Mycorrhizal Abundance and Its Interaction with Cereal Root Traits and Crop Productivity in Organically Managed Cereal/Legume Intercropping
Abstract
1. Introduction
2. Results
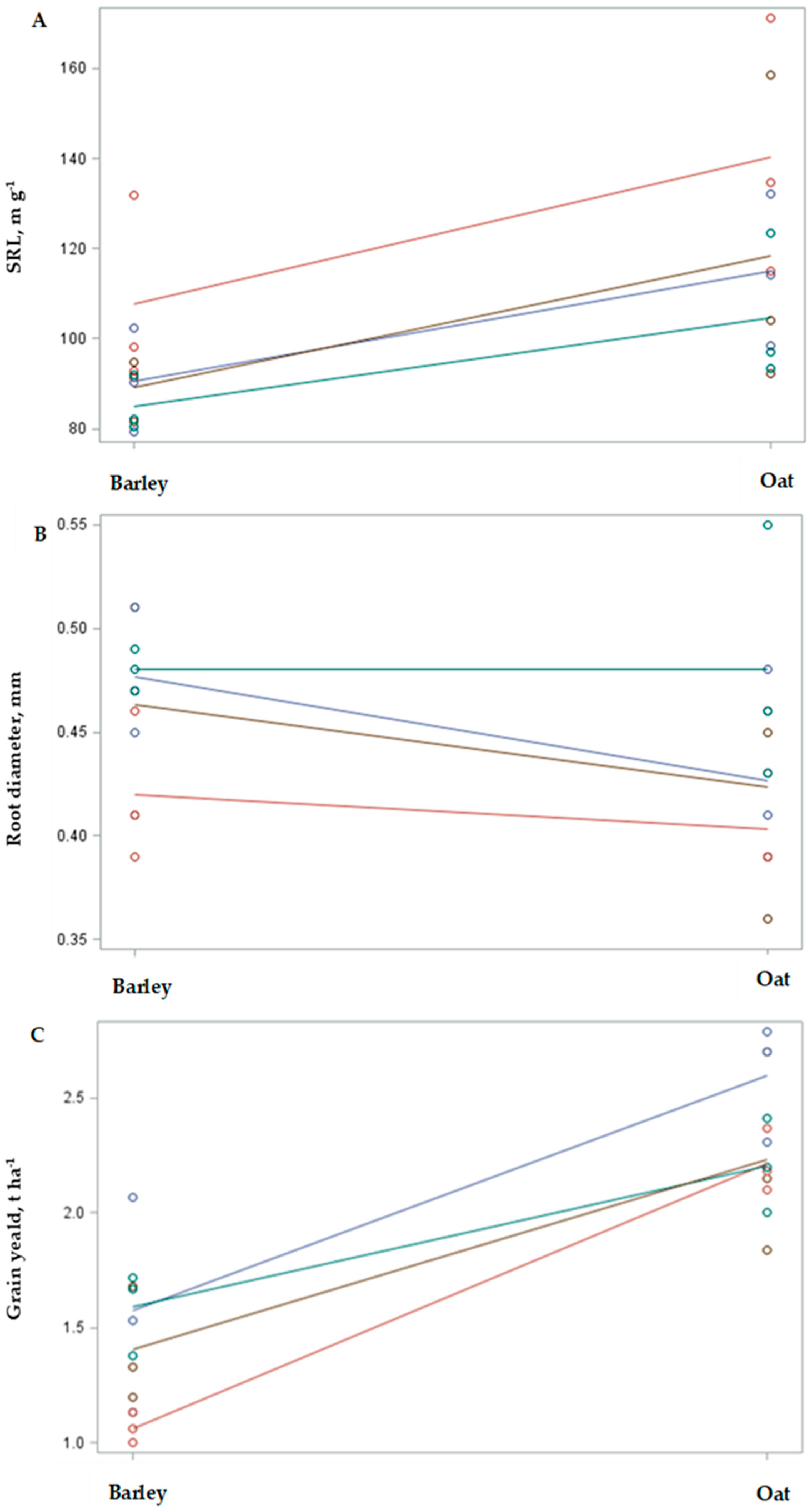
2.1. Intercropping Effect on Physical Root Parameters
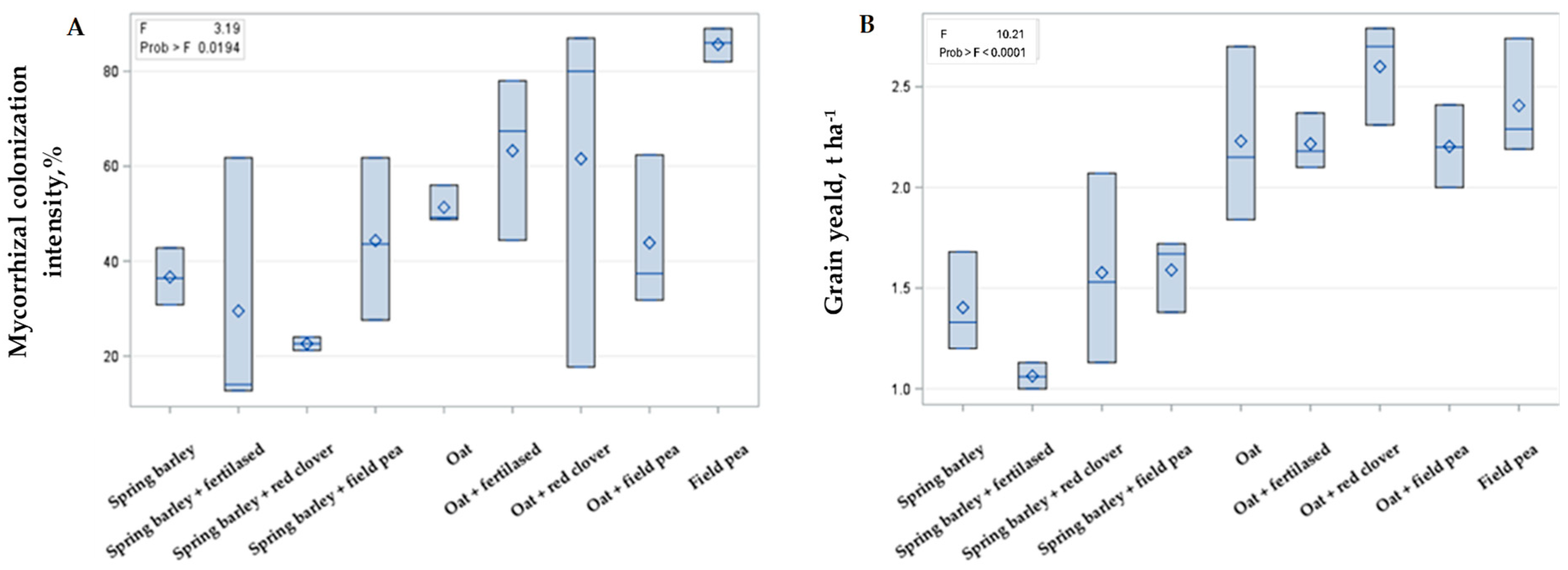
2.2. Intercropping Effect on Mycorrhization and Cereals Yield
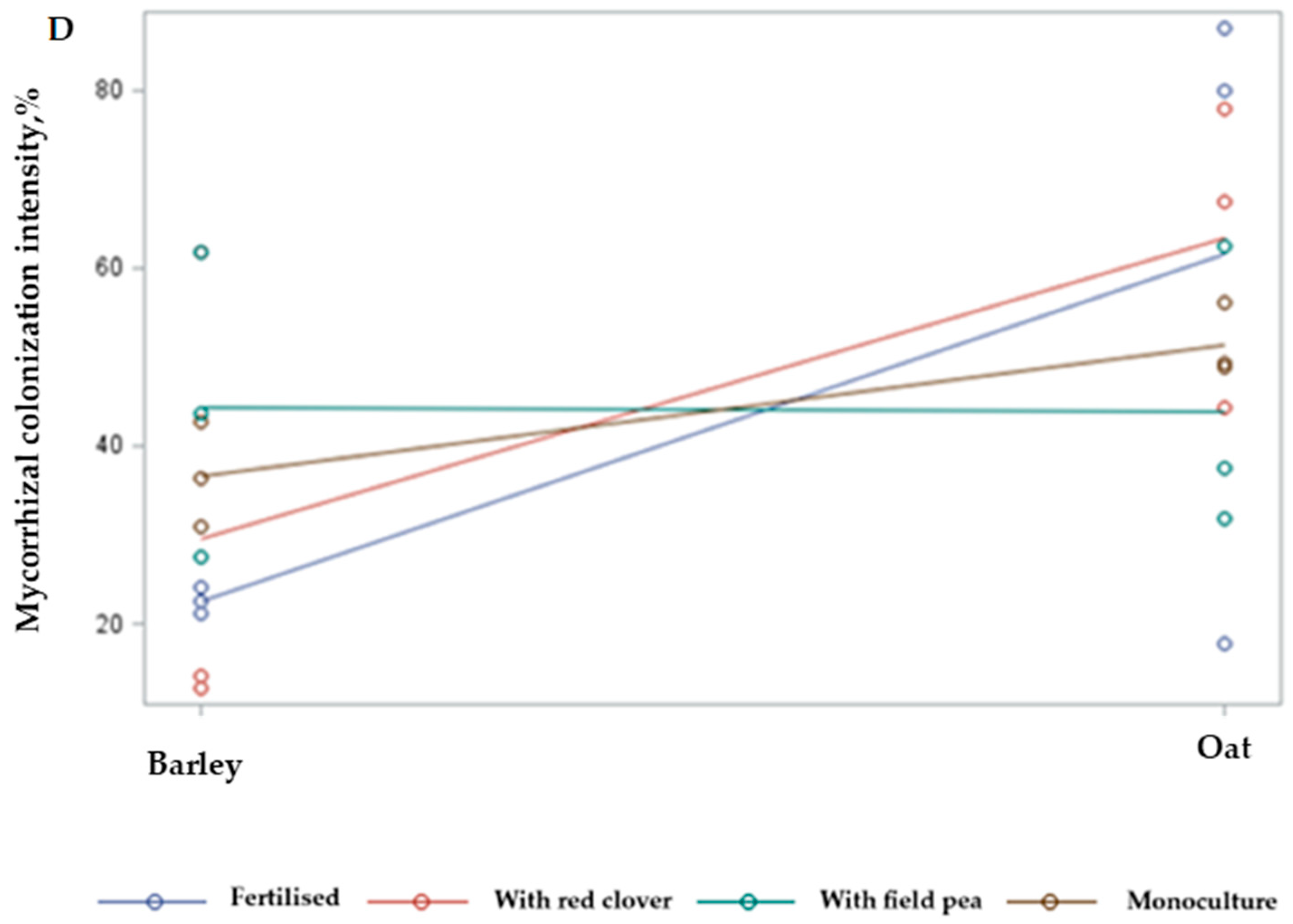
2.3. Interaction Effects of Selected Species and Management Practices
2.4. Relationship Between Physical Root Parameters, Mycorrhization Colonization, and Cereals Yield
3. Discussion
3.1. Intercropping Effect on the Physical Root Parameters of the Main Crop
3.2. Intercropping Effect on the Mycorrhization Intensity and Its Interaction with Main Crop Yield
4. Materials and Methods
4.1. Experimental Site
4.2. Meteorological Conditions
4.3. Experimental Design and Treatments
4.4. Mycorrhizal Colonization Intensity (M%)
4.5. Investigations of Physical Root Parameters
4.6. Crop Productivity
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bezner Kerr, R.; Liebert, J.; Kansanga, M.; Kpienbaareh, D. Human and social values in agroecology: A review. Elem. Sci. Anthr. 2022, 10, 00090. [Google Scholar] [CrossRef]
- Šarūnaitė, L.; Toleikienė, M.; Arlauskienė, A.; Razbadauskienė, K.; Deveikytė, I.; Supronienė, S.; Semaškienė, R.; Kadžiulienė, Ž. Effects of Pea (Pisum sativum L.) Cultivars for Mixed Cropping with Oats (Avena sativa L.) on Yield and Competition Indices in an Organic Production System. Plants 2022, 11, 2936. [Google Scholar] [CrossRef]
- Maitra, S.; Palai, J.B.; Manasa, P.; Kumar, D.P. Potential of Intercropping System in Sustaining Crop Productivity. Int. J. Agric. Environ. Biotechnol. 2019, 12, 39–45. [Google Scholar] [CrossRef]
- Ajal, J.; Jäck, O.; Vico, G.; Weih, M. Functional trait space in cereals and legumes grown in pure and mixed cultures is influenced more by cultivar identity than crop mixing. Perspect. Plant Ecol. Evol. Syst. 2021, 50, 125612. [Google Scholar] [CrossRef]
- Anil, L.; Park, R.; Phipps, R.H.; Miller, F.A. Temperate intercropping of cereals for forage: A review of the potential for growth and utilization with particular reference to the UK. Grass Forage Sci. 1998, 53, 301–317. [Google Scholar] [CrossRef]
- Alaru, M.; Talgre, L.; Luik, A.; Tein, B.; Eremeev, V.; Loit, E. Barley undersown with red clover in organic and conventional systems: Nitrogen aftereffect on legume growth. Zemdirb. Agric. 2017, 104, 131–138. [Google Scholar] [CrossRef]
- Stomph, T.J.; Dordas, C.; Baranger, A.; de Rijk, J.; Dong, B.; Evers, J.; Gu, C.; Li, L.; Simon, J.; Jensen, E.S. Designing intercrops for high yield, yield stability and efficient use of resources: Are there principles? Adv. Agron. 2020, 161, 1–50. [Google Scholar] [CrossRef]
