Seasonal and Regional Effects on the Yield and Bioactive Constituents of Torreya nucifera Essential Oils in South Korea
Abstract
1. Introduction
2. Results
2.1. The Aroma Characteristics of Leaf Essential Oil Extracted from T. nucifera
2.2. Seasonal and Regional Variations in the Oil Yield of T. nucifera
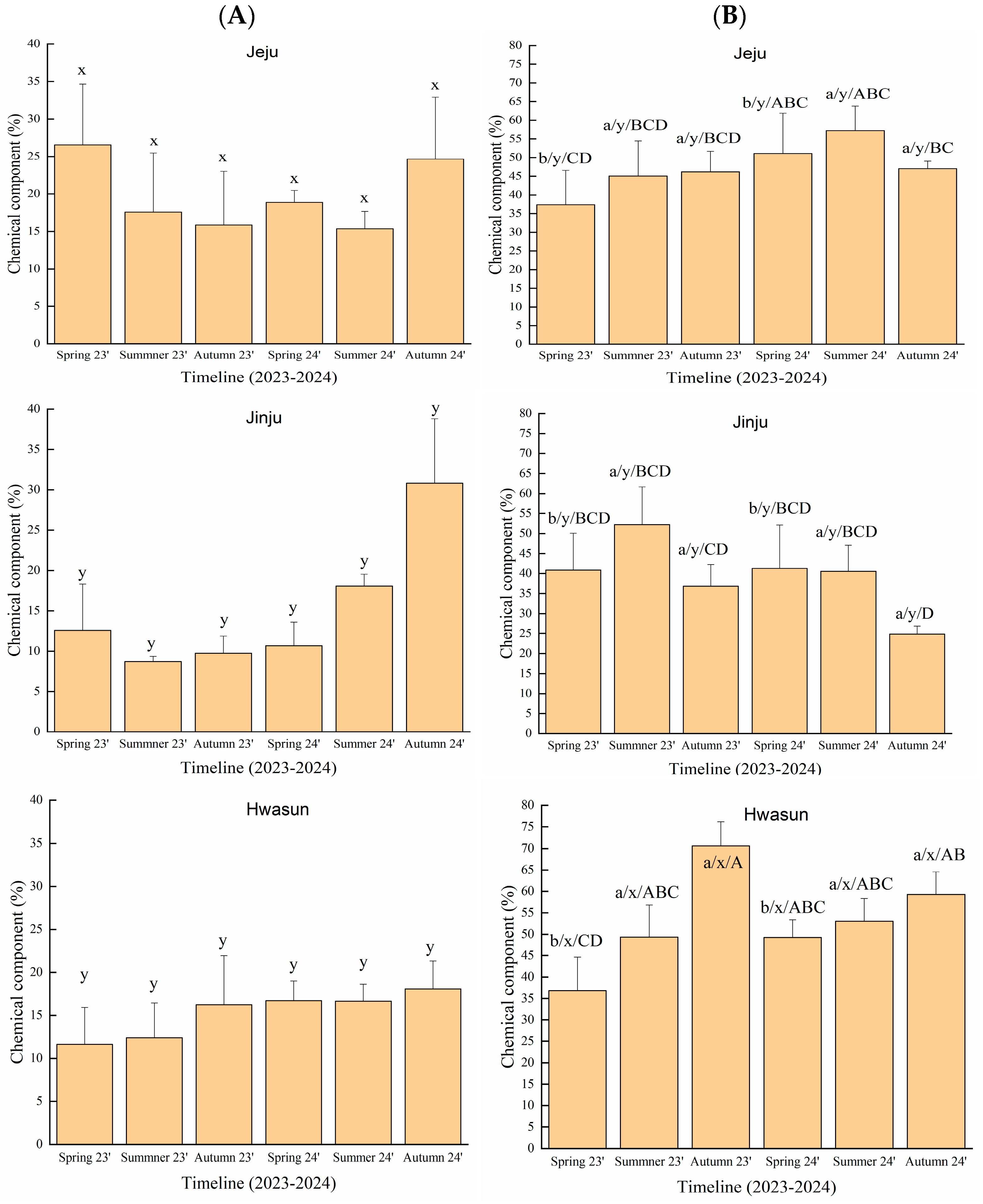
2.3. Effect of Season and Region on Targeted Bioactive Constituents (3-Carene and D-Limonene) in T. nucifera Essential Oil
3. Discussion
3.1. The Effect of Season and Region on the Oil Yield of T. nucifera
3.2. The Overall Chemical Profile of T. nucifera Essential Oils
3.3. Effect of Region and Season on Chemical Markers of T. nucifera Essential Oil
4. Materials and Methods
4.1. Chemicals and Reagents
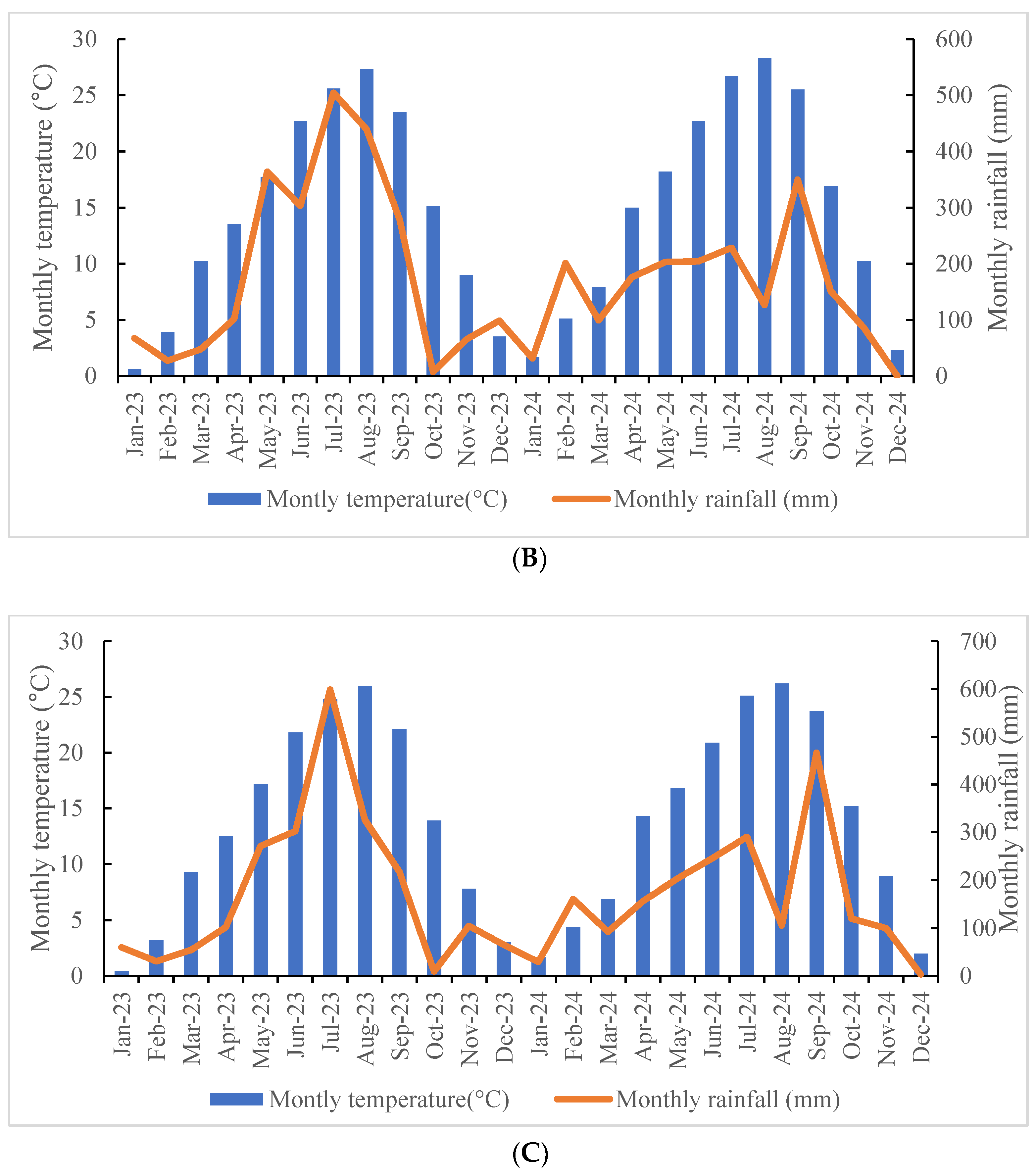
4.2. Experimental Site of Plant Material and Climate Characteristics
4.3. Hydrodistillation of Essential Oils
4.4. Assessment of Aroma Characteristics in Essential Oils
4.5. Analysis of Gas Chromatography-Mass Spectrometry (GC-MS)
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shin, H.C.; Lee, K.S.; Park, N.C.; Jung, S.Y. Vegetation structure of the Torreya nucifera stand in Korea. J. Korean Soc. For. Sci. 2010, 99, 312–322. [Google Scholar]
- Yoon, W.J.; Kim, S.S.; Oh, T.H.; Lee, N.H.; Hyun, C.G. Torreya nucifera essential oil inhibits skin pathogen growth and lipopolysaccharide-induced inflammatory effects. Int. J. Pharmacol. 2009, 5, 37–43. [Google Scholar] [CrossRef]
- Endo, Y.; Osada, Y.; Kimura, F.; Fujimoto, K. Effects of Japanese torreya (Torreya nucifera) seed oil on lipid metabolism in rats. Nutrition 2006, 22, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.; Kim, B.; Choi, K. Torreya nucifera seed oil improves 3T3-L1 adipocyte differentiation. BMC Complement. Med.Ther. 2021, 21, 1–7. