Study on Chemical Diversity, Antioxidant and Antibacterial Activities, and HaCaT Cytotoxicity of Camphora tenuipilis (a Traditional Aromatic Plant from Xishuangbanna)
Abstract
1. Introduction
2. Results
2.1. Chemical Composition Analysis of Five C. tenuipilis Essential Oils
2.2. Total Polyphenol Content and Antioxidant Capacity
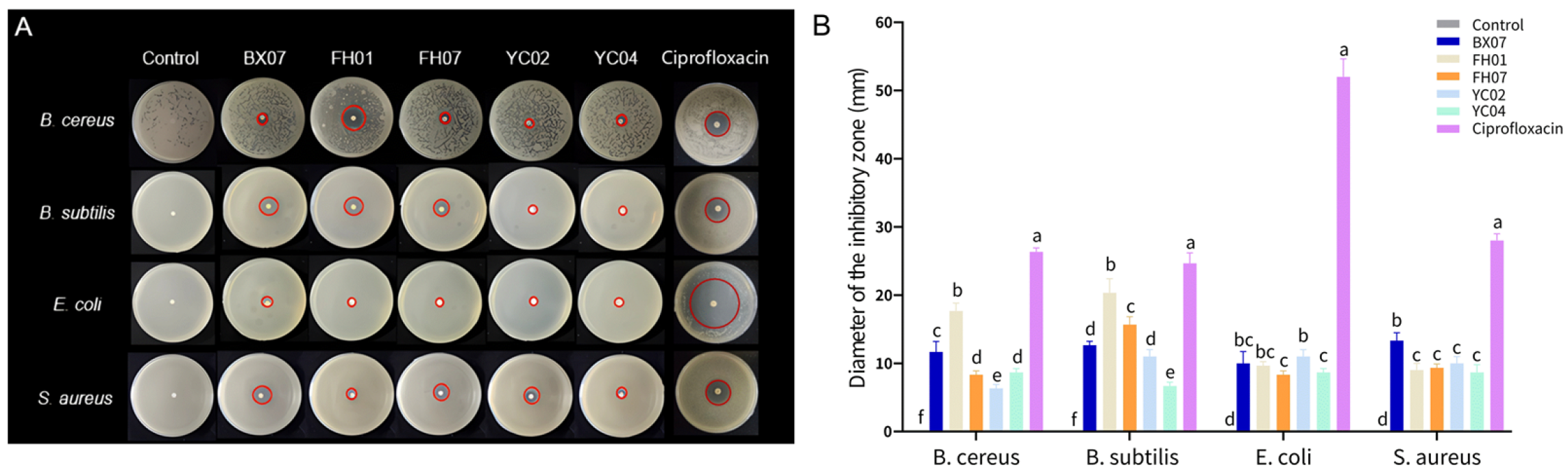
2.3. Antibacterial Activity
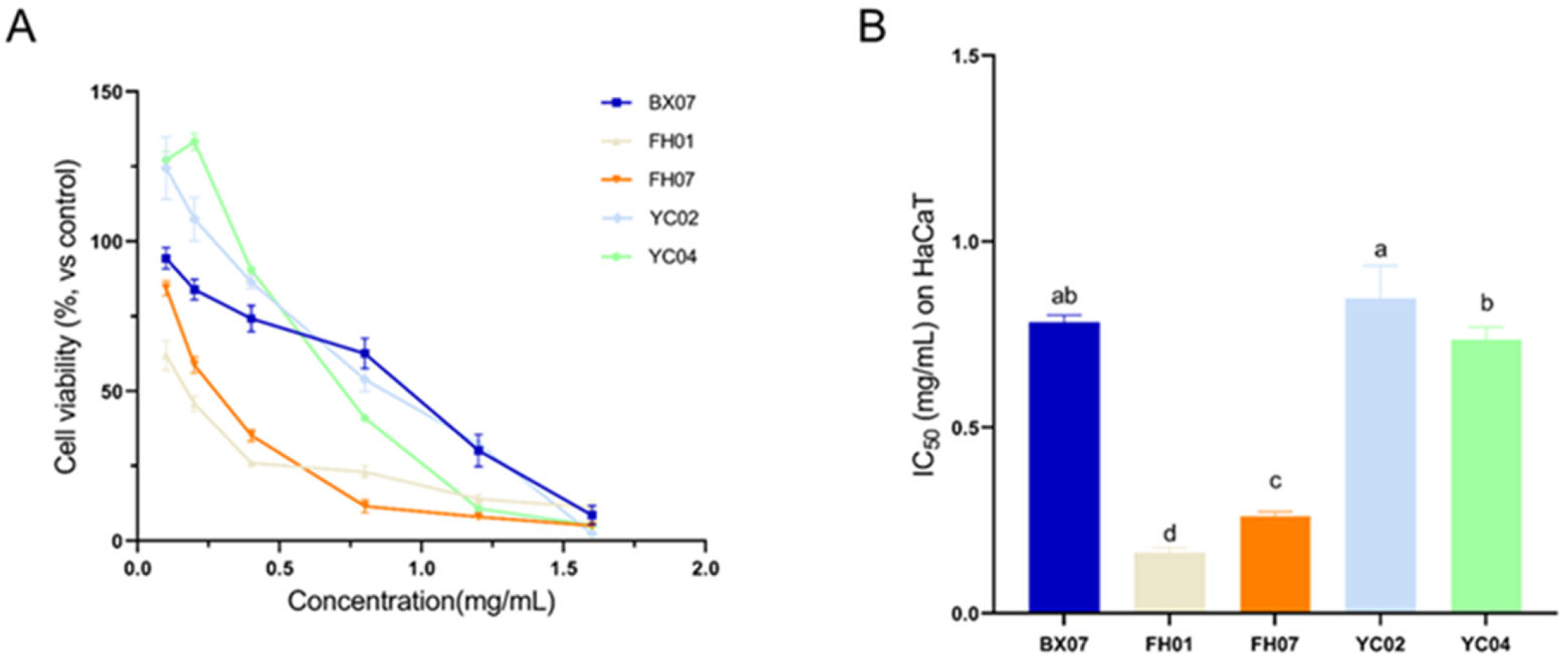
2.4. Cytotoxicity to Human Keratinocyte (HaCaT)
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Bacterial Strains
4.2. Isolation of Essential Oils
4.3. Analysis of the Essential Oil Components
4.4. Determination of Total Polyphenolic Content
4.5. Determination of Total Antioxidant Capacity
4.5.1. ABTS Radical Cation Scavenging Activity
4.5.2. DPPH Radical Scavenging Activity
4.5.3. Ferric Reducing Antioxidant Power
4.6. Antibacterial Activity Assay
4.7. Cytotoxicity Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-Azino-bis-3-ethylbenzthioazoline-6-sulfonic acid |
| ANOVA | Analysis of Variance |
| BHA | Butylated hydroxyanisole |
| BHT | Butylated hydroxytoluene |
| CCK-8 | Cell Counting Kit-8 |
| CFU | Colony Forming Unit |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| DIZ | Diameter of the Inhibitory Zone |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | Dimethyl Sulfoxide |
| EO | Essential Oil |
| FRAP | Ferric-Reducing Antioxidant Power |
| GAE | Gallic Acid Equivalents |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| IC50 | Half Maximal Inhibitory Concentration |
| INT | p-Iodonitrotetrazolium violet |
| LSD | Least Significant Difference |
| MBC | Minimum Bactericidal Concentration |
| MIC | Minimum Inhibitory Concentration |
| NIST | National Institute of Standards and Technology |
| RI | Retention Indices |
| RT | Retention Time |
| TAC | Total Antioxidant Capacity |
| TE | Trolox Equivalents |
| TP | Total Polyphenol |
References
- Damasceno, C.S.B.; Fabri Higaki, N.T.; Dias, J.F.G.; Miguel, M.D.; Miguel, O.G. Chemical Composition and Biological Activities of Essential Oils in the Family Lauraceae: A Systematic Review of the Literature. Planta Med. 2019, 85, 1054–1072. [Google Scholar] [CrossRef]
- Malabadi, R.B.; Kolkar, K.P.; Meti, N.T.; Chalannavar, R.K. Camphor tree, Cinnamomum camphora (L.); Ethnobotany and pharmacological updates. Biomedicine 2021, 41, 181–184. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, B.; Yang, Y.; Ferguson, D.K. Phylogeny and taxonomy of Cinnamomum (Lauraceae). Ecol. Evol. 2022, 12, 9378. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, H.; Liu, Y.; Zhu, M.; Wan, Z.; Yan, Y.; Wang, X.; Xiang, Y.; Gao, S.; Jiang, C.; et al. Potential Distribution and Response of Camphora longepaniculata Gamble (Lauraceae) to Climate Change in China. Forests 2025, 16, 338. [Google Scholar] [CrossRef]
- Fazmiya, M.J.A.; Sultana, A.; Rahman, K.; Heyat, M.B.B.; Sumbul; Akhtar, F.; Khan, S.; Appiah, S.C.Y. Current Insights on Bioactive Molecules, Antioxidant, Anti-Inflammatory, and Other Pharmacological Activities of Cinnamomum camphora Linn. Oxid. Med. Cell. Longev. 2022, 2022, 9354555. [Google Scholar] [CrossRef]