- Lithourgidis, A.S.; Vasilakoglou, I.B.; Dhima, K.V.; Dordas, C.A.; Yiakoulaki, M.D. Forage yield and quality of common vetch mixtures with oat and triticale in two seeding ratios. Field Crops Res. 2006, 99, 106–113. [Google Scholar] [CrossRef]
- Regehr, A.; Oelbermann, M.; Videla, C.; Echarte, L. Gross nitrogen mineralization and immobilization in temperate maize-soybean intercrops. Plant Soil 2015, 391, 353–365. [Google Scholar] [CrossRef]
- Chapagain, T.; Riseman, A. Barley–pea intercropping: Effects on land productivity, carbon and nitrogen transformations. Field Crops Res. 2014, 166, 18–25. [Google Scholar] [CrossRef]
- Alvey, S.; Bagayoko, M.; Neumann, G.; Buerkert, A. Cereal/legume rotations affect chemical properties and biological activities in two West African soils. Plant Soil 2001, 231, 45–54. [Google Scholar] [CrossRef]
- Bargaz, A.; Noyce, G.L.; Fulthorpe, R.; Carlsson, G.; Furze, J.R.; Jensen, E.S.; Dhiba, D.; Isaac, M.E. Species interactions enhance root allocation, microbial diversity and P acquisition in intercropped wheat and soybean under P deficiency. Appl. Soil Ecol. 2017, 120, 179–188. [Google Scholar] [CrossRef]
- Chamkhi, I.; Cheto, S.; Geistlinger Zeroual, Y.; Kouisni, L.; Bargaz, A.; Ghoulam, C. Legume-based intercropping systems promote beneficial rhizobacterial community and crop yield under stressing conditions. Ind. Crops Prod. 2022, 183, 114958. [Google Scholar] [CrossRef]
- Veršulienė, A.; Hirte, J.; Ciulla, F.; Camenzind, M.; Don, A.; Durand-Maniclas, F.; Heinemann, H.; Herrera, J.M.; Hund, A.; Seidel, F.; et al. Wheat varieties show consistent differences in root colonization by mycorrhiza across a European pedoclimatic gradient. Eur. J. Soil Sci. 2024, 75, e13543. [Google Scholar] [CrossRef]
- Trouvelot, S.; Bonneau, L.; Redecker, D.; van Tuinen, D.; Adrian, M.; Wipf, D. Arbuscular mycorrhiza symbiosis in viticulture: A review. Agron. Sustain. Dev. 2015, 35, 1449–1467. [Google Scholar] [CrossRef]
- Kakouridis, A.; Hagen, J.A.; Kan, M.P.; Mambelli, S.; Feldman, L.J.; Herman, D.J.; Weber, P.K.; Pett-Ridge, J.; Firestone, M.K. Routesto roots: Direct evidence of water transport by arbuscular mycorrhizal fungi to host plants. New Phytol. 2022, 236, 221. [Google Scholar] [CrossRef] [PubMed]
- Trinchera, A.; Testani, E.; Roccuzzo, G.; Campanelli, G.; Ciaccia, C. Agroecological Service Crops Drive Plant Mycorrhization in Organic Horticultural Systems. Microorganisms 2021, 9, 410. [Google Scholar] [CrossRef]