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Forest Science. The Anti-Asthmatic Effect of Torreya nucifera Leaf Essential Oil. Available online: https://nifos.forest.go.kr/kfsweb/cop/bbs/selectBoardArticle.do?nttId=3189281&bbsId=BBSMSTR_1036&pageUnit=10&pageIndex=1&searchtitle=title&searchcont=&searchkey=&searchwriter=&searchWrd=%eb%b9%84%ec%9e%90%eb%82%98%eb%ac%b4+%ec%b2%9c%ec%8b%9d&ctgryLrcls=CTGRY150&ctgryMdcls=&ctgrySmcls=&ntcStartDt=&ntcEndDt=&mn=UKFR_03_03_01&orgId=kfri (accessed on 18 September 2025).
- Kim, Y.; Lee, J.; Lee, M.; Ahn, C.; Park, M.; Na, H.; Jeung, E. 3-carene suppresses inflammatory cytokine interleukin-4, interleukin-5 and interleukin-13 in a murine model of asthma. J Physiol Pharmacol 2024, 75, 195–203. [Google Scholar]
- Oh, H.J.; Ahn, H.M.; So, K.H.; Kim, S.S.; Yun, P.Y.; Jeon, G.L.; Riu, K.Z. Chemical and antimicrobial properties of essential oils from three coniferous trees Abies koreana, Cryptomeria japonica, and Torreya nucifera. J. Appl. Biol. Chem. 2007, 50, 164–169. [Google Scholar]
- Akhavan-Mahdavi, S.; Sadeghi, R.; Esfanjani, A.F.; Hedayati, S.; Shaddel, R.; Dima, C.; Malekjani, N.; Boostani, S.; Jafari, S.M. Nanodelivery systems for d-limonene; techniques and applications. Food Chem. 2022, 384, 132479–133133. [Google Scholar] [CrossRef]
- Shu, H.; Chen, H.; Wang, X.; Hu, Y.; Yun, Y.; Zhong, Q.; Chen, W.; Chen, W. Antimicrobial activity and proposed action mechanism of 3-Carene against Brochothrix thermosphacta and Pseudomonas fluorescens. Molecules 2019, 24, 3246–3263. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.; Arruda, F.; Medeiros, J.; Baptista, J.; Madruga, J.; Lima, E. Variations in essential oil chemical composition and biological activities of Cryptomeria japonica (Thunb. ex Lf) D. Don from different geographical origins—A critical review. Appl. Sci. 2021, 11, 11097. [Google Scholar] [CrossRef]
