Abstract
As freshwater resources become increasingly scarce, seawater and brackish water represent alternative sources for crop irrigation, particularly in systems such as saltwater aquaponics. Red orache (Atriplex hortensis var. rubra) is a halophyte with high antioxidant content but also accumulates antinutrients like nitrate (NO3−) and oxalate. Oxalate helps plants cope with salinity stress but can cause health issues in humans. This study examined the growth of red orache baby greens in saline and nitrogen-limited hydroponic solutions to assess its adaptability and nutritional quality, focusing on the impact of salinity and reduced nitrogen on antinutrient levels. Four nutrient solutions differing in NaCl (0 or 428 mM) and NO3− (10 or 1 mM) were tested. Salinity significantly reduced red orache yield (by 75.5%), pigment levels, antioxidants, and nutrient uptake, while increasing leaf Na and oxalate concentration, ethylene production, and succulence. Salinity decreased NO3− concentration and oxalate oxidase (OxO) activity but boosted total ascorbic acid and oxalate accumulation. Low NO3− mildly reduced yield (by 25.7%), leaf area, and NO3− concentration in leaves, but had no effect on leaf moisture content, succulence, antioxidant capacity, and the concentration of antioxidants, pigments, and total oxalate. In addition, low NO3− increased OxO activity, only under non-saline conditions. The high salinity typical of aquaculture effluents strongly reduced red orache baby greens yield and quality to a greater extent than low NO3− levels. Both salinity and low NO3− reduced NO3− concentration in leaves, while salinity increased oxalate concentration, probably due to the reduced activity of OxO.
1. Introduction
As freshwater resources become scarce, brackish and highly saline waters, such as seawater, offer an alternative for crop irrigation after desalination or dilution with freshwater [1]. One specific application of seawater in agriculture is saltwater aquaponics, which utilizes water with salinities as high as 35 g L−1 (the salinity of seawater) [2]. Aquaponics is a technique that combines the hydroponic cultivation of crop plants with intensive fish farming. Growing plants in aquaponics presents different challenges compared to standard hydroponics. In aquaponic systems, fish, microorganisms, and plants have distinct water quality requirements. Water quality deterioration is mainly influenced by fish feeding rate, density, and feed composition. Consequently, macro- and microelement concentrations in aquaponic solutions are often below optimal levels for hydroponics, leading to plant nutrient deficiencies [3]. In marine aquaponic systems, crops must also tolerate high salinity (up to 35 g L−1 NaCl) and nutrient solutions that are relatively poor in nitrogen (1–2 mM NO3−) but rich in Mg, B, Na, and Cl compared to standard hydroponic formulations [3].
Orache (Atriplex hortensis), a member of the Amaranthaceae family, is a facultative halophyte naturally found in arid and semi-arid regions due to its ability to tolerate drought and salinity [4]. It can be consumed fresh or cooked, like spinach (Spinacia oleracea L.). Since ancient times, orache has been cultivated as a minor vegetable across Eurasia, from Central Asia to the Mediterranean, where it was originally domesticated. Today, its cultivation is expanding to other temperate and subtropical regions around the world, thanks to its salt tolerance and impressive nutritional profile, including its high-quality protein leaf and seed content [5]. Red orache (RO, Atriplex hortensis var. rubra) is distinguished by its leaves that are rich in betalains, which give them a red-purple color [6]. These compounds are typical of Amaranthaceae and have significant antioxidant properties [7], often exceeding those of anthocyanins [6]. Red orache can be utilized like other red-leafed plants, particularly as baby greens in mono- or mixed-species salads; these products can command high prices on the market [8]. Baby greens are immature and tender fresh products with higher antioxidant properties than mature plants of the same species [9].
Despite its beneficial nutritional profile, RO accumulates antinutrients such as nitrate (NO3−) and oxalate like other species in the Amaranthaceae [10]. Oxalic acid is naturally present in plants, with concentrations ranging from 3% to 80% of DW depending on genotype, organ, and growing conditions [11]. In plant tissues, it occurs both as free oxalic acid (H2C2O4) and as oxalate salts, forming insoluble complexes with Ca or Mg and soluble salts with Na or K [11]. The term “oxalate” refers to the deprotonated anion (C2O42−), which readily binds metal cations, whereas oxalic acid denotes the free, protonated form. Oxalic acid metabolism contributes to plant responses to abiotic and biotic stresses through ROS regulation, heavy metal detoxification, secondary metabolite production, hormonal interactions, and pathogen defense [11].
Oxalate can adversely affect human health [12]; when taken in excess, oxalate can bind to minerals such as Ca, forming insoluble crystals in the body and leading to kidney stones. Furthermore, soluble oxalate can interfere with the absorption of Ca, Mg, and Fe from food, leading to mineral deficiencies [13]. In plants, oxalic acid is an organic acid present in some plants in soluble and insoluble oxalate [14]. The synthesis of oxalate in plants is closely related to maintaining ionic balance between inorganic cations (K+, Na+, NH4+, Ca2+, Mg2+) and anions (NO3−, Cl−, H2PO4−, SO42−) [15]. Oxalate can help the plant’s tolerance to abiotic stresses, such as salinity stress. For instance, oxalate accumulation in vacuoles is pivotal for maintaining ion balance under salinity stress [16].
Oxalate metabolism in plants comprises both biosynthetic and degradative pathways, which are still not fully elucidated. Oxalate can arise from several metabolic routes, including glycolate and ascorbate catabolism [11], while its degradation is mainly mediated by enzymes such as oxalate oxidase (OxO), oxalate decarboxylase, and oxalyl-CoA synthetase [17]. Understanding the balance between these processes is essential for interpreting oxalate accumulation under varying nutritional or environmental conditions. Oxalate oxidase stands as a pivotal enzyme in plants, not only catalyzing the oxidation of oxalate to carbon dioxide and hydrogen peroxide but also playing multiple roles in plant physiology and defense mechanisms [11]. The levels of NO3− and ammonium (NH4+) in the growing medium are known to influence oxalate production in plant tissues [18]. Nitrate application is more likely to be responsible for oxalate buildup than NH4+ application as NO3− increases the activity of NO3− reductase and glutamine synthetase in leaves, thus promoting cation accumulation in plants and stimulating oxalate biosynthesis for intracellular pH homeostasis [11]. Additionally, NO3− may inhibit OxO activity by binding to its active site, reducing oxalate degradation [19].
This study aimed to evaluate the adaptability of RO to hydroponic baby green production under conditions of high salinity and reduced nitrogen availability, focusing particularly on their combined effects on leaf nutritional quality and antinutrient accumulation (NO3− and oxalate). Plants were grown in a floating raft system using nutrient solutions with different concentrations of NaCl (0 and 428 mM; 25 g L−1) and NO3− (10 and 1 mM) in a factorial design. The control represented standard hydroponic conditions, while the saline–low-nitrogen treatment simulated saltwater aquaponic environments. By linking plant performance and nutritional traits to combined salinity and nitrogen stress, this study provides new insights into the potential of RO as a resilient crop for saline or resource-limited soilless systems.
2. Results
Since ANOVA revealed no significant interaction between NaCl and NO3− concentration for almost all measured parameters, the results are reported and discussed focusing solely on the main effects of these two factors for clarity and brevity.
2.1. Effects of NaCl Salinity
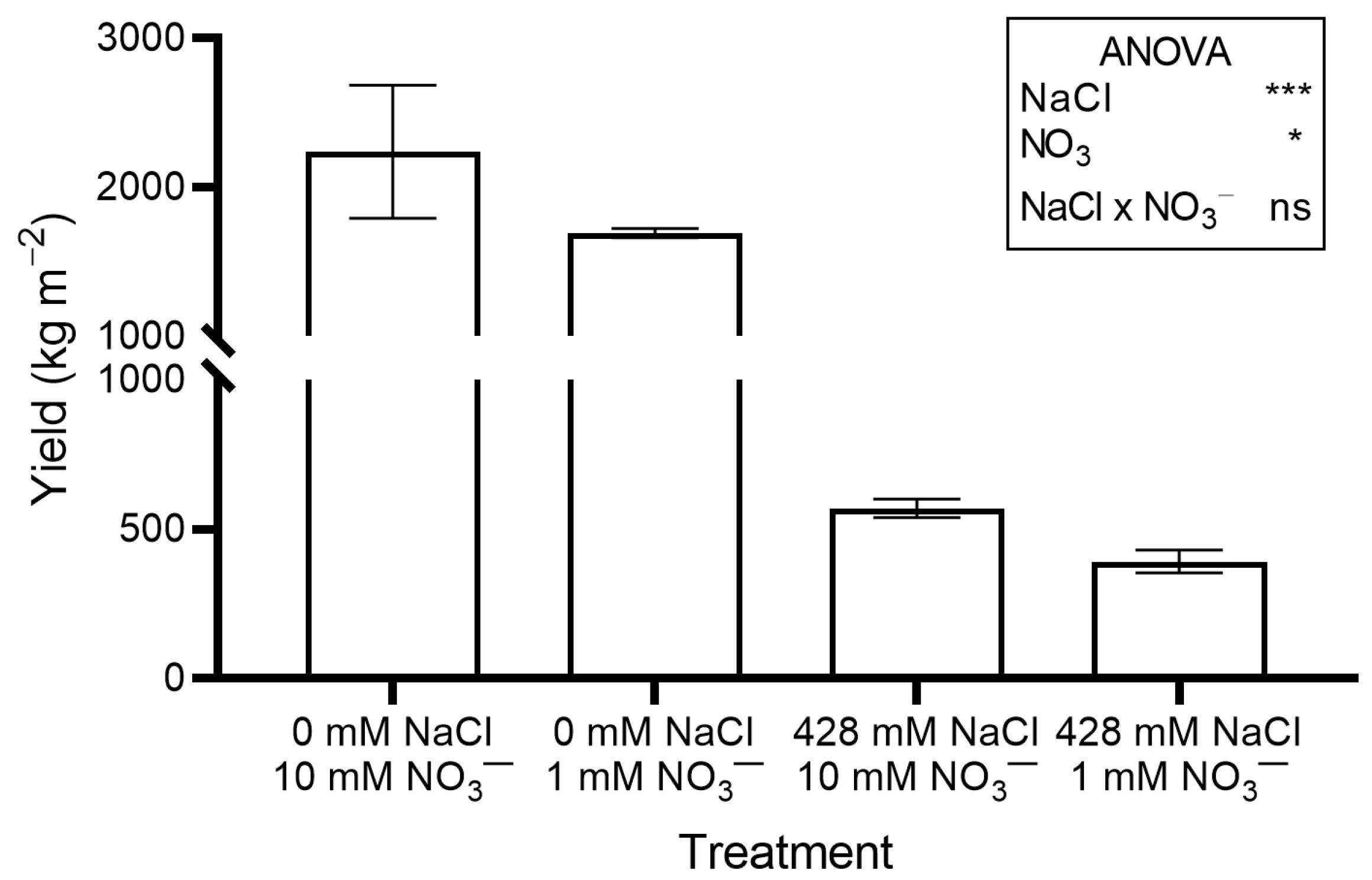
The use of a saline nutrient solution markedly reduced crop yield (Figure 1), leaf fresh weight (FW), leaf dry weight (DW), stem DW, root DW, total DW, and leaf area index (LAI) (Table 1), compared to the non-saline solution. The relative growth rate (RGR), leaf area ratio (LAR), and specific leaf area (SLA) were also reduced in salinized plants, while net assimilation rate (NAR) increased (Table S1).
