Effect of Different Temperatures on Herbicide Efficacy for the Management of the Invasive Weed Solanum rostratum Dunal (Family: Solanaceae)
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Herbicide Application
4.3. Statistical Analyses
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bassett, I.J.; Munro, D.B. The Biology of Canadian Weeds.: 78. Solunum carolinense L. and Solanum rostratum Dunal. Can. J. Plant Sci. 1986, 66, 977–991. [Google Scholar] [CrossRef]
- Zhao, J.; Solís-Montero, L.; Lou, A.; Vallejo-Marín, M. Population Structure and Genetic Diversity of Native and Invasive Populations of Solanum rostratum (Solanaceae). PLoS ONE 2013, 8, e79807. [Google Scholar] [CrossRef]
- Rushing, D.W.; Murray, D.S.; Verhalen, L.M. Weed Interference with Cotton (Gossypium hirsutum). I. Buffalobur (Solanum rostratum). Weed Sci. 1985, 33, 810–814. [Google Scholar] [CrossRef]
- Lyon, D.J.; Kniss, A.; Miller, S.D. Carfentrazone Improves Broadleaf Weed Control in Proso and Foxtail Millets. Weed Technol. 2007, 21, 84–87. [Google Scholar] [CrossRef]
- Danin, A. Flora Of Israel Online. Available online: https://flora.org.il/en/en/ (accessed on 12 January 2024).
- Abu-Nassar, J.; Matzrafi, M. Effect of Herbicides on the Management of the Invasive Weed Solanum rostratum Dunal (Solanaceae). Plants 2021, 10, 284. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Swanton, C.J.; Hall, J.C.; Mersey, B.G. The Influence of Temperature and Relative Humidity on the Efficacy of Glufosinate-Ammonium. Weed Res. 1993, 33, 139–147. [Google Scholar] [CrossRef]
- Caseley, J.C. Variation in Foliar Pesticide Performance Attributable to Humidity, Dew and Rain Effects. Asp. Appl. Biol. 1989, 21, 1215–1225. [Google Scholar]
- Hammerton, J.L. Environmental Factors and Susceptibility to Herbicides. Weed Sci. 1967, 15, 330–336. [Google Scholar] [CrossRef]
- Robinson, M.A.; Letarte, J.; Cowbrough, M.J.; Sikkema, P.H.; Tardif, F.J. Winter Wheat (Triticum aestivum L.) Response to Herbicides as Affected by Application Timing and Temperature. Can. J. Plant Sci. 2015, 95, 325–333. [Google Scholar] [CrossRef]
- Kleinman, Z.; Rubin, B. Non-Target-Site Glyphosate Resistance in Conyza bonariensis Is Based on Modified Subcellular Distribution of the Herbicide. Pest Manag. Sci. 2016, 73, 246–253. [Google Scholar] [CrossRef]
- Matzrafi, M.; Brunharo, C.; Tehranchian, P.; Hanson, B.D.; Jasieniuk, M. Increased Temperatures and elevated CO2 Levels Reduce the Sensitivity of Conyza canadensis and Chenopodium album to glyphosate. Sci. Rep. 2019, 9, 2228. [Google Scholar] [CrossRef]
- Viger, P.R.; Eberlein, C.V.; Fuerst, E.P.; Gronwald, J.W. Effects of CGA-154281 and Temperature on Metolachlor Absorption and Metabolism, Glutathione Content, and Glutathione-s-Transferase Activity in Corn. Weed Sci. 1991, 39, 324–328. [Google Scholar] [CrossRef]
- Mahan, J.R.; Dotray, P.A.; Light, G.G.; Dawson, K.R. Thermal Dependence of Bioengineered Glufosinate Tolerance in Cotton. Weed Sci. 2006, 54, 1–5. [Google Scholar] [CrossRef]
- Godar, A.S.; Varanasi, V.K.; Nakka, S.; Prasad, P.V.V.; Thompson, C.R. Physiological and Molecular Mechanisms of Differential Sensitivity of Palmer Amaranth (Amaranthus palmeri) to Mesotrione at Varying Growth Temperatures. PLoS ONE 2015, 10, e0126731. [Google Scholar] [CrossRef] [PubMed]
- Matzrafi, M.; Seiwert, B.; Reemtsma, T.; Rubin, B.; Peleg, Z. Climate Change Increases the Risk of Herbicide-Resistant Weeds Due to Enhanced Detoxification. Planta 2016, 244, 1217–1227. [Google Scholar] [CrossRef]
- Gafni, R.; Nassar, J.A.; Matzrafi, M.; Blank, L.; Eizenberg, H. Unraveling the Reasons for Failure to Control Amaranthus albus: Insights into Herbicide Application at Different Growth Stages, Temperature Effect, and Herbicide Resistance on a Regional Scale. Pest Manag. Sci. 2024, 80, 4757–4769. [Google Scholar] [CrossRef] [PubMed]