- Farias, K.S.; Alves, F.M.; Santos-Zanuncio, V.S.; de Sousa, P.T., Jr.; Silva, D.B.; Carollo, C.A. Global distribution of the chemical constituents and antibacterial activity of essential oils in Lauraceae family: A review. S. Afr. J. Bot. 2023, 155, 214–222. [Google Scholar] [CrossRef]
- Figueiredo, A.; Barroso, J. In Medicinal and Aromatic Plants of the World; Máthé, Á., Ed.; Medicinal and Aromatic Plants (MAP): How Do They Adapt to the Environment. Springer Nature: Berlin, Germany, 2015; pp. 87–112. [Google Scholar]
- Lourenco, S.C.; Moldao-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Wang, T.; Su, E. Electrospinning meets food packaging: A promising pathway towards novel opportunities in food preservation. Food Packag. Shelf Life 2024, 41, 101234. [Google Scholar] [CrossRef]
- Pires, S.M.; Desta, B.N.; Mughini-Gras, L.; Mmbaga, B.T.; Fayemi, O.E.; Salvador, E.M.; Gobena, T.; Majowicz, S.E.; Hald, T.; Hoejskov, P.S.; et al. Burden of foodborne diseases: Think global, act local. Curr. Opin. Food Sci. 2021, 39, 152–159. [Google Scholar] [CrossRef]
- Elbehiry, A.; Abalkhail, A.; Marzouk, E.; Elmanssury, A.E.; Almuzaini, A.M.; Alfheeaid, H.; Alshahrani, M.T.; Huraysh, N.; Ibrahem, M.; Alzaben, F.; et al. An Overview of the Public Health Challenges in Diagnosing and Controlling Human Foodborne Pathogens. Vaccines 2023, 11, 725. [Google Scholar] [CrossRef]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial resistance: Impacts, challenges, and future prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Antioxidants: Reviewing the chemistry, food applications, legislation and role as preservatives. Trends Food Sci. Technol. 2018, 71, 107–120. [Google Scholar] [CrossRef]
- Wojcik, M.; Burzynska-Pedziwiatr, I.; Wozniak, L.A. A review of natural and synthetic antioxidants important for health and longevity. Curr. Med. Chem. 2010, 17, 3262–3288. [Google Scholar] [CrossRef]
- Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martinez-Larranaga, M.R.; Wang, X.; Martinez, M.; Anadon, A.; Martinez, M.A. Synthetic phenolic antioxidants: Metabolism, hazards and mechanism of action. Food Chem. 2021, 353, 129488. [Google Scholar] [CrossRef]
- Mahady, G.B.; Huang, Y.; Doyle, B.J.; Locklear, T. Natural Products As Antibacterial Agents. Bioact. Nat. Prod. 2008, 35, 423–444. [Google Scholar]
- Lee, S.H.; Kim, D.S.; Park, S.H.; Park, H. Phytochemistry and Applications of Cinnamomum camphora Essential Oils. Molecules 2022, 27, 2695. [Google Scholar] [CrossRef]
- Bibow, A.; Oleszek, W. Essential Oils as Potential Natural Antioxidants, Antimicrobial, and Antifungal Agents in Active Food Packaging. Antibiotics 2024, 13, 1168. [Google Scholar] [CrossRef]
- Prakash, B.; Singh, P.P.; Gupta, V.; Raghuvanshi, T.S. Essential oils as green promising alternatives to chemical preservatives for agri-food products: New insight into molecular mechanism, toxicity assessment, and safety profile. Food Chem. Toxicol. 2024, 183, 114241. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Mohammed, D.M.; Korma, S.A.; Alshahrani, M.Y.; Ahmed, A.E.; Ibrahim, E.H.; Salem, H.M.; Alkafaas, S.S.; Saif, A.M.; et al. Medicinal plants: Bioactive compounds, biological activities, combating multidrug-resistant microorganisms, and human health benefits—A comprehensive review. Front. Immunol. 2025, 16, 1491777. [Google Scholar] [CrossRef]
