Nitrogen Fixation and Anoxygenic Photosynthesis in Filamentous Non-Heterocystous Cyanobacterium of the Genus Sodalinema Isolated from Soda Lake
Abstract
1. Introduction
2. Results
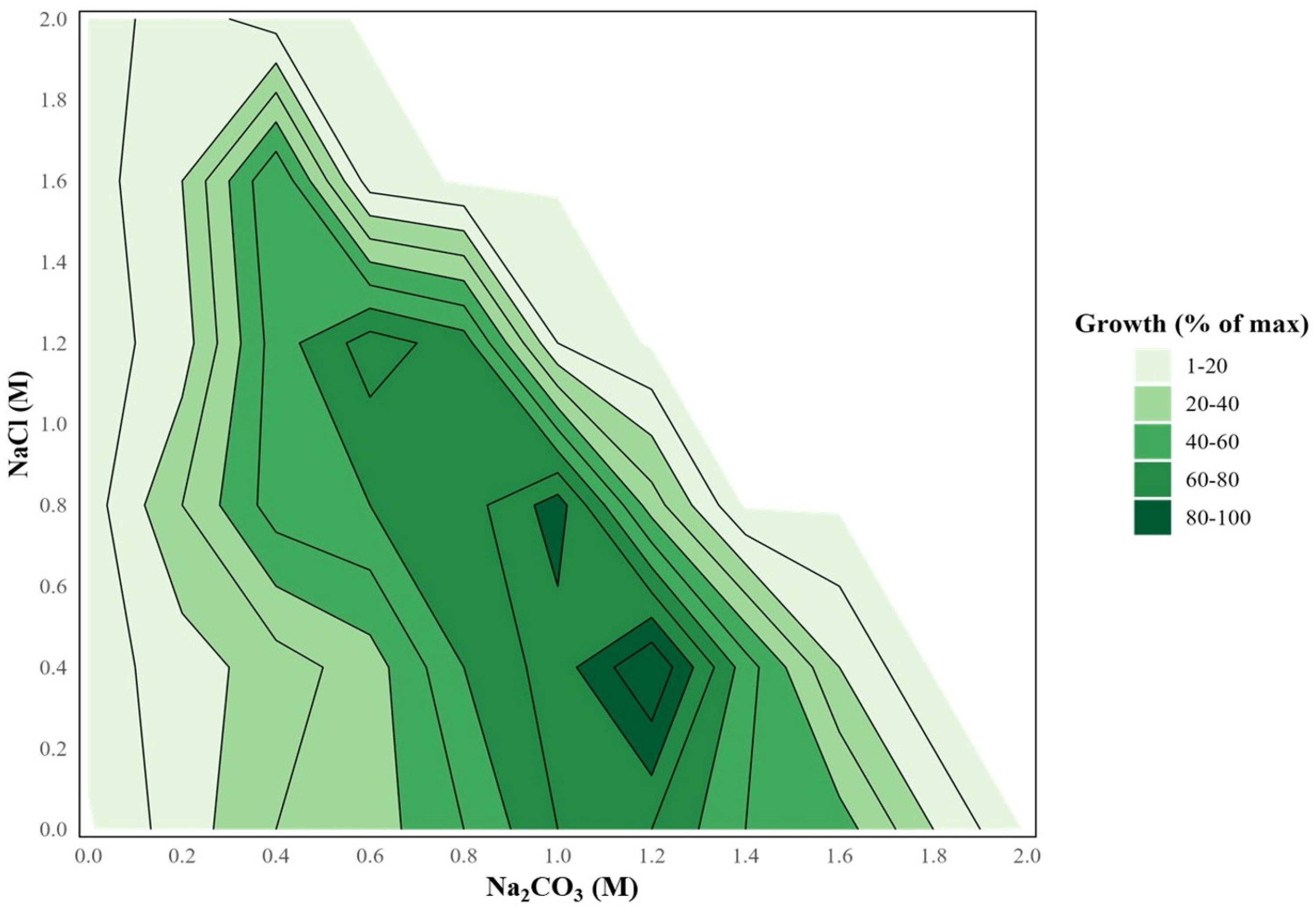
2.1. Ecophysiological Properties of the Strain Sodalinema sp. P-1104
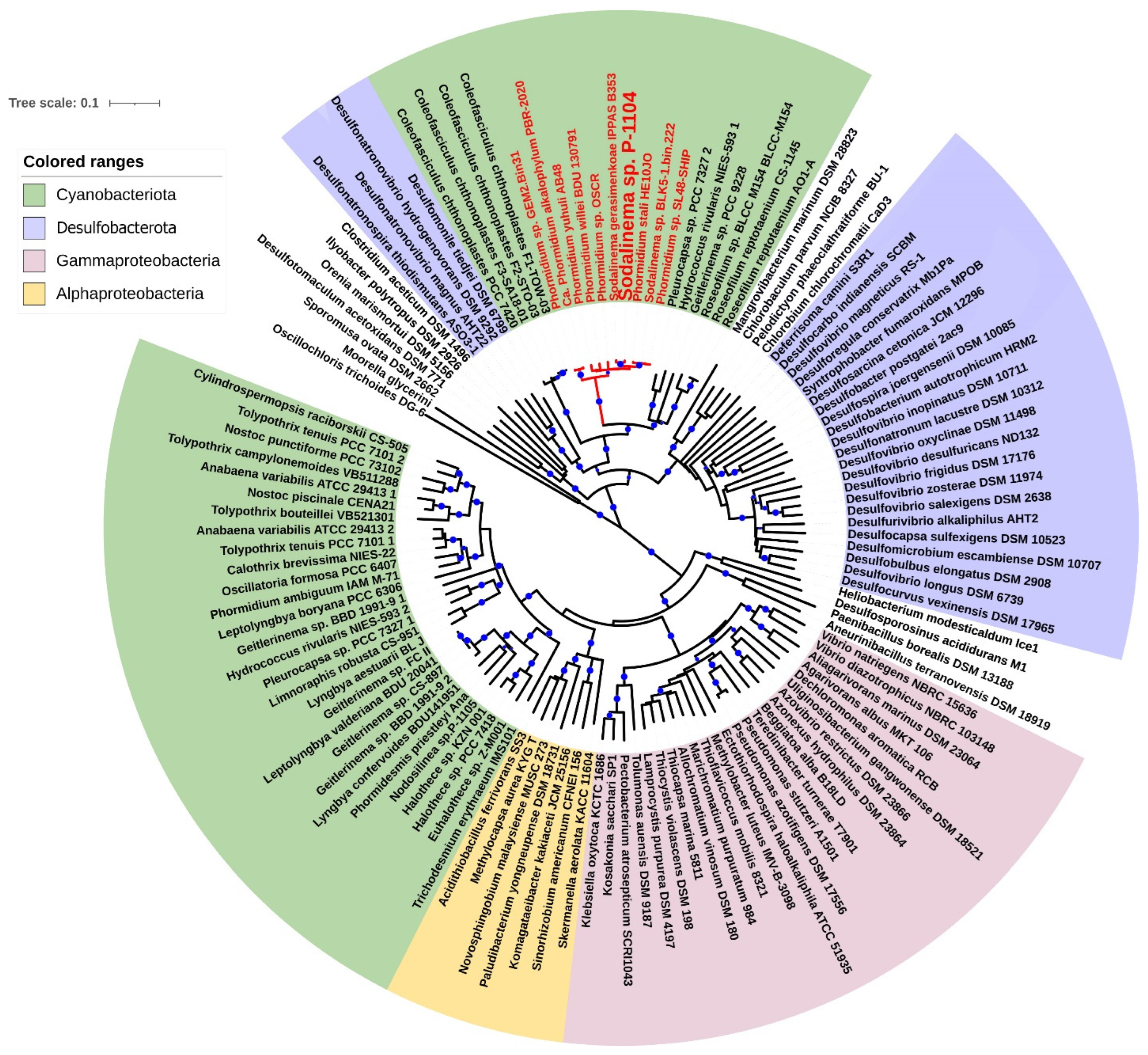
2.2. Nitrogenase Gene Cluster
2.3. Determining the Ability to Fix Nitrogen in Different Regimes of Cultivation
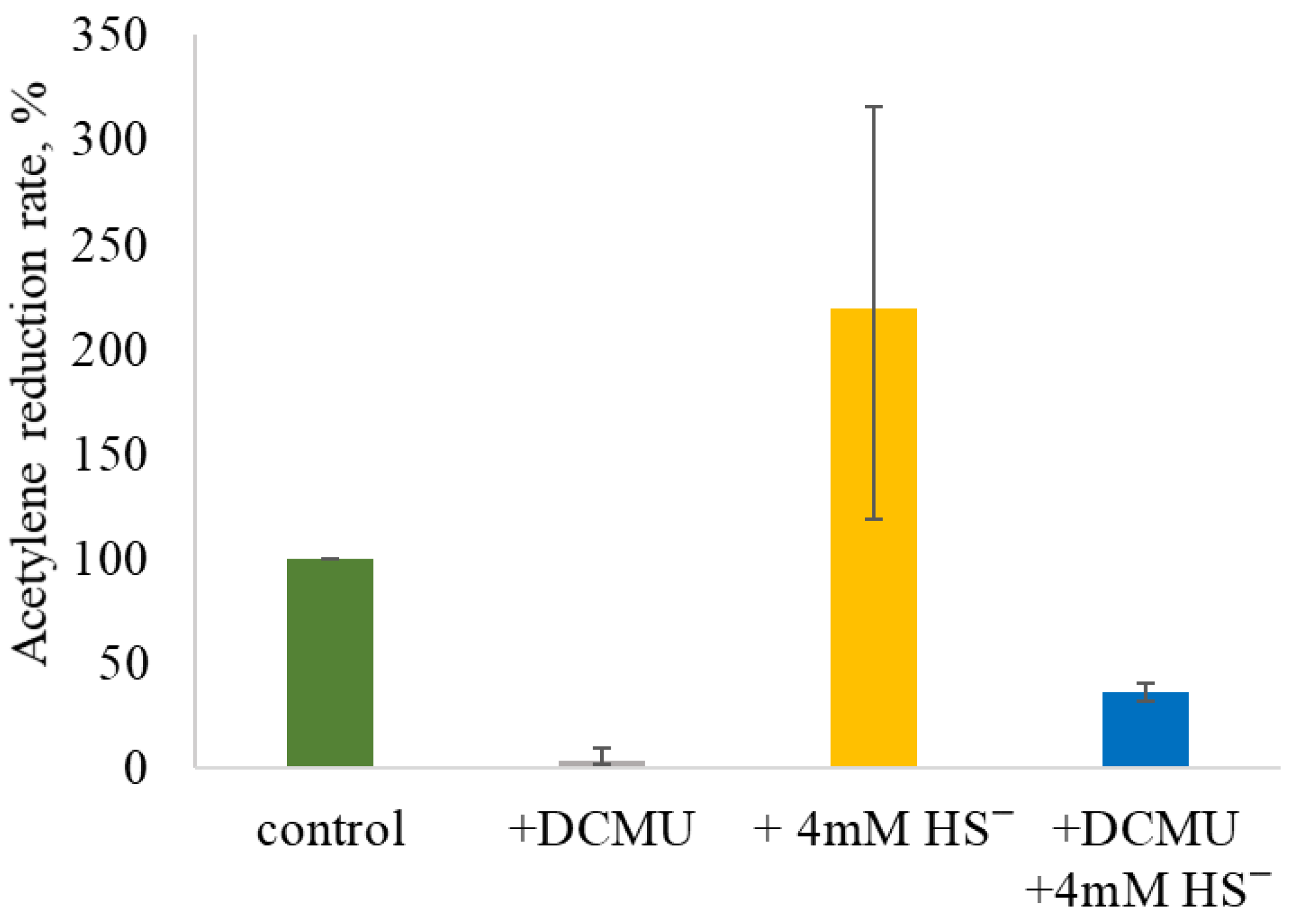
2.4. Effect of DCMU and Sulfide Addition on NF
2.5. Sulfide–Quinone Reductase
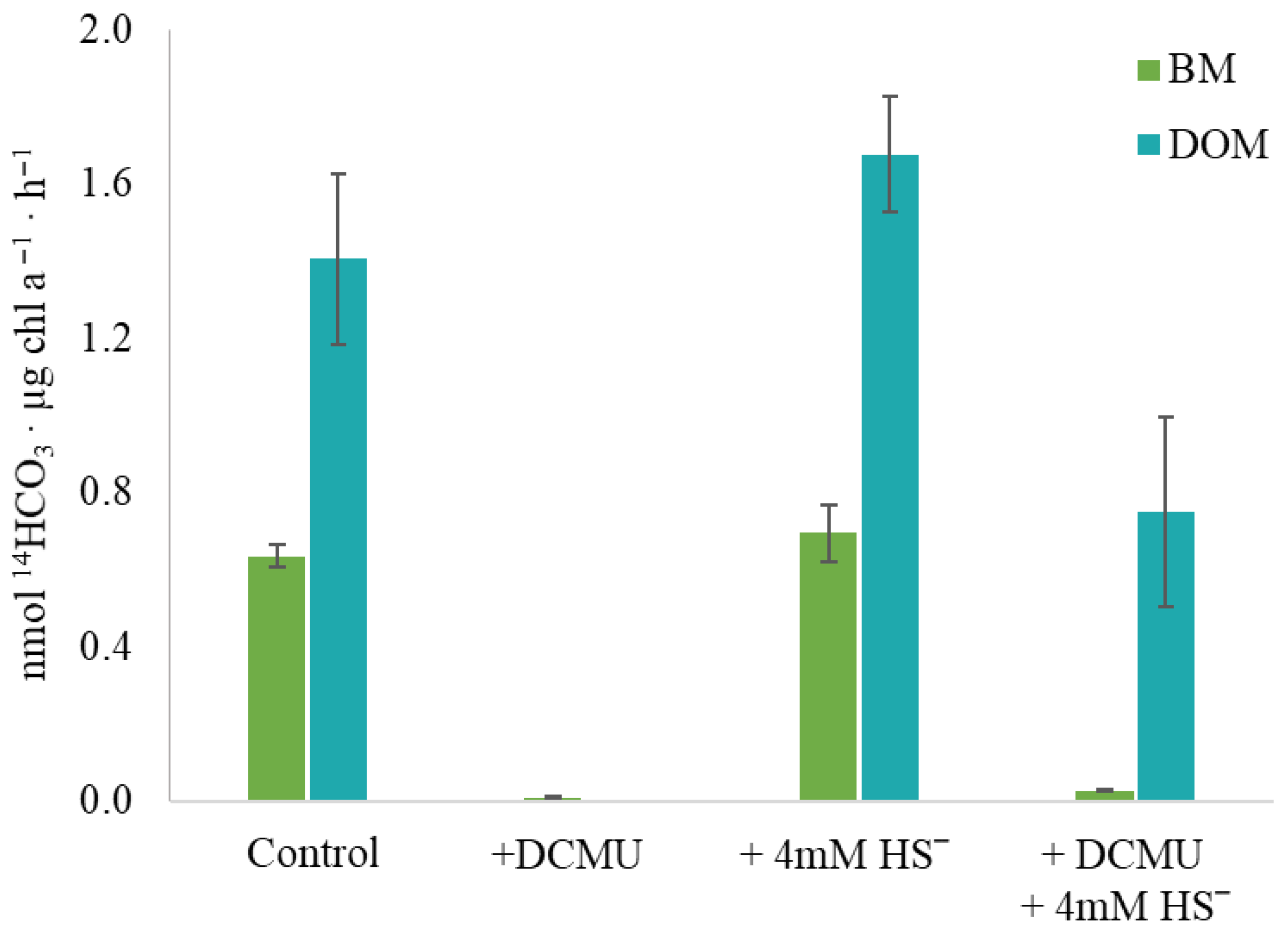
2.6. Anoxygenic Photosynthesis
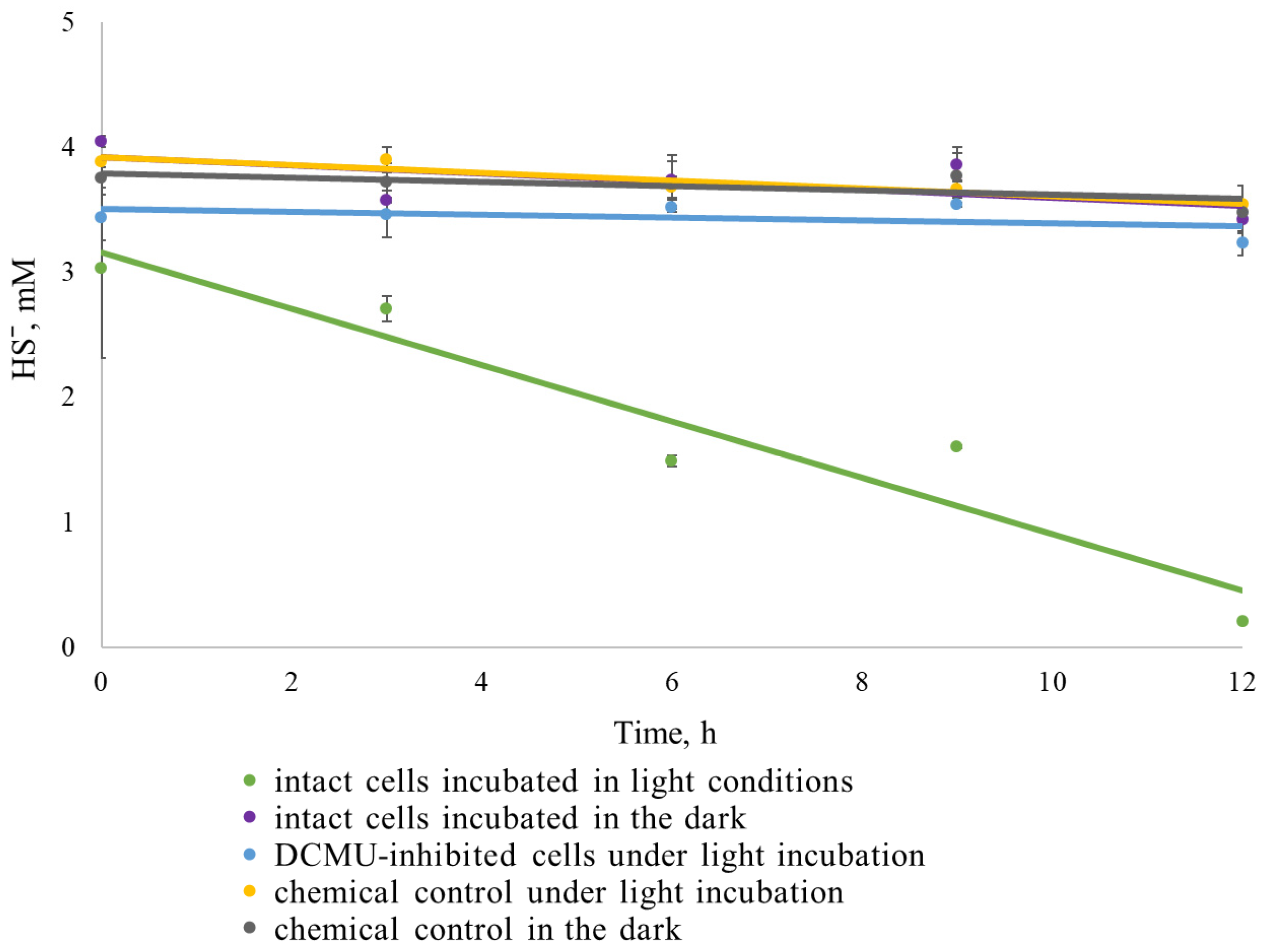
2.7. Biologically Mediated and Physiological Sulfide Oxidation
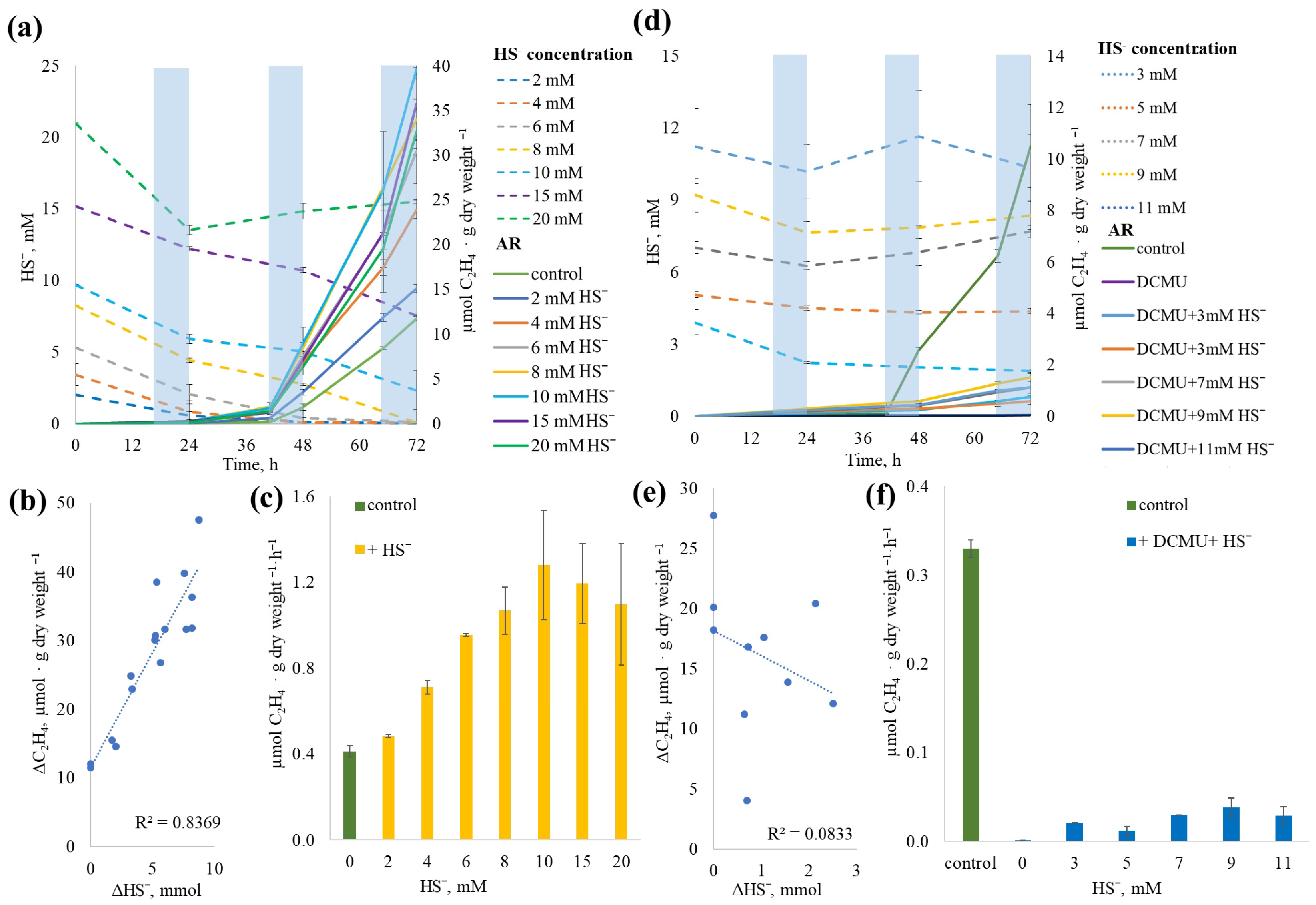
2.8. The Effect of Sulfide Concentration on NF
3. Discussion
3.1. Nitrogen Fixation Among Cyanobacteria with “Desulfo”-Type of nif Genes
3.2. Hypothesis on the Origin of the Nitrogenase Operon
3.3. Nitrogenase Activity
3.3.1. Nitrogenase Activity in Oxic Conditions
3.3.2. Anoxygenic Photosynthesis and Sulfide-Dependent Anaerobic Nitrogenase Activity
3.4. Absence of Growth in Nitrogen-Free Medium
3.5. Ecological Significance
4. Materials and Methods
4.1. Object of the Study
4.2. Cultivation and Biomass Measurements
4.3. Preparation of Sodium Sulfide Stock Solutions and Sulfide Analysis
4.4. Anoxygenic 14HCO3− Photoassimilation
4.5. Nitrogenase Activity Experiments
4.5.1. Preparation of Biomass
4.5.2. AR Assay
4.5.3. Experimental Setup
4.5.4. Data Processing and Normalization
4.6. Comparison of the Rate of Chemical and Biological Oxidation of Sulfide
4.7. Genome Analysis and Phylogenetic Tree Construction
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Howarth, R.W.; Marino, R.; Cole, J.J. Nitrogen Fixation in Freshwater, Estuarine, and Marine Ecosystems. 2. Biogeochemical Controls. Limnol. Oceanogr. 1988, 33, 688–701. [Google Scholar] [CrossRef]
- Capone, D.G.; Burns, J.A.; Montoya, J.P.; Subramaniam, A.; Mahaffey, C.; Gunderson, T.; Carpenter, E.J. Nitrogen Fixation by Trichodesmium Spp.: An Important Source of New Nitrogen to the Tropical and Subtropical North Atlantic Ocean. Glob. Biogeochem. Cycles 2005, 19, GB2024. [Google Scholar] [CrossRef]
- Sorokin, D.Y.; Banciu, H.L.; Muyzer, G. Functional Microbiology of Soda Lakes. Curr. Opin. Microbiol. 2015, 25, 88–96. [Google Scholar] [CrossRef]
