3. Results and Discussion
This study presents the first systematic evaluation comparing contrasting artificial lighting systems in hop cultivation under subtropical conditions in Brazil. Phenological, yield, and phytochemical traits of the Comet cultivar were assessed under conditions with and without supplemental lighting across two consecutive production cycles. This approach enabled precise quantification of agronomic and biochemical responses and advanced the experimental understanding of a cultivation practice already implemented by local growers.
The results presented below encompass climatic conditions, phenological stage duration, yield performance, and cone chemical composition, linking environmental, physiological, and biochemical variables that define the potential of supplemental lighting as a management strategy at lower latitudes. Climatic data from 2024 and 2025 revealed clear associations with hop phenology under contrasting photoperiod regimes (
Table 1).
In 2024, mean global radiation was 17.7 MJ m−2, with an average temperature of 22.8 °C (maximum 30.7 °C) and cumulative rainfall of 1016 mm. Under these conditions, plants without supplemental lighting completed their phenological cycle in 91 days, while those under an extended photoperiod maintained vegetative growth longer, reaching 108 days. In 2025, radiation was higher (18.9 MJ m−2), mean temperature slightly lower (21.5 °C), and precipitation greater (1202 mm), naturally extending the cycle to 97 days without lighting and to 113 days with lighting.
These results demonstrate that, although interannual climatic variability strongly influences the duration of budburst, elongation, flowering, and maturation, supplemental lighting is decisive for maintaining plants in a vegetative state for longer, thereby promoting phenological uniformity and productive potential. The prolonged cycle reflects enhanced vegetative growth and delayed flowering. As highlighted by Acosta-Rangel, Agehara, and Rechcigl (2024) [
18], photoperiod extension can even inhibit floral induction in hops, underscoring the critical role of supplemental light in modulating crop phenology.
By extending the phases of shoot elongation, floral initiation, and cone development, supplemental lighting positively affected both yield and technological quality, ensuring greater uniformity of maturation and higher accumulation of secondary metabolites. This effect is particularly relevant at lower latitudes, where natural photoperiods are insufficient to sustain longer cycles, as observed in traditional hop-growing regions.
Table 2 presents the accumulated growing degree day (GDD) values corresponding to the vegetative and reproductive phenological phases, as well as their total sum representing the complete growth cycle for the 2024 and 2025 harvests. The table also includes Pearson correlation coefficients between cycle duration and GDD accumulation for each harvest.
According to Fagherazzi (2020) [
24], the accumulation of growing degree days (GDD) is a key determinant of hop development, from the onset of bud emergence to cone maturation. In the 2024 season, both treatments required more than 2000 GDD to complete the growth cycle, totaling 2589.16 °C in light-supplemented plants and 2092.75 °C in non-supplemented plants. In the 2025 season, the cumulative heat requirement was 2115.77 °C for supplemented plants and 1728.29 °C for those grown under natural photoperiod conditions.
Across both harvests, plants exposed to supplemental lighting, which ensured attainment of the critical photoperiod, consistently required greater thermal accumulation to complete their developmental stages. Photoperiod management was decisive in extending the crop cycle, particularly the reproductive phase associated with cone formation and maturation. Consequently, higher energy input was necessary for these phenological transitions. Conversely, plants cultivated without supplemental light accumulated fewer degree days, which shortened the reproductive period and ultimately reduced yield potential.
As reported by Marovt (2007) [
25], the total GDD required for hop development varies among cultivars, typically ranging between 1751 °C and 2900 °C. This agrees with the present findings, in which similar values were recorded. Gonsaga (2021) [
26], when evaluating multiple cultivars, observed a range of approximately 1500 to 2200 GDD between early and late maturing varieties, emphasizing that reduced thermal accumulation is often associated with earlier phenology and lower cone yield due to limited reproductive development.