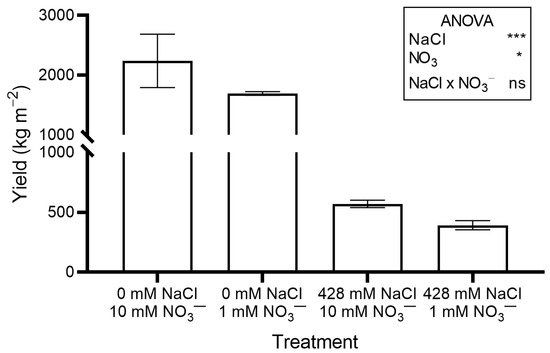
Figure 1.
Yield (shoot fresh weight) of red orache (Atriplex hortensis var. rubra) plants grown hydroponically for 17 days with different nutrient solutions, varying in the concentration of NaCl and NO3−. Mean values (±SE) of three replicates. Significance: *** p ≤ 0.001; * p ≤ 0.05; ns = not significant.

Table 1.
Leaf fresh weight (FW), leaf, stem, root and total dry weight (DW), leaf area index, leaf moisture content and leaf succulence in red orache (Atriplex hortensis var. rubra) plants grown hydroponically for 17 days with different nutrient solutions, varying in the concentration of NaCl and NO3−.
Leaf moisture content slightly decreased in salinized plants, while leaf succulence markedly increased in response to NaCl (Table 1).
The concentrations of total phenols, flavonoids, ascorbic acid and antioxidant capacity measured by Ferric Reducing Antioxidant Power (FRAP) and 2,2-difenil-1-picrilidrazile (DPPH) assays were reduced in salinized plants compared to the controls (Table 2). In contrast, NaCl salinity did not affect the total ascorbic acid concentration (Table 2).

Table 2.
Leaf concentration of total phenols and flavonoids, ascorbic acid, and antioxidant capacity measured by Ferric Reducing Antioxidant Power (FRAP) and 2,2-difenil-1-picrilidrazile (DPPH) assay in red orache (Atriplex hortensis var. rubra) plants grown hydroponically for 17 days with different nutrient solutions, varying in the concentration of NaCl and NO3−.
Salinity also significantly reduced leaf concentrations of total chlorophylls, carotenoids, and betalains, resulting in lighter leaf color as indicated by higher lightness (Table 3). No significant effects of salinity were observed on other color properties (Table 3).

Table 3.
Leaf concentration of total chlorophylls, carotenoids and betalains, and leaf color parameters in red orache (Atriplex hortensis var. rubra) plants grown hydroponically for 17 days with different nutrient solutions, varying in the concentration of NaCl and NO3−.
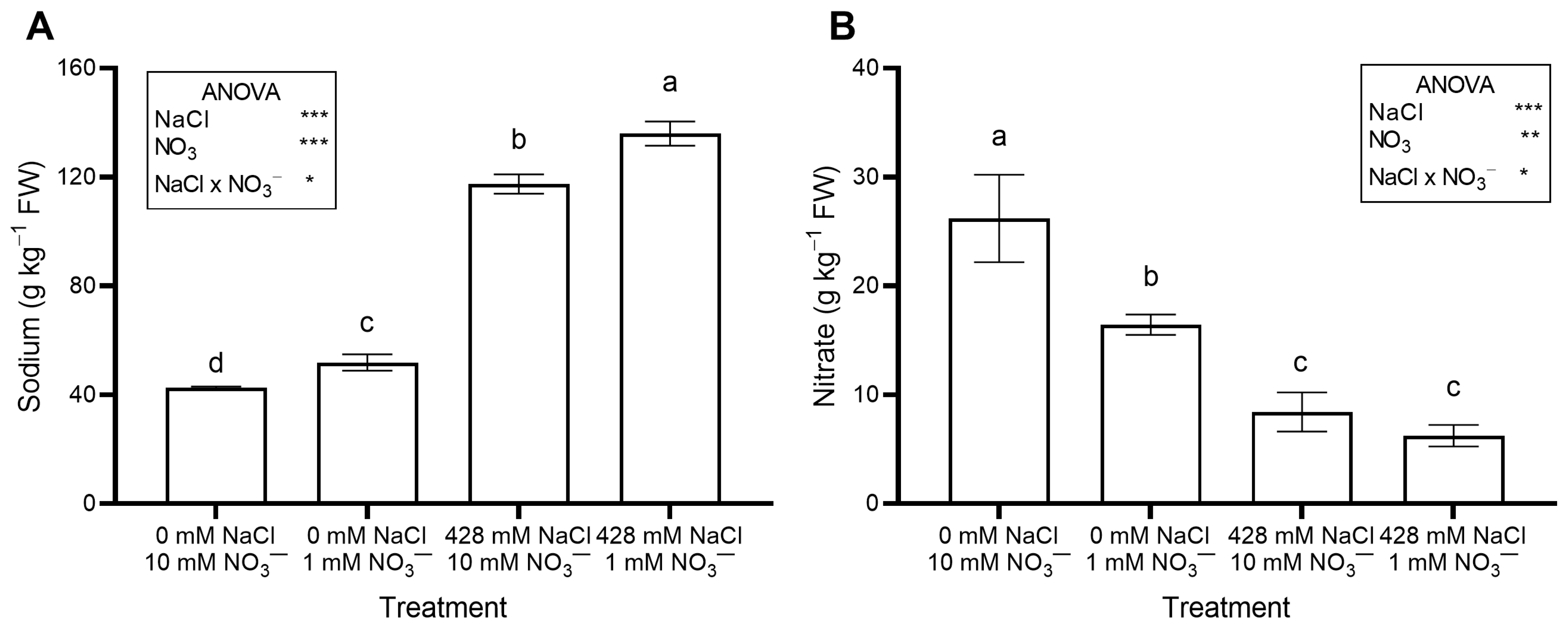
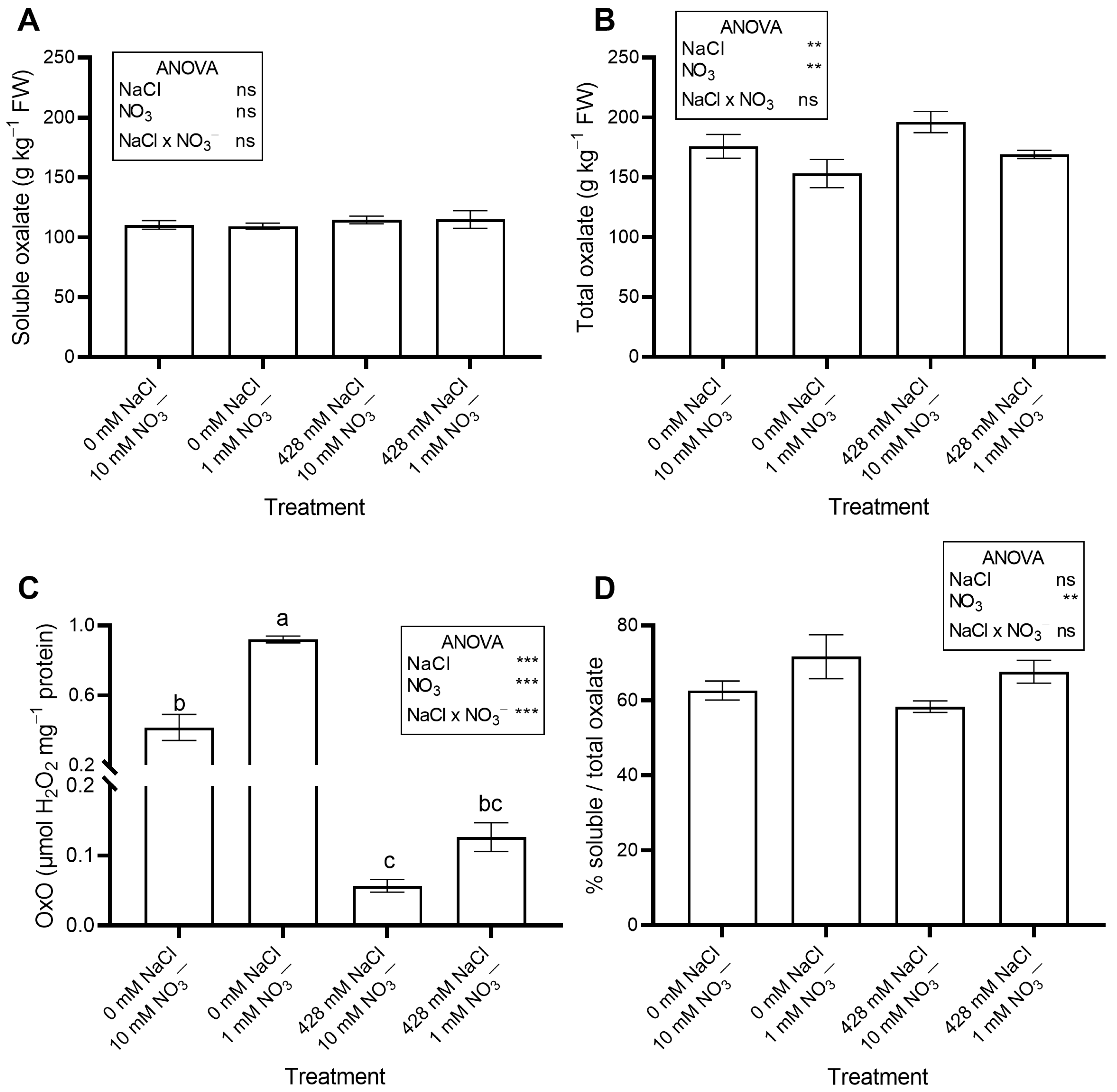
The saline treatments significantly increased leaf Na concentration (Figure 2A) and total oxalate (Figure 3B), while decreasing leaf NO3− concentration (Figure 2B) and OxO activity (Figure 3C). The percent ratio between soluble and total oxalate was not significantly affected by salinity, averaging a constant value across treatments (Figure 3D).