- Frey, S.D. Mycorrhizal fungi as mediators of soil organic matter dynamics. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 237–259. [Google Scholar] [CrossRef]
- Leake, J. Mycorrhizas and the terrestrial carbon cycle: Roles in global carbon sequestration and plant community composition. In Fungi in the Environment; Gadd, G., Watkinson, S., Dyer, P., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 161–185. [Google Scholar]
- Li, M.Y.; Wang, W.; Mo, F.; Ren, A.T.; Wang, Z.Y.; Zhu, Y.; Xiong, Y.-C. Seven-year long-term inoculation with Funneliformis mosseae increases maize yield and soil carbon storage evidenced by in situ 13C-labeling in a dryland. Sci. Total Environ. 2024, 944, e173975. [Google Scholar] [CrossRef]
- Beltrano, J.; Ruscitti, M.; Arango, M.C.; Ronco, M. Effects of arbuscular mycorrhiza inoculation on plant growth, biological and physiological parameters and mineral nutrition in pepper grown under different salinity and p levels. J. Soil Sci. Plant Nutr. 2013, 13, 123–141. [Google Scholar] [CrossRef]
- Paszkowski, U.; Boller, T. The growth defect of lrt1, a maize mutant lacking lateral roots, can be complemented by symbiotic fungi or high phosphate nutrition. Planta 2002, 214, 584–590. [Google Scholar] [CrossRef]
- Veršulienė, A.; Kadžienė, G.; Kochiieru, M.; Pranaitienė, S.; Meškauskienė, L.; Auškalnienė, O. Response of spring barley root and soil physical properties to changes under cover crop and different tillage. Zemdirb. Agric. 2022, 109, 291–296. [Google Scholar] [CrossRef]
- Hirte, J.; Walder, F.; Hess, J.; Büchi, L.; Colombi, T.; van der Heijden, M.G.; Mayer, J. Enhanced root carbon allocation through organic farming is restricted to topsoils. Sci. Total Environ. 2021, 755, 143551. [Google Scholar] [CrossRef]
- Ghafoor, A.; Poeplau, C.; Kätterer, T. Fate of straw- and root-derived carbon in a Swedish agricultural soil. Biol. Fertil. Soils 2017, 53, 257–267. [Google Scholar] [CrossRef]
- Paustian, K.; Lehmann, J.; Ogle, S.; Reay, D.; Robertson, G.P.; Smith, P. Climate-smart soils. Nature 2016, 532, 49–57. [Google Scholar] [CrossRef]
- Heinemann, H.; Durand-Maniclas, F.; Seidel, F.; Ciulla, F.; Bárcena, T.G.; Camenzind, M.; Corrado, S.; Csűrös, Z.; Czakó, Z.; Eylenbosch, D.; et al. Optimising Root and Grain Yield Through Variety Selection in Winter Wheat Across a European Climate Gradient. Eur. J. Soil Sci. 2025, 76, e70077. [Google Scholar] [CrossRef]
- Heinemann, H.; Hirte, J.; Seidel, F.; Don, A. Increasing Root Biomass Derived Carbon Input to Agricultural Soils by Genotype Selection—A Review. Plant Soil 2023, 490, 19–30. [Google Scholar] [CrossRef]