- Fernando Rolim de Almeida, L.; de Oliveira Portella, R.; Facanali, R.; Ortiz Mayo Marques, M.; Frei, F. Dry and wet seasons set the phytochemical profile of the Copaifera langsdorffii Desf. essential oils. J. Essent. Oil Res. 2014, 26, 292–300. [Google Scholar] [CrossRef]
- Aydın, C.; Özcan, M.M. Determination of nutritional and physical properties of myrtle (Myrtus communis L.) fruits growing wild in Turkey. J. Food Eng. 2007, 79, 453–458. [Google Scholar] [CrossRef]
- Flamini, G.; Cioni, P.L.; Morelli, I.; Maccioni, S.; Baldini, R. Phytochemical typologies in some populations of Myrtus communis L. on Caprione Promontory (East Liguria, Italy). Food Chem. 2004, 85, 599–604. [Google Scholar] [CrossRef]
- Rahimmalek, M.; Tabatabaei, B.E.S.; Etemadi, N.; Goli, S.A.H.; Arzani, A.; Zeinali, H. Essential oil variation among and within six Achillea species transferred from different ecological regions in Iran to the field conditions. Ind. Crops Prod. 2009, 29, 348–355. [Google Scholar] [CrossRef]
- Dlugoviet, T.F.; Ferriani, A.P.; Hendges, A.P.P.K.; Camargo, R.G.; Duarte, M.C.; Duarte, R.M.; Ruiz, A.L.T.G.; Nagata, N.; Marques, F.A.; Sales Maia, B.H. Seasonal Chemical Variability and Antimicrobial, Anti-Proliferative Potential of Essential Oils from Baccharis uncinella, B. retusa, and B. calvescens (Asteraceae). Plants 2025, 14, 1311. [Google Scholar] [CrossRef]
- Asghari, G.; Gholamali, H.; Mahmoudi, Z.; Asghari, M. Diurnal variation of essential of the oil components of Pycnocycla spinosa Decne. ex Boiss. Jundishapur J. Nat. Pharm. Prod. 2014, 9, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Lakušić, D.; Ristić, M.; Slavkovska, V.; Lakušić, B. Seasonal variations in the composition of the essential oils of rosemary (Rosmarinus officinalis, Lamiaceae). Nat. Prod. Commun. 2013, 8, 1–4. [Google Scholar] [CrossRef]