The correlation coefficients (r) between thermal accumulation and crop duration were positive (r > 0) in all cases, with stronger associations observed during the second season (values approaching 1), indicating very strong relationships. As described by Miot (2018) [
27], positive coefficients denote direct correlations, supporting the conclusion that cycle duration and heat demand are tightly coupled physiological parameters in hop growth.
Analysis of variance confirmed significant effects (p < 0.01) of supplemental lighting on several traits. Light significantly increased the number of lateral branches (NLB), lateral branch length (LBL), cone length (CL), and plant height (PH), while the insertion height of the first cone (IHC) remained unaffected. For yield traits, fresh plant mass (FPM), fresh cone mass per plant (FM), dry cone mass per plant (DM), and dry mass per cone (DMC) were all significantly higher (p < 0.01) under supplemental lighting. Phytochemically, α-acid and essential oil contents increased significantly (p < 0.01), while β-acid levels showed no difference. Year and year × light interactions were mostly non-significant, except for LBL, which responded to both main and interaction effects.
Overall, supplemental lighting had a pronounced impact on hop growth, proving to be a key factor for agronomic performance under subtropical conditions. Traits such as NLB, PH, and CL were significantly enhanced under lighting (
Table 3), reflecting greater vegetative vigor and a more balanced architecture that optimizes light interception and biomass accumulation. These responses indicate that supplemental light not only enhances vertical growth but also stimulates lateral branching, which is critical for maximizing yield potential.
The insertion height of the first cone (IHC) is an important agronomic trait in hops, as it reflects plant architecture and indicates the potential for cone distribution and yield. Lower values denote cone formation closer to the soil, increasing the number of fertile nodes and enhancing productivity. In this study, supplemental lighting significantly reduced IHC to 99.0 cm compared with 125.7 cm in plants without lighting. Similarly, Fortuna et al. (2023) [
28] reported values ranging from 1.51 to 1.95 m across cultivation systems and varieties in Brazil, with no significant differences, but highlighted that lower IHC values were associated with higher cone yields in cultivars such as ‘Chinook’, ‘Cascade’, and ‘Columbus’, reinforcing the link between this morphological trait and crop performance.
The number of lateral branches (NLB) was strongly influenced by lighting, averaging 70.05 under supplemental lighting versus only 13.0 without. This marked increase reflects the positive effect of photoperiod manipulation on fertile branch emission, expanding the number of reproductive sites and boosting yield potential. According to Leles et al. (2023) [
29], enhanced lateral branching is directly associated with greater cone production, especially after plants reach the top of the trellis, when resources are redirected to branch emission.
Plant height (PH) also differed significantly, with plants under lighting reaching 590.94 cm compared with 468.60 cm without. Photoperiod extension promoted greater vegetative growth and delayed floral induction, increasing radiation interception and the number of fertile nodes. Comparable results were reported by Neves et al. (2024) [
30], who found significant variation among cultivars grown under subtropical conditions: ‘Comet’ (561.12 cm) was the tallest, followed by ‘Chinook’ (512.04 cm) and ‘Nugget’ (498.50 cm), while ‘Cascade’ (442.12 cm) and ‘Columbus’ (375.87 cm) were shorter. In that study, Comet’s greater height was also linked to improved water-use efficiency, indicating superior biomass conversion and adaptability under subtropical conditions.
Cone length (CL) also responded positively, averaging 3.49 cm with lighting versus 2.84 cm without. Photoperiod extension thus promoted reproductive development. Cernea and Vâtcă (2013) [
31] reported a positive correlation between cone length and fresh mass, suggesting that this trait contributes to biomass accumulation. However, they noted that cone dry mass is a more robust and reliable indicator of productivity than length alone.
Lateral branch length (LBL) proved especially sensitive to the interaction between lighting and crop year, with averages of 78.98 cm in 2024 and 97.09 cm in 2025, compared with 42.80 cm and 43.60 cm, respectively, in plants without lighting (
Table 4).