- Aware, C.B.; Patil, D.N.; Suryawanshi, S.S.; Mali, P.R.; Rane, M.R.; Gurav, R.G.; Jadhav, J.P. Natural bioactive products as promising therapeutics: A review of natural product-based drug development. S. Afr. J. Bot. 2022, 151, 512–528. [Google Scholar] [CrossRef]
- Yu, X.-J.; Cheng, B.-Q. Study on The Chemical Constituents from the Essential Oil of Cinnamomum tenuipilis Kosterm. J. Integr. Plant Biol. 1987, 29, 537–540. [Google Scholar]
- Zheng, Y.; Wu, Y.; Xinliang, L.; Chen, Y.; Wang, Y. The complete chloroplast genome of the aromatic tree species Cinnamomum tenuipile Kosterm (Lauraceae). Mitochondrial DNA Part B Resour. 2022, 7, 312–313. [Google Scholar] [CrossRef]
- Cheng, B.; Xu, Y.; Yu, X.; Ding, J. Study on the new perfume plant Cinnamomum tenuipilum. Chem. Ind. For. Prod. 1993, 13, 57–63. [Google Scholar]
- Cheng, B.; Xu, Y.; Zeng, G.; Yu, X.; Ding, J. Ways of propagating Cinnamomum tenuipilum and variation in its leaf essential oil components. Acta Bot. Yunnanica 1993, 15, 78–82. [Google Scholar]
- Yang, T.; Li, J.; Wang, H.X.; Zeng, Y. A geraniol-synthase gene from Cinnamomum tenuipilum. Phytochemistry 2005, 66, 285–293. [Google Scholar] [CrossRef]
- Pucci, M.; Raimondo, S.; Zichittella, C.; Tinnirello, V.; Corleone, V.; Aiello, G.; Moschetti, M.; Conigliaro, A.; Fontana, S.; Alessandro, R. Biological Properties of a Citral-Enriched Fraction of Citrus limon Essential Oil. Foods 2020, 9, 1290. [Google Scholar] [CrossRef]
- Qian, W.; Liu, M.; Fu, Y.; Wang, T.; Zhang, J.; Yang, M.; Sun, Z.; Li, X.; Li, Y. Antimicrobial and Antibiofilm Activities of Citral Against Carbapenem-Resistant Enterobacter cloacae. Foodborne Pathog. Dis. 2020, 17, 459–465. [Google Scholar] [CrossRef]
- Vicenço, C.B.; Silvestre, W.P.; Pauletti, G.F.; Barros, N.M.d.; Schwambach, J. Cinnamomum camphora var. linaloolifera essential oil on pest control: Its effect on Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). Res. Soc. Dev. 2021, 10, 45710716216. [Google Scholar] [CrossRef]
- Ballout, R.; Toufeili, I.; Kharroubi, S.A.; Kassem, I.I. Raw Meat Consumption and Food Safety Challenges: A Survey of Knowledge, Attitudes, and Practices of Consumers in Lebanon. Foods 2023, 13, 118. [Google Scholar] [CrossRef]
- Dos Santos, E.R.Q.; Maia, J.G.S.; Fontes-Junior, E.A.; do Socorro Ferraz Maia, C. Linalool as a Therapeutic and Medicinal Tool in Depression Treatment: A Review. Curr. Neuropharmacol. 2022, 20, 1073–1092. [Google Scholar] [CrossRef]
- Ashaq, B.; Rasool, K.; Habib, S.; Bashir, I.; Nisar, N.; Mustafa, S.; Ayaz, Q.; Nayik, G.A.; Uddin, J.; Ramniwas, S.; et al. Insights into chemistry, extraction and industrial application of lemon grass essential oil -A review of recent advances. Food Chem. X 2024, 22, 101521. [Google Scholar] [CrossRef]
- Rai, A.; Subramaniyan, Y.; Fathima, F.; Rekha, P.D. Broad-spectrum antimicrobial properties of linalool: Supporting its pharmacological use in chronic wound infections by pathogens within the ESKAPE group and polymicrobial biofilms. World J. Microbiol. Biotechnol. 2025, 41, 99. [Google Scholar] [CrossRef]
- Baj, T.; Kowalska, G.; Kowalski, R.; Szymanska, J.; Kai, G.; Coutinho, H.D.M.; Sieniawska, E. Synergistic Antioxidant Activity of Four-Component Mixture of Essential Oils: Basil, Cedarwood, Citronella and Thyme for the Use as Medicinal and Food Ingredient. Antioxidants 2023, 12, 577. [Google Scholar] [CrossRef]