- Lasat, M.M.; DiTomaso, J.M.; Hart, J.J.; Kochian, L.V. Resistance to Paraquat in Hordeum glaucum Is Temperature Dependent and Not Associated with Enhanced Apoplasmic Binding. Weed Res. 1996, 36, 303–309. [Google Scholar] [CrossRef]
- Yu, Q.; Han, H.; Nguyen, L.; Forster, J.W.; Powles, S.B. Paraquat Resistance in a Lolium rigidum Population Is Governed by One Major Nuclear Gene. Theor. Appl. Genet. 2009, 118, 1601–1608. [Google Scholar] [CrossRef]
- Abu-Nassar, J.; Gal, S.; Shtein, I.; Distelfeld, A.; Matzrafi, M. Functional Leaf Anatomy of the Invasive Weed Solanum rostratum Dunal. Weed Res. 2022, 62, 172–180. [Google Scholar] [CrossRef]
- Matzrafi, M. Climate Change Exacerbates Pest Damage through Reduced Pesticide Efficacy. Pest Manag. Sci. 2019, 75, 9–13. [Google Scholar] [CrossRef]
- Trivedi, P.; Klavins, L.; Hykkerud, A.L.; Kviesis, J.; Elferts, D.; Martinussen, I.; Klavins, M.; Karppinen, K.; Häggman, H. Temperature Has a Major Effect on the Cuticular Wax Composition of Bilberry (Vaccinium myrtillus L.) Fruit. Front. Plant Sci. 2022, 13, 980427. [Google Scholar] [CrossRef]
- Sim, R.; Miranda, I.; Pereira, H. Effect of Seasonal Variation on Leaf Cuticular Waxes ’ Composition in the Mediterranean Cork Oak (Quercus suber L.). Forests 2022, 13, 1236. [Google Scholar] [CrossRef]
- Phatak, S.C.; Stephenson, G.R. Influence of Light and Temperature on Metribuzin Phytotoxicity To Tomato. Can. J. Plant Sci. 1973, 53, 843–847. [Google Scholar] [CrossRef][Green Version]
- Fortino, J.; Splittstoesser, W.E. Response of Tomato to Metribuzin. Weed Sci. 1974, 22, 460–463. [Google Scholar] [CrossRef]
- Alizade, S.; Keshtkar, E.; Mokhtasi-Bidgoli, A.; Sasanfar, H.R.; Streibig, J.C. Effect of Water Deficit Stress on Benzoylprop-Ethyl Performance and Physiological Traits of Winter Wild Oat (Avena sterilis subsp. ludoviciana). Crop Prot. 2020, 137, 105292. [Google Scholar] [CrossRef]
- Xie, H.S.; Hsiao, A.I.; Quick, W.A. Influence of Water Deficit on the Phytotoxicity of Imazamethabenz and Fenoxaprop among Five Wild Oat Populations. Environ. Exp. Bot. 1993, 33, 283–291. [Google Scholar] [CrossRef]
- Bossdorf, O.; Schröder, S.; Prati, D.; Auge, H. Palatability and Tolerance to Simulated Herbivory in Native and Introduced Populations of Alliaria petiolata (Brassicaceae). Am. J. Bot. 2004, 91, 856–862. [Google Scholar] [CrossRef]
- Sakai, A.K.; Allendorf, F.W.; Holt, J.S.; Lodge, M.; Molofsky, J.; With, K.A.; Cabin, R.J.; Cohen, J.E.; Norman, C.; Mccauley, D.E.; et al. The population biology of invasive species. Annu. Rev. Ecol. Syst. 2001, 32, 305–332. [Google Scholar] [CrossRef]
- Pazzaglia, J.; Reusch, T.B.H.; Terlizzi, A.; Marín-Guirao, L.; Procaccini, G. Phenotypic Plasticity under Rapid Global Changes: The Intrinsic Force for Future Seagrasses Survival. Evol. Appl. 2021, 14, 1181–1201. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.L.; Bossdorf, O.; Muth, N.Z.; Gurevitch, J.; Pigliucci, M. Jack of All Trades, Master of Some? On the Role of Phenotypic Plasticity in Plant Invasions. Ecol. Lett. 2006, 9, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S.M. The Genetics of Phenotypic Plasticity. VII. Evolution in a Spatially-Structured Environment. J. Evol. Biol. 1998, 11, 303–320. [Google Scholar] [CrossRef]
- Edelaar, P.; Jovani, R.; Gomez-Mestre, I. Should I Change or Should I Go? Phenotypic Plasticity and Matching Habitat Choice in the Adaptation to Environmental Heterogeneity. Am. Nat. 2017, 190, 506–520. [Google Scholar] [CrossRef]
- Ziska, L.H.; Blumenthal, D.M.; Franks, S.J. Understanding the Nexus of Rising CO2, Climate Change, and Evolution in Weed Biology. Invasive Plant Sci. Manag. 2019, 12, 79–88. [Google Scholar] [CrossRef]
- R Core Team. R Core Team at R Foundation for Statistical Computing, R: A Language and Environment for Statistical Computing. 2020. Available online: https://www.R-project.org (accessed on 12 January 2024).
