Physiological, Productive, and Soil Rhizospheric Microbiota Responses of ‘Santina’ Cherry Trees to Regulated Deficit Irrigation Applied After Harvest
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
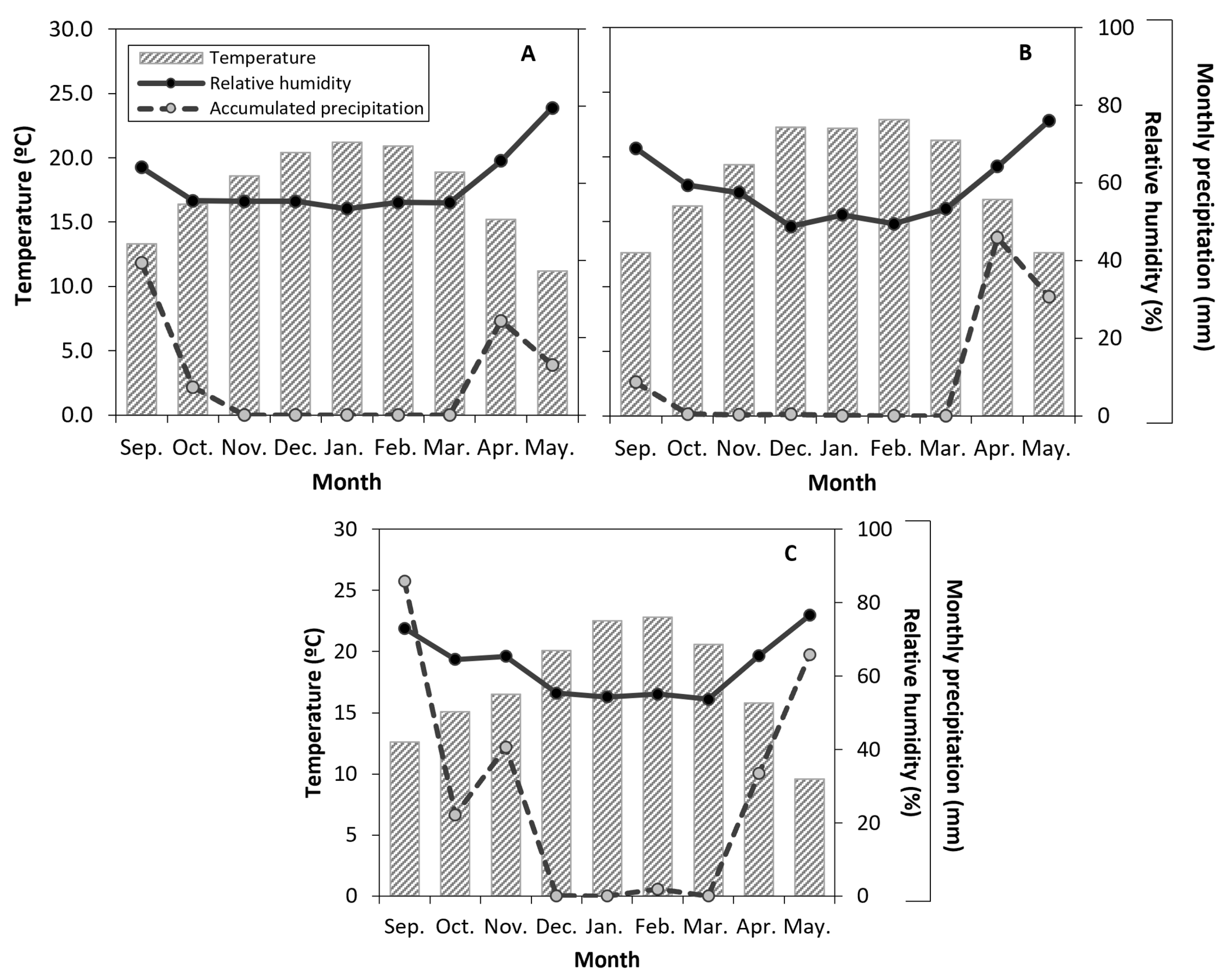
2.3. Environmental Conditions
2.4. Plant Water Status, Physiology, and Growth
2.5. Water Stress Integral
2.6. Intrinsic Water Use Efficiency
2.7. Yield Components and Fruit Quality
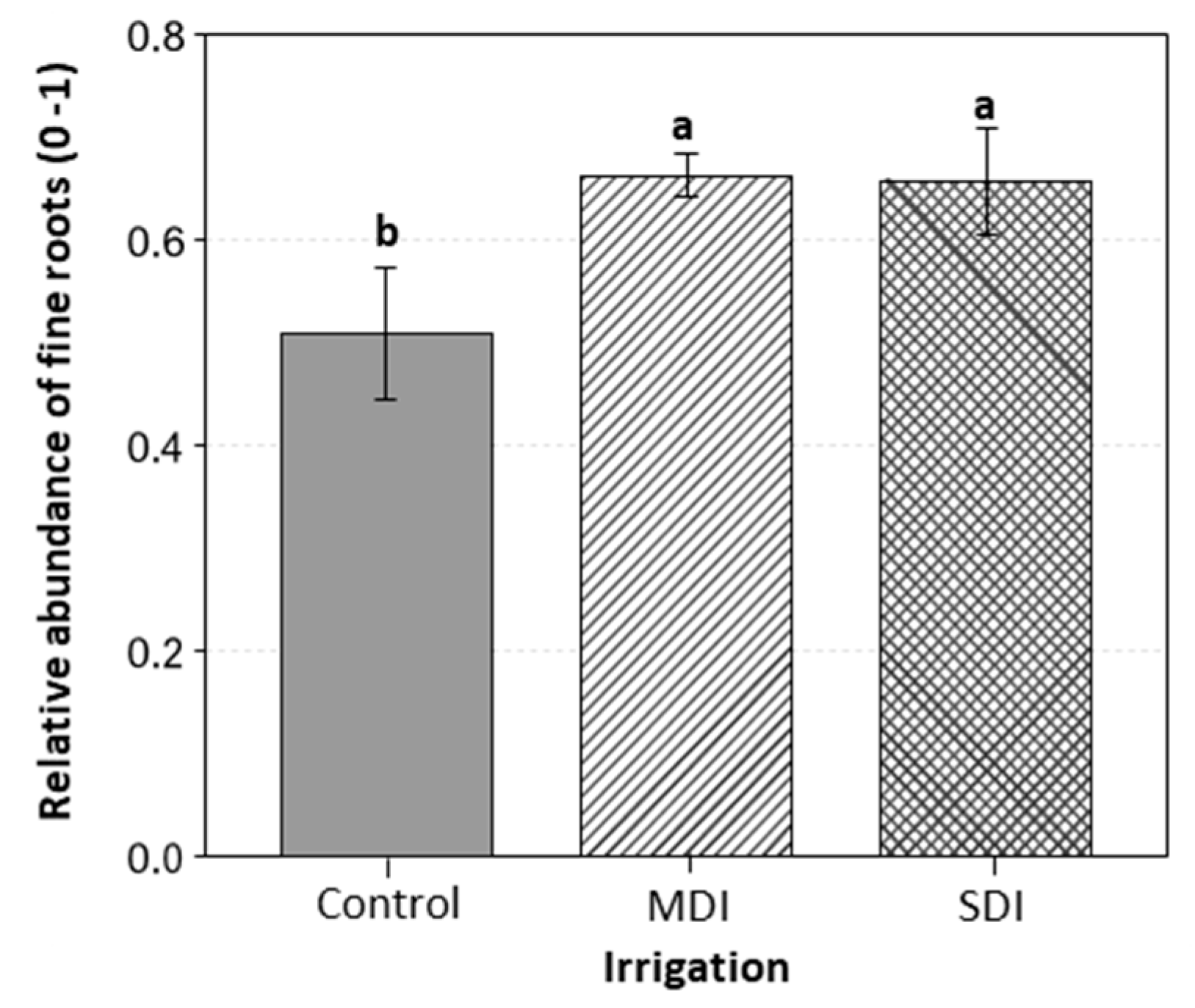
2.8. Root System Characterization
2.9. Evaluations of the Cultivable Soil Microbiota
2.10. Statistical Analysis
3. Results
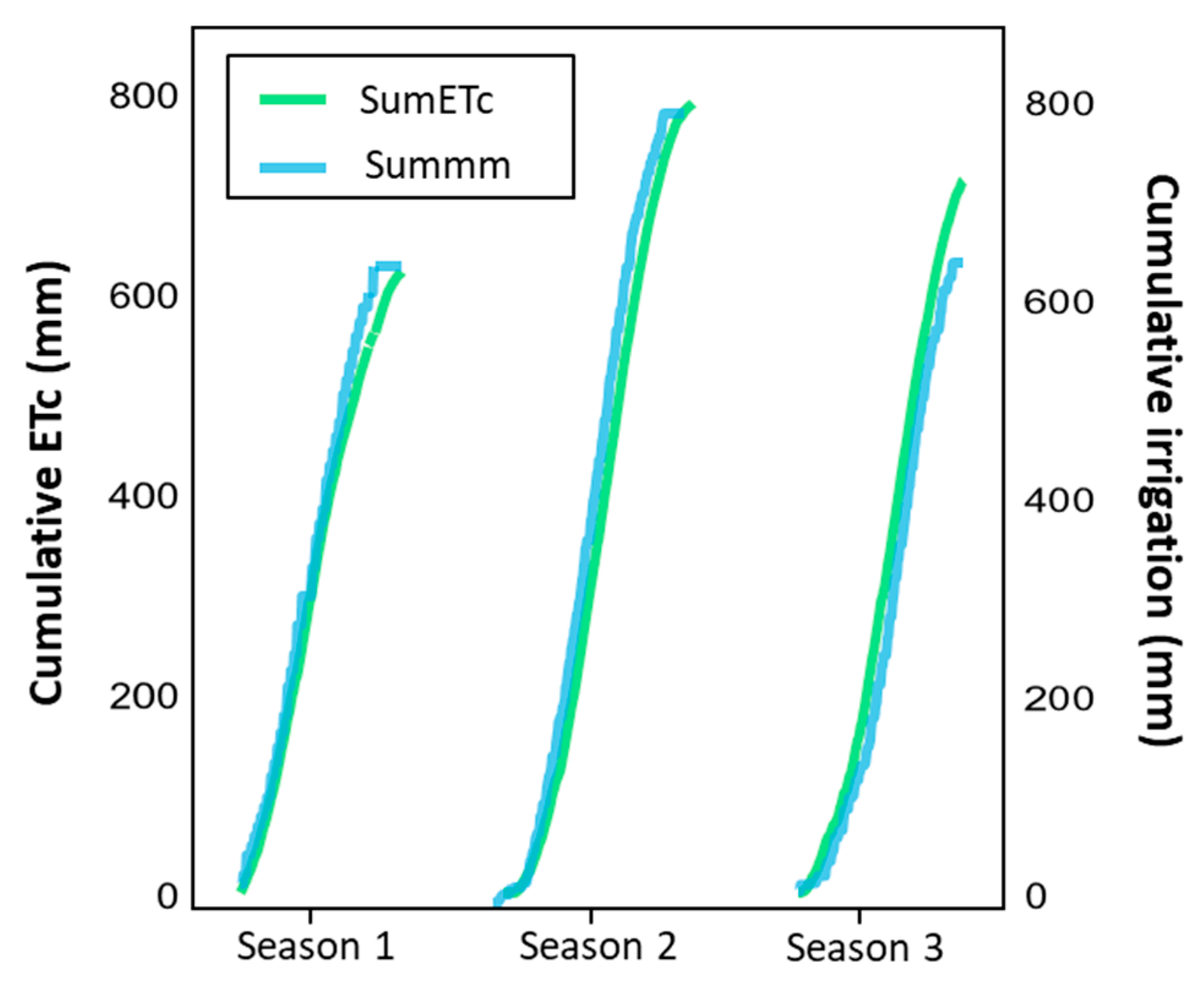
3.1. Environmental Conditions and Characterization of Irrigation
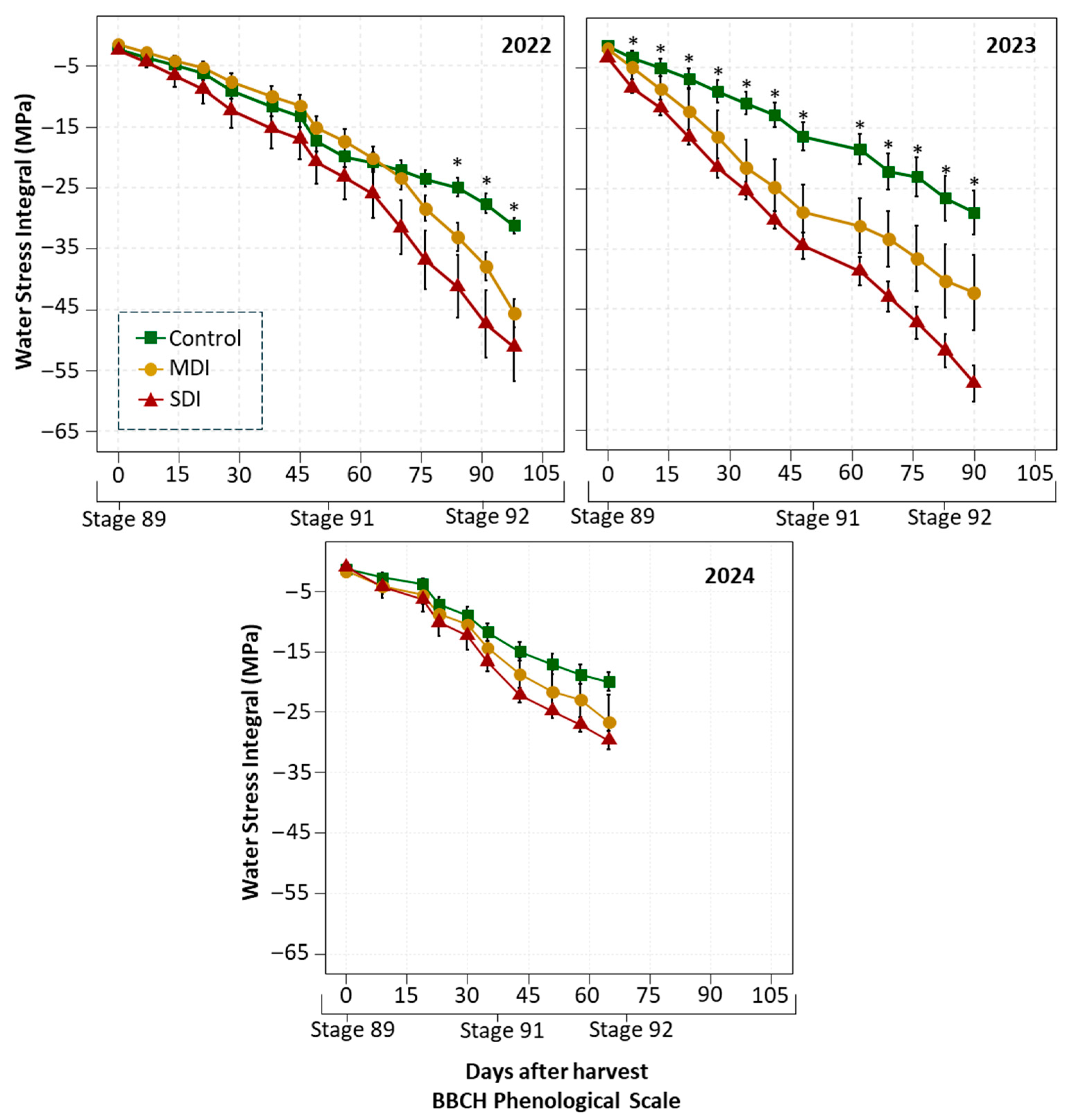
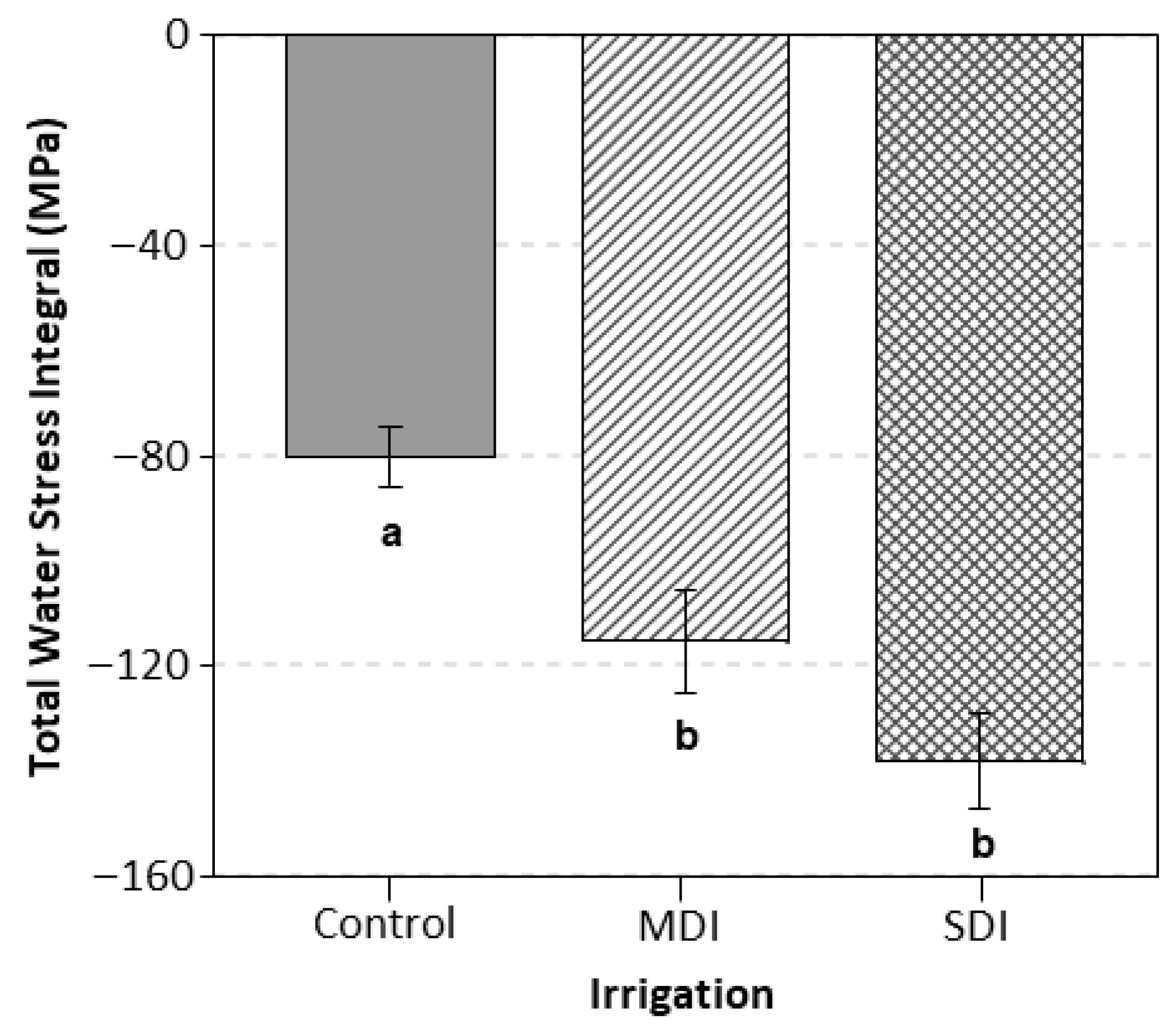
3.2. Water Stress Integral
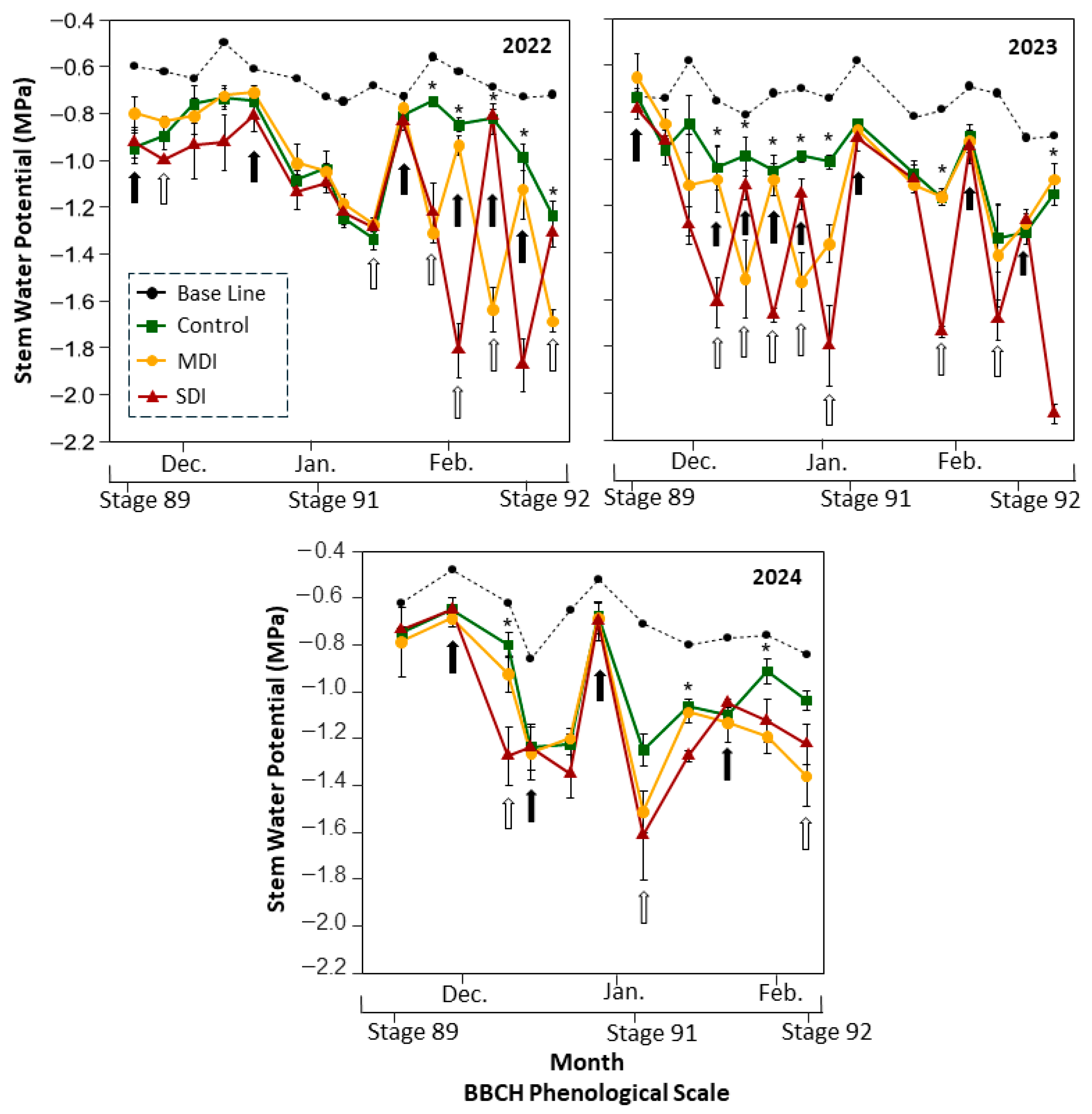
3.3. Physiological Responses
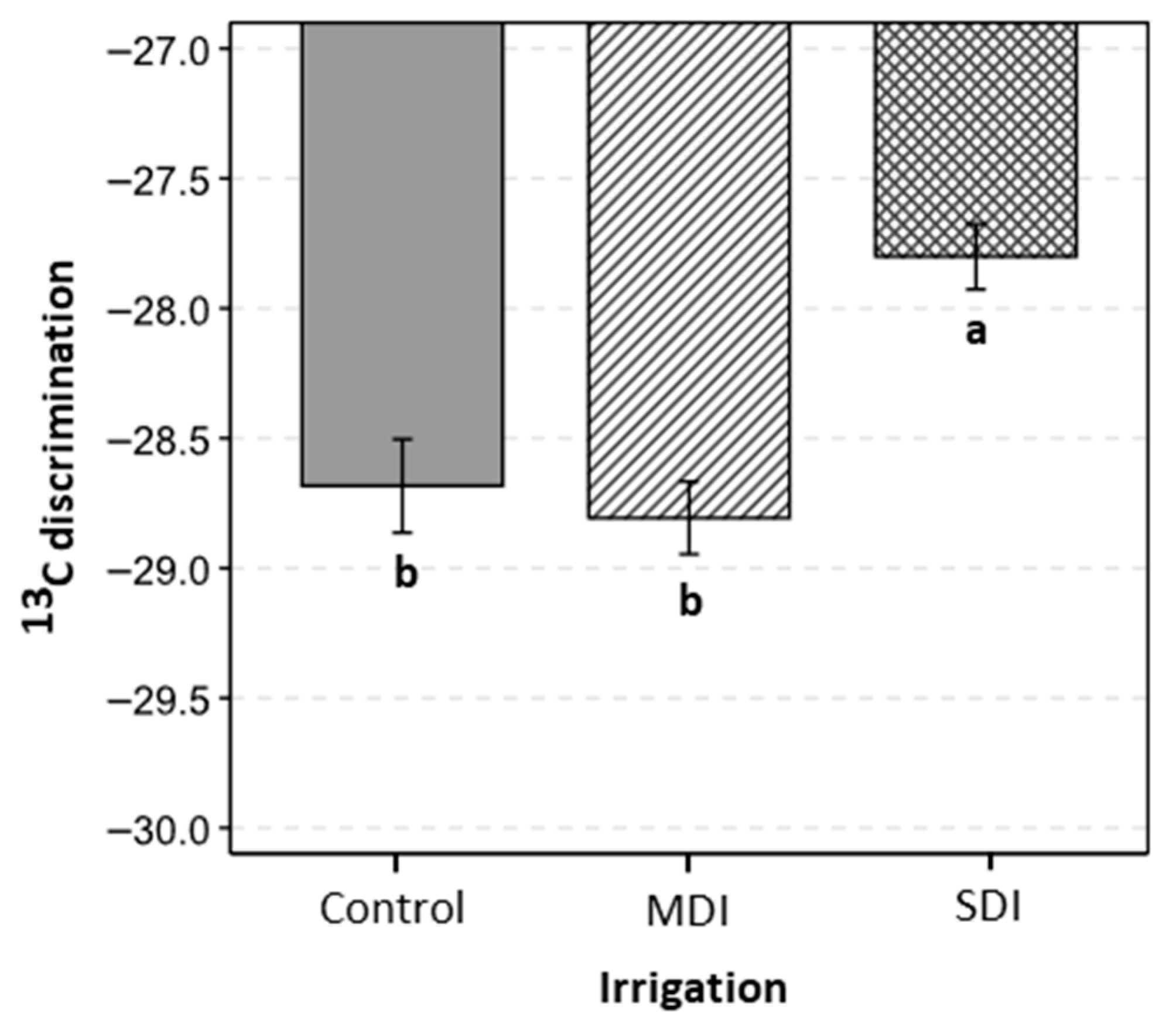
3.4. Intrinsic Water Use
3.5. Reproductive and Vegetative Growth Responses