- Al-Qahtani, W.H.; Dinakarkumar, Y.; Arokiyaraj, S.; Saravanakumar, V.; Rajabathar, J.R.; Arjun, K.; Gayathri, P.K.; Nelson Appaturi, J. Phyto-chemical and biological activity of Myristica fragrans, an ayurvedic medicinal plant in Southern India and its ingredient analysis. Saudi J. Biol. Sci. 2022, 29, 3815–3821. [Google Scholar] [CrossRef]
- De Vincenzi, M.; De Vincenzi, A.; Silano, M. Constituents of aromatic plants: Elemicin. Fitoterapia 2004, 75, 615–618. [Google Scholar] [CrossRef]
- Lal, M.; Dutta, S.; Munda, S.; Pandey, S.K. Novel high value elemicin-rich germplasm of lemon grass (Cymbopogon khasianus (Hack)Stapf(ex Bor) from North East India. Ind. Crop. Prod. 2018, 115, 98–103. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Tavaszi-Sarosi, S. Identification and quantification of essential oil content and composition, total polyphenols and antioxidant capacity of Perilla frutescens (L.) Britt. Food Chem. 2019, 275, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.T.; Moon, J.-Y.; Lee, Y.-C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Rahaman, M.M.; Hossain, R.; Herrera-Bravo, J.; Islam, M.T.; Atolani, O.; Adeyemi, O.S.; Owolodun, O.A.; Kambizi, L.; Dastan, S.D.; Calina, D.; et al. Natural antioxidants from some fruits, seeds, foods, natural products, and associated health benefits: An update. Food Sci. Nutr. 2023, 11, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- Parveen, B.; Rajinikanth, V.; Narayanan, M. Natural plant antioxidants for food preservation and emerging trends in nutraceutical applications. Discov. Appl. Sci. 2025, 7, 845. [Google Scholar] [CrossRef]
- Knez, E.; Kadac-Czapska, K.; Grembecka, M. Evaluation of Spectrophotometric Methods for Assessing Antioxidant Potential in Plant Food Samples—A Critical Approach. Appl. Sci. 2025, 15, 5925. [Google Scholar] [CrossRef]
- Tajammal, A.; Siddiqa, A.; Irfan, A.; Azam, M.; Hafeez, H.; Munawar, M.A.; Basra, M.A.R. Antioxidant, molecular docking and computational investigation of new flavonoids. J. Mol. Struct. 2022, 1254, 132189. [Google Scholar] [CrossRef]
- Alizadeh, Z.; Fattahi, M. Essential oil, total phenolic, flavonoids, anthocyanins, carotenoids and antioxidant activity of cultivated Damask Rose (Rosa damascena) from Iran: With chemotyping approach concerning morphology and composition. Sci. Hortic. 2021, 288, 110341. [Google Scholar] [CrossRef]
- Adukwu, E.C.; Bowles, M.; Edwards-Jones, V.; Bone, H. Antimicrobial activity, cytotoxicity and chemical analysis of lemongrass essential oil (Cymbopogon flexuosus) and pure citral. Appl. Microbiol. Biotechnol. 2016, 100, 9619–9627. [Google Scholar] [CrossRef]
- He, R.; Chen, W.; Chen, H.; Zhong, Q.; Zhang, H.; Zhang, M.; Chen, W. Antibacterial mechanism of linalool against L. monocytogenes, a metabolomic study. Food Control 2022, 132, 108533. [Google Scholar] [CrossRef]
- Li, Y.; Ren, F.; Chen, D.; Chen, H.; Chen, W. Antibacterial Mechanism of Linalool against Pseudomonas fragi: A Transcriptomic Study. Foods 2022, 11, 2058. [Google Scholar] [CrossRef] [PubMed]