These results indicate that the response to photoperiod extension is robust and reinforced by the physiological maturity of hops, a perennial species. The combination of additional light stimulus and reserve accumulation across production cycles enhanced branch elongation, improving light interception and the availability of reproductive sites. Thus, supplemental lighting acts synergistically with plant ontogeny, boosting vegetative vigor and yield potential in perennial systems, as also noted by Leles et al. (2025) [
32].
Supplemental lighting produced striking gains in biomass and cone yield, with fresh plant mass (FPM) of 6156.40 g compared with 505.85 g, fresh cone mass (FCM) of 1625.62 g compared with 39.73 g, and cone dry mass (CDM) of 374.61 g compared with 9.75 g in non-supplemented plants (
Table 5).
These gains confirm that photoperiod extension enhances both vegetative and reproductive growth, ensuring greater biomass accumulation and more abundant cone formation. Similar results were reported by Leles et al. (2023) [
29], who observed significant increases in cone number, fresh mass, and yield in cultivars exposed to 17 h of daily light under subtropical conditions. The authors emphasized that supplemental light, by delaying premature floral induction, strengthens vegetative vigor and promotes lateral branching, thereby expanding the availability of fertile sites. Together, these findings consolidate supplemental lighting as a decisive practice for enabling hop production in subtropical regions, achieving yields comparable to those in traditional growing areas.
Regarding chemical composition, supplemental lighting increased α-acid and essential oil contents by approximately 27.5% and 41.8%, respectively, compared with non-supplemented plants. In contrast, β-acid levels remained similar between treatments, with only a slight increase under lighting (
Table 6).
The α-acid content, the main contributor to beer bitterness and stability, was significantly higher in plants under photoperiod management (11.92%) compared with those without supplemental light (9.35%). This increase indicates that day-length extension stimulates the biosynthesis and accumulation of these compounds, reinforcing the potential of ‘Comet’ as a dual-purpose hop, capable of providing high bitterness without compromising its aromatic profile [
33].
For β-acids, no statistical differences were detected between treatments, with values of 2.88% under supplemental lighting and 2.34% without. A slight upward trend under light extension may contribute to secondary sensory attributes, such as smoother bitterness and antimicrobial activity in wort. Similar values were reported by Sabino et al. (2025) [
14] for ‘Comet’ cultivated under subtropical conditions without supplemental lighting, where α-acids averaged 10.54% and β-acids 4.30%, confirming the genetic potential of this cultivar for expansion into new production regions.
Across both seasons, α-acid levels were consistently higher in plants grown under supplemental lighting. However, even in the absence of this management practice, values remained within the reference range for Comet under temperate conditions (9.0–12.0%). For β-acids, concentrations also approached the expected range of 3.0–6.1% [
34]. The essential oil content obtained in this study was consistent with the general range of 0.5% to 3.0% of cone dry mass reported by Almaguer et al. (2014) [
35].
GC-MS/FID analysis allowed the identification of 31 compounds in the essential oils (
Table 7) across the evaluated harvests, under both lighting conditions (with and without supplemental light). The identified compounds included monoterpene and sesquiterpene hydrocarbons, esters, oxygenated sesquiterpenes, and oxygenated monoterpenes. No significant effects of the evaluated factors or their interaction were observed (
p < 0.01).
Chemical profiling of the essential oils revealed high stability across years and management regimes, with no significant variation in the overall distribution of compound classes. Monoterpene hydrocarbons predominated (69.74–79.03%), with β-myrcene as the principal constituent. Concentrations ranged from 76.36% to 69.74% in 2024 (without and with supplemental light, respectively) and from 79.03% to 73.25% in 2025, confirming β-myrcene as the primary chemical marker of hops.
According to Durello et al. (2019) [
6] and Teixeira (2024) [
36], β-myrcene content can vary widely among cultivars (0.5–70%), generally representing the dominant volatile compound and serving as an indicator of hop quality and freshness. However, its high volatility, susceptibility to oxidation, and low water solubility limit its stability and sensory persistence, resulting in a mild and short-lived “hoppy” aroma [
37,
38].