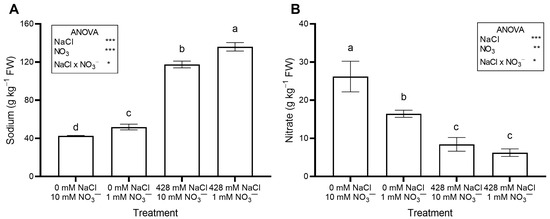
Figure 2.
Leaf concentration of Na (A) and NO3− (B) in red orache (Atriplex hortensis var. rubra) plants grown hydroponically for 17 days with different nutrient solutions, varying in the concentration of NaCl and NO3−. Mean values (n = 3; ±SE) flanked by the same letter are not statistically different at 5% level after Tukey’s post hoc test. Significance: *** p ≤ 0.001; ** p ≤ 0.01; * p ≤ 0.05; ns = not significant.
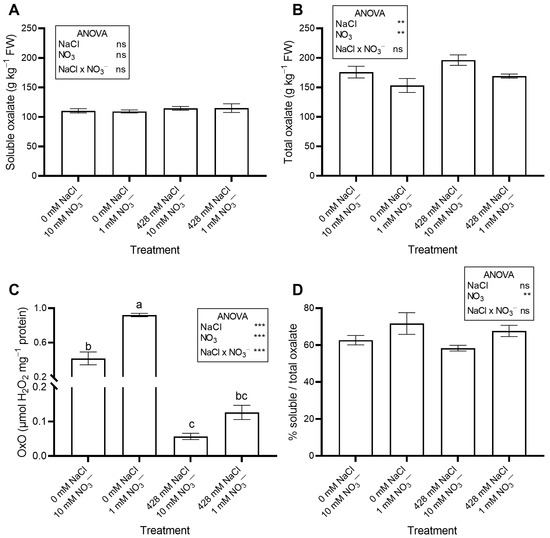
Figure 3.
Leaf concentration of soluble (A) and total oxalate (B), and oxalate oxidase (OxO) activity (C) of red orache (Atriplex hortensis var. rubra) plants grown hydroponically for 17 days with different nutrient solutions, varying in the concentration of NaCl and NO3−. The percent ratio between soluble and total oxalate is also shown (D). In (C), mean values (n = 3; ±SE) flanked by the same letter are not statistically different at 5% level after Tukey’s post hoc test. *** p ≤ 0.001; ** p ≤ 0.01; ns = not significant.
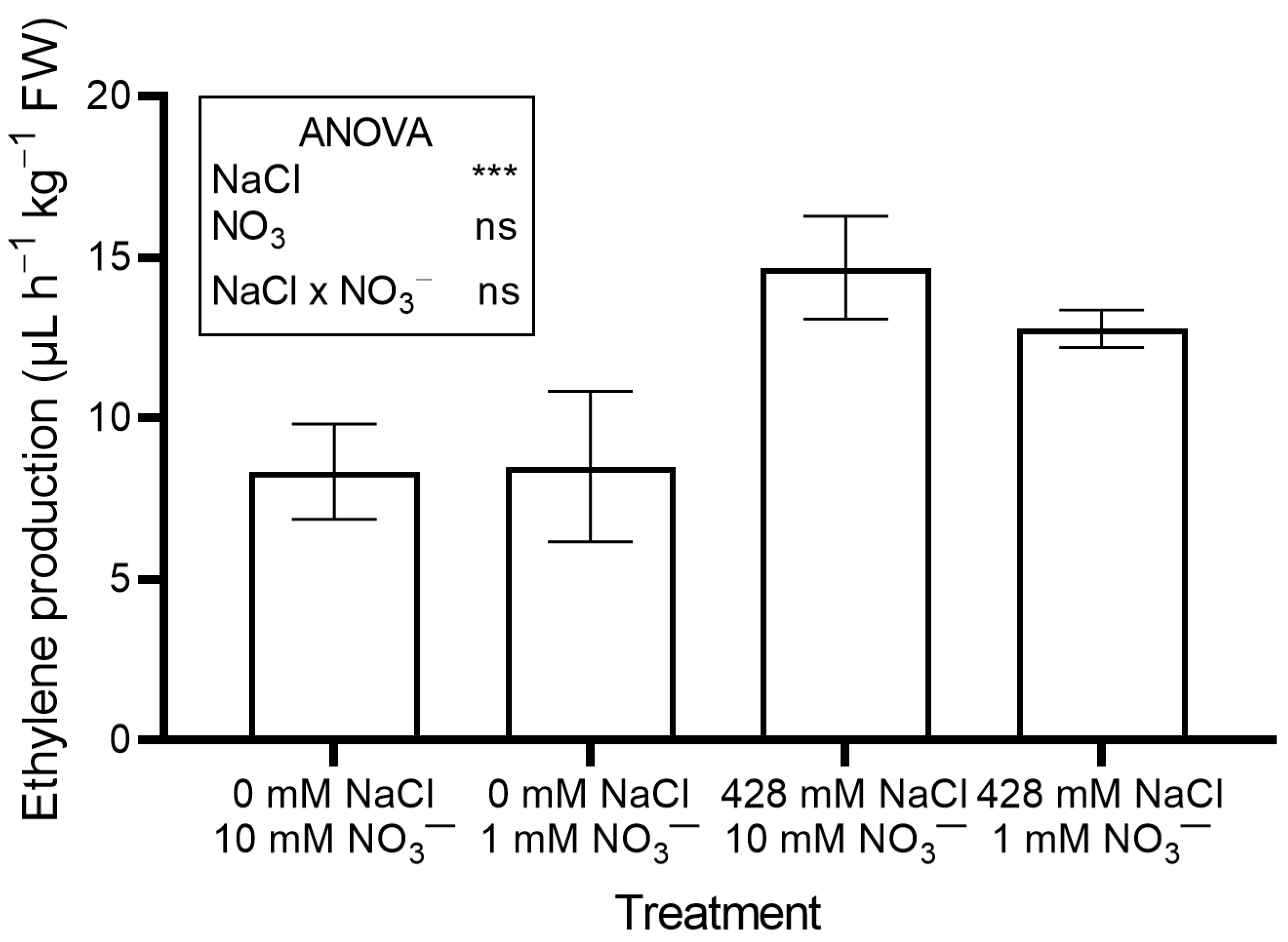
Ethylene production (Figure 4) and leaf performance index (PI) (Figure S1) also rose significantly in salinized plants compared to controls.
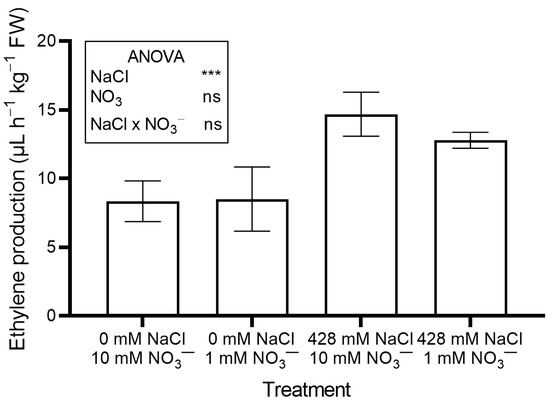
Figure 4.
Leaf ethylene production in red orache (Atriplex hortensis var. rubra) plants grown hydroponically for 17 days with different nutrient solutions, varying in the concentration of NaCl and NO3−. Significance: *** p ≤ 0.001; ns = not significant.
2.2. Effects of Nitrate Concentration
Reducing NO3− concentration in the nutrient solution inhibited plant growth, although the effects were less severe than those of NaCl salinity. Crop yield (Figure 1), leaf FW, leaf DW, total DW, and LAI (Table 1) were significantly lower in plants grown with 1 mM NO3− compared to those grown with the standard concentration. No significant differences were observed for stem and root DW (Table 1).
