Origanum dubium Boiss. growth, nutrient and water use efficiency, essential oil yield and composition. Ind. Crop. Prod. 2025, 224, 120291. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; El-Nouby, M.A.M.; Kimani, P.K.; Lim, L.W.; Rabea, E.I. A review of the modern principles and applications of solid-phase extraction techniques in chromatographic analysis. Anal. Sci. 2022, 38, 1457–1487. [Google Scholar] [CrossRef]
- Richardson, A.D.; Duigan, S.P.; Berlyn, G.P. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol. 2002, 153, 185–194. [Google Scholar] [CrossRef]
- Wellburn, A.R.A.R. The spectral determination of Chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Papakyriakou, E.; Petropoulos, S.A.S.A.; Tzortzakis, N. The combined and single effect of salinity and copper stress on growth and quality of Mentha spicata plants. J. Hazard. Mater. 2019, 368, 584–593. [Google Scholar] [CrossRef]
- Marinou, E.; Chrysargyris, A.; Tzortzakis, N. Use of sawdust, coco soil and pumice in hydroponically grown strawberry. Plant Soil Environ. 2013, 59, 452–459. [Google Scholar] [CrossRef]
- Meyers, K.J.; Watkins, C.B.; Pritts, M.P.; Liu, R.H. Antioxidant and Antiproliferative Activities of Strawberries. J. Agric. Food Chem. 2003, 51, 6887–6892. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Panayiotou, C.; Tzortzakis, N. Nitrogen and phosphorus levels affected plant growth, essential oil composition and antioxidant status of lavender plant (Lavandula angustifolia Mill.). Ind. Crops Prod. 2016, 83, 577–586. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Loreto, F.; Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef]
- De Azevedo Neto, A.D.; Prisco, J.T.; Enéas-Filho, J.; Abreu, C.E.B.D.; Gomes-Filho, E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. Exp. Bot. 2006, 56, 87–94. