Synergistic Effects of Salt-Tolerant PGPR and Foliar Silicon on Pak Choi Antioxidant Defense Under Salt Stress
Abstract
1. Introduction
2. Results
2.1. Soil Moisture and Salinity Dynamics
2.2. Antioxidant Enzyme Activities and Oxidative Stress
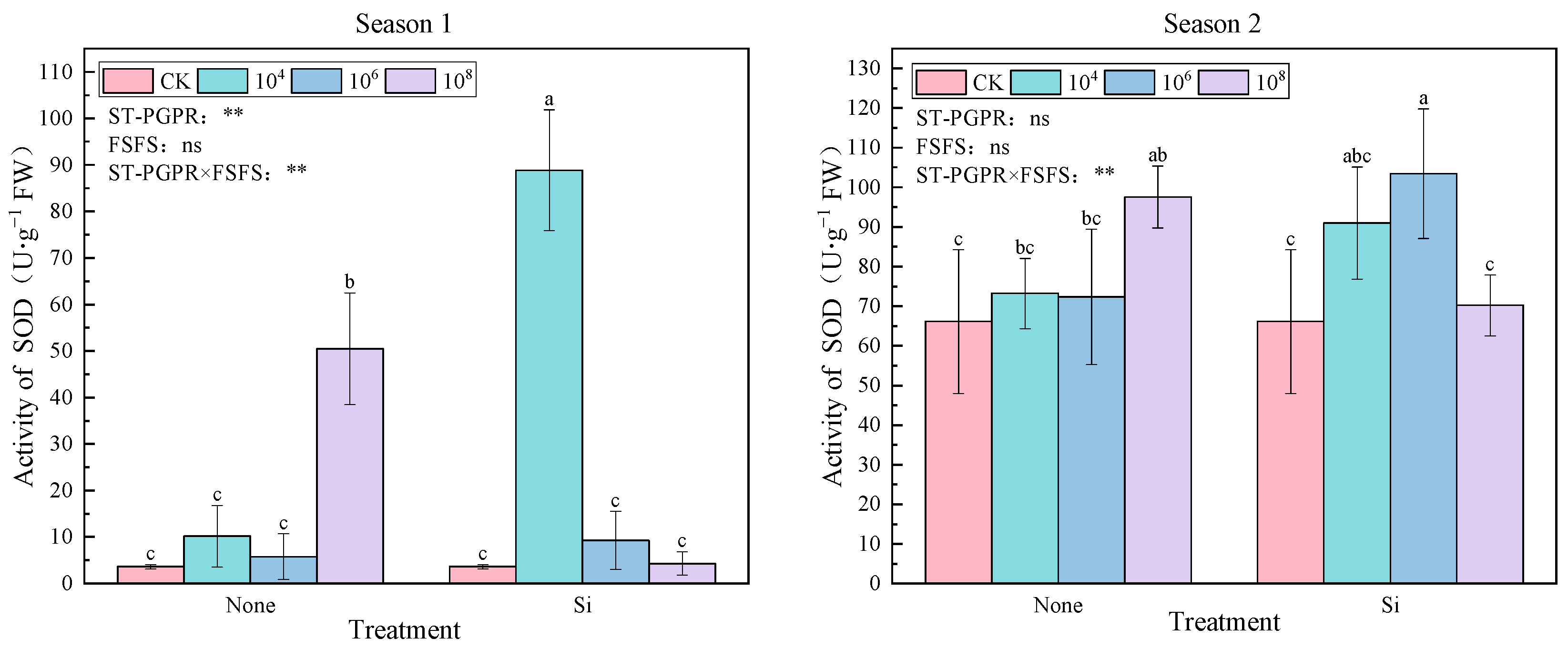
2.2.1. Superoxide Dismutase (SOD) Activity
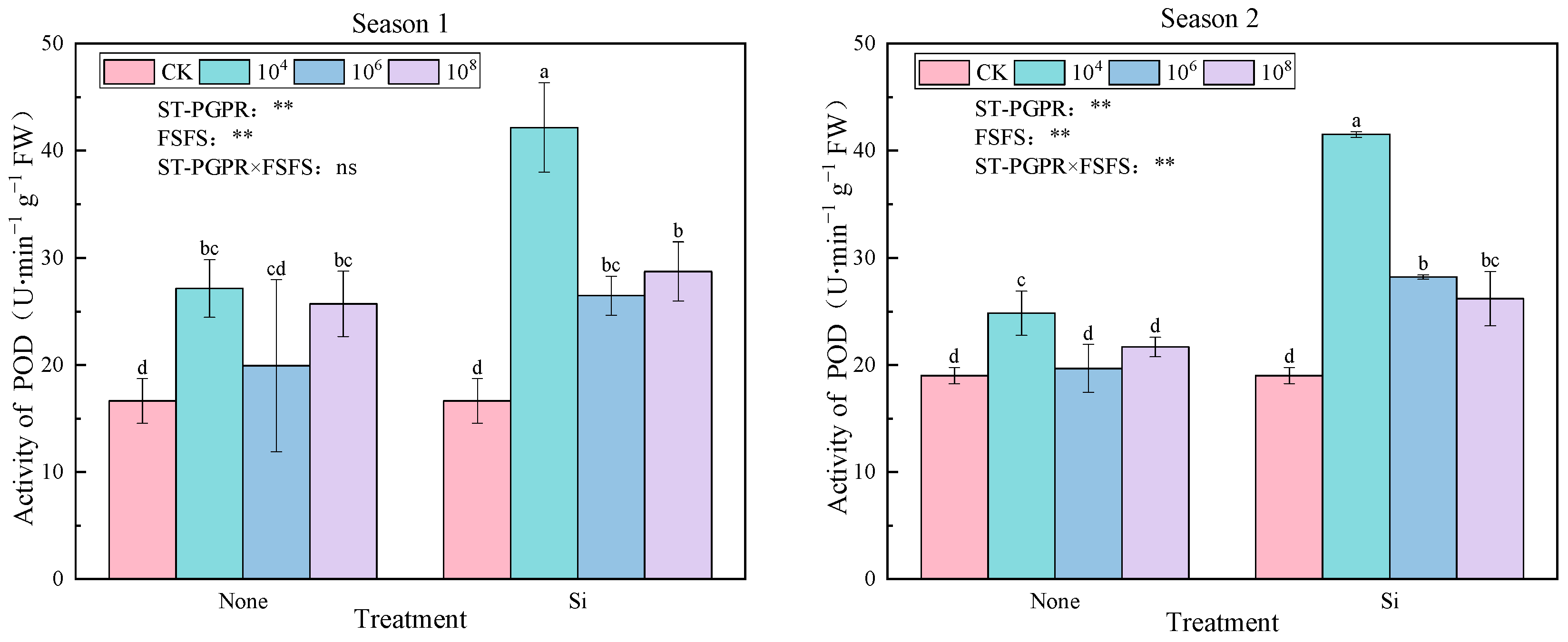