- Long, N.; Qiu, M.; Zuo, Y.; Deng, H. Antimicrobial Activity and Metabolomic Analysis of Linalool Against Pathogenic Bacteria Methicillin-Resistant Staphylococcus aureus. Infect. Drug Resist. 2025, 18, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Wang, Y.; Jiang, P.; Quek, S. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.G.; Hu, F.; Wei, Z.J. Antibacterial Activity and Mechanism of Ginger Essential Oil against Escherichia coli and Staphylococcus aureus. Molecules 2020, 25, 3955. [Google Scholar] [CrossRef]
- Proestos, C.; Lytoudi, K.; Mavromelanidou, O.K.; Zoumpoulakis, P.; Sinanoglou, V.J. Antioxidant Capacity of Selected Plant Extracts and Their Essential Oils. Antioxidants 2013, 2, 11–22. [Google Scholar] [CrossRef]
- Abbas, M.A.; Lee, G.-Y.; Sayem, S.A.J.; Lee, S.-J.; Park, S.-C. Integrated Phytochemical Profiling, GC-MS Characterization, and In Silico, In Vitro Evaluation of Synergistic Antimicrobial, Antioxidant, and Anti-Inflammatory Activities of Morus alba Bark and Pinus densiflora Extracts with Methyl Gallate. Antioxidants 2025, 14, 1114. [Google Scholar] [CrossRef]
- Lobel, B.T.; Baiocco, D.; Al-Sharabi, M.; Routh, A.F.; Zhang, Z.; Cayre, O.J. Current Challenges in Microcapsule Designs and Microencapsulation Processes: A Review. ACS Appl. Mater. Interfaces 2024, 16, 40326–40355. [Google Scholar] [CrossRef]
- Gupta, C.; Garg, A.P.; Uniyal, R.C.; Kumari, A. Comparative analysis of the antimicrobial activity of cinnamon oil and cinnamon extract on somefood-borne microbes. Afr. J. Microbiol. Res. 2008, 2, 247–251. [Google Scholar]
- Yasin, M.; Younis, A.; Javed, T.; Akram, A.; Ahsan, M.; Shabbir, R.; Ali, M.M.; Tahir, A.; El-Ballat, E.M.; Sheteiwy, M.S.; et al. River Tea Tree Oil: Composition, Antimicrobial and Antioxidant Activities, and Potential Applications in Agriculture. Plants 2021, 10, 2105. [Google Scholar] [CrossRef]
- Poudel, D.K.; Rokaya, A.; Ojha, P.K.; Timsina, S.; Satyal, R.; Dosoky, N.S.; Satyal, P.; Setzer, W.N. The Chemical Profiling of Essential Oils from Different Tissues of Cinnamomum camphora L. and Their Antimicrobial Activities. Molecules 2021, 26, 5132. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, M.; Wu, L.; Zhao, Y.; Wang, Y.; Chen, Y. Antimicrobial activity of essential oils extracted from Litsea cubeba. For. Res. 2022, 2, 2. [Google Scholar] [CrossRef]
- Yang, Y.; Xiang, Q.; Yang, Y.; Shu, D.; Yan, J.; Huang, L.; Yang, X.; Peng, J.; Chen, X.; Yang, G. Chemical Composition and Antimicrobial Activity of Essential Oil of Camphora glanduliferum ‘Honganzhang’. Horticulturae 2025, 11, 67. [Google Scholar] [CrossRef]
- Yang, L.T.Z.; Yang, X.; Li, X. Analysis of essential oil from the root of Litsea cubeba and its antibacterial and antioxidant activities. China Food Addit. 2021, 32, 140–146. [Google Scholar]
- He, J.; Fan, Z.; Jiang, Q.; Yang, X.; Sun, J.; Pang, Y.; Fang, X.; Zhang, D.; Li, Y.; Liu, Y.; et al. Sustainable natural bio-antimicrobial: Composition, bacteriostatic activity, and antibacterial strategy of Cinnamomum camphora essential oil. Ind. Crop. Prod. 2025, 233, 121493. [Google Scholar] [CrossRef]
- Manful, M.E.; Ahmed, L.; Barry-Ryan, C. Cosmetic Formulations from Natural Sources: Safety Considerations and Legislative Frameworks in the European Union. Cosmetics 2024, 11, 72. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Long, C. The significance of traditional culture for biodiversity conservation. Biodivers. Sci. 2025, 33, 25230. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, W. Conservation and applications of camphor tree (Cinnamomum camphora) in China: Ethnobotany and genetic resources. Genet. Resour. Crop Evol. 2015, 63, 1049–1061. [Google Scholar] [CrossRef]