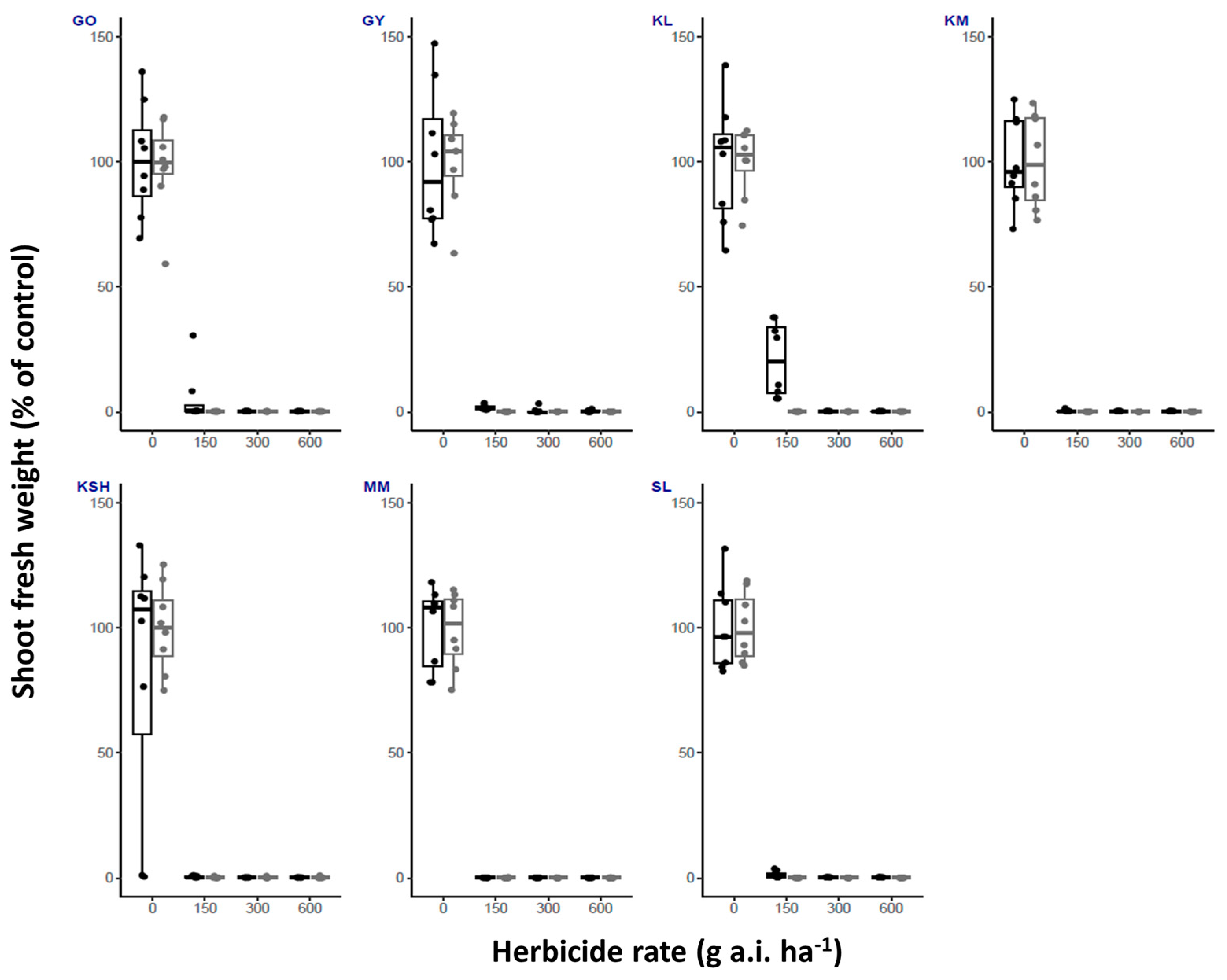
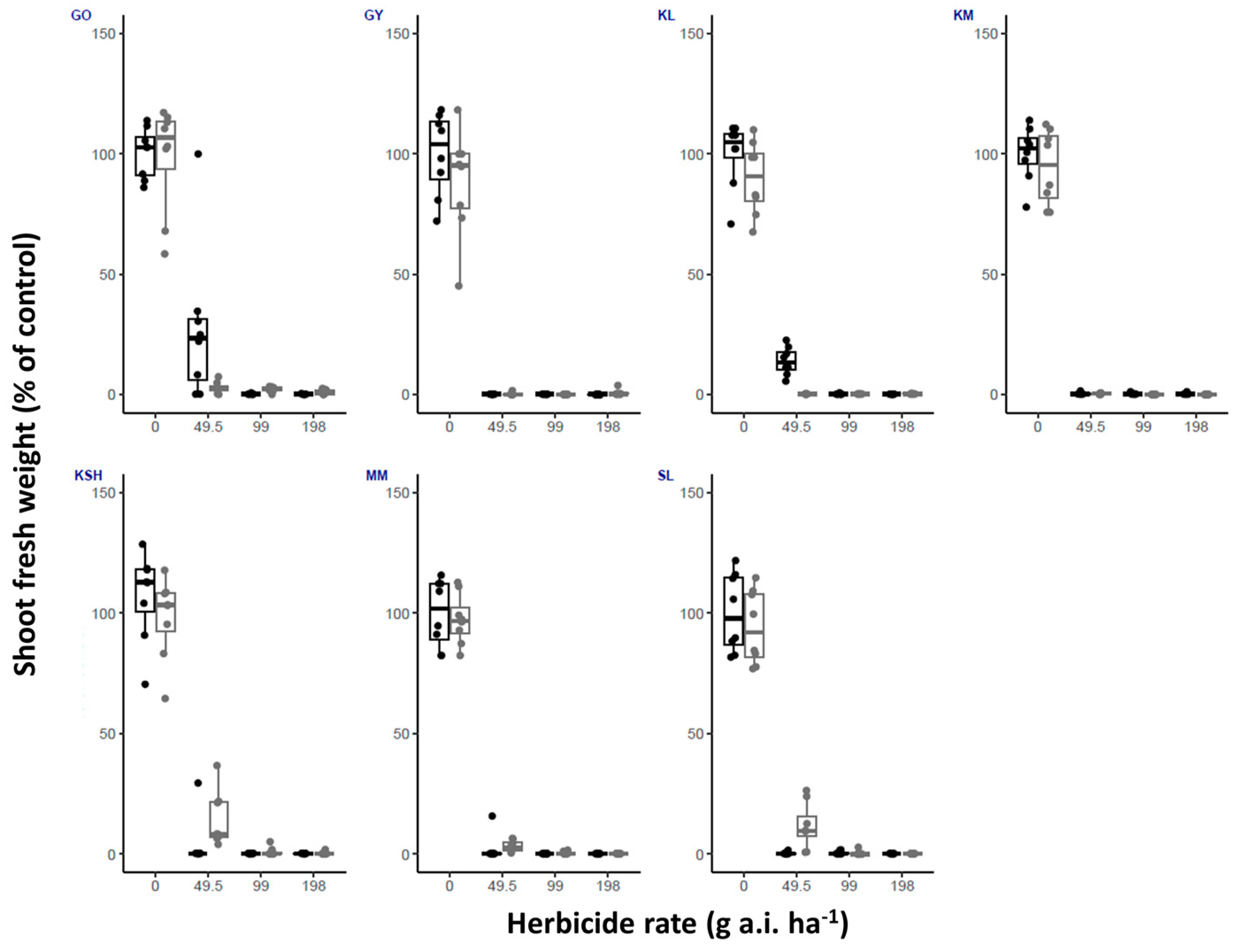
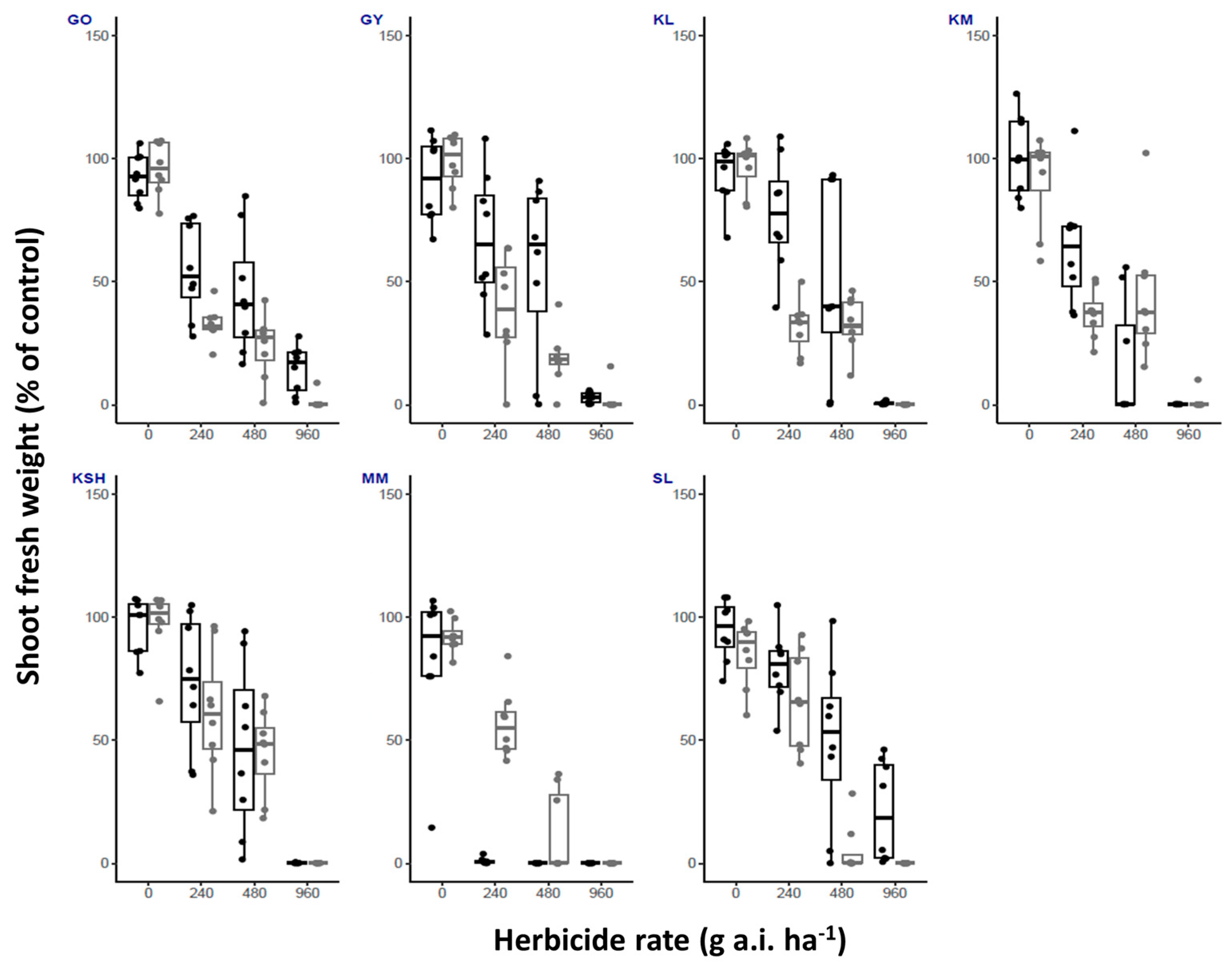
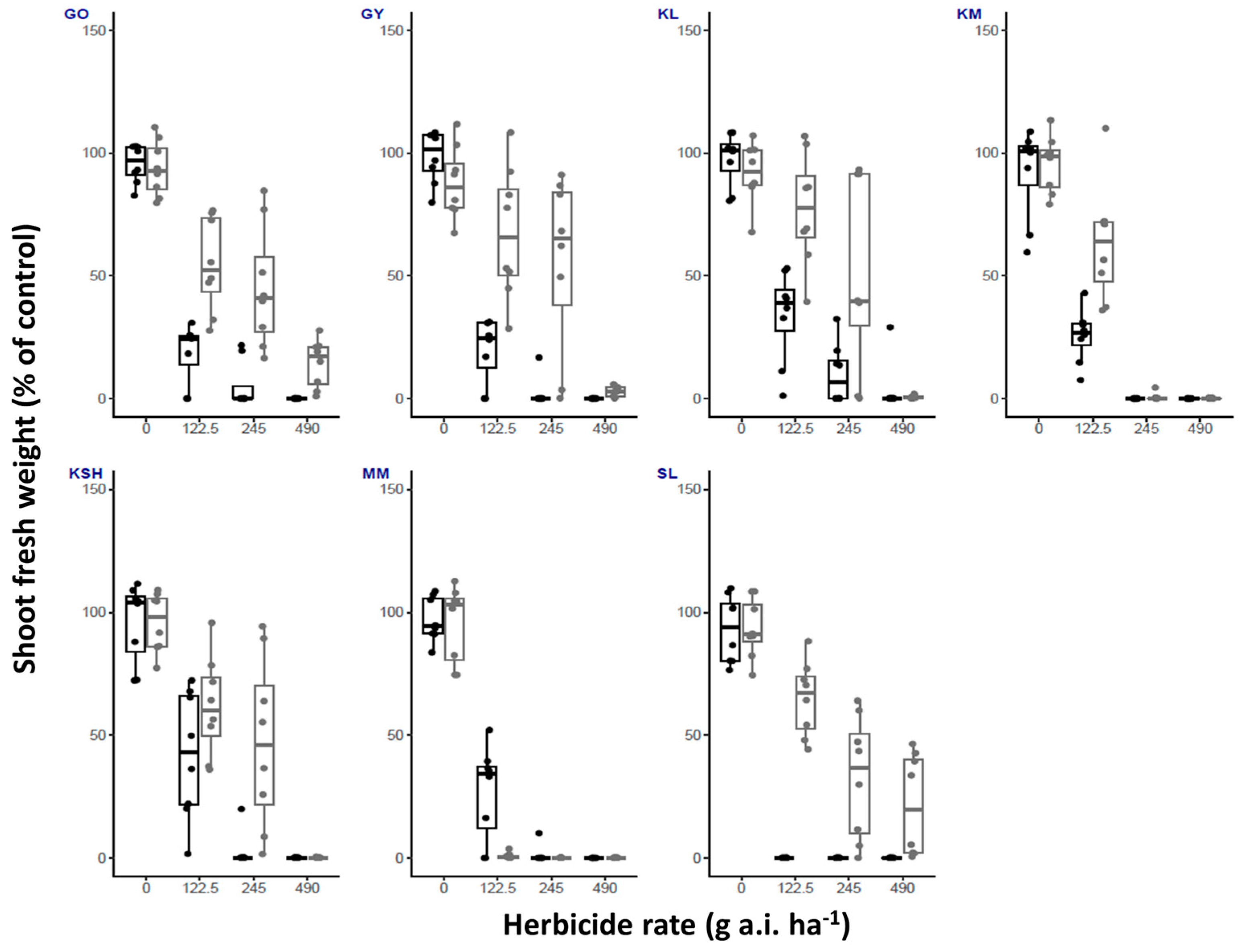
| Herbicide/ Temperature | Fluroxypyr | Metribuzin | Oxyfluorfen | Tembotrione | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pop ID | Rate (g a.i. ha−1) | 0 | 150 | 300 | 600 | 0 | 122.5 | 245 | 490 | 0 | 240 | 480 | 960 | 0 | 49.5 | 99 | 198 |
| GO | High | 100 | 25 | 0 | 0 | 100 | 37.5 | 25 | 0 | 100 | 100 | 100 | 87.5 | 100 | 12.5 | 0 | 0 |
| Low | 100 | 0 | 0 | 0 | 100 | 100 | 100 | 62.5 | 100 | 100 | 87.5 | 12.5 | 100 | 25 | 0 | 0 | |
| GY | High | 100 | 0 | 0 | 0 | 100 | 75 | 12.5 | 0 | 100 | 100 | 75 | 25 | 100 | 0 | 0 | 0 |
| Low | 100 | 0 | 0 | 0 | 100 | 100 | 25 | 12.5 | 100 | 87.5 | 75 | 12.5 | 100 | 0 | 0 | 0 | |
| KL | High | 100 | 0 | 0 | 0 | 100 | 100 | 50 | 0 | 100 | 100 | 50 | 0 | 100 | 50 | 0 | 0 |
| Low | 100 | 0 | 0 | 0 | 100 | 100 | 87.5 | 0 | 100 | 100 | 100 | 0 | 100 | 0 | 0 | 0 | |