3.6. Cultivable Microbiota Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oficina de Estudios y Políticas Agrarias (ODEPA). Catastro Frutícola: Principales Resultados Región de Ñuble; Ministerio de Agricultura: Madrid, Spain, 2024. Available online: https://bibliotecadigital.odepa.gob.cl/handle/20.500.12650/73934 (accessed on 12 September 2025).
- Oficina de Estudios y Políticas Agrarias (ODEPA). Boletín de la Fruta; Ministerio de Agricultura: Madrid, Spain, 2025. Available online: https://apps.odepa.gob.cl/powerBI/boletin_fruta.html (accessed on 12 September 2025).
- Ministerio del Medio Ambiente (MMA). Plan de Acción Regional de Cambio Climático. Región del Libertador General Bernardo O’Higgins (PARCC O’Higgins); Ministerio del Medio Ambiente: Santiago, Chile, 2024.
- Bambach, N.E.; Rhoades, A.M.; Hatchett, B.J.; Jones, A.D.; Ullrich, P.A.; Zarzycki, C.M. Projecting climate change in South America using variable-resolution Community Earth System Model: An application to Chile. Int. J. Climatol. 2021, 42, 2514–2542. [Google Scholar] [CrossRef]
- Departamento Meteorológico de Chile. Reporte Evolución Clima 2024; Departamento Meteorológico de Chile: Santiago, Chile, 2025. Available online: https://climatologia.meteochile.gob.cl/application/publicaciones/documentoPdf/reporteEvolucionClima/reporteEvolucionClima2024.pdf (accessed on 5 May 2025).