- Hammer, K.; Gladis, T.; Diederichsen, A. In situ and on-farm management of plant genetic resources. Eur. J. Agron. 2003, 19, 509–517. [Google Scholar] [CrossRef]
- Sinthumule, N.I. Traditional ecological knowledge and its role in biodiversity conservation: A systematic review. Front. Environ. Sci. 2023, 11, 1164900. [Google Scholar] [CrossRef]
- Li, R.; Yang, J.J.; Song, X.Z.; Wang, Y.F.; Corlett, R.T.; Xu, Y.K.; Hu, H.B. Chemical Composition and the Cytotoxic, Antimicrobial, and Anti-Inflammatory Activities of the Fruit Peel Essential Oil from Spondias pinnata (Anacardiaceae) in Xishuangbanna, Southwest China. Molecules 2020, 25, 343. [Google Scholar] [CrossRef]
- Michiu, D.; Socaciu, M.I.; Fogarasi, M.; Jimborean, A.M.; Ranga, F.; Muresan, V.; Semeniuc, C.A. Implementation of an Analytical Method for Spectrophotometric Evaluation of Total Phenolic Content in Essential Oils. Molecules 2022, 27, 1345. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Chu, C.; Du, Y.; Yu, X.; Shi, J.; Yuan, X.; Liu, X.; Liu, Y.; Zhang, H.; Zhang, Z.; Yan, N. Dynamics of antioxidant activities, metabolites, phenolic acids, flavonoids, and phenolic biosynthetic genes in germinating Chinese wild rice (Zizania latifolia). Food Chem. 2020, 318, 126483. [Google Scholar] [CrossRef]
- Sharma, K.; Guleria, S.; Razdan, V.K.; Babu, V. Synergistic antioxidant and antimicrobial activities of essential oils of some selected medicinal plants in combination and with synthetic compounds. Ind. Crop. Prod. 2020, 154, 112569. [Google Scholar] [CrossRef]
- Hao, Y.; Kang, J.; Yang, R.; Li, H.; Cui, H.; Bai, H.; Tsitsilin, A.; Li, J.; Shi, L. Multidimensional exploration of essential oils generated via eight oregano cultivars: Compositions, chemodiversities, and antibacterial capacities. Food Chem. 2022, 374, 131629. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, H.; Ding, M.; Li, J.; Wang, D.; Li, H.; Sun, M.; Xia, F.; Bai, H.; Wang, M.; et al. Screening of Rosemary Essential Oils with Different Phytochemicals for Antioxidant Capacity, Keratinocyte Cytotoxicity, and Anti-Proliferative Activity. Molecules 2023, 28, 586. [Google Scholar] [CrossRef]
- Corrêa, G.d.O.P.; Marcato, D.C.; Ramos, W.S.; Corrêa, M.A.; Cicarelli, R.M.B.; Isaac, V.L.B. In vitro evaluation of the cytotoxicity and eye irritation potential of preservatives widely used in cosmetics. Braz. J. Pharm. Sci. 2022, 58, 20039. [Google Scholar] [CrossRef]
- Ilieva, Y.; Dimitrova, L.; Zaharieva, M.M.; Kaleva, M.; Alov, P.; Tsakovska, I.; Pencheva, T.; Pencheva-El Tibi, I.; Najdenski, H.; Pajeva, I. Cytotoxicity and Microbicidal Activity of Commonly Used Organic Solvents: A Comparative Study and Application to a Standardized Extract from Vaccinium macrocarpon. Toxics 2021, 9, 92. [Google Scholar] [CrossRef]
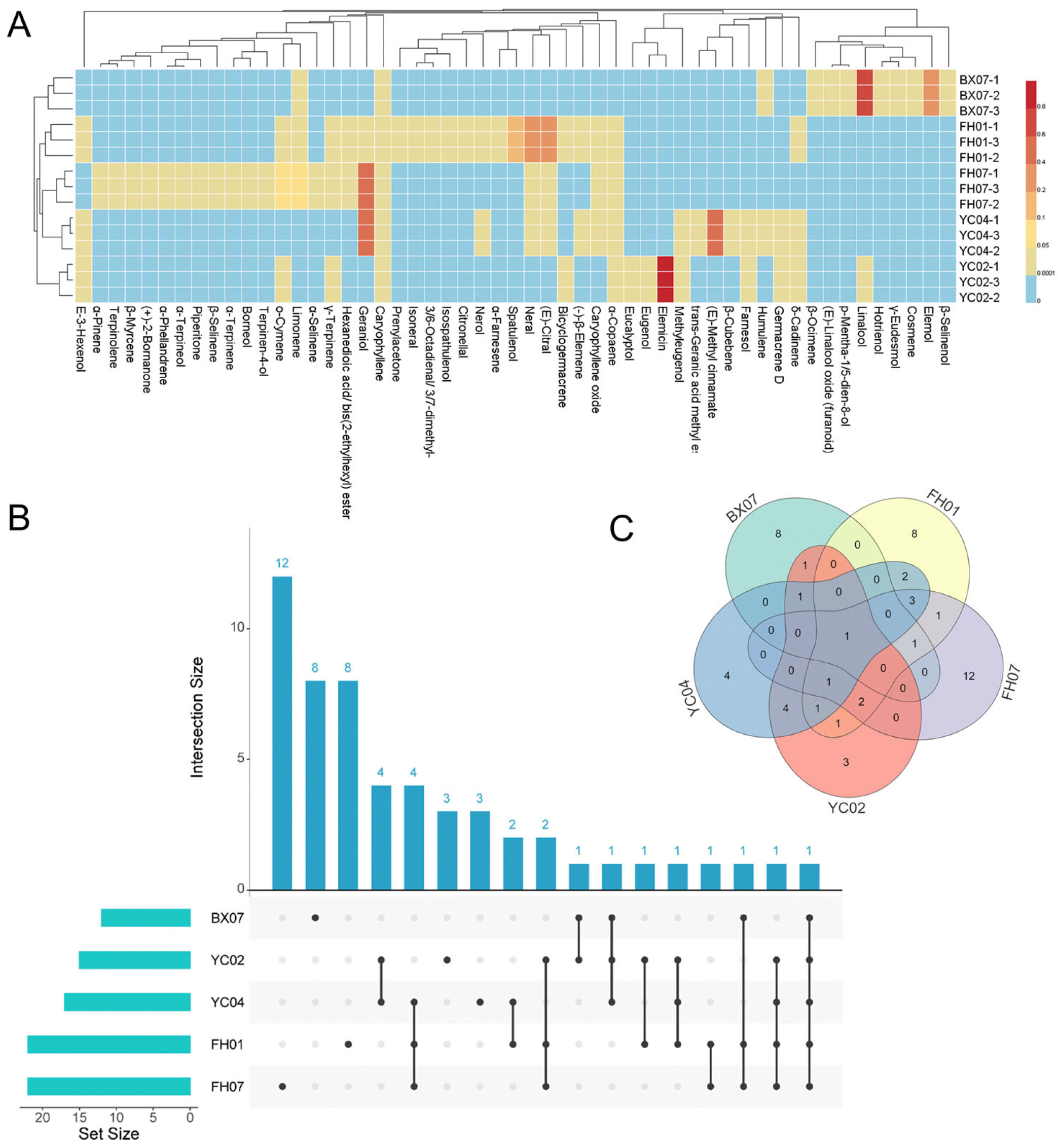

| No. | Compound | RT | RI | Area (%) | ||||
|---|---|---|---|---|---|---|---|---|
| BX07 | FH01 | FH07 | YC02 | YC04 | ||||
| 1 | (E)-3-Hexenol | 5.86 | 851 | - | 0.10 ± 0.01 | - | 0.05 ± 0.01 | 0.13 ± 0.01 |
| 2 | α-Pinene | 8.55 | 930 | - | - | 0.17 ± 0.01 | - | - |