- Anandhi, S.; Uchoi, A.; Rajamani, K. Study on estimation and seasonal variation of essential oil in tea tree by hydro distillation method. Indian J. Agric. Res. 2024, 58, 514–516. [Google Scholar] [CrossRef]
- Vokk, R.; Lõugas, T.; Mets, K.; Kravets, M. Dill (Anethum graveolens L.) and parsley (Petroselinum crispum (Mill.) Fuss) from Estonia: Seasonal differences in essential oil composition. Agron. J. 2011, 9, 515–520. [Google Scholar]
- Liebelt, D.J.; Jordan, J.T.; Doherty, C.J. Only a matter of time: The impact of daily and seasonal rhythms on phytochemicals. Phytochem. Rev. 2019, 18, 1409–1433. [Google Scholar] [CrossRef]
- Celiktas, O.Y.; Kocabas, E.H.; Bedir, E.; Sukan, F.V.; Ozek, T.; Baser, K. Antimicrobial activities of methanol extracts and essential oils of Rosmarinus officinalis, depending on location and seasonal variations. Food Chem. 2007, 100, 553–559. [Google Scholar] [CrossRef]
- Clark, R.; Menary, R. Effects of Photoperiod on the Yield and Composition of Peppermint Oil1. J. Am. Soc. Hortic. Sci. 1979, 104, 699–702. [Google Scholar] [CrossRef]
- Jnanesha, A.; Ashish, K.; Vanitha, T. Variation in the yield and chemical composition of eucalyptus species (Nilagiri) under different agro climatic condition of India. Int. J. Herbal. Med. 2019, 7, 4–7. [Google Scholar]
- Khaiper, M.; Poonia, P.K.; Dhanda, S.K.; Beniwal, R.; Verma, P.; Nasir, M. Seasonal variation in chemical composition and bioactivity of Eucalyptus tereticornis leaf essential oil. Biochem. Syst. Ecol. 2025, 121, 104988. [Google Scholar] [CrossRef]
- Jeong, S.-M.; Jin, S.-H.; Kim, M.-H.; Baek, K.-S.; Kim, C.-Y.; Ahn, Y.-S.; An, K.-W. Conservation management strategies of protected areas for genetic resources, Torreya nucifera forest of Bulhoesa (temple) in Naju. Korean J. Environ. Ecol. 2013, 27, 71–84. [Google Scholar]
- Plants For A Future. Available online: https://pfaf.org/user/plant.aspx?LatinName=Torreya+nucifera (accessed on 22 October 2025).