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Xylia, P.; Botsaris, G.; Tzortzakis, N. Antioxidant and antibacterial activities, mineral and essential oil composition of spearmint (Mentha spicata L.) affected by the potassium levels. Ind. Crops Prod. 2017, 103, 202–212. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Charalambous, S.; Xylia, P.; Litskas, V.; Stavrinides, M.; Tzortzakis, N. Assessing the biostimulant effects of a novel plant-based formulation on tomato crop. Sustainability 2020, 12, 8432. [Google Scholar] [CrossRef]

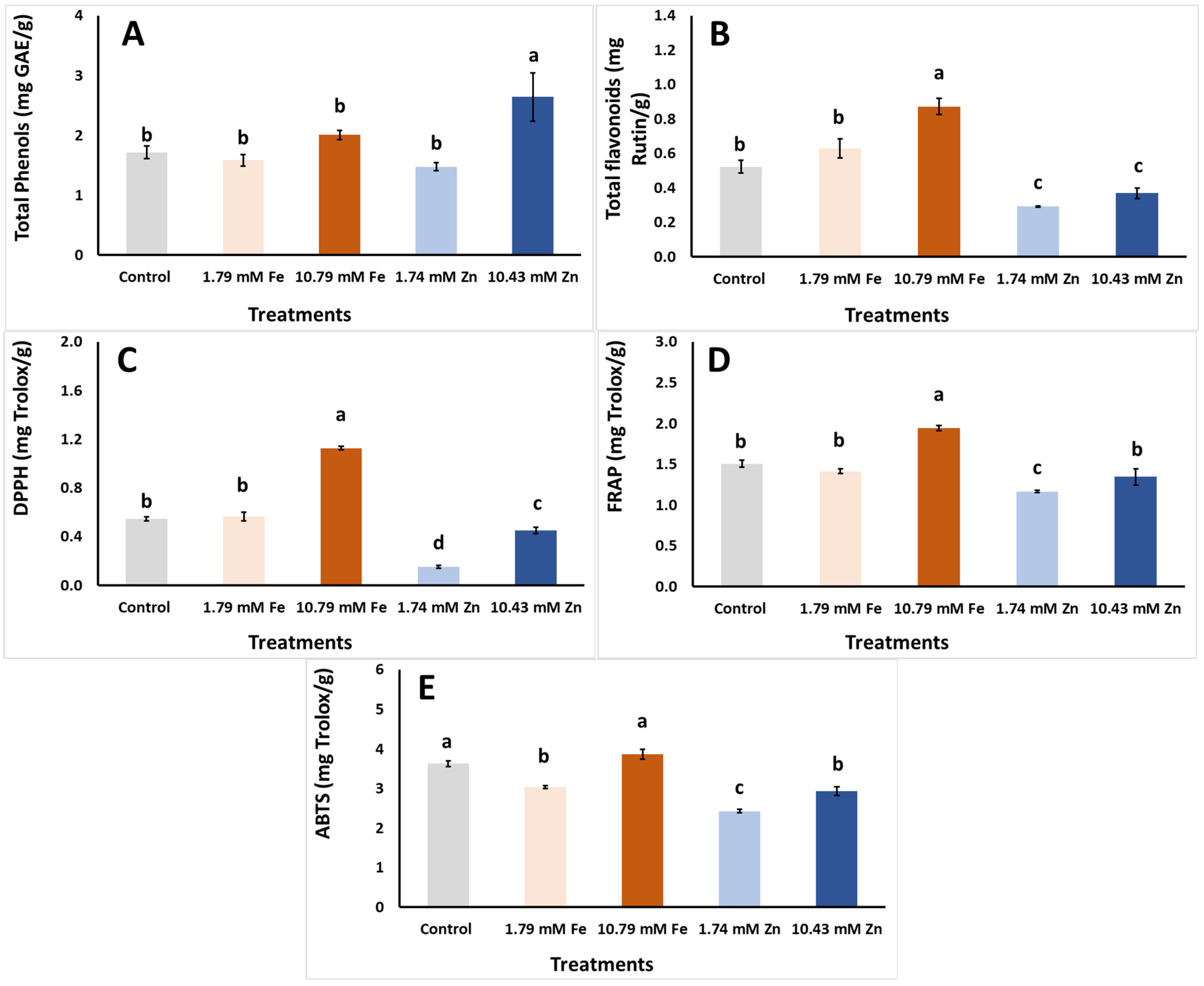
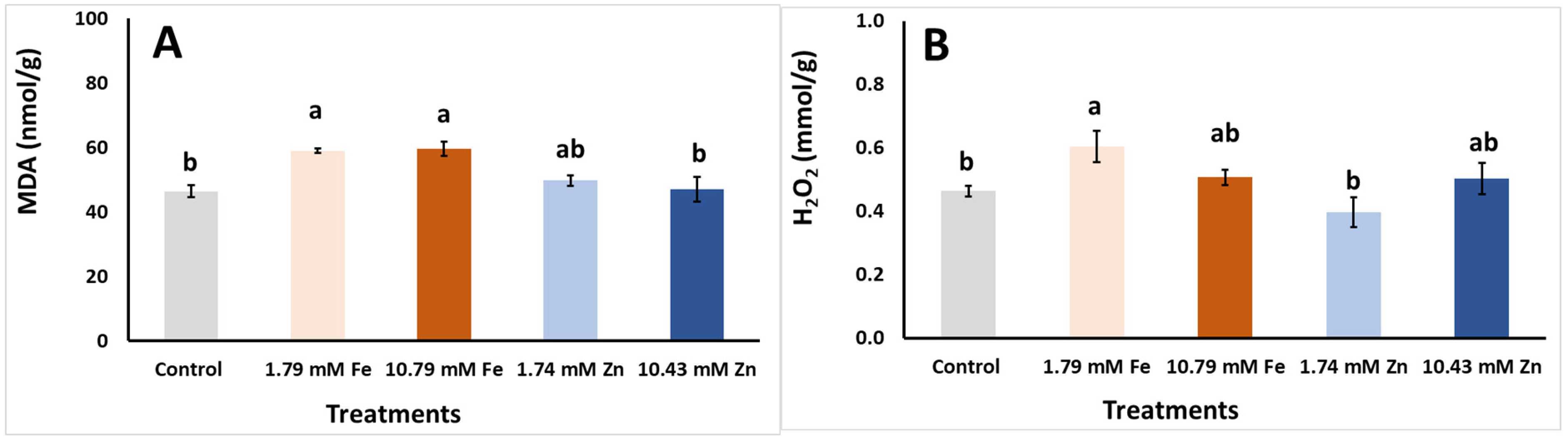
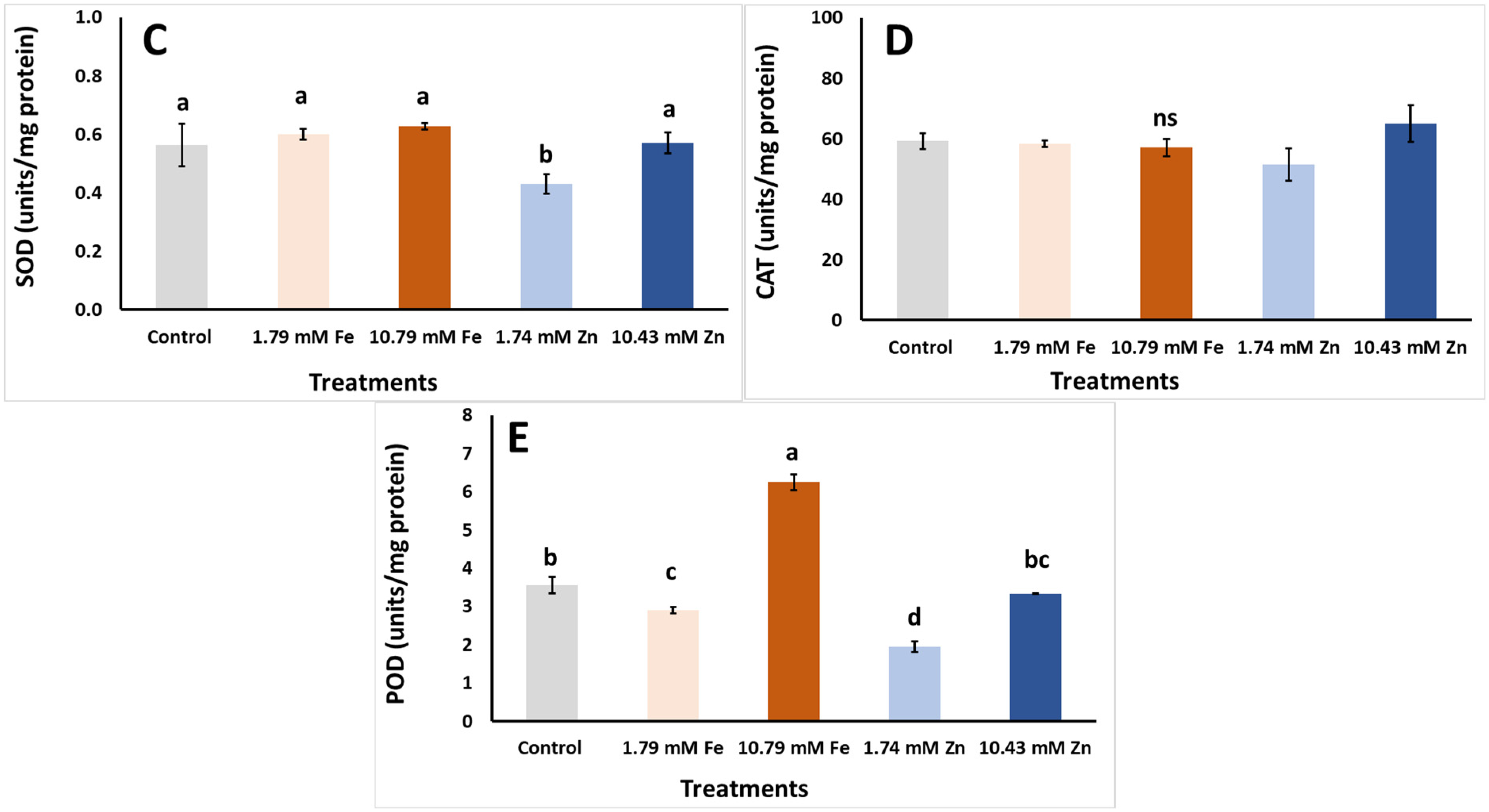
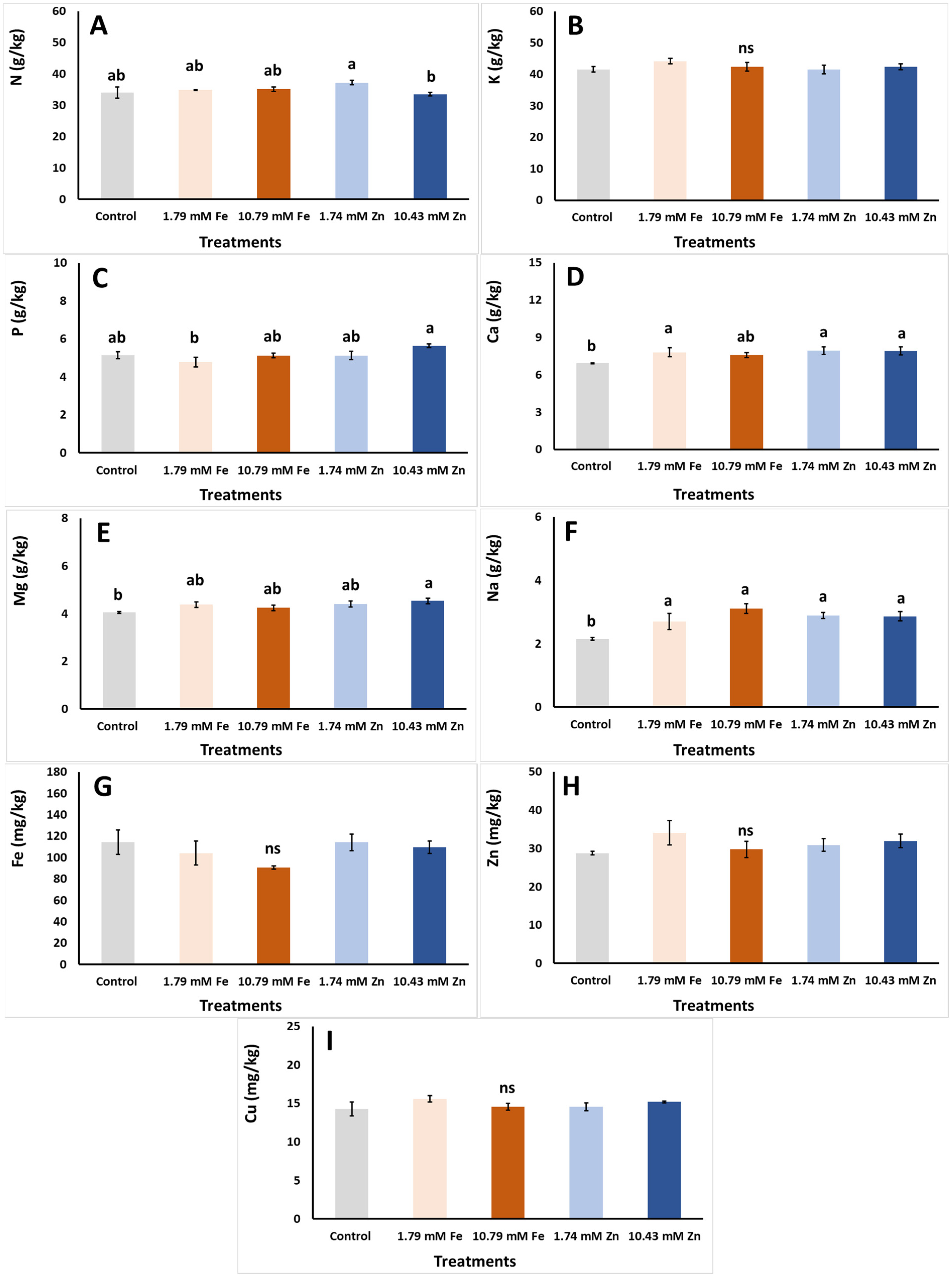
| Foliar Applic. | Biomass FW | Biomass DW | Biomass DM |
|---|---|---|---|
| Control | 84.83 ± 5.22 a | 9.80 ± 0.59 | 11.57 ± 0.24 b |
| 1.79 mM Fe | 68.13 ± 6.86 ab | 8.83 ± 0.89 | 12.97 ± 0.15 ab |
| 10.79 mM Fe | 57.18 ± 9.48 b | 7.81 ± 1.24 | 13.75 ± 1.09 a |
| 1.74 mM Zn | 60.00 ± 6.75 b | 8.54 ± 0.97 | 14.21 ± 0.34 a |