2.2.2. Peroxidase (POD) Activity
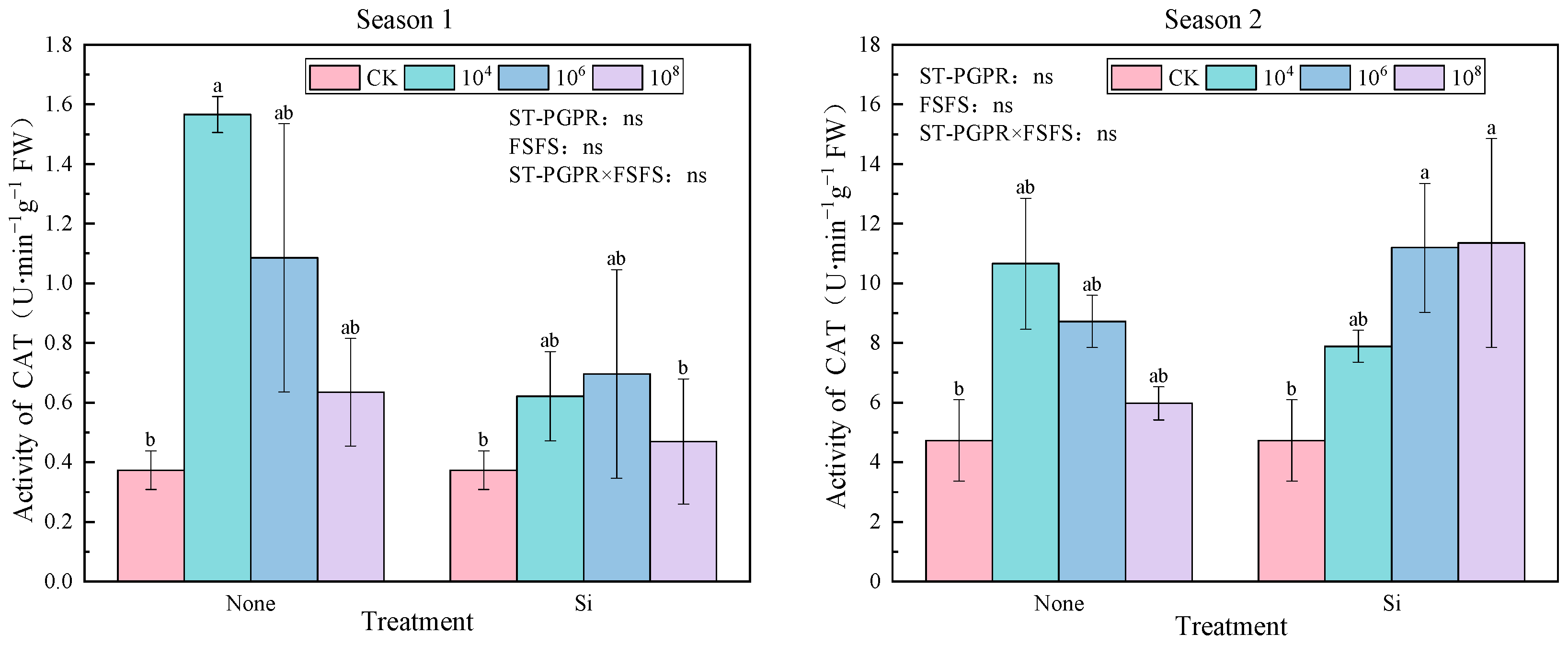
2.2.3. Catalase (CAT) Activity
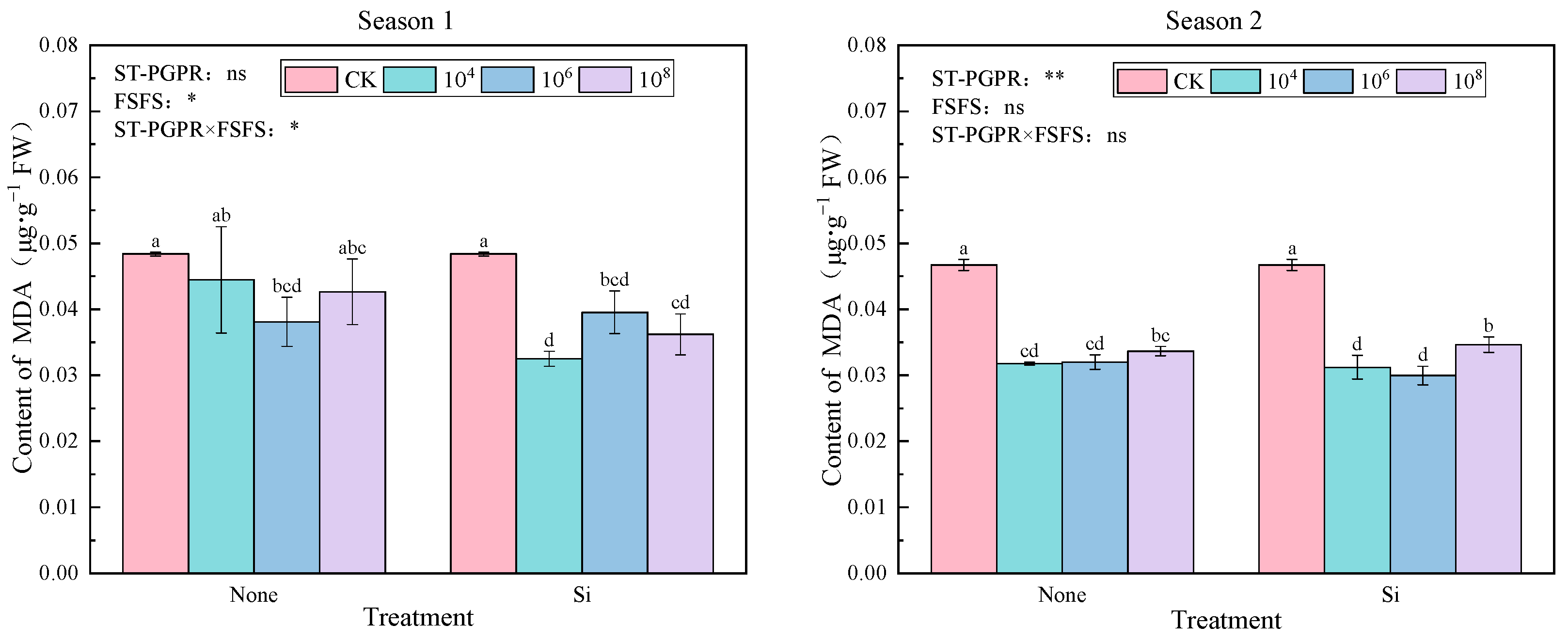
2.2.4. Malondialdehyde (MDA) Content
2.3. Osmolyte Accumulation and Hormonal Regulation
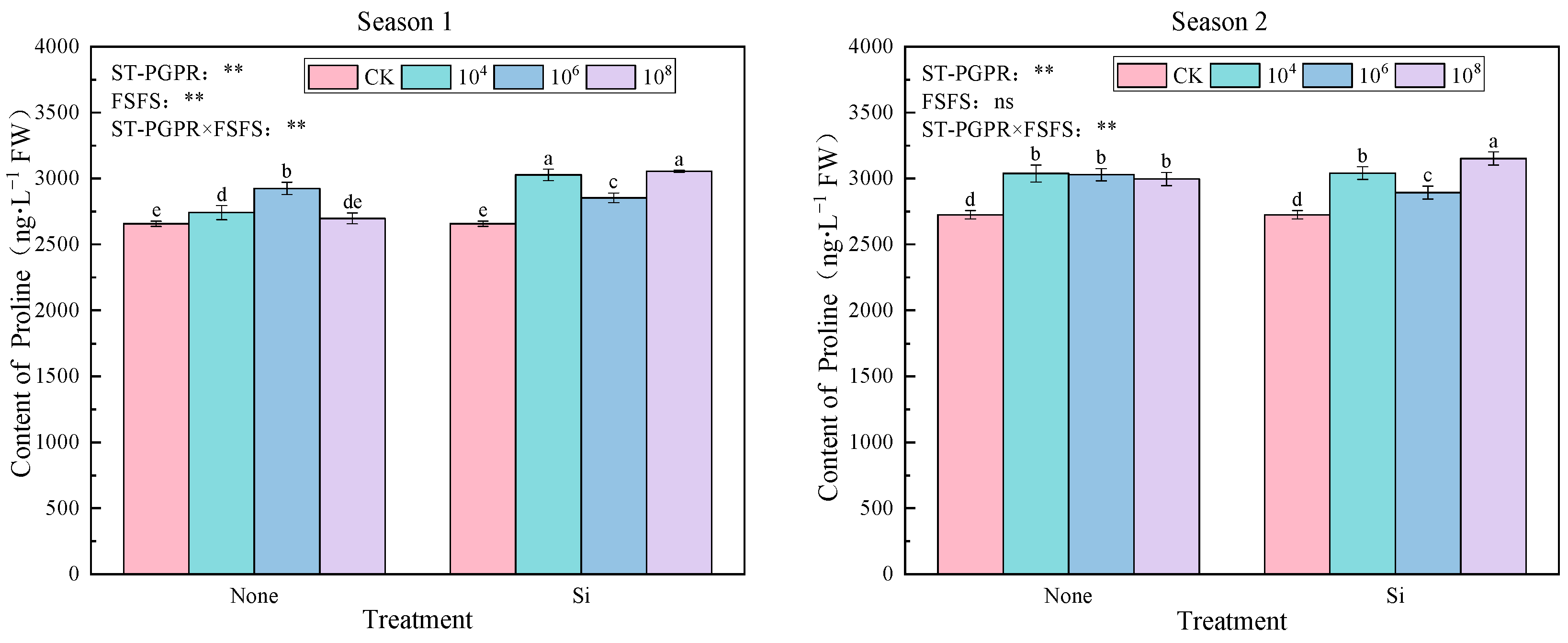
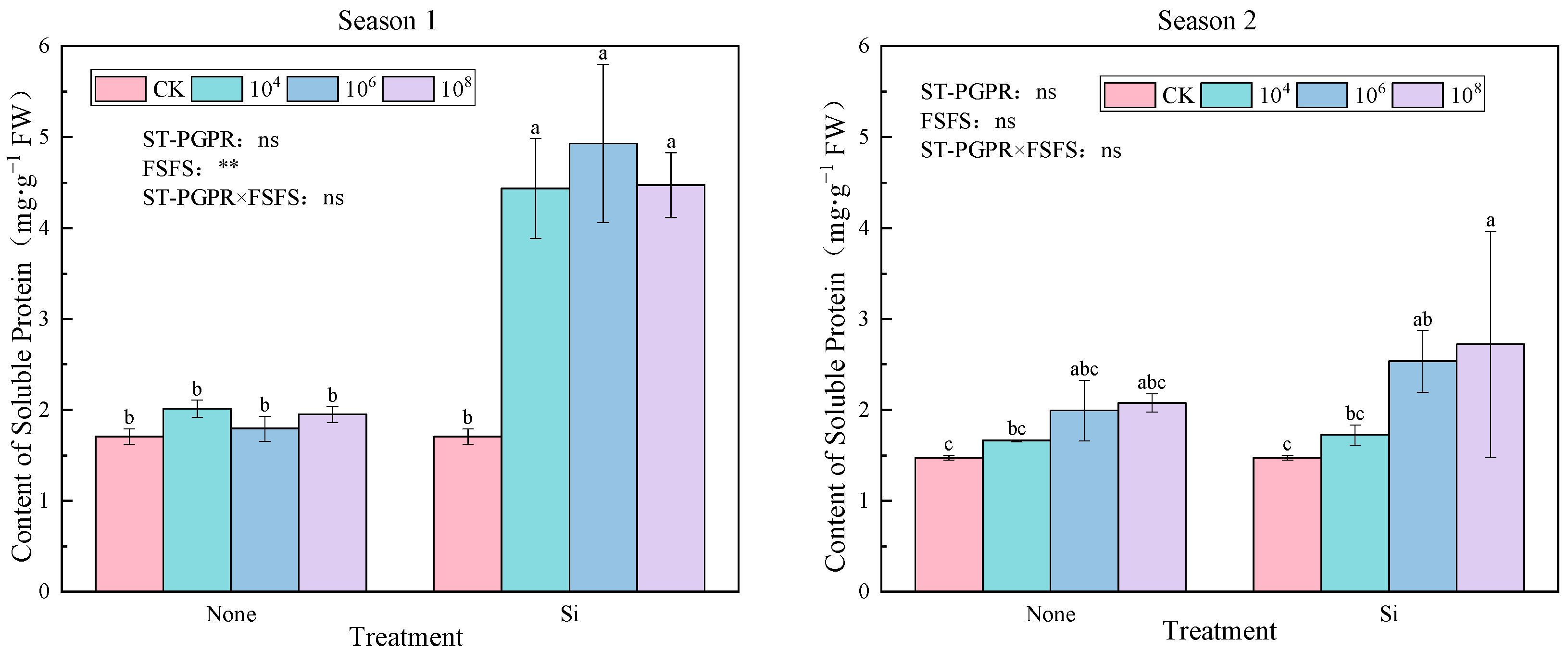
2.3.1. Proline and Soluble Protein
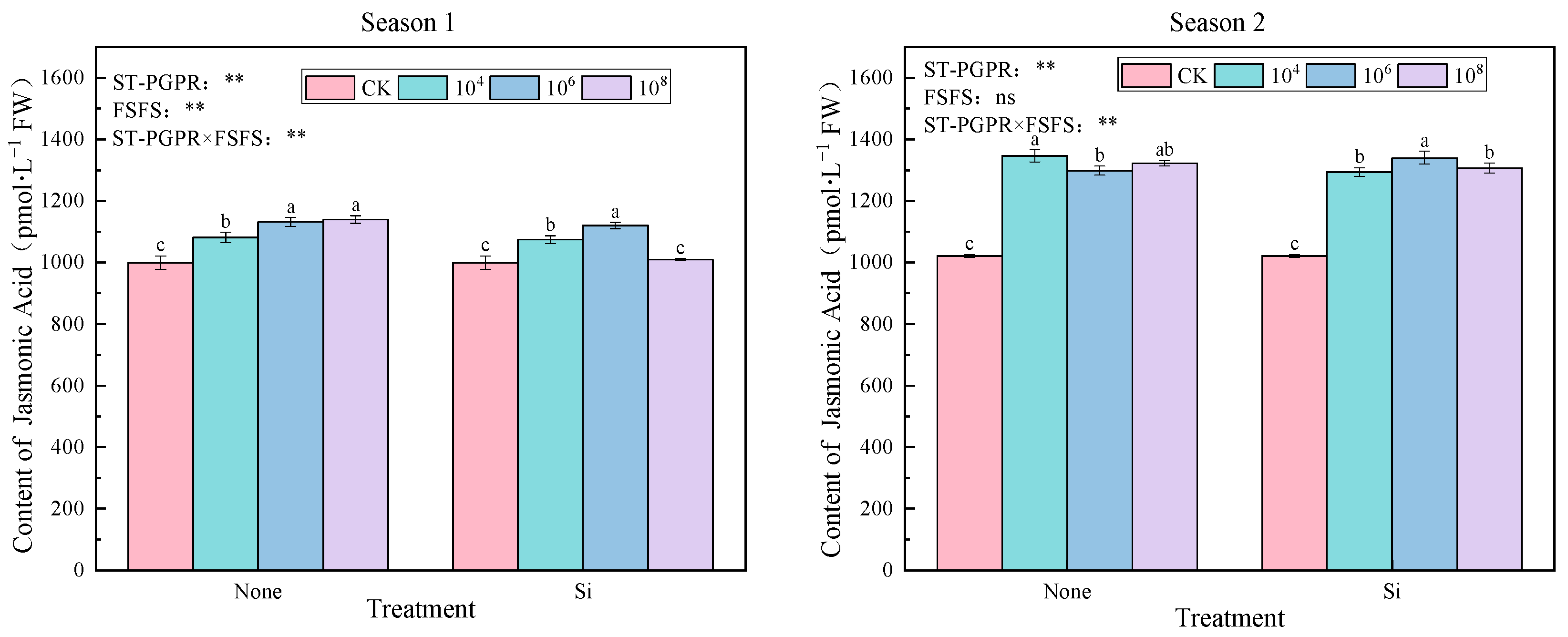