- Loziene, K.; Venskutonis, P. Influence of environmental and genetic factors on the stability of essential oil composition of Thymus pulegioides. Biochem. Syst. Ecol. 2005, 33, 517–525. [Google Scholar] [CrossRef]
- Basu, S.; Acharya, S.; Bandara, M.; Friebel, D.; Thomas, J. Effects of genotype and environment on seed and forage yield in fenugreek (‘Trigonella foenum-graecum L.’) grown in western Canada. Aust. J. Crop Sci. 2009, 3, 305–314. [Google Scholar]
- Shafie, M.S.B.; Zain Hasan, S.; Shah, R.M. Study of genetic variability of Wormwood capillary (Artemisia capillaris) using inter simple sequence repeat (ISSR) in Pahang region, Malaysia. Plant Omics J. 2009, 2, 127–134. [Google Scholar]
- Simmons, D.; Parsons, R. Seasonal variation in the volatile leaf oils of two Eucalyptus species. Biochem. Syst. Ecol. 1987, 15, 209–215. [Google Scholar] [CrossRef]
- Machado, C.A.; Oliveira, F.O.; de Andrade, M.A.; Hodel, K.V.S.; Lepikson, H.; Machado, B.A.S. Steam distillation for essential oil extraction: An evaluation of technological advances based on an analysis of patent documents. Sustainability 2022, 14, 7119. [Google Scholar] [CrossRef]
- Kant, R.; Kumar, A. Review on essential oil extraction from aromatic and medicinal plants: Techniques, performance and economic analysis. Sustain. Chem. Pharm. 2022, 30, 100829. [Google Scholar] [CrossRef]
- Sharafzadeh, S.; Zare, M. Influence of growth regulators on growth and secondary metabolites of some medicinal plants from Lamiaceae family. Adv. Environ. Biol. 2011, 5, 2296–2302. [Google Scholar]
- Li, S.; Han, Q.; Qiao, C.; Song, J.; Lung Cheng, C.; Xu, H. Chemical markers for the quality control of herbal medicines: An overview. Chin. Med. 2008, 3, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Liu, S.; Jin, G.; Yang, X.; Zhou, Y.J. Microbial production of limonene and its derivatives: Achievements and perspectives. Biotechnol. Adv. 2020, 44, 107628. [Google Scholar] [CrossRef]
- Erasto, P.; Viljoen, A.M. Limonene-a review: Biosynthetic, ecological and pharmacological relevance. Nat. Prod. Commun. 2008, 3, 1193–1202. [Google Scholar] [CrossRef]
- Uliana, M.P.; Fronza, M.; da Silva, A.G.; Vargas, T.S.; de Andrade, T.U.; Scherer, R. Composition and biological activity of Brazilian rose pepper (Schinus terebinthifolius Raddi) leaves. Ind. Crop. Prod. 2016, 83, 235–240. [Google Scholar] [CrossRef]
- Cao, J.Q.; Guo, S.S.; Wang, Y.; Pang, X.; Geng, Z.F.; Du, S.S. Toxicity and repellency of essential oil from Evodia lenticellata Huang fruits and its major monoterpenes against three stored-product insects. Ecotoxicol. Environ. Saf. 2018, 160, 342–348. [Google Scholar] [CrossRef]
- Guimarães, B.d.A.; Silva, R.C.; Andrade, E.H.d.A.; Setzer, W.N.; da Silva, J.K.; Figueiredo, P.L.B. Seasonality, composition, and antioxidant capacity of limonene/δ-3-carene/(E)-caryophyllene Schinus terebinthifolia essential oil chemotype from the brazilian Amazon: A chemometric approach. Plants 2023, 12, 2497. [Google Scholar] [CrossRef]