| KM | High | 100 | 0 | 0 | 0 | 100 | 100 | 0 | 0 | 100 | 100 | 50 | 0 | 100 | 0 | 0 | 0 |
| Low | 100 | 0 | 0 | 0 | 100 | 100 | 0 | 0 | 100 | 100 | 100 | 12.5 | 100 | 0 | 0 | 0 | |
| KSH | High | 100 | 0 | 0 | 0 | 100 | 100 | 12.5 | 0 | 100 | 100 | 75 | 0 | 100 | 0 | 0 | 0 |
| Low | 100 | 0 | 0 | 0 | 100 | 100 | 75 | 0 | 100 | 100 | 100 | 0 | 100 | 25 | 0 | 0 | |
| MM | High | 100 | 0 | 0 | 0 | 100 | 25 | 0 | 0 | 100 | 12.5 | 0 | 0 | 100 | 0 | 0 | 0 |
| Low | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 100 | 50 | 0 | 100 | 0 | 0 | 0 | |
| SL | High | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 100 | 87.5 | 50 | 100 | 0 | 0 | 0 |
| Low | 100 | 0 | 0 | 0 | 100 | 100 | 62.5 | 50 | 100 | 100 | 25 | 0 | 100 | 50 | 0 | 0 | |
| Pop ID | Herbicide/ Temperature | Fluroxypyr | Metribuzin | Oxyfluorfen | Tembotrione | ||||
|---|---|---|---|---|---|---|---|---|---|
| Rate (g a.i. ha−1) | 0 | 300 | 0 | 245 | 0 | 480 | 0 | 99 | |