- Cornejo-Castillo, F.M.; Zehr, J.P. Hopanoid Lipids May Facilitate Aerobic Nitrogen Fixation in the Ocean. Proc. Natl. Acad. Sci. USA 2019, 116, 18269–18271. [Google Scholar] [CrossRef]
- Stal, L.J. Nitrogen Fixation in Cyanobacteria. Encycl. Life Sci. ELS 2015, 1–9. [Google Scholar] [CrossRef]
- Fay, P. Oxygen Relations of Nitrogen Fixation in Cyanobacteria. Microbiol. Rev. 1992, 56, 340–373. [Google Scholar] [CrossRef]
- Helman, Y.; Tchernov, D.; Reinhold, L.; Shibata, M.; Ogawa, T.; Schwarz, R.; Ohad, I.; Kaplan, A. Genes Encoding A-Type Flavoproteins Are Essential for Photoreduction of O2 in Cyanobacteria. Curr. Biol. 2003, 13, 230–235. [Google Scholar] [CrossRef]
- Latifi, A.; Ruiz, M.; Zhang, C.-C. Oxidative Stress in Cyanobacteria. FEMS Microbiol. Rev. 2009, 33, 258–278. [Google Scholar] [CrossRef] [PubMed]
- Grim, S.L.; Dick, G.J. Photosynthetic Versatility in the Genome of Geitlerinema Sp. PCC 9228 (Formerly Oscillatoria limnetica ’Solar Lake’), a Model Anoxygenic Photosynthetic Cyanobacterium. Front. Microbiol. 2016, 7, 1546. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.; Jørgensen, B.B.; Revsbech, N.P.; Poplawski, R. Adaptation to Hydrogen Sulfide of Oxygenic and Anoxygenic Photosynthesis among Cyanobacteria. Appl. Environ. Microbiol. 1986, 51, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Belkin, S.; Arieli, B.; Padan, E. Sulfide Dependent Electron Transport in Oscillatoria limnetica. Isr. J. Bot. 1982, 31, 199–200. [Google Scholar]
- Belkin, S.; Shahak, Y.; Padan, E. Anoxygenic Photosynthetic Electron Transport. In Methods in Enzymology; Academic Press: Salt Lake City, UT, USA, 1988; Volume 167, pp. 380–386. [Google Scholar]
- Weisshaar, H.; Böger, P. Pathways of Hydrogen Uptake in the Cyanobacterium Nostoc muscorum. Arch. Microbiol. 1985, 142, 349–353. [Google Scholar] [CrossRef]
- Oremland, R.S. Nitrogen Fixation Dynamics of Two Diazotrophic Communities in Mono Lake, California. Appl. Environ. Microbiol. 1990, 56, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Villbrandt, M.; Stal, L.J. The Effect of Sulfide on Nitrogen Fixation in Heterocystous and Non-Heterocystous Cyanobacterial Mat Communities. Algol. Stud. Hydrobiol. Algol. Stud. 1996, 83, 549–563. [Google Scholar] [CrossRef]
- Chen, M.-Y.; Teng, W.-K.; Zhao, L.; Han, B.-P.; Song, L.-R.; Shu, W.-S. Phylogenomics Uncovers Evolutionary Trajectory of Nitrogen Fixation in Cyanobacteria. Mol. Biol. Evol. 2022, 39, msac171. [Google Scholar] [CrossRef]