- Mathew, I.; Shimelis, H.; Shayanowako, A.I.T.; Laing, M.; Chaplot, V. Genome-wide association study of drought tolerance and biomass allocation in wheat. PLoS ONE 2019, 14, e0225383. [Google Scholar] [CrossRef]
- Lynch, J.P. Rightsizing root phenotypes for drought resistance. J. Exp. Bot. 2018, 69, 3279–3292. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Lynch, J.P. Reduced crown root number improves water acquisition under water defi cit stress in maize (Zea mays L.). J. Exp. Bot. 2016, 67, 4545–4557. [Google Scholar] [CrossRef]
- Durand-Maniclas, F.; Heinemann, H.; Seidel, F.; Ciulla, F.; de la Bárcena, T.G.; Camenzind, M.; Corrado, S.; Csűrös, Z.; Czakó, Z.; Eylenbosch, D.; et al. Linking root length and surface area to yield: Variety-specific root plasticity in winter wheat 3 across contrasting European environments. Ann. Bot. 2025, mcaf155. [Google Scholar] [CrossRef]
- Kochiieru, M.; Veršulienė, A.; Shatkovska, K.; Feiza, V.; Seibutis, V. Mechanism of Interaction between Earthworms and Root Parameters on Cambisol. Agronomy 2024, 14, 1536. [Google Scholar] [CrossRef]
- Kochiieru, M.; Veršulienė, A.; Feiza, V.; Feizienė, D. Trend for Soil CO2 Efflux in Grassland and Forest Land in Relation with Meteorological Conditions and Root Parameters. Sustainability 2023, 15, 7193. [Google Scholar] [CrossRef]
- Kadžienė, G.; Munkholm, L.J.; Mutegi, J.K. Root growth conditions in the topsoil as affected by tillage intensity. Geoderma 2011, 166, 66–73. [Google Scholar] [CrossRef]
- Seethepalli, A.; Dhakal, K.; Griffiths, M.; Guo, H.; Freschet, G.T.; York, L.M. RhizoVision Explorer: Open-Source Software for Root Image Analysis and Measurement Standardization. AoB Plants 2021, 13, 056. [Google Scholar] [CrossRef] [PubMed]
- Bargaz, A.; Isaac, M.E.; Jensen, E.S.; Carlsson, G. Intercropping of faba bean with wheat under low water availability promotes faba bean nodulation and root growth in deeper soil layers. Procedia Environ. Sci. 2015, 29, 111–112. [Google Scholar] [CrossRef]
- Schiefelbein, J.W.; Benfey, P.N. The development of plant roots: New approaches to underground problems. Plant Cell 1991, 3, 1147–1154. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Badri, D.V.; Vivanco, J.M. Regulation and function of root exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef]
- Smith, S.; De Smet, I. Root system architecture: Insights from Arabidopsis and cereal crops. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1441–1452. [Google Scholar] [CrossRef]
- Li, L.; Tilman, D.; Lambers, H.; Zhang, F.-S. Plant diversity and overyielding: Insights from belowground facilitation of intercropping in agriculture. New Phytol. 2014, 203, 63–69. [Google Scholar] [CrossRef]