| GO | High | 100.69 ± 22.70 a | 0.28 ± 0.00 b | 95.61 ± 7.70 a | 5.23 ± 9.53 c,d | 92.62 ± 9.54 a | 45.29 ± 24.82 b,c | 100.42 ± 10.42 a | 0.35 ± 0.20 c |
| Low | 98.34 ± 18.49 a | 0.19 ± 0.00 b | 93.80 ± 11.29 a | 45.22 ± 24.79 b | 96.13 ± 10.67 a | 23.89 ± 12.93 d,e | 98.48 ± 17.11 a | 2.22 ± 0.00 c | |
| GY | High | 99.92 ± 29.46 a | 0.52 ± 1.16 b | 98.50 ± 10.67 a | 2.18 ± 5.89 c,d | 91.05 ± 9.54 a | 55.50 ± 24.82 b,c | 100.00 ± 13.88 a | 0.327 ± 0.20 c |
| Low | 99.91 ± 17.96 a | 0.11 ± 0.00 b | 87.84 ± 14.81 a | 55.62 ± 35.88 b | 96.13 ± 10.67 a | 18.78 ± 12.93 d | 88.27 ± 11.50 a | 0.45 ± 0.34 c | |
| KL | High | 100.03 ± 24.13 a | 0.26 ± 0.02 c | 97.43 ± 10.84 a | 10.05 ± 12.04 d | 93.81 ± 17.10 a,b | 49.60 ± 35.81 c | 96.39 ± 11.47 a | 0.35 ± 34.80 d |
| Low | 100.00 ± 13.63 a | 0.16 ± 0.01 c | 91.89 ± 12.39 a,b | 49.52 ± 38.76 c | 96.81 ± 10.97 a | 31.80 ± 11.30 c | 89.95 ± 13.91 b | 0.35 ± 0.01 d | |
| KM | High | 100.00 ± 17.77 a | 0.30 ± 0.00 b | 92.18 ± 18.50 a | 0.07 ± 0.01 d | 101.12 ± 12.70 a | 16.86 ± 38.82 d | 100.08 ± 16.27 a | 0.44 ± 0.70 b |