- Bolhuis, H.; Severin, I.; Confurius-Guns, V.; Wollenzien, U.I.; Stal, L.J. Horizontal Transfer of the Nitrogen Fixation Gene Cluster in the Cyanobacterium Microcoleus chthonoplastes. ISME J. 2010, 4, 121–130. [Google Scholar] [CrossRef]
- Nies, F.; Woerner, S.; Wunsch, N.; Armant, O.; Sharma, V.; Hesselschwerdt, A.; Lamparter, T. Characterization of Phormidium lacuna Strains from the North Sea and the Mediterranean Sea for Biotechnological Applications. Process Biochem. 2017, 59, 194–206. [Google Scholar] [CrossRef]
- Kuever, J. The Family Desulfovibrionaceae. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 75–86. [Google Scholar]
- Kuever, J. The Family Syntrophaceae. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 123–144. [Google Scholar]
- Marter, P.; Freese, H.M.; Ringel, V.; Brinkmann, H.; Pradella, S.; Rohde, M.; Jarek, M.; Spröer, C.; Wagner-Döbler, I.; Overmann, J.; et al. Superior Resolution Profiling of the Coleofasciculus Microbiome by Amplicon Sequencing of the Complete 16S rRNA Gene and ITS Region. Environ. Microbiol. Rep. 2025, 17, e70066. [Google Scholar] [CrossRef]
- Samylina, O.S.; Sinetova, M.A.; Kupriyanova, E.V.; Starikov, A.Y.; Sukhacheva, M.V.; Dziuba, M.V.; Tourova, T.P. Ecology and Biogeography of the ‘Marine Geitlerinema’ Cluster and a Description of Sodalinema orleanskyi sp. nov., Sodalinema gerasimenkoae sp. nov., Sodalinema stali sp. nov. and Baaleninema simplex gen. et sp. nov. (Oscillatoriales, Cyanobacteria). FEMS Microbiol. Ecol. 2021, 97, fiab104. [Google Scholar] [CrossRef]
- Stal, L.J.; Krumbein, W.E. Isolation and Characterization of Cyanobacteria from a Marine Microbial Mat. Bot. Mar. 1985, 28, 351–365. [Google Scholar] [CrossRef]
- Zorz, J.K.; Sharp, C.; Kleiner, M.; Gordon, P.M.; Pon, R.T.; Dong, X.; Strous, M. A Shared Core Microbiome in Soda Lakes Separated by Large Distances. Nat. Commun. 2019, 10, 4230. [Google Scholar] [CrossRef]
- Ataeian, M.; Vadlamani, A.; Haines, M.; Mosier, D.; Dong, X.; Kleiner, M.; Hawley, A.K. Proteome and Strain Analysis of Cyanobacterium Candidatus “Phormidium Alkaliphilum” Reveals Traits for Success in Biotechnology. iScience 2021, 24, 103405. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Noonan, A.J.; Qiu, Y.; Dofher, K.; Kieft, B.; Mottahedeh, S.; Hallam, S.J. The Survivor Strain: Isolation and Characterization of Phormidium Yuhuli AB48, a Filamentous Phototactic Cyanobacterium with Biotechnological Potential. Front. Bioeng. Biotechnol. 2022, 10, 932695. [Google Scholar] [CrossRef]