| 10.43 mM Zn | 57.43 ± 3.67 b | 8.04 ± 0.49 | 14.02 ± 0.57 a |
| Foliar Applic. | SPAD | Chlorophyll Fluorescence | Chlorophyll a | Chlorophyll b | Total Chlorophylls | Carotenoids | Chlorophyll a/Chlorophyll b | Carotenoids/Total Chlorophylls |
|---|---|---|---|---|---|---|---|---|
| Control | 51.97 ± 2.11 ab | 0.80 ± 0.004 | 1.05 ± 0.04 ab | 0.38 ± 0.02 | 1.43 ± 0.06 ab | 0.23 ± 0.010 a | 2.82 ± 0.07 | 0.16 ± 0.000 a |
| 1.79 mM Fe | 50.55 ± 5.79 ab | 0.82 ± 0.021 | 0.77 ± 0.03 d | 0.32 ± 0.06 | 1.09 ± 0.08 d | 0.14 ± 0.006 c | 2.54 ± 0.40 | 0.13 ± 0.013 b |
| 10.79 mM Fe | 47.17 ± 2.16 ab | 0.80 ± 0.025 | 0.95 ± 0.03 bc | 0.41 ± 0.03 | 1.35 ± 0.04 bc | 0.18 ± 0.012 b | 2.37 ± 0.21 | 0.13 ± 0.007 b |
| 1.74 mM Zn | 55.28 ± 3.77 a | 0.81 ± 0.007 | 1.15 ± 0.02 a | 0.41 ± 0.02 | 1.56 ± 0.04 a | 0.24 ± 0.003 a | 2.79 ± 0.09 | 0.15 ± 0.000 ab |