- Anjum, S.A.; Ashraf, U.; Zohaib, A.; Tanveer, M.; Naeem, M.; Ali, I.; Tabassum, T.; Nazir, U. Growth and development responses of crop plants under drought stress: A review. Zemdirbyste-Agriculture 2017, 104, 267–276. [Google Scholar] [CrossRef]
- Mitchell, P.D.; Chalmers, D.J. The effect of reduced water supply on peach tree growth and yields. J. Am. Soc. Hortic. Sci. 1982, 107, 853–856. [Google Scholar] [CrossRef]
- Calderón-Orellana, A. Challenges associated with a successful management of regulated deficit irrigation in commercial fresh-fruit production. Agric. Res. Technol. Open Access J. 2020, 24, 556268. [Google Scholar] [CrossRef]
- Girona, J.; Marsal, J.; Arbones, A.; DeJong, T.M. A comparison of the combined effect of water stress and crop load on fruit growth during different phenological stages in young peach trees. J. Hortic. Sci. Biotechnol. 2004, 79, 308–315. [Google Scholar] [CrossRef]
- Hajlaoui, H.; Maatallah, S.; Guizani, M.; Boughattas, N.E.; Guesmi, A.; Ennajeh, M.; Dabbou, S.; Lopez-Lauri, F. Effect of regulated deficit irrigation on agronomic parameters of three plum cultivars (Prunus salicina L.) under semi-arid climate conditions. Plants 2022, 11, 1545. [Google Scholar] [CrossRef]
- Calderón-Orellana, A.; Plaza-Rojas, G.; Gerding, M.; Huepe, G.; Kuschel-Otárola, M.; Bastías, R.M.; Calderón-Orellana, M. Productive, physiological, and soil microbiological responses to severe water stress during fruit maturity in a super high-density European plum orchard. Plants 2025, 14, 1222. [Google Scholar] [CrossRef]
- Leib, B.G.; Caspari, H.W.; Redulla, C.A.; Andrews, P.K.; Jabro, J.J. Partial rootzone drying and deficit irrigation of ‘Fuji’ apples in a semi-arid climate. Irrig. Sci. 2006, 24, 85–99. [Google Scholar] [CrossRef]
- Blanco, V.; Torres-Sánchez, R.; Blaya-Ros, P.J.; Pérez-Pastor, A.; Domingo, R. Vegetative and reproductive response of ‘Prime Giant’ sweet cherry trees to regulated deficit irrigation. Sci. Hortic. 2019, 249, 478–489. [Google Scholar] [CrossRef]
- De la Rosa, J.M.; Conesa, M.R.; Domingo, R.; Torres, R.; Pérez-Pastor, A. Feasibility of using trunk diameter fluctuation and stem water potential reference lines for irrigation scheduling of early nectarine trees. Agric. Water Manag. 2013, 126, 133–141. [Google Scholar] [CrossRef]