- de Wit, R.; van Gemerden, H. Oxidation of Sulfide to Thiosulfate by Microcoleus chtonoplastes. FEMS Microbiol. Ecol. 1987, 3, 7–13. [Google Scholar] [CrossRef]
- De Wit, R.; Van Boekel, W.H.; Van Gemerden, H. Growth of the Cyanobacterium Microcoleus chthonoplastes on Sulfide. FEMS Microbiol. Ecol. 1988, 4, 203–209. [Google Scholar] [CrossRef]
- Samylina, O.S.; Namsaraev, Z.B.; Grouzdev, D.S.; Slobodova, N.V.; Zelenev, V.V.; Borisenko, G.V.; Sorokin, D.Y. The Patterns of Nitrogen Fixation in Haloalkaliphilic Phototrophic Communities of Kulunda Steppe Soda Lakes (Altai, Russia). FEMS Microbiol. Ecol. 2019, 95, fiz174. [Google Scholar] [CrossRef] [PubMed]
- Kessler, P.S.; Daniel, C.; Leigh, J.A. Ammonia Switch-off of Nitrogen Fixation in the Methanogenic Archaeon Methanococcus maripaludis: Mechanistic Features and Requirement for the Novel GlnB Homologues, NifI1 and NifI2. J. Bacteriol. 2001, 183, 882–889. [Google Scholar] [CrossRef]
- Boyd, E.S.; Costas, A.M.; Hamilton, T.L.; Mus, F.; Peters, J.W. Evolution of Molybdenum Nitrogenase during the Transition from Anaerobic to Aerobic Metabolism. J. Bacteriol. 2015, 197, 1690–1699. [Google Scholar] [CrossRef]
- DeWeerd, K.A.; Mandelco, L.; Tanner, R.S.; Woese, C.R.; Suflita, J.M. Desulfomonile tiedjei gen. nov. and sp. nov., a Novel Anaerobic, Dehalogenating, Sulfate-Reducing Bacterium. Arch. Microbiol. 1990, 154, 23–30. [Google Scholar] [CrossRef]
- Rippka, R.; Waterbury, J.B. The Synthesis of Nitrogenase by Non-Heterocystous Cyanobacteria. FEMS Microbiol. Lett. 1977, 2, 83–86. [Google Scholar] [CrossRef]
- Marcia, M.; Ermler, U.; Peng, G.; Michel, H. A New Structure—Based Classification of Sulfide: Quinone Oxidoreductases. Proteins Struct. Funct. Bioinform. 2010, 78, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Bronstein, M.; Schütz, M.; Hauska, G.; Padan, E.; Shahak, Y. Cyanobacterial Sulfide-Quinone Reductase: Cloning and Heterologous Expression. J. Bacteriol. 2000, 182, 3336–3344. [Google Scholar] [CrossRef]
- Namsaraev, Z.B.; Kolganova, T.V.; Patutina, E.O.; Tsyrenova, D.D.; Samylina, O.S. Cyanobacterial Diversity in the Alkaline Lake Khilganta during the Dry and Wet Periods. Microbiology 2018, 87, 583–590. [Google Scholar] [CrossRef]