| 10.43 mM Zn | 43.3 ± 2.30 b | 0.82 ± 0.012 | 0.90 ± 0.05 c | 0.30 ± 0.03 | 1.19 ± 0.08 cd | 0.19 ± 0.010 b | 3.02 ± 0.18 | 0.16 ± 0.009 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrysargyris, A.; Tzortzakis, N. Iron and Zinc Foliar Spraying Affected Sideritis cypria Post. Growth, Mineral Content and Antioxidant Properties. Plants 2025, 14, 840. https://doi.org/10.3390/plants14060840
Chrysargyris A, Tzortzakis N. Iron and Zinc Foliar Spraying Affected Sideritis cypria Post. Growth, Mineral Content and Antioxidant Properties. Plants. 2025; 14(6):840. https://doi.org/10.3390/plants14060840
Chicago/Turabian StyleChrysargyris, Antonios, and Nikolaos Tzortzakis. 2025. "Iron and Zinc Foliar Spraying Affected Sideritis cypria Post. Growth, Mineral Content and Antioxidant Properties" Plants 14, no. 6: 840. https://doi.org/10.3390/plants14060840
APA StyleChrysargyris, A., & Tzortzakis, N. (2025). Iron and Zinc Foliar Spraying Affected Sideritis cypria Post. Growth, Mineral Content and Antioxidant Properties. Plants, 14(6), 840. https://doi.org/10.3390/plants14060840