The RGR, NAR, and SLA were not significantly affected by NO3− level, while a slight but significant reduction in LAR and LWR was observed in plants grown with 1 mM NO3− (Table S1).
No significant effects of NO3− level were observed on leaf moisture content, succulence (Table 1), antioxidant capacity, total phenols, flavonoids, ascorbic acid (Table 2), pigments (Table 3), soluble oxalate (Figure 3A), color properties (Table 3), ethylene evolution (Figure 4), or PI (Figure S1). In contrast, plants supplied with 1 mM NO3− showed a lower leaf concentration of NO3− (Figure 2B) and total oxalate (Figure 3B), and a higher Na concentration (Figure 2A). The percent ratio between soluble and total oxalate significantly increased under low NO3− conditions (Figure 3D). Reduced NO3− supply markedly increased OxO activity, especially under non-saline conditions (Figure 3C).
The leaf concentrations of N, P, K, Mg, and Mn were not significantly influenced by NO3− level. However, compared to plants grown with standard NO3−, those supplied with reduced NO3− showed higher Mn and Fe but lower Ca levels (Table S2). Leaf Na concentration was slightly higher in plants grown with 1 mM NO3−, regardless of NaCl level (Table S2).
3. Discussion
This study evaluated the adaptability of RO to hydroponic cultivation of baby greens under contrasting salinity and nitrogen conditions. Using a floating raft system, plants were exposed to nutrient solutions differing in NaCl and NO3− concentrations to reproduce the chemical environment typical of saltwater aquaponics. The effects on growth performance, pigment composition, antioxidant capacity, and the accumulation of antinutrients such as NO3− and oxalate were analyzed. The following discussion integrates these results to elucidate the physiological mechanisms underlying red orache responses to combined salinity and nitrogen stress, highlighting their implications for sustainable soilless cultivation in saline environments.
3.1. Adaptation of Red Orache to Hydroponic Cultivation
In this study, RO adapted well to hydroponic cultivation with a standard nutrient solution. The baby greens yield recorded 17 days after transplanting (DAP; 29 days after sowing, Figure 1) were similar to those reported for red amaranth (Amaranthus tricolor L.), a species in the Amaranthaceae closely related to RO, which was grown hydroponically for 26 days in a growth chamber under comparable temperature and light conditions [20].
In our work, the use of saline nutrient solution markedly inhibited crop growth and yield regardless of NO3− supply (Table 1 and Figure 1). In a previous study, the growth of RO plants grown in soil was markedly reduced by irrigation with saline water (5 to 15 g L−1 of NaCl) compared to those irrigated with NaCl-free water [21]. In contrast, no significant difference in DW was observed in RO and other Atriplex species grown hydroponically for 35 days under 0 and 360 mM NaCl salinity, and plant FW was even higher in NaCl-treated plants [22].
The leaf concentrations of N, P, K, Ca, Mg, and Mn were significantly decreased by high salinity (Table S2), consistent with previous results [22]. The antagonism between Na+ and other cations [23], and between Cl− and NO3− [24] likely accounted for the reduction in K, Ca, Mg and N uptake in plants grown with NaCl-enriched solution. However, the growth inhibition caused by high NaCl in RO plants was not attributable to leaf mineral deficiency, since no visible symptoms of salt toxicity (e.g., leaf scorch) or nutrient deficiency were observed. Leaf concentrations of all essential elements, except Ca, remained within the sufficiency ranges previously reported for related species such as table beet (Beta vulgaris subsp. vulgaris L.) and spinach (Table S3).
Growth analysis and the analysis of chlorophyll fluorescence transients (JIP test), indicate that differences in biomass production (Figure 1 and Table 1) and RGR (Table S1) between the two salinity treatments were not due to impaired leaf photosynthesis, since both NAR (Table S1) and PI (Figure S1) were higher in salinized than non-salinized plants. Similarly, Calone et al. [22] reported that NaCl salinity did not significantly affect leaf gas exchange or chlorophyll fluorescence parameters in RO and other Atriplex species.
In our work, NaCl-induced growth suppression was mainly attributable to a decrease in LAR, a component of RGR (RGR = NAR × LAR). SLA and LWR are the two components of LAR (LAR = SLA × LWR). SLA was significantly reduced under salinity, while LWR was unaffected (Table S1). This indicates that growth inhibition resulted from reduced leaf expansion per unit leaf DW rather than altered dry matter partitioning between leaves and other organs.
The reduction in leaf expansion is an adaptive response to salt stress, which leads to lower transpiration [25]. In our study, reduced leaf expansion in salt-treated plants was likely caused by osmotic stress [25], consistent with the observed decrease in leaf moisture content (Table 1).
Salt-induced stress was further confirmed by increased ethylene production (Figure 4). Salinity stress is known to trigger ethylene production [26]. Ethylene helps maintain Na+/K+ homeostasis, regulates mineral uptake (including NO3−), and activates antioxidant defenses [27]. It also interacts with other phytohormones such as abscisic acid, auxins, and jasmonic acid, to coordinate a broad stress response [27].
Reducing the NO3− concentration in the nutrient solution decreased crop growth and yield to a lesser extent than high NaCl salinity (Table 1 and Figure 1). Growth analysis and JIP test results suggest that differences in biomass (Figure 1 and Table 1) between the two NO3− treatments were not related to photosynthesis. This aligns with the lack of differences in leaf N content (Table S2) [28].
Growth suppression in plants fed with 1 mM NO3− was associated with reduced LAR, mainly due to lower SLA, whereas LWR was unaffected (Table S1). This suggests that growth inhibition resulted from smaller leaf area per unit mass rather than altered biomass allocation. In contrast, it was reported [29], that under N limitation, reduced leaf area was primarily due to decreased LWR rather than SLA. Measurements of growth traits (Table 2 and Table S2), leaf N (Table S2), and PI (Figure S1) in RO suggest that, under N limitation, RO maintained leaf N content but reduced leaf area, similar to other species such as sunflower (Helianthus annuus L.), maize (Zea mays L.) [30] and potato (Solanum tuberosum L.) [31]. In RO, high NaCl reduced leaf moisture content while increasing succulence (Table 1). Thus, harvested leaves from saline-grown plants may have improved shelf life compared to non-salinized plants. Increased succulence is a typical halophyte response to salt stress [32], which may positively affect product quality by altering texture [33].
The health benefits of leafy vegetables are largely attributed to antioxidants such as pigments, phenols, flavonoids, and ascorbic acid [34]. In this study, high NaCl reduced antioxidant capacity and concentrations of chlorophylls, carotenoids, betalains, and flavonoids, whereas NO3− supply had no effect on these traits (Table 2), nor on leaf moisture or succulence (Table 1).
The lower concentrations of pigments in salinized leaves accounted for their higher lightness compared to non-salinized leaves (Table 3).
Some results were expected. For example, leaf concentrations of chlorophylls and carotenoids generally decrease under salt stress [35]. However, the effect of salt stress on antioxidant compounds varies among Amaranthaceae halophytes. In Beta vulgaris spp. maritima [36], Chenopodium quinoa [37], Salicornia ramosissima [38], and Suaeda maritima [39], salinity increased phenol and flavonoid concentrations, whereas decreases were observed in S. europaea [40] and S. ramosissima [39], and in the facultative halophyte Portulaca oleracea L. [41].
The reduction in antioxidant compounds in RO under salinity may be explained by their use in scavenging ROS to protect against oxidative damage [42]. In our experiment, only total ascorbic acid slightly increased under 428 mM NaCl compared to 0 mM, likely due to a concentration effect.
3.2. Effect of Salinity and Nitrogen Nutrition on Leaf Concentration of Sodium and Antinutrients
Leaf Na concentration increased when the plants were irrigated with a saline nutrient solution (Figure 2A), as expected, due to the much higher Na concentration compared to the control solution. These results agree with previous studies on Swiss chard (Beta vulgaris var. cicla) [43], sea beet (Beta vulgaris subsp. maritima) [35,36,43], and spinach [44] grown with different NaCl concentrations in hydroponics.
A large Na intake raises cardiovascular risk, and in adults, the recommended maximum daily intake is 2 g per day [45]. In the current work, leaf Na concentration reached 12.47 g kg−1 FW in salinized RO grown at 1 mM NO3− (Figure 2A). Thus, consuming a 100 g serving of RO baby greens is safe, as it would provide much less Na than the daily intake limit.
The interaction between NaCl salinity and nitrogen nutrition and metabolism differs significantly between glycophytes and halophytes. In glycophytes, NaCl salinity can reduce the uptake and assimilation of NO3− through several mechanisms, such as specific inhibition of NO3− transport at the root plasma membrane, a reduced demand for N due to salinity-induced growth restriction, and direct inhibition of key enzymes involved in NO3− assimilation, such as nitrate reductase (NR) and glutamine synthetase (GS) [46]. On the other hand, optimal nitrogen fertilization can improve plant’s tolerance to salt stress by regulating antioxidant enzyme activities, as found in maize [47]. In contrast, in halophytes such as Suaeda physophora [48], Suaeda salsa [49], and Salicornia europaea [50], NO3− uptake and assimilation are enhanced under saline conditions.
In this study, there was no significant interaction between NaCl and NO3− levels in the growing medium with respect to leaf NO3− concentration (Figure 2B) and total N (Table S2), both of which were significantly reduced by high salinity and low NO3− supply.
Nitrate may negatively affect human health, and because leafy vegetables are among the primary dietary sources, the European Union (EU) has imposed limits for some leafy species such as lettuce, spinach, and rocket [51]. In this work, conducted in autumn, NO3− levels in RO were consistently well below the EU maximum for spinach (3.5 g kg−1 FW); the highest level measured was 1.70 g kg−1 FW in control plants (Figure 2B). Artificial lighting likely limited NO3− accumulation in RO leaves, as leaf NO3− levels generally decrease with increasing photosynthetically active radiation (PAR), which promotes NO3− assimilation [24].