- Novoselov, A.A.; Konstantinov, A.O.; Lim, A.G.; Goetschl, K.E.; Loiko, S.V.; Mavromatis, V.; Pokrovsky, O.S. Mg-Rich Authigenic Carbonates in Coastal Facies of the Vtoroe Zasechnoe Lake (Southwest Siberia): First Assessment and Possible Mechanisms of Formation. Minerals 2019, 9, 763. [Google Scholar] [CrossRef]
- Novoselov, A.; Konstantinov, A.; Konstantinova, E.; Simakova, Y.; Lim, A.; Kurasova, A.; Loiko, S.; Pokrovsky, O.S. Semiarid Lakes of Southwestern Siberia as Sentinels of On-Going Climate Change: Hydrochemistry, the Carbon Cycle, and Modern Carbonate Mineral Formation. Atmosphere 2023, 14, 1624. [Google Scholar] [CrossRef]
- Bok, F.; Moog, H.C.; Brendler, V. The Solubility of Oxygen in Water and Saline Solutions. Front. Nucl. Eng. 2023, 2, 1158109. [Google Scholar] [CrossRef]
- Debelius, B.; Gómez-Parra, A.; Forja, J.M. Oxygen Solubility in Evaporated Seawater as a Function of Temperature and Salinity. Hydrobiologia 2009, 632, 157–165. [Google Scholar] [CrossRef]
- Stal, L.J. Cyanobacterial Mats and Stromatolites. In Ecology of Cyanobacteria II: Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 65–125. [Google Scholar]
- Padan, E.; Cohen, Y. Anoxygenic Photosynthesis. Biol. Cyanobacteria 1982, 19, 215–235. [Google Scholar]
- Griesbeck, C.; Hauska, G.; Schütz, M. Biological sulfide oxidation: Sulfide–quinone reductase (SQR), the primary reaction. Recent Res. Dev. Microbiol. 2000, 4, 179–203. [Google Scholar]
- Nonaka, A.; Yamamoto, H.; Kamiya, N.; Kotani, H.; Yamakawa, H.; Tsujimoto, R.; Fujita, Y. Accessory Proteins of the Nitrogenase Assembly, NifW, NifX/NafY, and NifZ, Are Essential for Diazotrophic Growth in the Nonheterocystous Cyanobacterium Leptolyngbya boryana. Front. Microbiol. 2019, 10, 495. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Burgess, B.K. Evidence for the Direct Interaction of the NifW Gene Product with the MoFe Protein. J. Biol. Chem. 1996, 271, 9764–9770. [Google Scholar] [CrossRef]
- Rubio, L.M.; Ludden, P.W. Biosynthesis of the Iron-Molybdenum Cofactor of Nitrogenase. Annu. Rev. Microbiol. 2008, 62, 93–111. [Google Scholar] [CrossRef]
- Bergman, B.; Gallon, J.R.; Rai, A.N.; Stal, L.J. N2 Fixation by Non-Heterocystous Cyanobacteria1. FEMS Microbiol. Rev. 1997, 19, 139–185. [Google Scholar] [CrossRef]
- Boros, E.; Kolpakova, M. A Review of the Defining Chemical Properties of Soda Lakes and Pans: An Assessment on a Large Geographic Scale of Eurasian Inland Saline Surface Waters. PLoS ONE 2018, 13, e0202205. [Google Scholar] [CrossRef] [PubMed]