| Low | 100.04 ± 18.61 a | 0.09 ± 0.01 c | 95.61 ± 11.56 a | 0.76 ± 1.54 d | 91.56 ± 10.26 a | 37.07 ± 11.10 c | 94.41 ± 22.52 a | 0.15 ± 1.08 b | |
| KSH | High | 99.89 ± 73.50 a | 0.27 ± 0.03 b | 96.39 ± 11.47 a | 2.68 ± 34.80 d | 96.39 ± 16.74 a | 46.99± 4.52 c | 107.04 ± 22.15 a | 0.28 ± 0.00 c |
| Low | 100.13 ± 17.61 a | 0.25 ± 0.24 b | 96.39 ± 11.47 a | 46.99 ± 34.80 b,c | 97.67 ± 13.63 a | 45.14 ± 17.54 d | 98.7 ± 15.16 a | 1.00 ± 0.00 c | |
| MM | High | 100.17 ± 16.34 a | 0.16 ± 0.01 b | 97.01 ± 8.98 a | 1.35 ± 3.57 c | 83.02 ± 30.37 a | 0.16 ± 0.01 c | 100.41 ± 15.40 a | 0.16 ± 0.00 b |
| Low | 99.26 ± 15.04 a | 0.10 ± 0.06 b | 95.40 ± 15.56 a | 0.16 ± 0.00 c | 92.28 ± 13.63 a | 12.06 ± 17.54 c | 97.07 ± 16.94 a | 0.47 ± 1.78 b | |
| SL | High | 100.30 ± 17.13 a | 0.34 ± 0.02 b | 93.19 ± 13.63 a | 0.13 ± 0.01 d | 94.81 ± 12.54 a | 49.42 ± 33.71 c | 97.46 ± 10.51 a | 0.475 ± 0.59 b |