- Latati, M.; Bargaz, A.; Belarbi, B.; Lazali, M.; Benlahrech, S.; Tellah, S.; Kaci, G.; Drevon, J.J.; Ounane, S.M. The intercropping common bean with maize improves the rhizobial efficiency, resource use and grain yield under low phosphorus availability. Eur. J. Agron. 2016, 72, 80–90. [Google Scholar] [CrossRef]
- Latati, M.; Blavet, D.; Alkama, N.; Laoufi, H.; Drevon, J.J.; Gérard, F.; Pansu, M.; Ounane, S.M. The intercropping cowpea-maize improves soil phosphorus availability and maize yields in an alkaline soil. Plant Soil 2014, 385, 181–191. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; Jared, L.; Burton, A.J.; Allen, M.F.; Ruess, R.W.; Hendrick, R.L. Fine Root Architecture of Nine North American Trees. Ecol. Monogr. 2002, 72, 293–309. [Google Scholar] [CrossRef]
- Zamora, D.S.; Jose, S.; Nair, P.K.R. Morphological plasticity of cotton roots in response to interspecific competition with pecan in an alley cropping system in the southern United States. Agrofor. Syst. 2006, 69, 107–116. [Google Scholar] [CrossRef]
- Birouste, M.; Kazakou, E.; Blanchard, A.; Roumet, C. Plant traits and decomposition: Are the relationships for roots comparable to those for leaves? Ann. Bot. 2012, 109, 463–472. [Google Scholar] [CrossRef]
- Craine, J.M.; Froehle, J.; Tilman, D.G.; Wedin, D.A.; Chapin, F.S. The relationships among root and leaf traits of 76 grassland species and relative abundance along fertility and disturbance gradients. Oikos 2001, 93, 274–285. [Google Scholar] [CrossRef]
- Hummel, I.; Vile, D.; Violle, C. Relating root structure and anatomy to whole plant functioning in 14 herbaceous. Mediterranean species. New Phytol. 2007, 173, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Wahl, S.; Ryser, P. Root tissue structure is linked to ecological strategies of grasses. New Phytol. 2000, 148, 459–471. [Google Scholar] [CrossRef]
- Kammoun, B.; Journet, E.P.; Justes, E.; Bedoussac, L. Cultivar grain yield in durum wheat-grain legume intercrops could be estimated from sole crop yields and interspecific interaction index. Front. Plant Sci. 2021, 12, 733705. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, Z.; Liao, D.; Raza, M.A.; Wang, B.; Zhang, J.; Chen, J.; Feng, L.; Wu, X.; Liu, C.; et al. Uptake and utilization of nitrogen, phosphorus and potassium as related to yield advantage in maize-soybean intercropping under different row configurations. Sci. Rep. 2020, 10, 47–53. [Google Scholar] [CrossRef]
- Gong, X.; Dang, K.; Lv, S.; Zhao, G.; Tian, L.; Luo, Y.; Feng, B. Interspecific root interactions and water-use efficiency of intercropped proso millet and mung bean. Eur. J. Agron. 2020, 115, 1260342020. [Google Scholar] [CrossRef]