- Marsal, J. FAO Irrigation and Drainage Paper 66. Crop Yield Response to Water. Sweet Cherry; FAO: Rome, Italy, 2012; pp. 449–457. [Google Scholar]
- Marsal, J.; López, G.; Arbones, A.; Mata, M.; Vallverdú, X.; Girona, J. Influence of postharvest deficit irrigation and pre-harvest fruit thinning on sweet cherry (cv. New Star) fruit firmness and quality. J. Hortic. Sci. Biotechnol. 2009, 84, 273–278. [Google Scholar] [CrossRef]
- Blaya-Ros, P.J.; Blanco, V.; Torres-Sánchez, R.; Domingo, R. Drought-adaptive mechanisms of young sweet cherry trees in response to withholding and resuming irrigation cycles. Agronomy 2021, 11, 1812. [Google Scholar] [CrossRef]
- Kalefetoğlu, T.; Ekmekci, Y. The effects of drought on plants and tolerance mechanisms. Gazi Univ. J. Sci. 2005, 18, 723–740. [Google Scholar]
- AGV. Anuario Viveros 2014; Asociación de Viveros de Chile: Santiago, Chile, 2015; Available online: https://www.viverosdechile.cl/media/uploads/AGV_Anuario_Viveros_2014.pdf (accessed on 20 May 2025).