Although no official guidelines exist, adults are generally advised to limit oxalate intake to ~200 mg day−1 to prevent kidney stones [52]. In this work, leaf soluble oxalate ranged from 7.19 to 10.57 g kg−1 FW. Thus, consuming just 19–28 g of RO leaves would reach the daily limit.
Oxalic acid also impacts vegetable organoleptic traits, particularly causing the phenomenon known as “spinach teeth”, a gritty or chalky sensation caused by calcium oxalate crystal formation in saliva [13].
In our study, RO accumulated higher levels of oxalate (159.9–196.2 g kg−1 DW) than Atriplex halimus (76.7–106.7 g kg−1 DW) and A. nummularia (72.9–78.0 g kg−1 DW) [53]. At 17 DAP (Figure 3), soluble and total oxalate concentrations increased significantly in NaCl-treated plants, while OxO activity decreased.
Similar trends have been reported in halophytes such as Suaeda glauca [54], Portulaca oleracea [16], and Beta vulgaris ssp. maritima [36] grown under up to 400 mM NaCl, where oxalate accumulation contributed to osmotic adjustment and ionic balance between excess cations (Na+, K+) and anions (Cl−, SO42−).
Our results are partly consistent with the biochemical model that attributes oxalate accumulation to both de novo synthesis and limited degradation by OxO, which catalyzes oxidation of oxalate to CO2 and H2O2 [11]. Although oxalate breakdown is mediated by OxO, oxalate decarboxylase, and oxalyl-CoA synthase [11], we focused on OxO because our results directly relate to its activity. Since OxO alone only partially accounts for the observed changes in oxalate, further studies are needed to elucidate the contributions of other degrading enzymes.
The metabolism of ascorbate and oxalate are closely interconnected, as ascorbic acid is linked to mitochondrial and photosynthetic electron transport, influencing both production and conversion to oxalate [55]. Oxalate formation from ascorbic acid degradation has been reported in several species with structural, physiological, and biochemical roles [55]. In our experiment, total oxalate (Figure 3) increased, while ascorbic acid decreased (Table 1) in salinized plants and NO3− had no effect (Table 2). This decrease in ascorbic acid concentration might be related to its utilization as a substrate for oxalate synthesis.
Evidence indicates a relationship between N nutrition and oxalate biosynthesis [56,57]. Nitrate assimilation into amino acids requires reduction to NH4+, a process that alkalinizes the cytosol (one OH− released per NO3− reduced; [58]). To maintain cytosolic pH within an optimal range, plants synthesize organic acids such as oxalic acid, which release H+ ions and help counteract the alkalinization, leading to increased oxalate accumulation. In hydroponic spinach, soluble and total oxalate increased with NO3− concentration (up to a certain point) and decreased with the NO3−/NH4+ ratio. Similar results were reported in Atriplex nummularia [56].
As far as we know, no study has investigated N effects on oxalate accumulation in RO. In our work, low NO3− concentration had minor or insignificant effects on leaf oxalate at 17 DAP (Figure 3B). Indeed, in plants grown with 1 mM NO3−, total oxalate decreased slightly but significantly, while soluble oxalate was unaffected. In contrast, OxO activity increased significantly under 1 mM NO3−. According to our results, Çalişkan [15] hypothesized that NO3− inhibits OxO, promoting oxalate accumulation. Meeuse et al. [59] also found that NO3− inhibits oxalate breakdown by OxO in beet extracts. Thus, the reduction in total oxalate concentration observed in our experiment may have been partly driven by enhanced OxO activity under low NO3− conditions. Oxalate oxidase plays a central role in oxalate metabolism, catalyzing the oxidative degradation of oxalate into CO2 and H2O2. Its activity is essential for maintaining oxalate homeostasis and preventing excessive oxalate accumulation, which can lead to calcium oxalate crystal formation and reduced calcium bioavailability [60]. However, the relative contribution of OxO compared to other oxalate-degrading enzymes, such as oxalate decarboxylase and oxalyl-CoA synthetase, likely varies among species and environmental conditions [17]. Since the activity of these additional enzymes was not analyzed in this study, a complete understanding of the regulatory mechanisms requires further investigation. Hence, adjusting N levels and/or the NO3−/NH4+ ratio in hydroponics could lower leaf oxalate, benefiting human health, as shown by Fontana et al. [61] and Zhang et al. [57], although this strategy may also reduce biomass. Zhang et al. [57] suggested that a NO3−/NH4+ ratio of 1 can reduce oxalate without impairing growth. Moreover, blanching effectively removes oxalate, as reported for Portulaca oleracea [62] and Tetragonia expansa [63]. However, blanching is unsuitable for tender products like baby greens, as it causes wilting and nutrient loss [64].
4. Materials and Methods
4.1. Plant Material and Growing Conditions
Seeds were purchased from “De Bolster Organic Seeds” (https://www.bolster.eu/, accessed on 15 July 2023), sown into stonewool cubes and kept at 25 °C in a growth chamber until emergence. Twelve days after sowing, seedlings were transplanted into 13 L hydroponic tanks in a glasshouse at the University of Pisa (Pisa, Italy) at a plant density of 720 plants m−2. The experiment was conducted in autumn; lasting for 17 days DAP. In the glasshouse, average air temperature was 22.6 °C, with minimum and maximum temperatures of 17.9 °C and 31.5 °C, respectively; natural PAR averaged 8.50 mol m−2 d−1 and HPS lamps provided 100 µmol m−2 s−1 PAR for 12 h daily; therefore, total PAR was approximately 12.82 mol m−2 d−1.
4.2. Experimental Design and Nutrient Solutions
Four different nutrient solutions, varying in the concentration of NaCl (0 and 428 mM, which corresponds to 25 g L−1) and NO3− (10 and 1 mM), were compared in a completely randomized design with three replicates. Each replicate consisted of one hydroponic tank containing 30 individual plants. The mineral composition and electrical conductivity of the nutrient solutions are detailed in Table 4. The saline nutrient solutions were prepared using technical-grade salts—macronutrients from Haifa Chemicals Ltd. (Matam-Haifa, Haifa, Israel) and micronutrients from Vialca Srl (Uzzano, Pistoia, Italy)—dissolved in tap water: 5[Ca(NO3)2 · H2O]NH4NO3, KH2PO4, MgSO4 · 7H2O, KNO3, K2SO4, Fe EDDHA, boric acid, Cu EDTA, Zn EDTA, chelated Mn, Na2MoO4. Sodium chloride was added gradually over three consecutive days to prevent osmotic shock.

Table 4.
Mineral composition and electrical conductivity of the different nutrient solutions used in the experiment with red orache plants grown hydroponically in a glasshouse.
4.3. Determinations
4.3.1. Growth Analysis
Two samplings were performed, 10 and 17 days after the onset of the experiment (12 and 19 DAP). Stem and leaf FW and DW, root DW, and leaf area were determined in 18 plants collected from each tank. Dry weight was measured after drying fresh samples in a ventilated oven at 70 °C until constant weight. Leaf moisture content was calculated as the percentage of water in the fresh tissue. Leaf succulence was calculated as the amount of water per unit leaf area. Fresh yield corresponded to shoot FW. A digital planimeter was used to measure the leaf area, and LAI was calculated as individual plant leaf area multiplied by plant density. The leaf and whole-plant DW, and leaf area measured at the beginning of the experiment and 10 and 17 days later were used to calculate the following growth parameters [65]: RGR (g d−1), NAR (g m−2 d−1), LAR (m−2 g−1), SLA (m−2 g−1), and LWR (dimensionless). The equations are shown in Table S4.
4.3.2. Mineral Elements
The concentration of mineral elements and NO3− was determined in ground dry leaf samples. Samples were either mineralized with a mixture (5:2) of 65% HNO3 and 30% H2O2 at 240 °C for 1 h or extracted with distilled water at room temperature for 2 h. In the mineralized samples, K, Ca, Mg, Na, Cu, Fe, Mn, and Zn were quantified using atomic absorption spectroscopy (Varian Model Spectra AA240 FS, Agilent Technologies Australia [M]. Pty Ltd., Mulgrave, Australia), while P was measured by UV/VIS spectrometry using Olsen’s method. The NO3− concentration was analyzed spectrophotometrically in leaf water extracts using the salicylic sulfuric acid method as described by Puccinelli et al. [43]. Dry leaf samples were also used to determine organic N via the Kjeldahl method.
4.3.3. Plant Secondary Metabolites and Antioxidant Capacity
The leaf concentration of pigments, flavonoids, and phenols, and the total antioxidant capacity were analyzed in fresh samples (leaves from four plants per tank). Fresh samples were extracted with 99% (v/v) methanol, sonicated for 60 min, and then stored at −18 °C for 24 h; afterward, the concentration of total chlorophylls, carotenoids, phenols and flavonoids, and the antioxidant capacity (FRAP assay) were determined spectrophotometrically as reported by Puccinelli et al. [43].
To measure betalain concentration, 100 mg of fresh baby greens were extracted using 50% (v/v) ethanol [66]. The resulting extract was then diluted with 100 mM phosphate buffer (pH 6.5), and its absorbance was recorded at 538 nm, 476 nm, and 600 nm [67]. Betacyanin and betaxanthin concentrations were calculated [68] and expressed as mg per g of FW. Total betalains were the sum of betacyanins and betaxanthins.