Sesquiterpene hydrocarbons remained stable (10.09–14.87%), dominated by (
E)-caryophyllene (5.58%), α-selinene (3.47%), and β-selinene (2.33%). In contrast to monoterpenes, sesquiterpenes exhibit lower volatility and greater oxidative stability [
35]. Comparable patterns were observed by Sabino et al. (2025) [
14] and Campos et al. (2025) [
39] in ‘Comet’ hops cultivated under subtropical conditions, where β-myrcene was the predominant compound, followed by (
E)-caryophyllene and intermediate levels of α- and β-selinene. These sesquiterpenes have also been recurrent in Brazilian-grown hops, reinforcing their role as potential chemical markers of adaptation to local environments [
40].
Esters varied within a narrow range (5.17–7.06%), with geranyl isobutyrate as the main representative (1.91%), while oxygenated monoterpenes and sesquiterpenes remained below 1.3%. Ketones and unidentified compounds exhibited only minor fluctuations, without significant impact on the overall oil profile. From a brewing perspective, such compositional consistency is desirable, as it minimizes the risk of undesirable aromatic deviations during processing.
Overall, the ‘Comet’ cultivar displayed a highly stable aromatic profile across years and light regimes, characterized by β-myrcene dominance complemented by sesquiterpenes and esters. The resilience of its terpenoid metabolism under subtropical conditions ensures reproducible chemical composition and reliable sensory quality across harvests.
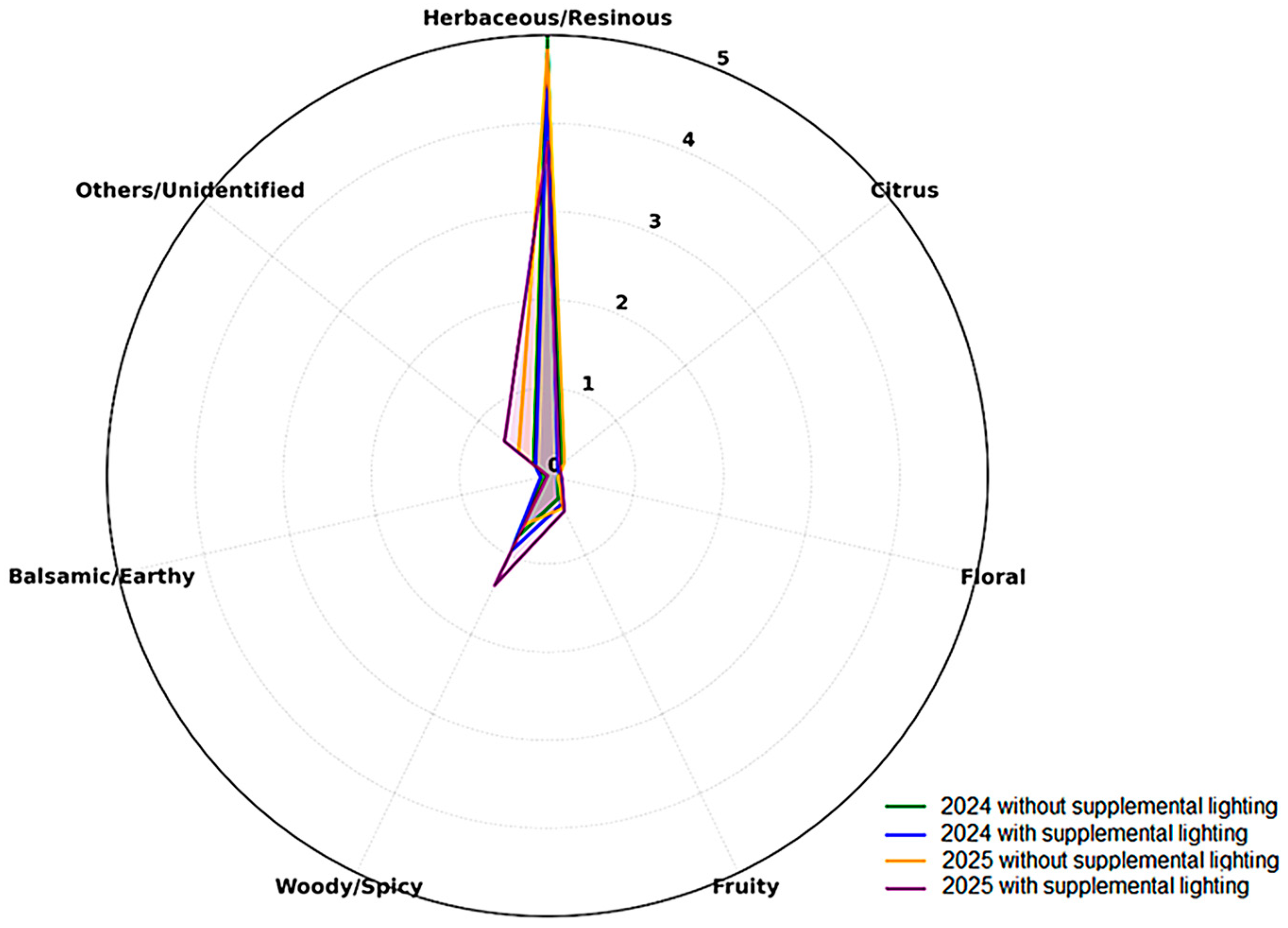
The aroma wheel (
Figure 2), based on Herkenhoff et al. (2024) [
22], further illustrates the common aromatic base of all treatments, defined by the herbaceous/resinous dimension driven by high β-myrcene abundance, reinforcing the green–resinous freshness as a hallmark of hops cultivated under subtropical environments.
The volatile profile of Humulus lupulus L. cones exhibited consistent patterns of secondary metabolite formation, irrespective of photoperiod management. Across all treatments, β-myrcene was the predominant compound, sustaining the herbaceous, resinous, and fresh character typical of hops cultivated at lower latitudes. This predominance defines a stable sensory identity in which vegetal freshness remains central to the overall aromatic expression.