- Lameck, A.S.; Skutai, J.; Boros, E. Review of Chemical Properties of Inland Soda and Saline Waters in East Africa (Rift Valley Region). J. Hydrol. Reg. Stud. 2023, 46, 101323. [Google Scholar] [CrossRef]
- Jones, B.E.; Grant, W.D.; Duckworth, A.W.; Owenson, G.G. Microbial Diversity of Soda Lakes. Extremophiles 1998, 2, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, D.Y.; Rusanov, I.I.; Pimenov, N.V.; Tourova, T.P.; Abbas, B.; Muyzer, G. Sulfidogenesis under Extremely Haloalkaline Conditions in Soda Lakes of Kulunda Steppe (Altai, Russia): Sulfidogenesis in Soda Lakes. FEMS Microbiol. Ecol. 2010, 73, 278–290. [Google Scholar] [CrossRef]
- Sorokin, D.Y.; Berben, T.; Melton, E.D.; Overmars, L.; Vavourakis, C.D.; Muyzer, G. Microbial Diversity and Biogeochemical Cycling in Soda Lakes. Extremophiles 2014, 18, 791–809. [Google Scholar] [CrossRef] [PubMed]
- Herbst, D.B. Potential Salinity Limitations on Nitrogen Fixation in Sediments from Mono Lake, California. Int. J. Salt Lake Res. 1998, 7, 261–274. [Google Scholar] [CrossRef]
- Samylina, O.S.; Sapozhnikov, F.V.; Gainanova, O.Y.; Ryabova, A.V.; Nikitin, M.A.; Sorokin, D.Y. Algo-Bacterial Communities of the Kulunda Steppe (Altai Region, Russia) Soda Lakes. Microbiology 2014, 83, 849–860. [Google Scholar] [CrossRef]
- Namsaraev, Z.B. Application of Extinction Coefficients for Quantification of Chlorophylls and Bacteriochlorophylls. Microbiology 2009, 78, 794–797. [Google Scholar] [CrossRef]
- Cline, J.D. Spectrophotometric Determination of Hydrogen Sulfide in Natural Waters. Limnol. Oceanogr. 1969, 14, 454–458. [Google Scholar] [CrossRef]
- Hardy, R.W.; Holsten, R.D.; Jackson, E.K.; Burns, R.C. The Acetylene-Ethylene Assay for N₂ Fixation: Laboratory and Field Evaluation. Plant Physiol. 1968, 43, 1185–1207. [Google Scholar] [CrossRef] [PubMed]
- Rzhetsky, A.; Nei, M. A Simple Method for Estimating and Testing Minimum-Evolution Trees. Mol. Biol. Evol. 1992, 9, 945–967. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v3: An Online Tool for the Display and Annotation of Phylogenetic and Other Trees. Nucleic Acids Res. 2016, 44, W242–W245. [Google Scholar] [CrossRef] [PubMed]
- van den Belt, M.; Gilchrist, C.; Booth, T.J.; Chooi, Y.H.; Medema, M.H.; Alanjary, M. CAGECAT: The CompArative GEne Cluster Analysis Toolbox for Rapid Search and Visualisation of Homologous Gene Clusters. BMC Bioinform. 2023, 24, 181. [Google Scholar] [CrossRef]