For DPPH radical scavenging activity, 0.1 mL of methanolic extract was mixed with 2.9 mL of DPPH solution (20 mg L−1 in methanol), incubated in the dark for 45 min at room temperature, and absorbance measured at 515 nm. Antioxidant capacity was expressed as Trolox equivalent antioxidant capacity, using a Trolox calibration curve [69].
For the determination of ascorbic acid, 500 mg of leaf samples were stored at −80 °C, then extracted with 5 mL of a cold of 50 mM KH2PO4 solution (pH 7.0) under dim light conditions. The extracts were filtered using Chromafil® Xtra 20/25 H-PTFE syringe filters (Macherey–Nagel, Duren, Germany) and analyzed without further treatment. For the determination of total ascorbic acid, which is the sum of ascorbic acid and dehydroascorbic acids, 50 µL of 2 mM dithiothreitol (DTT, pH 7.0), were added to 500 µL of filtered extract and the solution was stored 20 min in the dark at room temperature before HPLC analysis. Dehydroascorbic acid was determined by the difference between total ascorbic acid and ascorbic acid.
The concentration of total oxalate was measured in dried leaf samples extracted with 0.25 M HCl (50 mg DW in 6 mL) at 100 °C for 30 min. The mixture was allowed to cool, filled to a volume of 10 mL with 0.25 M HCl, and then filtered through filter paper. The soluble oxalate content in each leaf sample was determined as above, using distilled water instead of 0.25 M HCl.
Both ascorbic acid and oxalate were determined with a HPLC-DAD system (Jasco, Tokyo, Japan) consisting of a PU-2089 four-solvent low-pressure gradient pump and a MD-4010 diode array detector. The separation was performed using a C18 Atlantis® T3 5 µm 4.6 × 250 mm column (Waters, Milford, MA, USA). Ascorbic acid and oxalate were determined using 50 mM KH2PO4 (pH 2.8) in isocratic mode with a flow of 0.8 mL min−1, followed by 5 min washing with 95% acetonitrile (CH3CN) and 5 min column re-equilibration. The injection volume was 20 µL and the chromatograms were recorded at 243 or 214 nm for ascorbic acid and oxalate, respectively. Ascorbic acid or oxalate standard solutions were used for calibration.
4.3.4. Oxalate Oxidase
Crude extracts were obtained by homogenizing fresh samples in 0.4 M phosphate buffer (pH 7.0), followed by centrifugation. Assays were performed in foil-wrapped tubes containing succinate buffer (0.05 M, pH 5.0), CuSO4 (0.01 M), and oxalic acid (0.01 M), incubated at 40 °C for 5 min. A 4-aminophenazone reagent was added, and color developed after 30 min in darkness. Absorbance was measured at 520 nm against a standard H2O2 curve. One unit of OxO was defined as the amount of enzyme producing 1 µmol of H2O2 per 5 min under assay conditions [70]. Specific activity was expressed as µmol H2O2 mg−1 protein, with protein determined by using the Bradford method with bovine serum albumin standards [71].
4.3.5. Chlorophyll a Fluorescence
Chlorophyll a fluorescence transients were determined in dark-adapted leaves maintained for 30 min at room temperature, using a portable Handy PEA fluorimeter (Hansatech, UK). Measurements were taken on the leaf surface (4 mm diameter) exposed to an excitation light of 3000 μmol m−2 s−1 (600 W m−2) emitted by three ultrabright red LEDs with a peak at 650 nm. Leaf fluorescence detection was measured by a fast-response PIN photodiode with an RG9 long-pass filter (Hansatech, technical manual). The JIP test was performed to determine the PI, which integrates several parameters related to photosynthesis and provides an integrative parameter of leaf functionality and vitality [72].
Leaf color variations were determined by measuring CIELAB color space coordinates L*, a*, b* with a Konica Minolta CR-400 colorimeter with D65 illuminant (Konica Minolta Sensing, Inc., Osaka, Japan). The colorimeter was automatically calibrated with an internal standard before each measurement. Lightness was measured directly while HUE angle and Chroma Index were derived from a* and b* values according to Hirschler et al. [73] and Nambi et al. [74].
4.3.6. Ethylene Evolution
Ethylene production was measured by enclosing approximately 1 g of intact fresh leaves in airtight containers (50 mL). Two mL gas samples were taken from the headspace of the containers after 1 h incubation at room temperature. The ethylene concentration was measured with a gas chromatograph (HP 8890) using a flame ionization detector (FID), a stainless-steel column (150 × 0.4 cm ø packed with Hysep T) (Hewlett-Packard, Menlo Park, CA, USA), column and detector temperatures of 70° and 350 °C, respectively, and helium as carrier gas at a flow rate of 30 mL min−1. Quantification was performed against an external standard and results were expressed as nL h−1 g−1 FW.
4.4. Statistical Analysis
Data were analyzed with JMP Pro 17 (SAS Institute, Cary, NC, USA). Normality was tested using the Shapiro–Wilk test and homogeneity of variances with Levene’s test. Data were then subjected to two-way ANOVA, with NaCl and NO3− concentration as independent variables. Means were compared using Tukey’s HSD test (p < 0.05). Percent ratios of soluble to total oxalate were arcsine-transformed prior to analysis but are presented in tables as untransformed values.
5. Conclusions
In conclusion, the hydroponic production of red orache baby greens was markedly reduced when grown with solutions containing the typical salinity and nitrogen levels of marine aquaculture effluents. Such solutions should therefore be diluted with freshwater or supplemented with a standard nutrient solution before use. High salinity negatively affected plant growth and leaf quality to a much greater extent than reduced NO3− availability. From a nutraceutical perspective, high salinity reduced the leaf concentrations of pigments and flavonoids, as well as total antioxidant capacity. Leaf sodium levels increased under saline conditions but remained within safe limits for moderate daily consumption, i.e., less than 160 g day−1, of red orache baby leaves. Both high salinity and low nitrogen supply led to decreased leaf NO3− concentrations. High salinity increased leaf oxalate levels, whereas nitrogen availability had only minor effects. Overall, red orache leaves were high in oxalate and should therefore be consumed in moderation. The pattern of oxalate accumulation observed supports the hypothesis that increased oxalate levels are linked to reduced catabolic activity of OxO. To our knowledge, this is the first report of OxO activity in a dicot species.
Future research should focus on developing cultivation strategies to lower oxalate concentrations in hydroponically grown red orache. However, as this study was conducted under controlled hydroponic conditions for a limited growth period, further research is needed to confirm the long-term performance of red orache in diverse saline cultivation systems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants14213292/s1, Figure S1. Leaf performance index in red orache L. plants grown hydroponically for 17 days with different nutrient solutions, varying in the concentration of NaCl and nitrate; Table S1. Relative growth rate (RGR), net assimilation rate (NAR), leaf area ratio (LAR), specific leaf area (SLA), and leaf weight ratio (LWR) in red orache plants grown hydroponically for 17 days with different nutrient solutions, varying in the concentration of NaCl and NO3−. Mean values (n = 6; ±SE) of the parameters calculated for the first and second growth phase, which ended 10 and 17 days after the onset of the experiment; Table S2. Leaf mineral concentration (on dry weight basis) in red orache L. plants grown hydroponically for 17 days with different nutrient solutions for total salinity and nitrate concentration; Table S3. Adequate ranges for the leaf concentration of macronutrients and micronutrients in Beta vulgaris var. vulgaris and Spinacia oleracea; Table S4. Equations used to calculate the growth parameters of red orache plants grown hydroponically for 17 days with different nutrient solutions, varying in the concentration of NaCl and NO3−. The parameters were calculated based on the leaf (L) and whole-plant (W) dry weight (g), and leaf area (A, m2) measured at the beginning of the experiment and 10 and 17 days later; Table S5. Table of abbreviations.