- Latati, M.; Dokukin, P.; Aouiche, A.; Rebouh, N.Y.; Takouachet, R.; Hafnaoui, E.; Hamdani, F.Z.; Bacha, F.; Ounane, S.M. Species interactions improve above-ground biomass and land use efficiency in intercropped wheat and chickpea under low soil inputs. Agronomy 2019, 9, 765. [Google Scholar] [CrossRef]
- Lu, X. Effect of intercropping soybean on the diversity of the rhizosphere soil arbuscular mycorrhizal fungi communities in wheat fields. Clean Soil Air Water 2022, 50, 2100014. [Google Scholar] [CrossRef]
- Xiao, D.; Tan, Y.; Liu, X.; Yang, R.; Zhang, W.; He, X.; Wang, K. Effects of different legume species and densities on arbuscular mycorrhizal fungal communities in a karst grassland ecosystem. Sci. Total Environ. 2019, 678, 551–558. [Google Scholar] [CrossRef]
- Higo, M.; Isobe, K.; Miyazawa, Y.; Matsuda, Y.; Drijber, A.R.; Torigoe, Y. Molecular diversity and distribution of indigenous arbuscular mycorrhizal communities colonizing roots of two different winter cover crops in response to their root proliferation. J. Microbiol. 2016, 54, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Korniłłowicz-Kowalska, T.; Wojdyło-Kotwica, B.; Kwiatkowska, E. Changes in the spore numbers of am fungi and in am colonisation of roots of clovers and grasses on a peat-muck soil with respect to mineral fertilisation. Pak. J. Bot. 2016, 48, 729–738. [Google Scholar]
- Jannouraa, R.; Brunsb, C.; Joergensena, R.G. Organic fertilizer effects on pea yield, nutrient uptake, microbial root colonization and soil microbial biomass indices in organic farming systems. Eur. J. Agron. 2013, 49, 32–41. [Google Scholar] [CrossRef]
- Bilalis, D.; Karkanis, A.; Angelopoulou, F.; Travlos, I.; Ntoniadis, A.; Ntatsi, G.; Lazaridi, E.; Savvas, D. Effect of Organic and Mineral Fertilization on Root Growth and Mycorrhizal Colonization of Pea Crops (Pisum sativum L.). Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Hortic. 2015, 72, 1843–5254. [Google Scholar] [CrossRef]
- Zhang, R.; Mu, Y.; Li, X.; Li, S.; Sang, P.; Wang, X.; Wu, H.; Xu, N. Response of the arbuscular mycorrhizal fungi diversity and community in maize and soybean rhizosphere soil and roots to intercropping systems with different nitrogen application rates. Sci. Total Environ. 2020, 740, 139810. [Google Scholar] [CrossRef]
- Bini, D.; dos Santos, C.A.; da Silva, M.C.P.; Bonfim, J.A.; Cardoso, E.J.B.N. Intercropping Acacia mangium stimulates AMF colonization and soil phosphatase activity in Eucalyptus grandis. Sci. Agric. 2018, 75, 102–110. [Google Scholar] [CrossRef]