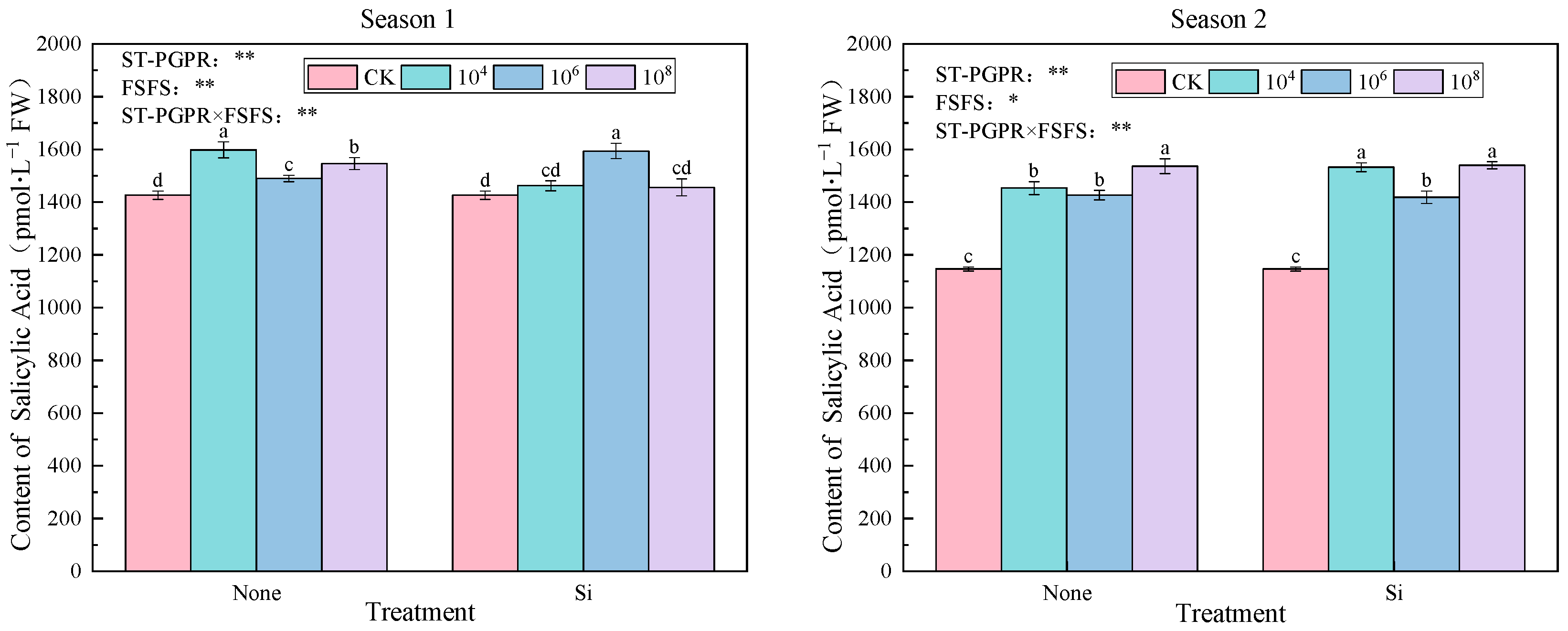
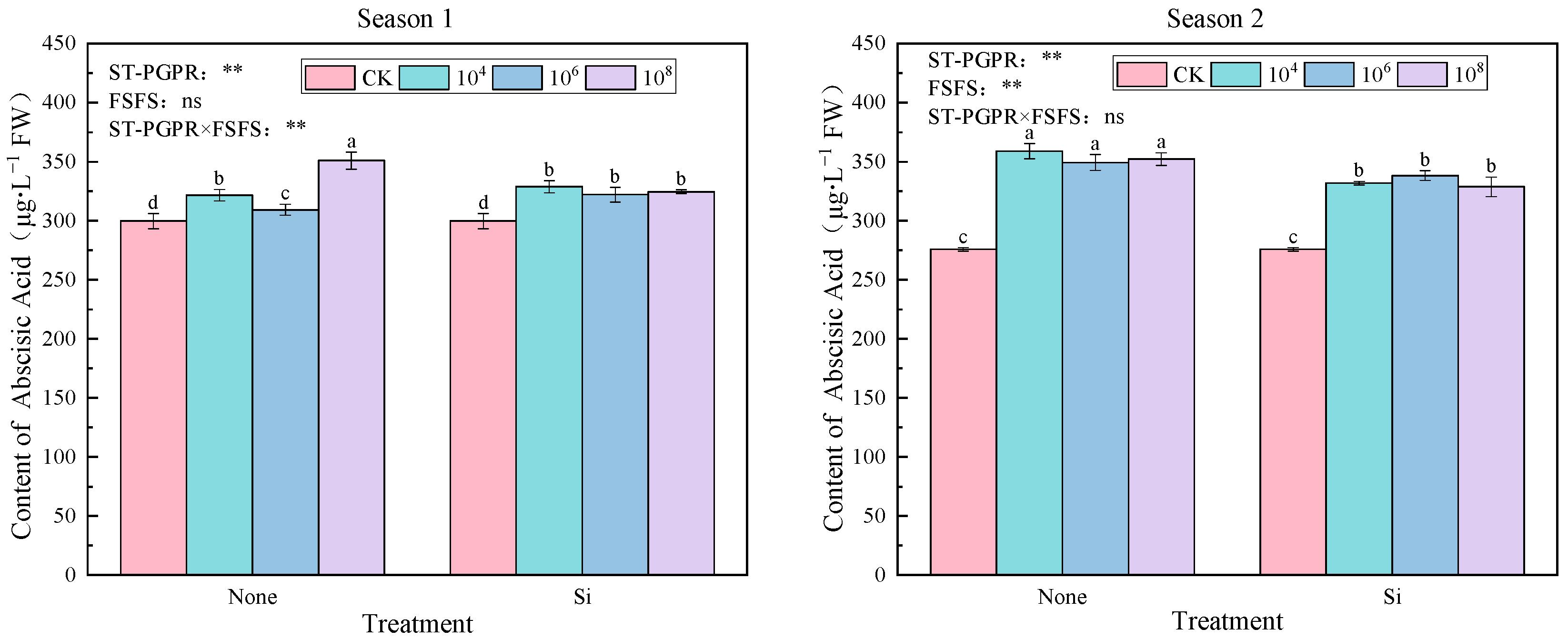
2.3.2. Phytohormones
2.4. Comprehensive Evaluation Based on PCA
3. Discussion
3.1. Synergistic Regulation of Osmolytes by Microbes and Silicon Fertilization
3.2. Effects of ST-PGPR and FSFS on Antioxidant Enzyme Activities
3.3. Interactions Between Hormonal Networks Regulated by Microbes and Silicon
4. Materials and Methods
4.1. Experimental Site and Soil Preparation
4.2. Experimental Design and Treatments
4.3. Agronomic Management
4.4. Data Collection and Analysis
- (1)
- Soil parameters: Soil moisture was measured using the gravimetric drying method. Electrical conductivity (EC, soil-to-water ratio 1:5) was assessed using a conductivity meter (DDB-303A).