- Cabrera, D.; Gomes, G.; Flach, A.; da Costa, L.; Rosa, G.; de Moura, N. Evaluation of climatic factors on the yield and chemical composition of the essential oil of Myrocarpus frondosus. Nat. Prod. Res. 2015, 29, 667–670. [Google Scholar] [CrossRef]
- Darwish, R.S.; Shawky, E.; El Naggar, E.M.B.; Hammoda, H.M.; Harraz, F.M. Evaluation of the effect of seasonal variation and organ selection on the chemical composition and antimicrobial activity of the essential oil of oriental-cedar (Platyclaudus orientalis (L.) Franco). J. Essent. Oil Res. 2021, 33, 69–79. [Google Scholar] [CrossRef]
- Barra, A. Factors affecting chemical variability of essential oils: A review of recent developments. Nat. Prod. Commun. 2009, 4, 1147–1154. [Google Scholar] [CrossRef]
- Bhatt, S.; Tewari, G.; Pande, C.; Prakash, O.; Tripathi, S. Aroma profile and antioxidant potential of Origanum vulgare L.: Impact of drying. J. Essent. Oil-Bear. Plant 2019, 22, 214–230. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Sherazi, S.T.H.; Przybylski, R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef]
- Lee, L.S.; Brooks, L.O.; Homer, L.E.; Rossetto, M.; Henry, R.J.; Baverstock, P.R. Geographic variation in the essential oils and morphology of natural populations of Melaleuca alternifolia (Myrtaceae). Biochem. Syst. Ecol. 2002, 30, 343–360. [Google Scholar] [CrossRef]
- Khan, M.; Khan, S.T.; Khan, M.; Mousa, A.A.; Mahmood, A.; Alkhathlan, H.Z. Chemical diversity in leaf and stem essential oils of Origanum vulgare L. and their effects on microbicidal activities. AMB Express 2019, 9, 176–191. [Google Scholar] [CrossRef]
- Thomas, J.; Narkowicz, C.; Jacobson, G.; Davies, N. An examination of the essential oils of Tasmanian Kunzea ambigua, other Kunzea spp. and commercial Kunzea oil. J. Essent. Oil Res. 2010, 22, 381–385. [Google Scholar] [CrossRef]
- Gobbo-Neto, L.; Lopes, N.P. Plantas medicinais: Fatores de influência no conteúdo de metabólitos secundários. Química Nova 2007, 30, 374–381. [Google Scholar] [CrossRef]
- Prinsloo, G.; Nogemane, N. The effects of season and water availability on chemical composition, secondary metabolites and biological activity in plants. Phytochem. Rev. 2018, 17, 889–902. [Google Scholar] [CrossRef]
- Grulova, D.; De Martino, L.; Mancini, E.; Salamon, I.; De Feo, V. Seasonal variability of the main components in essential oil of Mentha× piperita L. J. Sci. Food Agric. 2015, 95, 621–627. [Google Scholar] [CrossRef]
- Bejenaru, L.E.; Segneanu, A.-E.; Bejenaru, C.; Biţă, A.; Tuţulescu, F.; Radu, A.; Ciocîlteu, M.V.; Mogoşanu, G.D. Seasonal Variations in Chemical Composition and Antibacterial and Antioxidant Activities of Rosmarinus officinalis L. Essential Oil from Southwestern Romania. Appl. Sci. 2025, 15, 681. [Google Scholar] [CrossRef]
- Administration, K.M. Available online: https://data.kma.go.kr/cmmn/main.do (accessed on 30 March 2025).