Author Contributions
Conceptualization, M.P., A.P. and A.T.; Data curation, M.P., S.C., R.M., G.C. and A.T.; Formal analysis, M.P., S.C., R.M., G.C. and A.T.; Investigation, M.P., S.C. and A.T.; Methodology, M.P., R.M. and A.T.; Resources, A.P.; Supervision, M.P., A.P. and A.T.; Writing—original draft, M.P., R.M. and A.T.; Writing—review & editing, M.P., R.M., G.C., A.P. and A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Atzori, G.; Mancuso, S.; Masi, E. Seawater Potential Use in Soilless Culture: A Review. Sci. Hortic. 2019, 249, 199–207. [Google Scholar] [CrossRef]
- Kotzen, B.; Emerenciano, M.G.C.; Moheimani, N.; Burnell, G.M. Aquaponics: Alternative Types and Approaches. In Aquaponics Food Production Systems; Springer Nature: Cham, Switzerland, 2019; pp. 301–330. ISBN 9783030159436. [Google Scholar]
- Rossi, L.; Puccinelli, M.; Marchioni, I.; Incrocci, L.; Fronte, B.; Bibbiani, C. Aquaponics: Challenges and Opportunities for Commercial Application. In Developing Circular Agricultural Production Systems; Amon, B., Ed.; Burleigh Dodds Science Publishing: Sawston, UK, 2024; p. 480. ISBN 9781801462563. [Google Scholar]
- Sai Kachout, S.; Ennajah, A.; Guenni, K.; Ghorbel, N.; Zoghlami, A. Potential of Halophytic Plant Atriplex Hortensis for Phytoremediation of Metal-Contaminated Soils in the Mine of Tamra. Soil Sediment Contam. Int. J. 2024, 33, 139–154. [Google Scholar] [CrossRef]
- Hunt, S.P.; Jarvis, D.E.; Larsen, D.J.; Mosyakin, S.L.; Kolano, B.A.; Jackson, E.W.; Martin, S.L.; Jellen, E.N.; Maughan, P.J. A Chromosome-Scale Assembly of the Garden Orach (Atriplex hortensis L.) Genome Using Oxford Nanopore Sequencing. Front. Plant Sci. 2020, 11, 624. [Google Scholar] [CrossRef] [PubMed]
- Kumorkiewicz-Jamro, A.; Pachulicz, R.J.; Fitter, S.; Górska, R.; Duggan, J.; Vandyke, K.; Pukala, T.L.; Wybraniec, S.; Zannettino, A.C.W. Atriplex hortensis Var. “rubra” Extracts and Purified Amaranthin-Type Pigments Reduce Oxidative Stress and Inflammatory Response in LPS-Stimulated RAW264.7 Cells. Food Chem. 2025, 462, 140920. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.G. Betalains in Some Species of the Amaranthaceae Family: A Review. Antioxidants 2018, 7, 53. [Google Scholar] [CrossRef]
- Arbour, A.J.; Chu, Y.T.; Brown, P.B.; Huang, J.Y. Life Cycle Assessment on Marine Aquaponic Production of Shrimp, Red Orache, Minutina and Okahajiki. J. Environ. Manag. 2024, 353, 120208. [Google Scholar] [CrossRef]
- Prakash, P.; Kaur, R.; Shekhar, S.; Prasad, K. Technological and Analytical Aspects of Bioactive Compounds and Nutraceuticals from Plant (Vegetable) Sources. In Bioactive Compounds and Nutraceuticals from Plant Sources; Apple Academic Press: Burlington, ON, Canada, 2024; pp. 3–41. [Google Scholar]
- Sharma, P.; Mahongnao, S.; Gupta, A.; Nanda, S. Health Risk Assessment for Potentially Toxic Elements Accumulation in Amaranthaceae Family Cultivars and Their Correlation with Antioxidants and Antinutrients. Arch. Environ. Contam. Toxicol. 2024, 87, 187–207. [Google Scholar] [CrossRef]
- Li, P.; Liu, C.; Luo, Y.; Shi, H.; Li, Q.; Pinchu, C.; Li, X.; Yang, J.; Fan, W. Oxalate in Plants: Metabolism, Function, Regulation, and Application. J. Agric. Food Chem. 2022, 2022, 16037–16049. [Google Scholar] [CrossRef]
- Salgado, N.; Silva, M.A.; Figueira, M.E.; Costa, H.S.; Albuquerque, T.G. Oxalate in Foods: Extraction Conditions, Analytical Methods, Occurrence, and Health Implications. Foods 2023, 12, 3201. [Google Scholar] [CrossRef]
- Huynh, N.K.; Nguyen, D.H.M.; Nguyen, H.V.H. Effects of Processing on Oxalate Contents in Plant Foods: A Review. J. Food Compos. Anal. 2022, 112, 104685. [Google Scholar] [CrossRef]
- Ghanati, K.; Oskoei, V.; Rezvani-Ghalhari, M.; Shavali-Gilani, P.; Mirzaei, G.; Sadighara, P. Oxalate in Plants, Amount and Methods to Reduce Exposure; a Systematic Review. Toxin Rev. 2024, 43, 411–422. [Google Scholar] [CrossRef]
- Çalişkan, M. The Metabolism of Oxalic Acid. Turk. J. Zool. 2000, 24, 103–106. [Google Scholar]
- Camalle, M.; Standing, D.; Jitan, M.; Muhaisen, R.; Bader, N.; Bsoul, M.; Ventura, Y.; Soltabayeva, A.; Sagi, M. Effect of Salinity and Nitrogen Sources on the Leaf Quality, Biomass, and Metabolic Responses of Two Ecotypes of Portulaca Oleracea. Agronomy 2020, 10, 656. [Google Scholar] [CrossRef]
- Svedružić, D.; Jónsson, S.; Toyota, C.G.; Reinhardt, L.A.; Ricagno, S.; Lindqvist, Y.; Richards, N.G.J. The Enzymes of Oxalate Metabolism: Unexpected Structures and Mechanisms. Arch. Biochem. Biophys. 2005, 433, 176–192. [Google Scholar] [CrossRef]
- Tian, H.; Jiang, L.; Liu, E.; Zhang, J.; Liu, F.; Peng, X. Dependence of Nitrate-induced Oxalate Accumulation on Nitrate Reduction in Rice Leaves. Physiol. Plant. 2008, 133, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Palaniswamy, U.R.; Bible, B.B.; McAvoy, R.J. Oxalic Acid Concentrations in Purslane (Portulaca oleraceae L.) Is Altered by the Stage of Harvest and the Nitrate to Ammonium Ratios in Hydroponics. Sci. Hortic. 2004, 102, 267–275. [Google Scholar] [CrossRef]
- Wittayathanarattana, T.; Wanichananan, P.; Supaibulwatana, K.; Goto, E. A Short-Term Cooling of Root-Zone Temperature Increases Bioactive Compounds in Baby Leaf Amaranthus tricolor L. Front. Plant Sci. 2022, 13, 944716. [Google Scholar] [CrossRef]
- Kachout, S.S. The Effect of Salinity on the Growth of the Halophyte Atriplex Hortensis (Chenopodiaceae). Appl. Ecol. Environ. Res. 2009, 7, 319–332. [Google Scholar] [CrossRef]
- Calone, R.; Cellini, A.; Manfrini, L.; Lambertini, C.; Gioacchini, P.; Simoni, A.; Barbanti, L. The C4 Atriplex Halimus vs. The C3 Atriplex Hortensis: Similarities and Differences in the Salinity Stress Response. Agronomy 2021, 11, 1967. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Marschner, H., Ed.; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Colla, G.; Kim, H.J.; Kyriacou, M.C.; Rouphael, Y. Nitrate in Fruits and Vegetables. Sci. Hortic. 2018, 237, 221–238. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity Induced Physiological and Biochemical Changes in Plants: An Omic Approach Towards Salt Stress Tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef]
- Boorboori, M.R.; Li, J. The Effect of Salinity Stress on Tomato Defense Mechanisms and Exogenous Application of Salicylic Acid, Abscisic Acid, and Melatonin to Reduce Salinity Stress. Soil. Sci. Plant Nutr. 2024, 71, 93–110. [Google Scholar] [CrossRef]
- Riyazuddin, R.; Verma, R.; Singh, K.; Nisha, N.; Keisham, M.; Bhati, K.K.; Kim, S.T.; Gupta, R. Ethylene: A Master Regulator of Salinity Stress Tolerance in Plants. Biomolecules 2020, 10, 959. [Google Scholar] [CrossRef]
- Walker, A.P.; Beckerman, A.P.; Gu, L.; Kattge, J.; Cernusak, L.A.; Domingues, T.F.; Scales, J.C.; Wohlfahrt, G.; Wullschleger, S.D.; Woodward, F.I. The Relationship of Leaf Photosynthetic Traits—Vcmax and Jmax—To Leaf Nitrogen, Leaf Phosphorus, and Specific Leaf Area: A Meta-Analysis and Modeling Study. Ecol. Evol. 2014, 4, 3218–3235. [Google Scholar] [CrossRef]
- Wang, P.; Grimm, B. Connecting Chlorophyll Metabolism with Accumulation of the Photosynthetic Apparatus. Trends Plant Sci. 2021, 26, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Massignam, A.M.; Chapman, S.C.; Hammer, G.L.; Fukai, S. Effects of Nitrogen Supply on Canopy Development of Maize and Sunflower. Crop Pasture Sci. 2011, 62, 1045–1055. [Google Scholar] [CrossRef]
- Vos, J.; Van Der Putten, P.E.L. Effect of Nitrogen Supply on Leaf Growth, Leaf Nitrogen Economy and Photosynthetic Capacity in Potato. Field Crops Res. 1998, 59, 63–72. [Google Scholar] [CrossRef]
- Lombardi, T.; Bertacchi, A.; Pistelli, L.; Pardossi, A.; Pecchia, S.; Toffanin, A.; Sanmartin, C. Biological and Agronomic Traits of the Main Halophytes Widespread in the Mediterranean Region as Potential New Vegetable Crops. Horticulturae 2022, 8, 195. [Google Scholar] [CrossRef]
- Damerum, A.; Chapman, M.A.; Taylor, G. Innovative Breeding Technologies in Lettuce for Improved Post-Harvest Quality. Postharvest Biol. Technol. 2020, 168, 111266. [Google Scholar] [CrossRef]
- Yang, X.; Gil, M.I.; Yang, Q.; Tomás-Barberán, F.A. Bioactive Compounds in Lettuce: Highlighting the Benefits to Human Health and Impacts of Preharvest and Postharvest Practices. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4–45. [Google Scholar] [CrossRef]
- Yolcu, S.; Alavilli, H.; Ganesh, P.; Panigrahy, M.; Song, K. Salt and Drought Stress Responses in Cultivated Beets (Beta vulgaris L.) and Wild Beet (Beta maritima L.). Plants 2021, 10, 1843. [Google Scholar] [CrossRef]