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Ilyas, M.; Nisar, M.; Khan, N.; Hazrat, A.; Khan, A.H.; Hayat, K.; Ullah, A. Drought tolerance strategies in plants: A mechanistic approach. J. Plant Growth Regul. 2021, 40, 926–944. [Google Scholar] [CrossRef]
- Gregory, P.; Atkinson, C.; Glyn, A.; Else, M.; Fernandez-Fernandez, F.; Harrison, R.; Schmidt, S. Contributions of roots and rootstocks to sustainable, intensified crop production. J. Exp. Bot. 2013, 64, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C.; González, F.; Santander, C.; Pérez, R.; Gallardo, V.; Santos, C.; Aponte, H.; Ruiz, A.; Cornejo, P. Management of Rhizosphere Microbiota and Plant Production under Drought Stress: A Comprehensive Review. Plants 2022, 11, 2437. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Stress control and ACC deaminase. In Principles of Plant-Microbe Interactions: Microbes for Sustainable Agriculture; Springer International Publishing: Cham, Switzerland, 2014; pp. 257–264. [Google Scholar] [CrossRef]
- Ullah, A. Zinc Nutrition and Microbial Allelopathy for Improving the Productivity, Grain Biofortification and Tolerance Against Abiotic Stresses in Chickpea. Ph.D. Thesis, University of Agriculture, Faisalabad, Pakistan, 2019. [Google Scholar]
- Finkel, O.M.; Castrillo, G.; Paredes, S.H.; González, I.S.; Dangl, J.L. Understanding and exploiting plant beneficial microbes. Curr. Opin. Plant Biol. 2017, 38, 155–163. [Google Scholar] [CrossRef]
- CIREN. Recursos Naturales Comuna de Placilla. 2022. Available online: https://www.sitrural.cl/wp-content/uploads/2022/06/Placilla_rec_nat.pdf (accessed on 5 May 2025).
- Blanco, V.; Domingo, R.; Pérez-Pastor, A.; Blaya-Ros, P.J.; Torres-Sánchez, R. Soil and plant water indicators for deficit irrigation management of field-grown sweet cherry trees. Agric. Water Manag. 2018, 208, 83–94. [Google Scholar] [CrossRef]
- Fadón, E.; Herrero, M.; Rodrigo, J. Flower development in sweet cherry framed in the BBCH scale. Sci. Hortic. 2015, 192, 141–147. [Google Scholar] [CrossRef]
- Allen, R.G. Crop Evapotranspiration: Guidelines for Computing Crop Water Requirements; FAO: Rome, Italy, 1998. [Google Scholar]
- D’Urso, G.; Richter, K.; Calera, A.; Osann, M.A.; Escadafal, R.; Garatuza-Pajan, J.; Hanich, L.; Perdigão, A.; Tapia, J.B.; Vuolo, F. Earth observation products for operational irrigation management in the context of the PLEIADeS project. Agric. Water Manag. 2010, 98, 271–282. [Google Scholar] [CrossRef]
- Balbontín, N.C. Plataforma Agrícola Satelital (PLAS): Coeficientes de Cultivo Satelitales para Aumentar Eficiencia Hídrica; PLAS: Santiago, Chile, 2020; Available online: https://hdl.handle.net/20.500.14001/68930 (accessed on 10 May 2025).
- Grujić, N.; Golubović, M.; Jovanović, D. The use of satellite images in the field of agriculture. Zb. Rad. Departmana Geogr. Turiz. Hotel. 2018, 47, 11–22. [Google Scholar] [CrossRef]
- FAO. CROPWAT: Programa de Ordenador Para Planificar y Manejar el Riego. Estudio Riego y Drenaje No. 46; FAO: Rome, Italy, 1993. [Google Scholar]
- Howell, T.A.; Dusek, D.A. Comparison of vapor-pressure-deficit calculation methods—Southern High Plains. J. Irrig. Drain. Eng. 1995, 121, 191–198. [Google Scholar] [CrossRef]
- McCutchan, H.; Shackel, K.A. Stem-water potential as a sensitive indicator of water stress in prune trees (Prunus domestica L. cv. French). J. Am. Soc. Hortic. Sci. 1992, 117, 607–611. [Google Scholar] [CrossRef]
- Reyes-Díaz, M.; Ivanov, A.G.; Huner, N.P.; Alberdi, M.; Corcuera, L.J.; Bravo, L.A. Thermal energy dissipation and its components in two developmental stages of a shade-tolerant species, Nothofagus nitida, and a shade-intolerant species, Nothofagus dombeyi. Tree Physiol. 2009, 29, 651–662. [Google Scholar] [CrossRef]
- Myers, B.J. Water stress integral—A link between short-term stress and long-term growth. Tree Physiol. 1988, 4, 315–323. [Google Scholar] [CrossRef]
- Bchir, A.; Escalona, J.M.; Gallé, A.; Hernández-Montes, E.; Tortosa, I.; Braham, M.; Medrano, H. Carbon isotope discrimination (δ13C) as an indicator of vine water status and water use efficiency (WUE): Looking for the most representative sample and sampling time. Agric. Water Manag. 2016, 167, 11–20. [Google Scholar] [CrossRef]
- Brugnoli, E.; Farquhar, G.D. Photosynthetic fractionation of carbon isotopes. In Photosynthesis: Physiology and Metabolism; Springer: Dordrecht, The Netherlands, 2000; pp. 399–434. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Hubick, K.T.; Condon, A.G.; Richards, R.A. Carbon isotope fractionation and plant water-use efficiency. In Stable Isotopes in Ecological Research; Rundel, P.W., Ehleringer, J.R., Nagy, K.A., Eds.; Springer: New York, NY, USA, 1989; pp. 21–40. [Google Scholar] [CrossRef]