| Essential Oils Yield (ODW, %) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Jeju | Jinju | Hwasun | |||||||
| Season | 2023 | 2024 | Average | 2023 | 2024 | Average | 2023 | 2024 | Average |
| Spring | 1.1 ± 0.2 y | 1.1 ± 0.2 y | 1.1 ± 0.2 | 1.2 ± 0.2 y | 0.9 ± 0.1 y | 1.1 ± 0.2 | 1.1 ± 0.1 x | 1.3 ± 0.1 x | 1.2 ± 0.1 |
| Summer | 1.2 ± 0.2 y | 1.2 ± 0.2 y | 1.2 ± 0.2 | 1.4 ± 0.3 y | 1.0± 0.1 y | 1.2 ± 0.3 | 1.4 ± 0.3 x | 1.6 ± 0.3 x | 1.5 ± 0.3 |
| Autumn | 0.9 ± 0.2 y | 1.5 ± 0.5 y | 1.2 ± 0.5 | 1.3 ± 0.1 y | 1.2 ± 0.3 y | 1.3 ± 0.2 | 1.4 ± 0.3 x | 1.6 ± 0.5 x | 1.5 ± 0.4 |
| Essenti al oils yield: factor 1 (season): ns, factor 2 (region): * and factor 1 X factor 2 (interaction): NS | |||||||||
| Relative Area Percentage (%) | |||||||
|---|---|---|---|---|---|---|---|
| RT * | Compound | KI ** | KI Ref *** | Jeju | Jinju | Hwasun | |
| 1 | 16.65 | Tricyclene | 919 | 927 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| 2 | 16.89 | α-Thujene | 921 | 930 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.1 |
| 3 | 17.6 | α-Pinene | 927 | 931 | 22.6 ± 6.3 | 26.0 ± 0.1 | 33.0 ± 3.2 |
| 4 | 19.17 | (+)-Camphene | 941 | 946 | 0.7 ± 0.3 | 0.4 ± 0.2 | 0.6 ± 0.1 |
| 5 | 22.67 | (-)-β-Pinene | 972 | 980 | 0.8 ± 0.3 | 1.1 ± 0.0 | 1.2 ± 0.1 |
| 6 | 23.07 | 1-Octen-3-ol | 976 | 981 | 0.1 ± 0.0 | bdl | 0.1 ± 0.0 |
| 7 | 24.28 | β-Myrcene | 987 | 991 | 1.6 ± 0.1 | 2.2 ± 0.2 | 1.8 ± 0.2 |
| 8 | 27.24 | 3-Carene | 1009 | 1005 | 26.6 ± 5.2 | 12.6 ± 3.3 | 11.6 ± 3.1 |
| 9 | 31.2 | D-Limonene | 1032 | 1031 | 37.4 ± 8.4 | 40.9 ± 5.9 | 36.8 ± 5.8 |
| 10 | 37.43 | β-cis-Ocimene | 1042 | 1034 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| 11 | 44.64 | 3-Octyl_acetate | 1126 | 1123 | 0.1 ± 0.0 | bdl | 0.1 ± 0.0 |
| 12 | 48.58 | Terpinen-4-ol | 1184 | 1177 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| 13 | 49.54 | α-Terpineol | 1198 | 1189 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| 14 | 51.54 | Citronellol | 1225 | 1228 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| 15 | 55.88 | (-)-Bornyl_acetate | 1284 | 1285 | bdl | bdl | 0.1 ± 0.0 |
| 16 | 60.07 | δ-Eiemene | 1346 | 1338 | 0.1 ± 0.0 | 0.3 ± 0.1 | 0.1 ± 0.0 |
| 17 | 65.27 | β-Copaene | 1439 | 1435 | bdl | 0.2 ± 0.0 | 0.1 ± 0.0 |
| 18 | 66.06 | cis-β-Farnesene | 1457 | 1455 | 0.1 ± 0.0 | 1.0 ± 0.2 | 0.3 ± 0.1 |
| 19 | 66.4 | Humulene | 1465 | 1457 | bdl | 0.5 ± 0.1 | 0.5 ± 0.2 |
| 20 | 67.14 | γ-Muurolene | 1483 | 1480 | bdl | 0.3 ± 0.1 | 0.1 ± 0.0 |
| 21 | 67.86 | β-Cubebene | 1500 | 1396 | 0.1 ± 0.0 | 0.4 ± 0.1 | 0.1 ± 0.0 |
| 22 | 68.25 | γ-Cadinene | 1512 | 1513 | 0.1 ± 0.0 | 0.9 ± 0.2 | 0.5 ± 0.1 |