- Schütz, M.; Shahak, Y.; Padan, E.; Hauska, G. Sulfide-Quinone Reductase from Rhodobacter capsulatus: Purification, Cloning, and Expression. J. Biol. Chem. 1997, 272, 9890–9894. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]

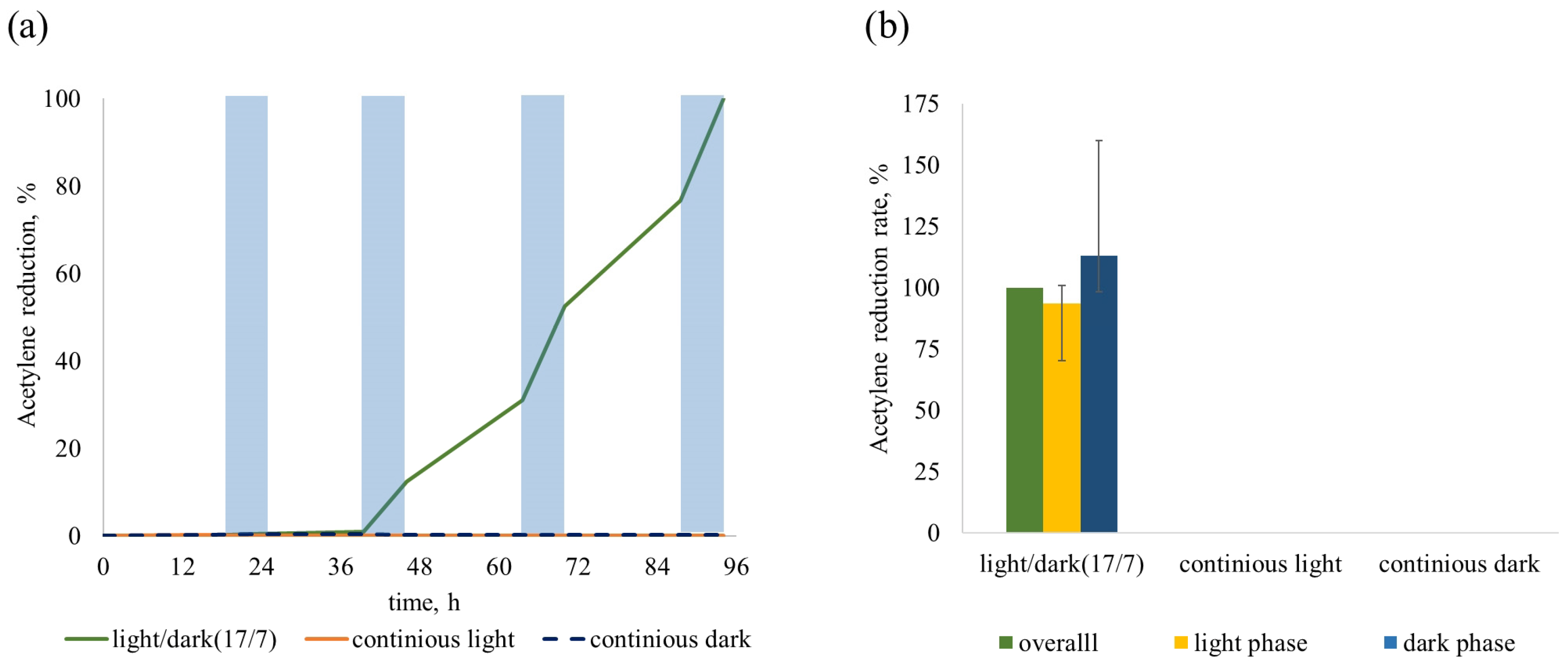

| Day of Measurements | Light/Dark | Continuous | Continuous | ||
|---|---|---|---|---|---|
| (17/7 h) Regime | Light | Darkness | |||
| 24 h | Light Phase ARR | Dark Phase ARR | 24 h ARR | 24 h ARR | |
| ARR | |||||
| 1 | 0.02 ± 0.02 | 0 | 0.05 ± 0.05 | 0 | 0.02 ± 0.02 |
| 2 | 0.41 ± 0.14 | 0.02 ± 0.01 | 1.45 ± 0.09 | 0 | 0 |
| 3 | 1.37 ± 0.07 | 0.88 ± 0.11 | 2.7 ± 0.23 | 0 | 0 |
| 4 | 1.63 ± 0.11 | 1.14 ± 0.08 | 2.94 ± 0.15 | 0 | 0 |
| Strains | Ecology (Geography) | Nitrogen Fixation Evidence | Refs. | |||
|---|---|---|---|---|---|---|
| Original Name | Phylogenetic Affiliation | Genetics | Nitrogenase Activity | Growth in Nitrogen-Free Medium | ||
| Microcoleus chthonoplastes ‘strain 11’ | Sodalinema stali CCY9619 (t.a. species, type strain) | Marine (microbial mats of Mellum, North Sea, Germany) | nifH gene belongs to “desulfo”-type | AR: Anaerobically with DCMU in the light during 24 h-long experiment | No | [22,23] |
| Phormidium lacuna HE10JO | Sodalinema stali HE10JO (t.a. species) | Marine (rockpools in Helgoland, North Sea, Germany) | nif-gene cluster nifVBSUHDKENB | n/d | Weak (2–3 fold increase in OD750 in 7 days both in light and light/dark regimes), statistically insignificant | [18] |
| Phormidium lacuna HE10DO | Sodalinema sp. HE10DO (ph.c.) | Marine (rockpools in Helgoland, North Sea, Germany) | n/d | n/d | Weak (about 2,5 fold increase in OD750 in 7 days both in light and light/dark regimes), statistically significant | [18] |
| Phormidium yuhuli AB48 | Sodalinema sp. AB48 (ph.c.) | Saline alkaline (industrial photobioreactor environment initially designed to grow Spirulina, Canada) | nif-gene cluster nifVBSUHDKENB | Proteome: nifBSUHDK-genes expression in culture | No (under aerobic conditions with either continuous lighting or a 12 h light/dark regime) | [26] |
| Сa. “Phormidium alkaliphilum” PBR-2020 | Sodalinema sp. PBR-2020 (ph.c.) | Soda lake, (Cariboo Plateau, Canada) | nif-gene cluster nifVBSUHDKENB | Proteome: nifHDK-genes expression in photobioreactor and natural environment | n/d | [24,25,26] |