- (2)
- Crop antioxidant indicators:
- (1)
- Enzyme activities: The activities of catalase (CAT), superoxide dismutase (SOD), and peroxidase (POD), expressed in U·min−1g−1 FW (fresh weight), U·g−1 FW, and U·min−1g−1 FW, were measured by employing the UV absorption method, the nitrogen blue tetrazolium photochemical reduction method, and the guaiacol method at wavelengths of 240 nm, 560 nm, and 470 nm, respectively [88].
- (2)
- Osmolytes: The soluble protein content was determined using the Coomassie brilliant blue method at 595 nm [88], and the Proline content was measured using an ELISA kit (LabRe).
- (3)
- Oxidative Damage: Malondialdehyde (MDA) content was quantified using the thiobarbituric acid (TBA) method at 452 nm, 532 nm, and 600 nm wavelengths [88].
- (4)
- Phytohormone Quantification: The contents of Jasmonic Acid (JA), Salicylic Acid (SA), and Abscisic Acid (ABA) were determined using an ELISA kit (LabRe).
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rao, Y.; Peng, T.; Xue, S. Mechanisms of Plant Saline-Alkaline Tolerance. J. Plant Physiol. 2023, 281, 153916. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Chen, Q.; Li, Y.; Zhuo, Y.Q.; Xu, L.Z. Research on Saline-Alkali Soil Amelioration with FGD Gypsum. Resour. Conserv. Recycl. 2017, 121, 82–92. [Google Scholar] [CrossRef]
- Han, M.; Gao, M.; Yang, J. Three Dimensional Evaluation on Soil Improvement Effect of Saline Alkali Soil in Yellow River Delta. IOP Conf. Ser. Earth Environ. Sci. 2021, 734, 012036. [Google Scholar] [CrossRef]
- Qadir, M.; Oster, J.D.; Schubert, S.; Noble, A.D.; Sahrawat, K.L. Phytoremediation of Sodic and Saline-Sodic Soils. Advances in Agronomy; Academic Press: San Diego, CA, USA, 2007; Volume 96, pp. 197–247. [Google Scholar]
- Etesami, H.; Beattie, G.A. Mining Halophytes for Plant Growth-Promoting Halotolerant Bacteria to Enhance the Salinity Tolerance of Non-Halophytic Crops. Front. Microbiol. 2018, 9, 148. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, X.; Banerjee, S.; Hartmann, M.; Peng, B.; Elvers, R.; Zhao, Z.-Y.; Zhou, N.; Liu, J.-J.; Wang, B.; et al. Continuous Planting of Euhalophyte Suaeda salsa Enhances Microbial Diversity and Multifunctionality of Saline Soil. Appl. Environ. Microbiol. 2024, 90, e02355-23. [Google Scholar] [CrossRef]
- Numan, M.; Bashir, S.; Khan, Y.; Mumtaz, R.; Shinwari, Z.K.; Khan, A.L.; Khan, A.; AL-Harrasi, A. Plant Growth Promoting Bacteria as an Alternative Strategy for Salt Tolerance in Plants: A Review. Microbiol. Res. 2018, 209, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Bacteria with ACC Deaminase Can Promote Plant Growth and Help to Feed the World. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-Tolerant Plant Growth Promoting Rhizobacteria for Enhancing Crop Productivity of Saline Soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef]
- Ruzzi, M.; Aroca, R. Plant Growth-Promoting Rhizobacteria Act as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 124–134. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant Growth-Promoting Bacteria: Biological Tools for the Mitigation of Salinity Stress in Plants. Front. Microbiol. 2020, 11, 1216. [Google Scholar] [CrossRef]
- Liang, Y.; Sun, W.; Zhu, Y.-G.; Christie, P. Mechanisms of Silicon-Mediated Alleviation of Abiotic Stresses in Higher Plants: A Review. Environ. Pollut. 2007, 147, 422–428. [Google Scholar] [CrossRef]
- Jinger, D.; Dhar, S.; Dass, A.; Sharma, V.K.; Shukla, L.; Paramesh, V.; Parihar, M.; Joshi, N.; Joshi, E.; Gupta, G.; et al. Residual Silicon and Phosphorus Improved the Growth, Yield, Nutrient Uptake and Soil Enzyme Activities of Wheat. Silicon 2022, 14, 8949–8964. [Google Scholar] [CrossRef]
- Zhu, Y.-X.; Gong, H.-J.; Yin, J.-L. Role of Silicon in Mediating Salt Tolerance in Plants: A Review. Plants 2019, 8, 147. [Google Scholar] [CrossRef]
- Rhoades, J.D.; Corwin, D.L. Determining Soil Electrical Conductivity-Depth Relations Using an Inductive Electromagnetic Soil Conductivity Meter. Soil Sci. Soc. Am. J. 1981, 45, 255–260. [Google Scholar] [CrossRef]
- Naseem, H.; Ahsan, M.; Shahid, M.A.; Khan, N. Exopolysaccharides Producing Rhizobacteria and Their Role in Plant Growth and Drought Tolerance. J. Basic Microbiol. 2018, 58, 1009–1022. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene Networks Involved in Drought Stress Response and Tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef]
- Hurkman, W.J.; Tanaka, C.K. Effect of Salt Stress on Germin Gene Expression in Barley Roots. Plant Physiol. 1996, 110, 971–977. [Google Scholar] [CrossRef]
- Rangseekaew, P.; Barros-Rodríguez, A.; Pathom-aree, W.; Manzanera, M. Deep-Sea Actinobacteria Mitigate Salinity Stress in Tomato Seedlings and Their Biosafety Testing. Plants 2021, 10, 1687. [Google Scholar] [CrossRef] [PubMed]
- Rangseekaew, P.; Barros-Rodríguez, A.; Pathom-aree, W.; Manzanera, M. Plant Beneficial Deep-Sea Actinobacterium, Dermacoccus abyssi MT1.1T Promote Growth of Tomato (Solanum lycopersicum) under Salinity Stress. Biology 2022, 11, 191. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, Z.; Wang, L.; Li, M.; Lang, D.; Zhang, X. Silicon Alleviates Salt and Drought Stress of Glycyrrhiza Uralensis Seedling by Altering Antioxidant Metabolism and Osmotic Adjustment. J. Plant Res. 2017, 130, 611–624. [Google Scholar] [CrossRef]
- Chinachanta, K.; Shutsrirung, A.; Santasup, C.; Pathom-Aree, W.; Luu, D.T.; Herrmann, L.; Lesueur, D.; Prom-u-thai, C. Rhizoactinobacteria Enhance Growth and Antioxidant Activity in Thai Jasmine Rice (Oryza sativa) KDML105 Seedlings under Salt Stress. Plants 2023, 12, 3441. [Google Scholar] [CrossRef]
- Hoque, M.A.; Banu, M.N.A.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Proline and Glycinebetaine Enhance Antioxidant Defense and Methylglyoxal Detoxification Systems and Reduce NaCl-Induced Damage in Cultured Tobacco Cells. J. Plant Physiol. 2008, 165, 813–824. [Google Scholar] [CrossRef]