- Koskey, G.; Avio, L.; Turrini, A.; Sbrana, C.; Bàrberi, P. Durum wheat-lentil relay intercropping enhances soil mycorrhizal activity but does not alter structure of arbuscular mycorrhizal fungal community within roots. Agric. Ecosyst. Environ. Vol. 2023, 357, 108696. [Google Scholar] [CrossRef]
- Stefani, F.; Dupont, S.; Laterrière, M.; Knox, R.; Ruan, Y.; Hamel, C.; Hijri, M. Similar arbuscular mycorrhizal fungal communities in 31 durum wheat cultivars (Triticum turgidum L. var. durum) under field conditions in Eastern Canada. Front. Plant Sci. 2020, 11, 1206. [Google Scholar] [CrossRef]
- Boudabbous, K.; Bouhaouel, I.; Jerbi, M.; Chamekh, Z.; Karmous, C.; Benaissa, N.; Trifa, Y.; Sahli, A.; Amara, H.S.; Araus, J.L. Relationships between Mycorrhizal attributes and stable carbon and azote isotopes in a semi-arid environment as influenced by durum wheat cultivars and salinity level. J. Soil Sci. Plant Nutr. 2022, 22, 4327–4343. [Google Scholar] [CrossRef]
- Ryan, M.H.; Graham, J.H. Little evidence that farmers should consider abundance or diversity of arbuscular mycorrhizal fungi when managing crops. New Phytol. 2018, 220, 1092–1107. [Google Scholar] [CrossRef]
- Kirk, A.; Entz, M.; Fox, S.; Tenuta, M. Mycorrhizal colonization, P uptake and yield of older and modern wheats under organic management. Can. J. Plant Sci. 2011, 91, 663–667. [Google Scholar] [CrossRef]
- Rillig, M.C.; Aguilar-Trigueros, C.A.; Camenzind, T.; Cavagnaro, T.R.; Degrune, F.; Hohmann, P.; Lammel, D.R.; Mansour, I.; Roy, J.; Heijden, M.G.A.; et al. Why farmers should manage the 449 arbuscular mycorrhizal symbiosis. New Phytol. 2019, 222, 1171–1175. [Google Scholar] [CrossRef]
- Grace, C.; Stribely, D.P. A safer procedure for routine staining of vesicular-arbuscular mycorrhizal fungi. Mycol. Res. 1991, 95, 1160–1162. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kouch, J.; Gianinazzi-Pearson, V. Mesure dutaux de mycorhization VA d’un systeme radiculaire. Recherche demethods d’estimation ayant une signification fonctionnelle. In Physiological and Genetical Aspects of Mycorrhizae; Gianinazzi-Pearson, V., Gianinazzi, S., Eds.; INRA: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Trinchera, A.; Testani, E.; Ciaccia, C.; Campanelli, G.; Leteo, F.; Canali, S. Effects induced by living mulch on rhizosphere interactions in organic artichoke: The cultivar’s adaptive strategy. Renew. Agric. Food Syst. 2016, 32, 214–223. [Google Scholar] [CrossRef]
- Nuixe, M.; Traoré, A.S.; Blystone, S.; Bonny, J.M.; Pages, G.; Picon-Cochard, C. Specific root length as an indicator of environmental change. Plant Biosyst. 2007, 141, 426–442. [Google Scholar] [CrossRef]

| Correlation Matrix | ||||||||
|---|---|---|---|---|---|---|---|---|