| 3 | Prenylacetone | 10.85 | 985 | - | 1.10 ± 0.06 | - | - | - |
| 4 | β-Myrcene | 11.02 | 990 | - | - | 1.17 ± 0.21 g | - | - |
| 5 | α-Phellandrene | 11.49 | 1001 | - | - | 4.86 ± 0.43 | - | - |
| 6 | α-Terpinene | 12.04 | 1014 | - | - | 0.92 ± 0.05 | - | - |
| 7 | o-Cymene | 12.40 | 1024 | - | 0.16 ± 0.01 | 6.24 ± 0.11 c | 0.04 ± 0.01 | - |
| 8 | Limonene | 12.57 | 1026 | 0.08 ± 0.01 | 0.18 ± 0.02 | 7.18 ± 0.09 b | - | - |
| 9 | Eucalyptol | 12.66 | 1030 | - | - | - | 0.11 ± 0.01 | - |
| 10 | β-Ocimene | 13.57 | 1048 | 0.17 ± 0.01 | - | - | - | - |
| 11 | γ-Terpinene | 13.97 | 1057 | - | 0.12 ± 0.01 | 0.54 ± 0.01 | 0.05 ± 0.01 | - |
| 12 | Terpinolene | 15.31 | 1088 | - | - | 0.26 ± 0.02 | - | - |
| 13 | (E)-Linalool oxide (furanoid) | 15.35 | 1088 | 0.25 ± 0.02 | - | - | - | - |
| 14 | Linalool | 16.08 | 1100 | 68.64 ± 1.15 a | - | - | - | - |
| 15 | Hotrienol | 16.18 | 1107 | 0.62 ± 0.02 | - | - | - | - |
| 16 | Cosmene | 17.29 | 1132 | 0.12 ± 0.01 | - | - | - | - |
| 17 | (+)-2-Bornanone | 17.82 | 1140 | - | - | 2.02 ± 0.13 f | - | - |
| 18 | Citronellal | 18.42 | 1153 | - | 0.18 ± 0.03 | - | - | - |
| 19 | Borneol | 18.83 | 1162 | - | - | 0.46 ± 0.01 | - | - |
| 20 | p-Mentha-1,5-dien-8-ol | 18.94 | 1161 | 0.16 ± 0.01 | - | - | - | - |
| 21 | Isoneral | 18.95 | 1165 | - | 1.00 ± 0.09 g | - | - | - |
| 22 | Terpinen-4-ol | 19.39 | 1172 | - | - | 0.42 ± 0.06 | - | - |
| 23 | 3,6-Octadienal, 3,7-dimethyl- | 19.80 | 1183 | - | 1.77 ± 0.12 ef | - | - | - |
| 24 | α-Terpineol | 20.02 | 1188 | - | - | 2.77 ± 0.33 e | - | - |
| 25 | Nerol | 21.83 | 1229 | - | 2.22 ± 0.11 e | - | - | 0.09 ± 0.01 |
| 26 | Neral | 22.44 | 1240 | - | 30.75 ± 0.09 b | 1.88 ± 0.17 f | - | 0.21 ± 0.02 |
| 27 | Piperitone | 22.89 | 1251 | - | - | 0.82 ± 0.03 | - | - |
| 28 | Geraniol | 23.17 | 1255 | - | 2.90 ± 0.11 d | 54.46 ± 0.56 a | - | 46.15 ± 0.12 a |
| 29 | (E)-Citral | 23.81 | 1270 | - | 35.80 ± 0.17 a | 0.52 ± 0.09 | - | 0.50 ± 0.06 |
| 30 | Trans-Geranic acid methyl ester | 26.13 | 1321 | - | - | - | - | 0.28 ± 0.03 |
| 31 | Eugenol | 27.48 | 1353 | - | - | - | 0.18 ± 0.02 | - |
| 32 | α-Copaene | 28.22 | 1372 | - | 0.47 ± 0.02 | 0.23 ± 0.01 | 0.07 ± 0.01 | 0.23 ± 0.02 |
| 33 | (E)-Methyl cinnamate | 28.61 | 1380 | - | - | - | - | 46.20 ± 0.44 a |
| 34 | β-Cubebene | 28.87 | 1387 | - | - | - | - | 0.25 ± 0.02 |
| 35 | (-)-β-Elemene | 28.96 | 1389 | - | 0.79 ± 0.08 | - | - | 0.07 ± 0.01 |
| 36 | Methyleugenol | 29.58 | 1408 | - | - | - | 1.50 ± 0.27 c | 0.05 ± 0.01 |
| 37 | Caryophyllene | 30.01 | 1415 | 1.69 ± 0.21 c | 1.49 ± 0.25 fg | 2.27 ± 0.26 f | 2.11 ± 0.19 b | 0.60 ± 0.07 |
| 38 | Humulene | 31.41 | 1445 | 0.10 ± 0.01 | - | - | - | 0.19 ± 0.03 |
| 39 | Germacrene D | 32.56 | 1478 | - | - | - | 0.09 ± 0.01 | 0.14 ± 0.01 |
| 40 | β-Selinene | 32.74 | 1482 | - | - | 2.84 ± 0.19 e | - | - |
| 41 | α-Selinene | 33.11 | 1493 | - | - | 0.78 ± 0.10 | - | - |
| 42 | Bicyclogermacrene | 33.18 | 1493 | - | 3.03 ± 0.25 d | - | 0.25 ± 0.03 | - |
| 43 | α-Farnesene | 33.80 | 1508 | - | 0.44 ± 0.02 | - | - | - |
| 44 | δ-Cadinene | 34.29 | 1521 | - | 0.28 ± 0.01 | - | 0.09 ± 0.01 | 0.48 ± 0.03 |
| 45 | Elemol | 35.32 | 1550 | 20.72 ± 0.73 b | - | - | - | - |
| 46 | Elemicin | 35.86 | 1558 | - | - | - | 94.56 ± 0.98 a | - |