These results corroborate those of Campos et al. (2025) [
39], who demonstrated that in ‘Comet’ hops subjected to different hydrodistillation durations, the herbal descriptor was the most prominent among treatments, reflecting the high monoterpene content, particularly β-myrcene, responsible for fresh and green notes. Similarly, Bonfim et al. (2025) [
41] reported stability in the volatile composition of ‘Fuggle’ hops grown under subtropical conditions, identifying β-myrcene, (
E)-β-farnesene, α-selinene, β-selinene, and (
E)-caryophyllene as key constituents. β-Myrcene consistently emerged as the dominant metabolite over three consecutive harvests (2021–2023), imparting resinous and herbaceous notes complemented by woody, floral, and citrus nuances, thereby maintaining aromatic typicity despite environmental variability. Such stability reflects metabolic resilience and ensures predictable sensory quality.
Sesquiterpenes, including (
E)-caryophyllene, and selinene isomers, were recurrent across years and treatments, reinforcing woody, spicy, and peppery dimensions. Although their relative proportions varied, their consistent occurrence alongside β-myrcene established a shared aromatic framework, contributing to both typicity and complexity. Esters imparted subtle fruity notes that softened the dominant green freshness, while oxygenated volatiles such as linalool, methyl geranate, and geranyl acetate introduced floral and citrus nuances, enhancing aromatic balance. This interaction suggests that hops cultivated under subtropical conditions maintain a fundamental equilibrium among herbaceous, floral, fruity, and spicy dimensions [
22].
Collectively, these findings demonstrate that all treatments preserved a stable aromatic identity, characterized by herbaceous and resinous freshness enriched with complementary woody, floral, and fruity layers. This convergence indicates that hop cultivation under subtropical conditions, whether with or without supplemental lighting, supports a consistent sensory profile, providing the aromatic stability and technological reliability required by the brewing industry.