| Root Length, m m−2 | Root Diameter, mm | Root Volume, cm3 | Root Dry Mass, mg m−2 | SRL, m g−1 | RTD, g cm−3 | M, % | Cereals Yield, t ha−1 | |
| Root length, m m−2 | 1.0000 | −0.5014 * | 0.5834 * | 0.6325 * | 0.6830 * | −0.2758 | −0.1739 | 0.0806 |
| Root diameter, mm | −0.5014 * | 1.0000 | 0.2676 | 0.1858 | −0.7752 ** | −0.1124 | 0.1747 | 0.0917 |
| Root volume, mm3 | 0.5834 * | 0.2676 | 1.0000 | 0.7596 ** | 0.0989 | −0.6507 * | −0.1747 | 0.0954 |
| Dry mass, mg m−2 | 0.6325 * | 0.1858 | 0.7596 ** | 1.0000 | −0.0915 | −0.0694 | −0.1847 | 0.0270 |
| SRL, m g−1 | 0.6830 * | −0.7752 ** | 0.0989 | −0.0915 | 1.0000 | −0.3892 | −0.0242 | 0.1195 |
| RTD, g cm−3 | −0.2758 | −0.1124 | −0.6507 * | −0.0694 | −0.3892 | 1.0000 | 0.0713 | −0.1222 |
| M, % | −0.1739 | 0.1747 | −0.1747 | −0.1847 | −0.0242 | 0.0713 | 1.0000 | 0.6273 * |
| Cereals yield, t ha−1 | 0.0806 | 0.0917 | 0.0954 | 0.0270 | 0.1195 | −0.1222 | 0.6273 * | 1.0000 |
| Year | 2024 | Long—Term Mean (1924–2024) |
|---|---|---|
| Annual mean air temperature, °C | 9.6 | 6.6 |
| Difference from long—term mean, °C | +3.0 | − |
| Average air temperature during the plant’s vegetation period, °C | 18.2 | 15.8 |
| Difference from long—term mean, °C | +2.4 | − |
| Total annual precipitation, mm | 586.2 | 569.3 |
| Difference from long—term mean, mm | +16.9 | − |
| Total amount of precipitation during the plant’s vegetation period, mm | 200.3 | 263.6 |
| Difference from long—term mean, mm | −63.3 | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veršulienė, A.; Garbaras, A.; Kadžienė, G.; Shamshitov, A.; Toleikienė, M. Mycorrhizal Abundance and Its Interaction with Cereal Root Traits and Crop Productivity in Organically Managed Cereal/Legume Intercropping. Plants 2025, 14, 3561. https://doi.org/10.3390/plants14233561
Veršulienė A, Garbaras A, Kadžienė G, Shamshitov A, Toleikienė M. Mycorrhizal Abundance and Its Interaction with Cereal Root Traits and Crop Productivity in Organically Managed Cereal/Legume Intercropping. Plants. 2025; 14(23):3561. https://doi.org/10.3390/plants14233561
Chicago/Turabian StyleVeršulienė, Agnė, Andrius Garbaras, Gražina Kadžienė, Arman Shamshitov, and Monika Toleikienė. 2025. "Mycorrhizal Abundance and Its Interaction with Cereal Root Traits and Crop Productivity in Organically Managed Cereal/Legume Intercropping" Plants 14, no. 23: 3561. https://doi.org/10.3390/plants14233561
APA StyleVeršulienė, A., Garbaras, A., Kadžienė, G., Shamshitov, A., & Toleikienė, M. (2025). Mycorrhizal Abundance and Its Interaction with Cereal Root Traits and Crop Productivity in Organically Managed Cereal/Legume Intercropping. Plants, 14(23), 3561. https://doi.org/10.3390/plants14233561