- Dobereiner, J.; Baldani, V.; Baldani, J. Como Isolar e Identificar Bactérias Diazotróficas de Plantas Não-Leguminosas; Embrapa-SPI: Brasília, Brazil, 1995. [Google Scholar]
- Rodriguez, E. Improved medium for isolation of Azospirillum spp. Appl. Environ. Microbiol. 1982, 44, 990–999. [Google Scholar]
- Kappel, F.; Lane, W.D. Recent sweet cherry introductions from the breeding program at Summerland, British Columbia, Canada. In Proceedings of the III International Cherry Symposium, British Columbia, Canada, July 1997. Acta Hortic. 1998, 468, 105–110. [Google Scholar] [CrossRef]
- Bhusal, N.; Park, I.H.; Jeong, S.; Choi, B.H.; Han, S.G.; Yoon, T.M. Photosynthetic traits and plant hydraulic dynamics in ‘Gamhong’ apple cultivar under drought, waterlogging, and stress recovery periods. Sci. Hortic. 2023, 321, 112276. [Google Scholar] [CrossRef]
- Parent, C.; Capelli, N.; Berger, A.; Crèvecoeur, M.; Dat, J.F. An overview of plant responses to soil waterlogging. Plant Stress 2008, 2, 20–27. [Google Scholar]
- Liu, R.Q.; Xu, X.J.; Wang, S.; Shan, C.J. Lanthanum improves salt tolerance of maize seedlings. Photosynthetica 2016, 54, 148–151. [Google Scholar] [CrossRef]
- Greer, D.H.; Weston, C. Seasonal patterns of shoot growth in sweet cherry (Prunus avium L.) trees. Sci. Hortic. 2018, 234, 270–277. [Google Scholar] [CrossRef]
- Küçükyumuk, C. Responses of sweet cherry trees to regulated deficit irrigation applied before and after harvesting. J. Agric. Sci. 2024, 162, 91–104. [Google Scholar] [CrossRef]
- Blanco, V.; Blaya-Ros, P.J.; Torres-Sánchez, R.; Domingo, R. Influence of regulated deficit irrigation and environmental conditions on reproductive response of sweet cherry trees. Plants 2020, 9, 94. [Google Scholar] [CrossRef]
- Lang, G.A. Critical concepts for sweet cherry training systems. Compact Fruit Tree 2001, 34, 70–75. [Google Scholar]
- Ayala, M.; Lang, G.A. 13C photoassimilate partitioning in sweet cherry (Prunus avium) during early spring. Cienc. Investig. Agrar. 2015, 42, 191–203. [Google Scholar] [CrossRef]
- Koumanov, K.S.; Staneva, I.N.; Kornov, G.D.; Germanova, D.R. Intensive sweet cherry production on dwarfing rootstocks revisited. Sci. Hortic. 2018, 229, 193–200. [Google Scholar] [CrossRef]
- Measham, P.F.; Bound, A.; Gracie, J.; Wilson, S.J. Incidence and type of cracking in sweet cherry (Prunus avium L.) are affected by genotype and season. Crop Pasture Sci. 2009, 60, 1002–1008. [Google Scholar] [CrossRef]
- Meisner, A.; Jacquiod, S.; Snoek, B.L.; Ten Hooven, F.C.; Van der Putten, W.H. Drought legacy effects on the composition of soil fungal and prokaryote communities. Front. Microbiol. 2018, 9, 3235. [Google Scholar] [CrossRef] [PubMed]
- Meisner, A.; Bååth, E.; Rousk, J. Microbial growth responses upon rewetting soil dried for four days or one year. Soil Biol. Biochem. 2013, 66, 188–192. [Google Scholar] [CrossRef]
- Canarini, A.; Schmidt, H.; Fuchslueger, L.; Martin, V.; Herbold, C.W.; Zezula, D.; Richter, A. Ecological memory of recurrent drought modifies soil processes via changes in soil microbial community. Nat. Commun. 2021, 12, 5308. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhao, J.; Yuan, J.; Hale, L.; Wen, T.; Huang, Q.; Shen, Q. Root exudates drive soil–microbe–nutrient feedbacks in response to plant growth. Plant Cell Environ. 2021, 44, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Chen, X.F.; Zheng, Z.C.; Huang, W.L.; Guo, J.; Yang, L.T.; Chen, L.S. Characterization of copper-induced release of exudates by Citrus sinensis roots and their possible roles in copper tolerance. Chemosphere 2022, 308, 136348. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Bano, A. Exopolysaccharide-producing rhizobacteria and their impact on growth and drought tolerance of wheat grown under rainfed conditions. PLoS ONE 2019, 14, e0222302. [Google Scholar] [CrossRef]
- Pedraza, L.A.; López, C.E.; Uribe-Vélez, D. Mecanismos de acción de Bacillus spp. (Bacillaceae) contra microorganismos fitopatógenos durante su interacción con plantas. Acta Biológica Colomb. 2020, 25, 112–125. [Google Scholar] [CrossRef]
- Goswami, D.; Dhandhukia, P.; Patel, P.; Thakker, J.N. Screening of PGPR from saline desert of Kutch: Growth promotion in Arachis hypogea by Bacillus licheniformis A2. Microbiol. Res. 2014, 169, 66–75. [Google Scholar] [CrossRef]
- Instituto de Investigaciones Agropecuarias INIA. Formación y Sistemas de Conducción del Cerezo Dulce. Boletín INIA N°247. Available online: https://bibliotecadigital.ciren.cl/server/api/core/bitstreams/81a43de6-20ea-491e-86a0-01bb2574ab1c/content (accessed on 10 May 2025).