- Ben Ahmed, C.; Ben Rouina, B.; Sensoy, S.; Boukhriss, M.; Ben Abdullah, F. Exogenous Proline Effects on Photosynthetic Performance and Antioxidant Defense System of Young Olive Tree. J. Agric. Food Chem. 2010, 58, 4216–4222. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.J.; Wang, W.W.; Hu, G.Q.; Chen, W.F.; Zhuge, Y.P.; Wang, Z.L.; He, M.R. Role of Exogenous 24-Epibrassinolide in Enhancing the Salt Tolerance of Wheat Seedlings. J. Soil Sci. Plant Nutr. 2017, 17, 554–569. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant Responses to Salt Stress: Adaptive Mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Munns, R. A Leaf Elongation Assay Detects an Unknown Growth Inhibitor in Xylem Sap From Wheat and Barley. Funct. Plant Biol. 1992, 19, 127–135. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A Multifunctional Amino Acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, Perception, Signal Transduction and Action in Plant Stress Response, Growth and Development. An Update to the 2007 Review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. Genetic Strategies for Improving Crop Yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef]
- Zhu, J.-K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox Homeostasis and Antioxidant Signaling: A Metabolic Interface between Stress Perception and Physiological Responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of Superoxide Dismutases (SODs) in Controlling Oxidative Stress in Plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Anwar-ul-Haq, M.; Iftikhar, I.; Akhtar, J.; Maqsood, M. Role of Exogenous Osmolyte Supplementation in Ameliorating Osmotic and Oxidative Stress and Promoting Growth in Salinity-Stressed Soybean Genotypes. J. Soil. Sci. Plant Nutr. 2023, 23, 3682–3694. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. REACTIVE OXYGEN SPECIES: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Zandi, P.; Schnug, E. Reactive Oxygen Species, Antioxidant Responses and Implications from a Microbial Modulation Perspective. Biology 2022, 11, 155. [Google Scholar] [CrossRef]
- Stephenie, S.; Chang, Y.P.; Gnanasekaran, A.; Esa, N.M.; Gnanaraj, C. An Insight on Superoxide Dismutase (SOD) from Plants for Mammalian Health Enhancement. J. Funct. Foods 2020, 68, 103917. [Google Scholar] [CrossRef]
- Pan, L.; Luo, Y.; Wang, J.; Li, X.; Tang, B.; Yang, H.; Hou, X.; Liu, F.; Zou, X. Evolution and Functional Diversification of Catalase Genes in the Green Lineage. BMC Genom. 2022, 23, 411. [Google Scholar] [CrossRef]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.-M.; Qian, P.; Xin, W.; Li, H.-Y.; Burritt, D.J.; Fujita, M.; Tran, L.-S.P. Hydrogen Peroxide Priming Modulates Abiotic Oxidative Stress Tolerance: Insights from ROS Detoxification and Scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative Stress, Antioxidants and Stress Tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Abogadallah, G.M. Differential Regulation of Photorespiratory Gene Expression by Moderate and Severe Salt and Drought Stress in Relation to Oxidative Stress. Plant Sci. 2011, 180, 540–547. [Google Scholar] [CrossRef]
- Gowtham, H.G.; Singh, S.B.; Shilpa, N.; Aiyaz, M.; Nataraj, K.; Udayashankar, A.C.; Amruthesh, K.N.; Murali, M.; Poczai, P.; Gafur, A.; et al. Insight into Recent Progress and Perspectives in Improvement of Antioxidant Machinery upon PGPR Augmentation in Plants under Drought Stress: A Review. Antioxidants 2022, 11, 1763. [Google Scholar] [CrossRef]
- Rabhi, N.E.H.; Silini, A.; Cherif-Silini, H.; Yahiaoui, B.; Lekired, A.; Robineau, M.; Esmaeel, Q.; Jacquard, C.; Vaillant-Gaveau, N.; Clément, C.; et al. Pseudomonas knackmussii MLR6, a Rhizospheric Strain Isolated from Halophyte, Enhances Salt Tolerance in Arabidopsis thaliana. J. Appl. Microbiol. 2018, 125, 1836–1851. [Google Scholar] [CrossRef]
- Aloo, B.N.; Dessureault-Rompré, J.; Tripathi, V.; Nyongesa, B.O.; Were, B.A. Signaling and Crosstalk of Rhizobacterial and Plant Hormones That Mediate Abiotic Stress Tolerance in Plants. Front. Microbiol. 2023, 14, 1171104. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Y. Unraveling Salt Stress Signaling in Plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zeng, W.; Ao, C.; Luo, Y.; Wang, Z.; Hou, M.; Huang, J. Bacillus Atrophaeus WZYH01 and Planococcus Soli WZYH02 Improve Salt Tolerance of Maize (Zea mays L.) in Saline Soil. Front. Plant Sci. 2022, 13, 891372. [Google Scholar] [CrossRef]
- Barnawal, D.; Bharti, N.; Pandey, S.S.; Pandey, A.; Chanotiya, C.S.; Kalra, A. Plant Growth-Promoting Rhizobacteria Enhance Wheat Salt and Drought Stress Tolerance by Altering Endogenous Phytohormone Levels and TaCTR1/TaDREB2 Expression. Physiol. Plant. 2017, 161, 502–514. [Google Scholar] [CrossRef]
- Lee, H.-J.; Seo, P.J. Ca2+talyzing Initial Responses to Environmental Stresses. Trends Plant Sci. 2021, 26, 849–870. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.H.; Kausar, H.; Saud, H.M. Plant Growth-Promoting Rhizobacteria Enhance Salinity Stress Tolerance in Okra through ROS-Scavenging Enzymes. BioMed Res. Int. 2016, 2016, 6284547. [Google Scholar] [CrossRef]
- Gururani, M.A.; Upadhyaya, C.P.; Baskar, V.; Venkatesh, J.; Nookaraju, A.; Park, S.W. Plant Growth-Promoting Rhizobacteria Enhance Abiotic Stress Tolerance in Solanum Tuberosum Through Inducing Changes in the Expression of ROS-Scavenging Enzymes and Improved Photosynthetic Performance. J. Plant Growth Regul. 2013, 32, 245–258. [Google Scholar] [CrossRef]
- Egamberdieva, D. Alleviation of Salt Stress by Plant Growth Regulators and IAA Producing Bacteria in Wheat. Acta Physiol. Plant 2009, 31, 861–864. [Google Scholar] [CrossRef]