| 47 | Spatulenol | 36.33 | 1571 | - | 10.62 ± 0.77 c | - | - | - |
| 48 | Caryophyllene oxide | 36.52 | 1576 | - | 3.21 ± 0.21 d | 0.72 ± 0.07 | - | 0.18 ± 0.01 |
| 49 | γ-Eudesmol | 38.32 | 1630 | 1.07 ± 0.11 d | - | - | - | - |
| 50 | Isospathulenol | 38.53 | 1640 | - | 0.88 ± 0.04 | - | - | - |
| 51 | β-Selinenol | 38.86 | 1649 | 1.45 ± 0.28 cd | - | - | - | - |
| 52 | Farnesol | 40.63 | 1721 | - | - | - | 0.26 ± 0.05 | 1.64 ± 0.09 b |
| 53 | Hexanedioic acid, bis(2-ethylhexyl) ester | 48.51 | 2398 | - | 0.23 ± 0.01 | 0.56 ± 0.01 | - | - |
| Total | 94.73 ± 0.66 | 97.48 ± 0.87 | 97.8 ± 1.13 | 99.27 ± 0.15 | 97.31 ± 0.56 | |||
| NO of compounds | 12 | 22 | 22 | 15 | 17 | |||
| EO yields (%) | 0.68 | 0.49 | 0.77 | 0.39 | 1.02 | |||
| Numbers | ABTS (µmol TE/g DW) | DPPH (µmol TE/g DW) | FRAP (µmol TE/g DW) | TAC (% Inhibition) | TP (mg GAE/g DW) |
|---|---|---|---|---|---|
| BX07 | 20.82 ± 1.75 e | 25.65 ± 0.57 e | 1.33 ± 0.67 e | 28.90 ± 3.22 e | 35.85 ± 1.13 e |
| FH01 | 52.09 ± 0.66 c | 75.99 ± 1.65 b | 4.53 ± 0.54 b | 83.83 ± 3.92 b | 153.8 ± 1.58 b |
| FH07 | 62.33 ± 0.49 a | 49.55 ± 1.01 c | 3.71 ± 0.16 d | 72.73 ± 13.94 c | 113.4 ± 1.87 c |
| YC02 | 57.27 ± 0.82 b | 83.75 ± 0.15 a | 5.87 ± 0.13 a | 97.29 ± 2.71 a | 239.4 ± 6.87 a |
| YC04 | 45.33 ± 0.60 d | 40.44 ± 0.85 d | 3.79 ± 0.42 c | 61.86 ± 7.18 d | 80.01 ± 1.07 d |
| p-value | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.05 | p < 0.01 |
| Bacteria | C. tenuipilis Essential Oils (μL/mL) | Antibiotic (μg/mL) | |||||
|---|---|---|---|---|---|---|---|
| BX07 | FH01 | FH07 | YC02 | YC04 | Ciprofloxacin | ||
| B. cereus | MIC | 6.25 | 3.13 | 6.25 | 12.5 | 6.25 | 0.25 |
| MBC | 12.5 | 6.25 | 12.5 | 25 | 12.5 | 0.25 | |
| B. subtilis | MIC | 6.25 | 3.13 | 12.5 | 12.5 | 6.25 | 0.13 |
| MBC | 12.5 | 6.25 | 12.5 | 25 | >25 | 0.25 | |
| E. coli | MIC | 12.5 | 6.25 | 12.5 | 12.5 | 12.5 | 0.06 |
| MBC | 12.5 | 6.25 | >25 | 25 | 12.5 | 0.13 | |
| S. aureus | MIC | 3.13 | 6.25 | 12.5 | >25 | 12.5 | 0.50 |
| MBC | 6.25 | 12.5 | 12.5 | >25 | 12.5 | 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Fan, X.; Qi, H.; Chen, S.-G.; Li, R.; Liu, Y.-J. Study on Chemical Diversity, Antioxidant and Antibacterial Activities, and HaCaT Cytotoxicity of Camphora tenuipilis (a Traditional Aromatic Plant from Xishuangbanna). Plants 2025, 14, 3409. https://doi.org/10.3390/plants14223409
Chen L, Fan X, Qi H, Chen S-G, Li R, Liu Y-J. Study on Chemical Diversity, Antioxidant and Antibacterial Activities, and HaCaT Cytotoxicity of Camphora tenuipilis (a Traditional Aromatic Plant from Xishuangbanna). Plants. 2025; 14(22):3409. https://doi.org/10.3390/plants14223409
Chicago/Turabian StyleChen, Long, Xuan Fan, Hao Qi, Shi-Guo Chen, Ren Li, and Yu-Jing Liu. 2025. "Study on Chemical Diversity, Antioxidant and Antibacterial Activities, and HaCaT Cytotoxicity of Camphora tenuipilis (a Traditional Aromatic Plant from Xishuangbanna)" Plants 14, no. 22: 3409. https://doi.org/10.3390/plants14223409
APA StyleChen, L., Fan, X., Qi, H., Chen, S.-G., Li, R., & Liu, Y.-J. (2025). Study on Chemical Diversity, Antioxidant and Antibacterial Activities, and HaCaT Cytotoxicity of Camphora tenuipilis (a Traditional Aromatic Plant from Xishuangbanna). Plants, 14(22), 3409. https://doi.org/10.3390/plants14223409