- Puccinelli, M.; Galati, D.; Carmassi, G.; Rossi, L.; Pardossi, A.; Incrocci, L. Leaf Production and Quality of Sea Beet (Beta vulgaris Subsp. Maritima) Grown with Saline Drainage Water from Recirculating Hydroponic or Aquaculture Systems. Sci. Hortic. 2023, 322, 112416. [Google Scholar] [CrossRef]
- del Carmen Rodríguez-Hernández, M.; Garmendia, I. Leaf Production and Quality of Quinoa ‘Titicaca’ Is Enhanced Under Moderate Salinity. Chil. J. Agric. Res. 2023, 83, 732–741. [Google Scholar] [CrossRef]
- Lima, A.R.; Castañeda-Loaiza, V.; Salazar, M.; Nunes, C.; Quintas, C.; Gama, F.; Pestana, M.; Correia, P.J.; Santos, T.; Varela, J.; et al. Influence of Cultivation Salinity in the Nutritional Composition, Antioxidant Capacity and Microbial Quality of Salicornia Ramosissima Commercially Produced in Soilless Systems. Food Chem. 2020, 333, 127525. [Google Scholar] [CrossRef]
- Benjamin, J.J.; Lucini, L.; Jothiramshekar, S.; Parida, A. Metabolomic Insights into the Mechanisms Underlying Tolerance to Salinity in Different Halophytes. Plant Physiol. Biochem. 2019, 135, 528–545. [Google Scholar] [CrossRef] [PubMed]
- Puccinelli, M.; Marchioni, I.; Botrini, L.; Carmassi, G.; Pardossi, A.; Pistelli, L. Growing Salicornia europaea L. with Saline Hydroponic or Aquaculture Wastewater. Horticulturae 2024, 10, 196. [Google Scholar] [CrossRef]
- He, J.; You, X.; Qin, L. High Salinity Reduces Plant Growth and Photosynthetic Performance but Enhances Certain Nutritional Quality of C4 Halophyte Portulaca oleracea L. Grown Hydroponically Under LED Lighting. Front. Plant Sci. 2021, 12, 651341. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of Reactive Oxygen Species and Antioxidant Defense in Plants under Salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef] [PubMed]
- Puccinelli, M.; Carmassi, G.; Botrini, L.; Bindi, A.; Rossi, L.; Fierro-sañudo, J.F.; Pardossi, A.; Incrocci, L. Growth and Mineral Relations of Beta vulgaris Var. Cicla and Beta vulgaris Ssp. Maritima Cultivated Hydroponically with Diluted Seawater and Low Nitrogen Level in the Nutrient Solution. Horticulturae 2022, 8, 638. [Google Scholar] [CrossRef]
- de Carvalho Leal, L.Y.; de Souza, E.R.; Santos Júnior, J.A.; Dos Santos, M.A. Comparison of Soil and Hydroponic Cultivation Systems for Spinach Irrigated with Brackish Water. Sci. Hortic. 2020, 274, 109616. [Google Scholar] [CrossRef]
- Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Pelaez, C.; et al. Dietary Reference Values for Sodium. EFSA J. 2019, 17, e05778. [Google Scholar] [CrossRef]
- Zayed, O.; Hewedy, O.A.; Abdelmoteleb, A.; Ali, M.; Youssef, M.S.; Roumia, A.F.; Seymour, D.; Yuan, Z.C. Nitrogen Journey in Plants: From Uptake to Metabolism, Stress Response, and Microbe Interaction. Biomolecules 2023, 13, 1443. [Google Scholar] [CrossRef]
- Javed, S.A.; Arif, M.S.; Shahzad, S.M.; Ashraf, M.; Kausar, R.; Farooq, T.H.; Hussain, M.I.; Shakoor, A. Can Different Salt Formulations Revert the Depressing Effect of Salinity on Maize by Modulating Plant Biochemical Attributes and Activating Stress Regulators Through Improved N Supply? Sustainability 2021, 13, 8022. [Google Scholar] [CrossRef]
- Yuan, J.-F.; Feng, G.; Ma, H.-Y.; Tian, C.-Y. Effect of Nitrate on Root Development and Nitrogen Uptake of Suaeda physophora Under NaCl Salinity. Pedosphere 2010, 20, 536–544. [Google Scholar] [CrossRef]
- Liu, R.; Cui, B.; Lu, X.; Song, J. The Positive Effect of Salinity on Nitrate Uptake in Suaeda salsa. Plant Physiol. Biochem. 2021, 166, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Feng, J.; Fan, P.; Chen, X.; Guo, J.; Lv, S.; Bao, H.; Jia, W.; Tai, F.; Jiang, P.; et al. Comparative Proteomics of Root Plasma Membrane Proteins Reveals the Involvement of Calcium Signalling in NaCl-Facilitated Nitrate Uptake in Salicornia Europaea. J. Exp. Bot. 2015, 66, 4497–4510. [Google Scholar] [CrossRef]
- European Parliament and Council of the European Union Commission Regulation (EU) No 1258/2011 of 2 December 2011 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels for Nitrates in Foodstuffs. Off. J. Eur. Union 2011, 320, 15–17.
- Bargagli, M.; Tio, M.C.; Waikar, S.S.; Ferraro, P.M. Dietary Oxalate Intake and Kidney Outcomes. Nutrients 2020, 12, 2673. [Google Scholar] [CrossRef]
- Abu-Zanat, M.M.; Al-Hassanat, F.M.; Alawi, M.; Ruyle, G.B. Oxalate and Tannins Assessment in Atriplex halimus L. and A. nummularia L. J. Range Manag. 2003, 56, 370–374. [Google Scholar] [CrossRef]
- Yang, C.; Shi, D.; Wang, D. Comparative Effects of Salt and Alkali Stresses on Growth, Osmotic Adjustment and Ionic Balance of an Alkali-Resistant Halophyte Suaeda glauca (Bge.). Plant Growth Regul. 2008, 56, 179–190. [Google Scholar] [CrossRef]
- Ford, C.M.; Sweetman, C.; Fry, S.C. Ascorbate Degradation: Pathways, Products, and Possibilities. J. Exp. Bot. 2024, 75, 2733–2739. [Google Scholar] [CrossRef]
- Al Daini, H.; Norman, H.C.; Young, P.; Barrett-Lennard, E.G. The Source of Nitrogen (NH4+ or NO3−) Affects the Concentration of Oxalate in the Shoots and the Growth of Atriplex Nummularia (Oldman Saltbush). Funct. Plant Biol. 2013, 40, 1057–1064. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, X.; Zhang, Y.; Shao, J.Z.; Du, S. Effects of Nitrogen Levels and Nitrate/Ammonium Ratios on Oxalate Concentrations of Different Forms in Edible Parts of Spinach. J. Plant Nutr. 2005, 28, 2011–2025. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. Nitrogen Acquisition, PEP Carboxylase, and Cellular PH Homeostasis: New Views on Old Paradigms. Plant Cell Environ. 2005, 28, 1396–1409. [Google Scholar] [CrossRef]
- Meeuse, B.J.D.; Campbell, J.M. An Inhibitor of Oxalic Acid Oxidase in Beet Extracts. Plant Physiol. 1959, 34, 583. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Espinoza, O.; Rojas-Villalta, D.; Zúñiga-Pereira, A.M.; Chacón-Díaz, C.; Bravo, L.A.; Reyes-Díaz, M. Plant Oxalate Oxidases: Key Enzymes in Redox and Stress Regulation. J. Exp. Bot. 2025, eraf317. [Google Scholar] [CrossRef]
- Fontana, E.; Hoeberechts, J.; Nicola, S.; Cros, V.; Palmegiano, G.B.; Peiretti, P.G. Nitrogen Concentration and Nitrate/Ammonium Ratio Affect Yield and Change the Oxalic Acid Concentration and Fatty Acid Profile of Purslane (Portulaca oleracea L.) Grown in a Soilless Culture System. J. Sci. Food Agric. 2006, 86, 2417–2424. [Google Scholar] [CrossRef]
- Poeydomenge, G.Y.; Savage, G.P. Oxalate Content of Raw and Cooked Purslane. J. Food Agric. Environ. 2007, 5, 124–128. [Google Scholar]
- Savage, G.P.; Vanhanen, L.; Mason, S.M.; Ross, A.B. Effect of Cooking on the Soluble and Insoluble Oxalate Content of Some New Zealand Foods. J. Food Compos. Anal. 2000, 13, 201–206. [Google Scholar] [CrossRef]
- Lee, S.; Choi, Y.; Jeong, H.S.; Lee, J.; Sung, J. Effect of Different Cooking Methods on the Content of Vitamins and True Retention in Selected Vegetables. Food Sci. Biotechnol. 2018, 27, 333–342. [Google Scholar] [CrossRef]
- Hunt, R. Plant Growth Analysis. Stud. Biol. 1978, 96, 245–249. [Google Scholar]
- Prieto-Santiago, V.; Cavia, M.M.; Alonso-Torre, S.R.; Carrillo, C. Relationship between Color and Betalain Content in Different Thermally Treated Beetroot Products. J. Food Sci. Technol. 2020, 57, 3305–3313. [Google Scholar] [CrossRef]
- Nilsson, T. Beta vulgaris L. Ssp. Vulgaris Var Rubra L. Lantbrukshogskolans Ann. 1970, 36, 179–219. [Google Scholar]
- Ravichandran, K.; Saw, N.M.M.T.; Mohdaly, A.A.A.; Gabr, A.M.M.; Kastell, A.; Riedel, H.; Cai, Z.; Knorr, D.; Smetanska, I. Impact of Processing of Red Beet on Betalain Content and Antioxidant Activity. Food Res. Int. 2013, 50, 670–675. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Sathishraj, R.; Augustin, A. Oxalic Acid and Oxalate Oxidase Enzyme in Costus Pictus D. Don. Acta Physiol. Plant 2012, 34, 657–667. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The Fluorescence Transient as a Tool to Characterize and Screen Photosynthetic Samples. In Probing Photosynthesis: Mechanism, Regulation & Adaptation; CRC Press: London, UK, 2000; pp. 443–480. [Google Scholar]
- Hirschler, R. Whiteness, Yellowness, and Browning in Food Colorimetry: A Critical Review. In Color in Food, Technological and Psychophysical Aspects; CRC Press: Boca Raton, FL, USA, 2012; pp. 118–129. [Google Scholar]
- Eyarkai Nambi, V.; Thangavel, K.; Shahir, S.; Geetha, V. Evaluation of Colour Behavior During Ripening of Banganapalli Mango Using CIE-Lab and RGB Colour Coordinates. J. Appl. Hortic. 2015, 17, 205–209. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).