- Kang, S.-M.; Shahzad, R.; Bilal, S.; Khan, A.L.; Park, Y.-G.; Lee, K.-E.; Asaf, S.; Khan, M.A.; Lee, I.-J. Indole-3-Acetic-Acid and ACC Deaminase Producing Leclercia Adecarboxylata MO1 Improves Solanum lycopersicum L. Growth and Salinity Stress Tolerance by Endogenous Secondary Metabolites Regulation. BMC Microbiol. 2019, 19, 80. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Waqas, M.; Kang, S.-M.; Lee, I.-J. Inoculation of Abscisic Acid-Producing Endophytic Bacteria Enhances Salinity Stress Tolerance in Oryza Sativa. Environ. Exp. Bot. 2017, 136, 68–77. [Google Scholar] [CrossRef]
- Kumawat, K.C.; Sharma, B.; Nagpal, S.; Kumar, A.; Tiwari, S.; Nair, R.M. Plant Growth-Promoting Rhizobacteria: Salt Stress Alleviators to Improve Crop Productivity for Sustainable Agriculture Development. Front. Plant Sci. 2023, 13, 1101862. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.; Guo, J.; Liu, F.; Song, F.; Li, X. Effect of the Transgenerational Exposure to Elevated CO2 on Low Temperature Tolerance of Winter Wheat: Chloroplast Ultrastructure and Carbohydrate Metabolism. J. Agron. Crop Sci. 2020, 206, 773–783. [Google Scholar] [CrossRef]
- Lin, T.; Haider, F.U.; Liu, T.; Li, S.; Zhang, P.; Zhao, C.; Li, X. Salt Tolerance Induced by Plant Growth-Promoting Rhizobacteria Is Associated with Modulations of the Photosynthetic Characteristics, Antioxidant System, and Rhizosphere Microbial Diversity in Soybean (Glycine max (L.) Merr.). Agronomy 2025, 15, 341. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Al-Ghamdi, A.A.; Ali, H.M.; Alayafi, A.A. Azospirillum lipoferum FK1 Confers Improved Salt Tolerance in Chickpea (Cicer arietinum L.) by Modulating Osmolytes, Antioxidant Machinery and Stress-Related Genes Expression. Environ. Exp. Bot. 2019, 159, 55–65. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Silicon Uptake and Accumulation in Higher Plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Passardi, F.; Cosio, C.; Penel, C.; Dunand, C. Peroxidases Have More Functions than a Swiss Army Knife. Plant Cell Rep. 2005, 24, 255–265. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Breusegem, F.V. Reactive Oxygen Gene Network of Plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Liang, Y.; Wong, J.W.C.; Wei, L. Silicon-Mediated Enhancement of Cadmium Tolerance in Maize (Zea mays L.) Grown in Cadmium Contaminated Soil. Chemosphere 2005, 58, 475–483. [Google Scholar] [CrossRef]
- Yoshioka, H.; Sugie, K.; Park, H.-J.; Maeda, H.; Tsuda, N.; Kawakita, K.; Doke, N. Induction of Plant Gp91 Phox Homolog by Fungal Cell Wall, Arachidonic Acid, and Salicylic Acid in Potato. MPMI 2001, 14, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Cárdenas, M.L.; Narváez-Vásquez, J.; Ryan, C.A. Hydrogen Peroxide Acts as a Second Messenger for the Induction of Defense Genes in Tomato Plants in Response to Wounding, Systemin, and Methyl Jasmonate. Plant Cell 2001, 13, 179–191. [Google Scholar] [CrossRef]
- Sevgi, B.; Leblebici, S. Exogenous Sucrose Alleviates Salt Stress in Sunflower (Helianthus annuus L.) and Canola (Brassica napus L.) by Modulating Osmotic Adjustment and Antioxidant Defense System. Physiol. Mol. Biol. Plants 2025, 31, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Samad, A.; Shaukat, K.; Ansari, M.; Ahmad, H.; Nizar, M.; Hakeem, A.; Fiaz, S.; Alafari, H.; Jalal, A.; Attia, K.; et al. Plant Growth Regulators Ameliorate Biochemical and Molecular Parameters in Plantago Ovata Forssk. under Salt Stress. Turk. J. Agric. For. 2023, 47, 676–687. [Google Scholar] [CrossRef]
- Gomez, M.Y.; Schroeder, M.M.; Chieb, M.; McLain, N.K.; Gachomo, E.W. Bradyrhizobium Japonicum IRAT FA3 Promotes Salt Tolerance through Jasmonic Acid Priming in Arabidopsis thaliana. BMC Plant Biol. 2023, 23, 60. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, K.; Hafez, E.M.; Omara, A.E.-D.; Rashwan, E.; Alshaal, T. Zinc Oxide Nanoparticles and PGPR Strengthen Salinity Tolerance and Productivity of Wheat Irrigated with Saline Water in Sodic-Saline Soil. Plant Soil 2023, 493, 475–495. [Google Scholar] [CrossRef]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Bélanger, R.R. The Controversies of Silicon’s Role in Plant Biology. New Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of Iron-Free Fenton-like Systems for Activating H2O2 in Advanced Oxidation Processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How Plant Hormones Mediate Salt Stress Responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef]
- Terzi, R.; Kalaycıoglu, E.; Demiralay, M.; Saglam, A.; Kadioglu, A. Exogenous Ascorbic Acid Mitigates Accumulation of Abscisic Acid, Proline and Polyamine under Osmotic Stress in Maize Leaves. Acta Physiol. Plant 2015, 37, 43. [Google Scholar] [CrossRef]
- Wasti, S.; Mimouni, H.; Smiti, S.; Zid, E.; Ben Ahmed, H. Enhanced Salt Tolerance of Tomatoes by Exogenous Salicylic Acid Applied Through Rooting Medium. OMICS A J. Integr. Biol. 2012, 16, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Prakriti; Sridevi, T.; Sarita, D. SATPAL Salinity Stress Alleviation in Fodder Crops through Foliar Application of Jasmonic Acid—A Review. Forage Res. 2022, 48, 11–21. [Google Scholar]
- Lata, C.; Prasad, M. Role of DREBs in Regulation of Abiotic Stress Responses in Plants. J. Exp. Bot. 2011, 62, 4731–4748. [Google Scholar] [CrossRef]
- Akhtar, N.; Ilyas, N.; Mashwani, Z.-R.; Hayat, R.; Yasmin, H.; Noureldeen, A.; Ahmad, P. Synergistic Effects of Plant Growth Promoting Rhizobacteria and Silicon Dioxide Nano-Particles for Amelioration of Drought Stress in Wheat. Plant Physiol. Biochem. 2021, 166, 160–176. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant Hormone-Mediated Regulation of Stress Responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Bari, R.; Jones, J.D.G. Role of Plant Hormones in Plant Defence Responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Hao, S.; Wang, Y.; Yan, Y.; Liu, Y.; Wang, J.; Chen, S. A Review on Plant Responses to Salt Stress and Their Mechanisms of Salt Resistance. Horticulturae 2021, 7, 132. [Google Scholar] [CrossRef]