| 23 | 68.74 | δ-Cadinene | 1527 | 1524 | 0.9 ± 0.3 | 3.6 ± 0.8 | 1.7 ± 0.1 |
| 24 | 68.91 | trans-Calamenene_ | 1532 | 1529 | 0.1 ± 0.0 | 0.3 ± 0.1 | 0.2 ± 0.1 |
| 25 | 70.81 | Spathulenol | 1590 | 1591 | bdl | bdl | 0.1 ± 0.0 |
| 26 | 71.02 | Gleenol | 1597 | 1587 | 0.6 ± 0.2 | 0.7 ± 0.1 | 0.7 ± 0.1 |
| 27 | 72.3 | α-Copaene | 1627 | 1380 | bdl | 0.2 ± 0.0 | 0.1 ± 0.0 |
| 28 | 72.38 | Epicubenol | 1646 | 1629 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| 29 | 72.5 | δ-Cedrol | 1650 | 1645 | 2.2 ± 0.3 | 2.1 ± 0.2 | 2.1 ± 0.3 |
| 30 | 72.8 | T-MuuroloI | 1679 | 1657 | 0.3 ± 0.0 | 0.3 ± 0.1 | 0.3 ± 0.0 |
| 31 | 73.81 | Levomenol | 1698 | 1682 | 0.8 ± 0.1 | 0.7 ± 0.2 | bdl |
| 32 | 74.39 | (Z, E)-Farnesol | 1722 | 1722 | bdl | 0.1 ± 0.0 | bdl |
| Monoterpene hydrocarbons | 89.8 ± 1.5 | 83.5 ± 1.0 | 85.3 ± 1.2 | ||||
| Oxygenated monoterpenes | 0.6 ± 0.1 | 0.2 ± 0.1 | 0.5 ± 0.1 | ||||
| Sesquiterpene hydrocarbons | 1.4 ± 0.1 | 7.7 ± 1.0 | 3.5 ± 0.8 | ||||
| Oxygenated sesquiterpenes | 4.0 ± 0.6 | 3.9 ± 0.5 | 3.2 ± 0.4 | ||||
| Total identified (%) | 95.9 ± 2.0 | 95.4 ± 1.2 | 92.8 ± 1.5 | ||||
| The Chemical Markers of T. nucifera Essential Oil | ||
|---|---|---|
| 3-Carene | D-Limonene | |
| Season | NS | ** |
| Region | *** | ** |
| Season × Region | NS | ** |
| 3-Carene | Temperature | Rainfall | |
| 3-Carene | 1.000 | 0.1933 | 0.0099 |
| Temperature | 0.1933 | 1.000 | 0.571 |
| Rainfall | 0.0099 | 0.571 | 1.000 |
| [3-carene] factor 1: temperature, factor 2: rainfall | |||
| D-Limonene | Temperature | Rainfall | |
| D-Limonene | 1.000 | 0.2125 | 0.3107 |
| Temperature | 0.2125 | 1.000 | 0.571 |
| Rainfall | 0.3107 | 0.571 | 1.000 |
| [D-limonene] factor 1: rainfall, factor 2: temperature | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.; Kim, N.; Park, M.-J. Seasonal and Regional Effects on the Yield and Bioactive Constituents of Torreya nucifera Essential Oils in South Korea. Plants 2025, 14, 3370. https://doi.org/10.3390/plants14213370
Park C, Kim N, Park M-J. Seasonal and Regional Effects on the Yield and Bioactive Constituents of Torreya nucifera Essential Oils in South Korea. Plants. 2025; 14(21):3370. https://doi.org/10.3390/plants14213370
Chicago/Turabian StylePark, Chanjoo, Nahyun Kim, and Mi-Jin Park. 2025. "Seasonal and Regional Effects on the Yield and Bioactive Constituents of Torreya nucifera Essential Oils in South Korea" Plants 14, no. 21: 3370. https://doi.org/10.3390/plants14213370
APA StylePark, C., Kim, N., & Park, M.-J. (2025). Seasonal and Regional Effects on the Yield and Bioactive Constituents of Torreya nucifera Essential Oils in South Korea. Plants, 14(21), 3370. https://doi.org/10.3390/plants14213370