| Low | 100.30 ± 17.13 a | 0.34 ± 0.02 b | 93.45 ± 12.11 a | 32.75 ± 24.92 c | 85.12 ± 13.43 a | 5.19 ± 10.25 d,e | 94.22 ± 15.32 a | 0.36 ± 1.00 c | |
| Pop ID | GPS Coordinates | *Specific Crop/Habitat |
|---|---|---|
| Kibbutz Ginegar (GO) | 32.6543271802, 35.2488696828 | watermelon |
| Moshav Givat Yoav (GY) | 32.8012266548, 35.6977272346 | watermelon |
| Kibbutz Lavi (KL) | 32.7874152565, 35.4297385362 | maize |
| Kfar Masaryk (KM) | 32.8851355473, 35.109928859 | tomato |
| Kibbutz Sha’alabim (KSH) | 31.8722352816, 34.9683375942 | corn |
| Kibbutz Ma’agan Michael (MM) | 32.5472419936, 34.9184483341 | Fallow |
| Kfar Sold (SL) | 33.194514, 35.642185 | Fallow |
| Common Name | Trade Name | MOA a | Manufacturer | Rate (g a.i. ha−1) |
|---|---|---|---|---|
| Fluroxypyr | Tomahawk ® | PPO inhibitors | ADAMA-Agan | 300 |
| Metribuzin | Sencor® | PSII inhibitor | Bayer | 245 |
| Oxyfluorfen | Galigan® | PPO inhibitors | ADAMA-Agan | 480 |
| Tembotrione | Laudis® | HPPD | Bayer | 99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Nassar, J.; Matzrafi, M. Effect of Different Temperatures on Herbicide Efficacy for the Management of the Invasive Weed Solanum rostratum Dunal (Family: Solanaceae). Plants 2025, 14, 574. https://doi.org/10.3390/plants14040574
Abu-Nassar J, Matzrafi M. Effect of Different Temperatures on Herbicide Efficacy for the Management of the Invasive Weed Solanum rostratum Dunal (Family: Solanaceae). Plants. 2025; 14(4):574. https://doi.org/10.3390/plants14040574
Chicago/Turabian StyleAbu-Nassar, Jackline, and Maor Matzrafi. 2025. "Effect of Different Temperatures on Herbicide Efficacy for the Management of the Invasive Weed Solanum rostratum Dunal (Family: Solanaceae)" Plants 14, no. 4: 574. https://doi.org/10.3390/plants14040574
APA StyleAbu-Nassar, J., & Matzrafi, M. (2025). Effect of Different Temperatures on Herbicide Efficacy for the Management of the Invasive Weed Solanum rostratum Dunal (Family: Solanaceae). Plants, 14(4), 574. https://doi.org/10.3390/plants14040574