- Blumwald, E.; Aharon, G.S.; Apse, M.P. Sodium Transport in Plant Cells. Biochim. Biophys. Acta (BBA)—Biomembr. 2000, 1465, 140–151. [Google Scholar] [CrossRef]
- Liu, J.; Moore, S.; Chen, C.; Lindsey, K. Crosstalk Complexities between Auxin, Cytokinin, and Ethylene in Arabidopsis Root Development: From Experiments to Systems Modeling, and Back Again. Mol. Plant 2017, 10, 1480–1496. [Google Scholar] [CrossRef]
- Kazan, K.; Manners, J.M. MYC2: The Master in Action. Mol. Plant 2013, 6, 686–703. [Google Scholar] [CrossRef] [PubMed]
- Vlot, A.C.; Dempsey, D.A.; Klessig, D.F. Salicylic Acid, a Multifaceted Hormone to Combat Disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef]
- Zheng, J.; Ma, X.; Zhang, X.; Hu, Q.; Qian, R. Salicylic Acid Promotes Plant Growth and Salt-Related Gene Expression in Dianthus superbus L. (Caryophyllaceae) Grown under Different Salt Stress Conditions. Physiol. Mol. Biol. Plants 2018, 24, 231–238. [Google Scholar] [CrossRef]
- Nemhauser, J.L.; Hong, F.; Chory, J. Different Plant Hormones Regulate Similar Processes through Largely Nonoverlapping Transcriptional Responses. Cell 2006, 126, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Li, Q.; Zhou, D.; Jia, M.; Liu, Z.; Hou, Z.; Ren, Q.; Ji, S.; Sang, S.; Lu, S.; et al. B. Subtilis CNBG-PGPR-1 Induces Methionine to Regulate Ethylene Pathway and ROS Scavenging for Improving Salt Tolerance of Tomato. Plant J. 2024, 117, 193–211. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, S.; Ali, S.; Ahmad, R.; Ercisli, S.; Anjum, M.A. Foliar Application of Silicon Enhances Growth, Flower Yield, Quality and Postharvest Life of Tuberose (Polianthes tuberosa L.) under Saline Conditions by Improving Antioxidant Defense Mechanism. Silicon 2022, 14, 1511–1518. [Google Scholar] [CrossRef]
- Liu, C.; Cui, B.; Hu, C.; Wu, H.; Gao, F. Effects of Mixed Irrigation Using Brackish Water with Different Salinities and Reclaimed Water on a Soil-Crop System. Water Reuse 2021, 11, 632–648. [Google Scholar] [CrossRef]
| Treatment | SMC/% | |||
|---|---|---|---|---|
| Season 1 | Season 2 | |||
| 0–10 cm | 10–20 cm | 0–10 cm | 10–20 cm | |
| CK | 11.67 ± 1.72 ab | 10.99 ± 1.71 abc | 14.15 ± 1.42 ab | 12.94 ± 2.18 a |
| M4 | 12.20 ± 0.80 ab | 11.09 ± 1.09 abc | 14.49 ± 1.33 ab | 14.14 ± 1.70 a |
| M6 | 15.43 ± 3.58 a | 13.98 ± 2.67 ab | 13.09 ± 0.70 b | 12.25 ± 0.61 a |
| M8 | 11.96 ± 1.89 ab | 11.06 ± 1.88 abc | 13.70 ± 0.06 ab | 13.25 ± 1.42 a |
| SiM4 | 10.07 ± 0.18 b | 9.46 ± 0.42 c | 15.00 ± 0.69 a | 14.49 ± 0.52 a |
| SiM6 | 10.78 ± 0.79 b | 9.84 ± 0.85 bc | 14.50 ± 0.93 ab | 14.42 ± 1.01 a |
| SiM8 | 15.92 ± 4.45 a | 14.19 ± 4.37 a | 12.98 ± 0.31 b | 12.26 ± 0.94 a |
| ST-PGPR | ns | ns | ns | ns |
| FSFS | ns | ns | ns | ns |
| ST-PGPR × FSFS | * | * | ns | ns |
| Treatment | EC/(μS·cm−1) | |||
|---|---|---|---|---|
| Season 1 | Season 2 | |||
| 0–10 cm | 10–20 cm | 0–10 cm | 10–20 cm | |
| CK | 1462.00 ± 10.39 b | 10.99 ± 1.71 abc | 14.15 ± 1.42 ab | 12.94 ± 2.18 a |
| M4 | 1403.00 ± 24.25 c | 11.09 ± 1.09 abc | 14.49 ± 1.33 ab | 14.14 ± 1.70 a |
| M6 | 1058.00 ± 10.15 f | 13.98 ± 2.67 ab | 13.09 ± 0.70 b | 12.25 ± 0.61 a |
| M8 | 1338.00 ± 11.53 d | 11.06 ± 1.88 abc | 13.70 ± 0.06 ab | 13.25 ± 1.42 a |
| SiM4 | 1598.33 ± 6.51 a | 9.46 ± 0.42 c | 15.00 ± 0.69 a | 14.49 ± 0.52 a |
| SiM6 | 1570.00 ± 48.88 a | 9.84 ± 0.85 bc | 14.50 ± 0.93 ab | 14.42 ± 1.01 a |
| SiM8 | 1232.00 ± 20.42 e | 14.19 ± 4.37 a | 12.98 ± 0.31 b | 12.26 ± 0.94 a |
| ST-PGPR | ** | ** | ns | ns |
| FSFS | ** | ** | ns | ns |
| ST-PGPR × FSFS | ** | ** | ns | ns |
| Growing Season | Principal Component | Eigenvalue | Contribution Rate (%) | Cumulative Contribution Rate (%) |
|---|---|---|---|---|
| Season 1 | 1 | 3.260 | 36.227 | 36.227 |
| 2 | 2.243 | 24.925 | 61.152 | |
| 3 | 1.232 | 13.689 | 74.841 | |
| Season 2 | 1 | 4.904 | 54.490 | 54.490 |
| 2 | 1.183 | 13.142 | 67.632 | |
| 3 | 1.029 | 11.432 | 79.065 |
| Growing Season | Treatment | Comprehensive Scores | Ranking | Growing Season | Treatment | Comprehensive Scores | Ranking |
|---|---|---|---|---|---|---|---|
| Season 1 | CK | −1.473 | 7 | Season 2 | CK | −1.807 | 7 |
| M4 | −0.070 | 5 | M4 | 0.357 | 3 | ||
| M6 | −0.127 | 6 | M6 | 0.023 | 6 | ||
| M8 | 0.173 | 3 | M8 | 0.083 | 4 | ||
| SiM4 | 1.003 | 1 | SiM4 | 0.597 | 2 | ||
| SiM6 | 0.393 | 2 | SiM6 | 0.723 | 1 | ||
| SiM8 | 0.100 | 4 | SiM8 | 0.027 | 5 |
| Treatment | ST-PGPR Concentration | Silicon Fertilizer |
|---|---|---|
| CK | - | - |
| M4 | 104 cfu·mL−1 | - |
| M6 | 106 cfu·mL−1 | - |
| M8 | 108 cfu·mL−1 | - |
| SiM4 | 104 cfu·mL−1 | Sodium metasilicate pentahydrate |
| SiM6 | 106 cfu·mL−1 | Sodium metasilicate pentahydrate |
| SiM8 | 108 cfu·mL−1 | Sodium metasilicate pentahydrate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Han, Q.; Cui, B.; Wang, J.; Hu, C.; Li, R.; Lin, Y.; Xu, Y.; Liu, C. Synergistic Effects of Salt-Tolerant PGPR and Foliar Silicon on Pak Choi Antioxidant Defense Under Salt Stress. Plants 2025, 14, 2065. https://doi.org/10.3390/plants14132065
Zhao J, Han Q, Cui B, Wang J, Hu C, Li R, Lin Y, Xu Y, Liu C. Synergistic Effects of Salt-Tolerant PGPR and Foliar Silicon on Pak Choi Antioxidant Defense Under Salt Stress. Plants. 2025; 14(13):2065. https://doi.org/10.3390/plants14132065
Chicago/Turabian StyleZhao, Jieru, Qibiao Han, Bingjian Cui, Juan Wang, Chao Hu, Rui Li, Yanyu Lin, Ying Xu, and Chuncheng Liu. 2025. "Synergistic Effects of Salt-Tolerant PGPR and Foliar Silicon on Pak Choi Antioxidant Defense Under Salt Stress" Plants 14, no. 13: 2065. https://doi.org/10.3390/plants14132065
APA StyleZhao, J., Han, Q., Cui, B., Wang, J., Hu, C., Li, R., Lin, Y., Xu, Y., & Liu, C. (2025). Synergistic Effects of Salt-Tolerant PGPR and Foliar Silicon on Pak Choi Antioxidant Defense Under Salt Stress. Plants, 14(13), 2065. https://doi.org/10.3390/plants14132065