| Geitlerinema sp. P-1104 | Sodalinema sp. P-1104 (t.a. genus) | Soda lake Petukhovskoe (Kulunda steppe, Russia) | nifH gene belongs to “desulfo”-type; nif-gene cluster nifVBSUHDKE | AR: Only in the light/dark regime aerobically or anaerobically (with DCMU + sulfide or sulfide alone) during 96 h-long experiment | No (under aerobic conditions under natural light, continuous lighting and light/dark regime) | [22], this study |
| Microcoleus chthonoplastes PCC7420 | Coleofasciculus chthonoplastes PCC7420 (t.a. species, type strain) | Marine (Sippewissett Salt Marsh, USA) | nifH gene belongs to “desulfo”-type; nif-gene cluster nifBSUHDKENB | AR: No (anaerobically with DCMU during 30 h-long experiment) qRT-PCR: No nifHDK genes expression in culture | No | [17] |
| Microcoleus sp. CCY0002 | Coleofasciculus sp. CCY0002 (ph.c.) | Marine (Schiermonnikoog, North Sea, The Netherlands) | nifHDK genes belong to “desulfo”-type | AR: No (anaerobically with DCMU during 30 h-long experiment) qRT-PCR: No nifHDK genes expression in culture, but positive result on nifH gene expression in natural environment | No | [17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosyakova, A.I.; Rusanov, I.I.; Tourova, T.P.; Zakharova, E.E.; Sorokin, D.Y.; Pimenov, N.V.; Burakova, O.S. Nitrogen Fixation and Anoxygenic Photosynthesis in Filamentous Non-Heterocystous Cyanobacterium of the Genus Sodalinema Isolated from Soda Lake. Plants 2025, 14, 3558. https://doi.org/10.3390/plants14233558
Kosyakova AI, Rusanov II, Tourova TP, Zakharova EE, Sorokin DY, Pimenov NV, Burakova OS. Nitrogen Fixation and Anoxygenic Photosynthesis in Filamentous Non-Heterocystous Cyanobacterium of the Genus Sodalinema Isolated from Soda Lake. Plants. 2025; 14(23):3558. https://doi.org/10.3390/plants14233558
Chicago/Turabian StyleKosyakova, Anastasia I., Igor I. Rusanov, Tatiana P. Tourova, Elena E. Zakharova, Dimitry Y. Sorokin, Nikolay V. Pimenov, and Olga S. Burakova. 2025. "Nitrogen Fixation and Anoxygenic Photosynthesis in Filamentous Non-Heterocystous Cyanobacterium of the Genus Sodalinema Isolated from Soda Lake" Plants 14, no. 23: 3558. https://doi.org/10.3390/plants14233558
APA StyleKosyakova, A. I., Rusanov, I. I., Tourova, T. P., Zakharova, E. E., Sorokin, D. Y., Pimenov, N. V., & Burakova, O. S. (2025). Nitrogen Fixation and Anoxygenic Photosynthesis in Filamentous Non-Heterocystous Cyanobacterium of the Genus Sodalinema Isolated from Soda Lake. Plants, 14(23), 3558. https://doi.org/10.3390/plants14233558