| Phenological Stages * | Season | ||
|---|---|---|---|
| 2021–2022 | 2022–2023 | 2023–2024 | |
| Stage 65 | September 12th | September 10th | September 9th |
| Stage 67 | September 26th | September 27th | September 29th |
| Stage 81 | October 25th | October 28th | October 31st |
| Stage 89 | November 19th | November 17th | November 23rd |
| Stage 95 | May 1st | May 1st | May 1st |
| Cumulative Values | Irrigation Treatment | ||
|---|---|---|---|
| Control | MDI | SDI | |
| Season 2022 | |||
| Crop evaporation (m3 ha−1) | 6403 | ||
| Irrigation (m3 ha−1) | 6474 | 5425 | 5197 |
| Effective Precipitation (m3 ha−1) | 454 | ||
| Applied water (m3 ha−1) | 6928 | 5879 | 5651 |
| Difference in Applied Water (%) | - | −15.1% | −18.4% |
| Season 2023 | |||
| Crop evaporation (m3 ha−1) | 7889 | ||
| Irrigation (m3 ha−1) | 7972 | 6388 | 5648 |
| Effective Precipitation (m3 ha−1) | 250 | ||
| Applied water (m3 ha−1) | 8222 | 6638 | 5898 |
| Difference in Applied Water (%) | - | −19.3% | −28.3% |
| Season 2024 | |||
| Crop evaporation (m3 ha−1) | 7138 | ||
| Irrigation (m3 ha−1) | 6370 | 5583 | 5477 |
| Precipitation (m3 ha−1) | 1552 | ||
| Applied water (m3 ha−1) | 7922 | 7135 | 7029 |
| Difference in Applied Water (%) | - | −9.9% | −11.3% |
| Photosystem II Efficiency | Irrigation Treatment | |||
|---|---|---|---|---|
| Season | Control | MDI | SDI | p-Value |
| 2021–2022 | 0.67 ± 0.14 | 0.66 ± 0.14 | 0.65 ± 0.18 | ns |
| 2022–2023 | 0.68 ± 0.14 | 0.69 ± 0.10 | 0.69 ± 0.11 | ns |
| 2023–2024 | 0.76 ± 0.07 | 0.75 ± 0.09 | 0.73 ± 0.14 | ns |
| Leaf Area Index | ||||
| 2021–2022 | 5.70 ± 1.48 | 5.98 ± 0.78 | 5.47 ± 1.27 | ns |
| 2022–2023 | 5.67 ± 1.24 | 5.97 ± 1.08 | 5.67 ± 1.49 | ns |
| 2023–2024 | 6.47 ± 0.98 | 5.93 ± 0.85 | 5.52 ± 0.26 | ns |
| Irrigation Treatment | ||||
|---|---|---|---|---|
| Control | MDI | SDI | p-Value | |
| Season 2023 | ||||
| Orchard yield (Mg ha−1) | 22.6 ± 2.5 | 19.8 ± 3.9 | 21.5 ± 2.7 | ns |
| Plant yield (kg tree−1) | 24.5 ± 2.7 | 20.6 ± 5.4 | 23.3 ± 3.0 | ns |
| Crop load (fruits tree−1) | 2472 ± 268 | 1825 ± 360 | 2261 ± 287 | ns |
| Season 2024 | ||||
| Orchard yield (Mg ha−1) | 14.6 ± 2.7 | 12.4 ± 2.5 | 15.0 ± 2.9 | ns |
| Plant yield (kg tree−1) | 15.8 ± 2.9 | 13.4 ± 2.7 | 16.2 ± 3.1 | ns |
| Crop load (fruits tree−1) | 1435 ± 263 | 1183 ± 242 | 1571 ± 303 | ns |
| Irrigation Treatment | ||||
|---|---|---|---|---|
| Control | MDI | SDI | p-Value | |
| Season 2023 | ||||
| Color | ||||
| C* | 24.4 ± 1.0 | 23.7 ± 6.9 | 24.7 ± 5.5 | ns |
| h° | 12.0 ± 0.7 | 11.7 ± 2.0 | 12.2 ± 1.8 | ns |
| Weight (g) | 9.9 b ± 1.0 | 11.7 a ± 1.0 | 10.3 ab ± 0.5 | p ≤ 0.05 |
| Polar diameter (mm) | 24.5 ± 0.5 | 25.6 ± 1.7 | 24.6 ± 1.4 | ns |
| Equatorial diameter (mm) | 25.7 ± 0.9 | 27.2 ± 2.2 | 26.0 ± 1.8 | ns |
| Soluble solids (°Brix) | 14.3 ± 0.2 | 15.0 ± 1.6 | 15.0 ± 0.9 | ns |
| Season 2024 | ||||
| Color | ||||
| C* | 24.5 ± 3.6 | 25.9 ± 6.4 | 28.6 ± 11.5 | ns |
| h° | 13.8 ± 1.1 | 13.9 ± 1.0 | 14.3 ± 2.3 | ns |
| Weight (g) | 11.0 ± 0.9 | 11.3 ± 0.9 | 10.3 ± 0.3 | ns |
| Polar diameter (mm) | 25.9 ± 1.0 | 25.8 ± 0.4 | 24.5 ± 0.6 | ns |
| Equatorial diameter (mm) | 27.6 ± 1.3 | 27.6 ± 0.9 | 26.4 ± 0.4 | ns |
| Soluble solids (°Brix) | 15.4 ± 0.8 | 15.9 ± 1.5 | 15.4 ± 1.7 | ns |
| Apical cracking (%) | 21.6% ± 2.3% | 27.5% ± 2.5% | 21.3% ± 8.9% | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvear, T.; Gerding, M.; Bastías, R.M.; Contreras, C.; Antileo-Mellado, S.; Olivos, A.; Calderón-Orellana, M.; Calderón-Orellana, A. Physiological, Productive, and Soil Rhizospheric Microbiota Responses of ‘Santina’ Cherry Trees to Regulated Deficit Irrigation Applied After Harvest. Plants 2025, 14, 3611. https://doi.org/10.3390/plants14233611
Alvear T, Gerding M, Bastías RM, Contreras C, Antileo-Mellado S, Olivos A, Calderón-Orellana M, Calderón-Orellana A. Physiological, Productive, and Soil Rhizospheric Microbiota Responses of ‘Santina’ Cherry Trees to Regulated Deficit Irrigation Applied After Harvest. Plants. 2025; 14(23):3611. https://doi.org/10.3390/plants14233611
Chicago/Turabian StyleAlvear, Tamara, Macarena Gerding, Richard M. Bastías, Carolina Contreras, Silvia Antileo-Mellado, Andrés Olivos, Mauricio Calderón-Orellana, and Arturo Calderón-Orellana. 2025. "Physiological, Productive, and Soil Rhizospheric Microbiota Responses of ‘Santina’ Cherry Trees to Regulated Deficit Irrigation Applied After Harvest" Plants 14, no. 23: 3611. https://doi.org/10.3390/plants14233611
APA StyleAlvear, T., Gerding, M., Bastías, R. M., Contreras, C., Antileo-Mellado, S., Olivos, A., Calderón-Orellana, M., & Calderón-Orellana, A. (2025). Physiological, Productive, and Soil Rhizospheric Microbiota Responses of ‘Santina’ Cherry Trees to Regulated Deficit Irrigation Applied After Harvest. Plants, 14(23), 3611. https://doi.org/10.3390/plants14233611









