Optimization of the Extraction of Bioactive Compounds and Metabolomic Profile of Licaria armeniaca
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of the Extraction of Bioactive Compounds
2.2. Optimization by Desirability Tool
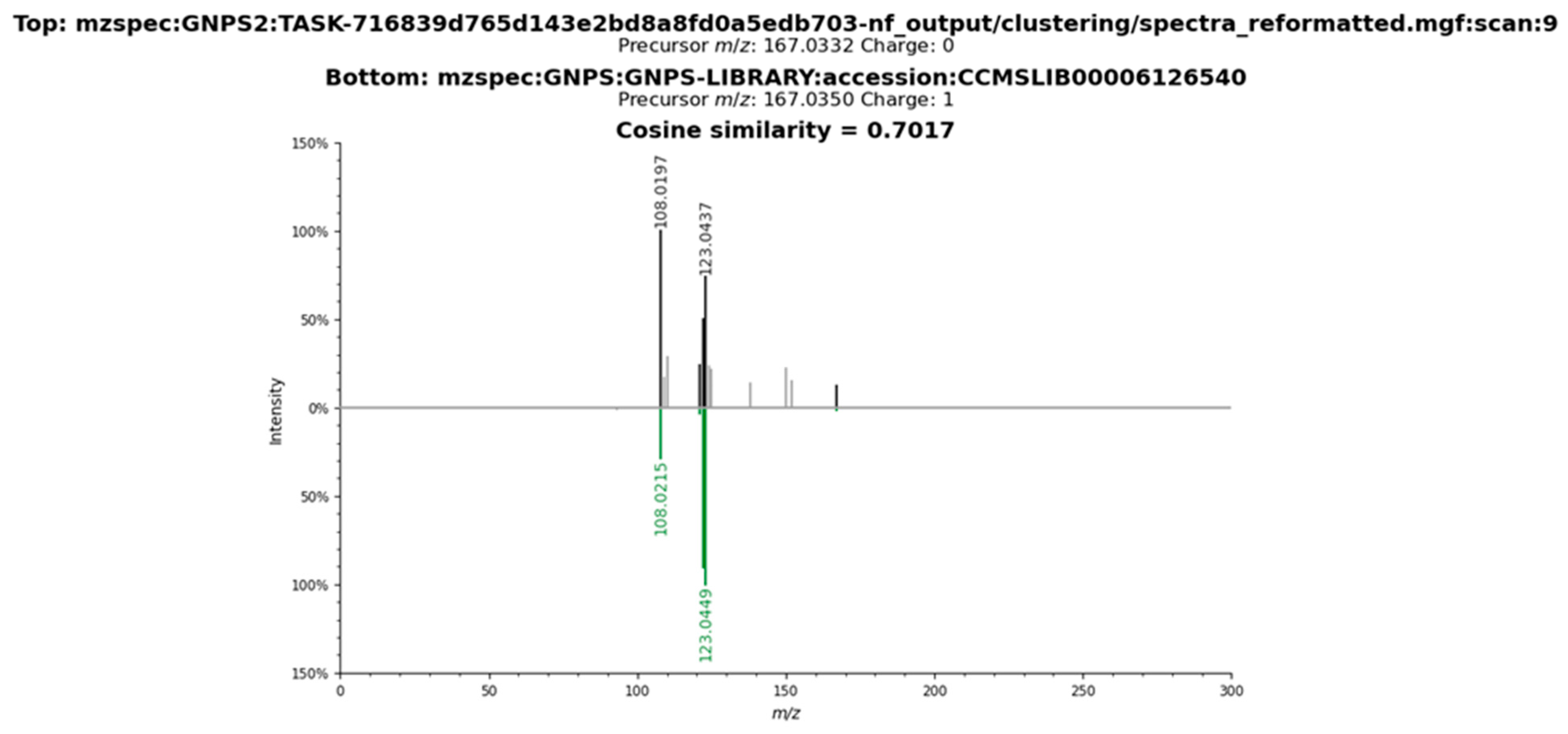
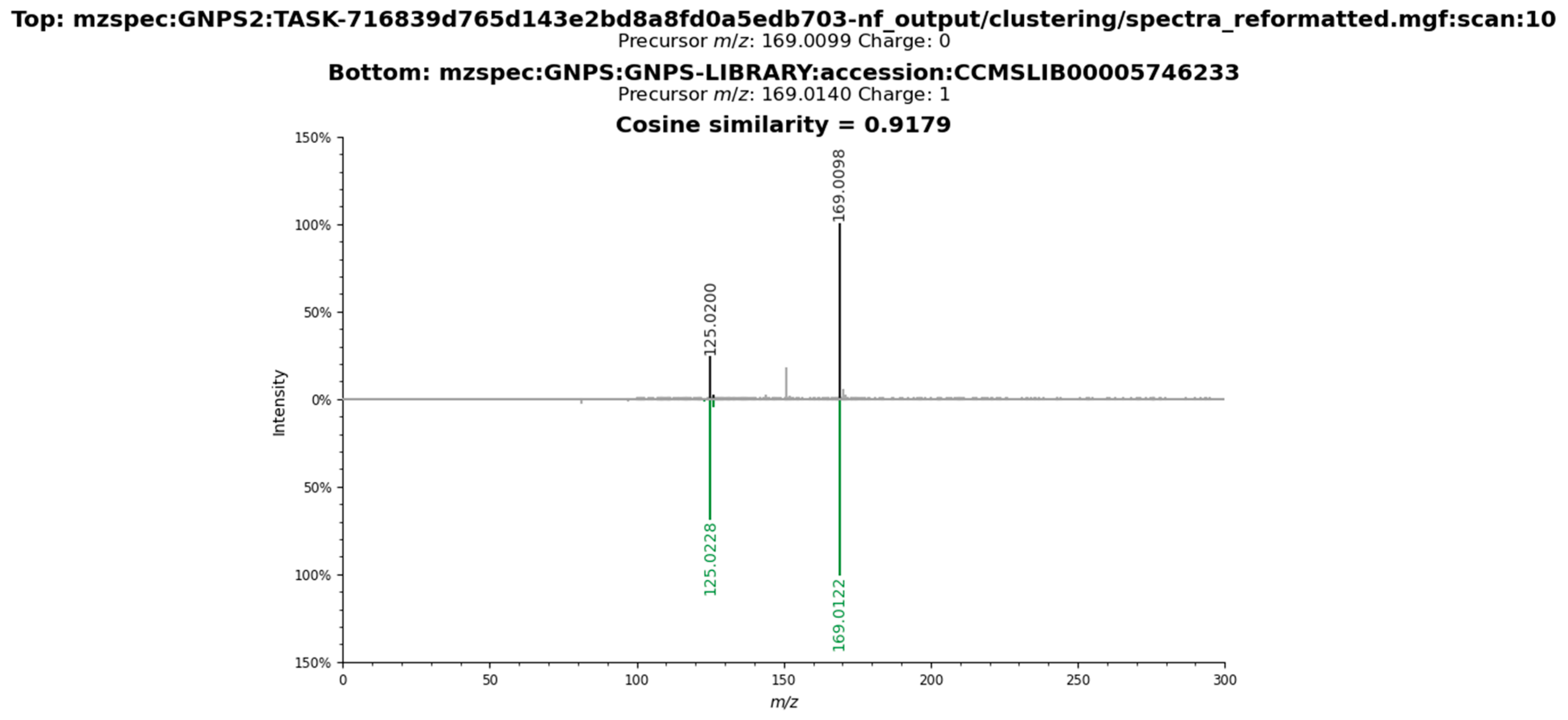
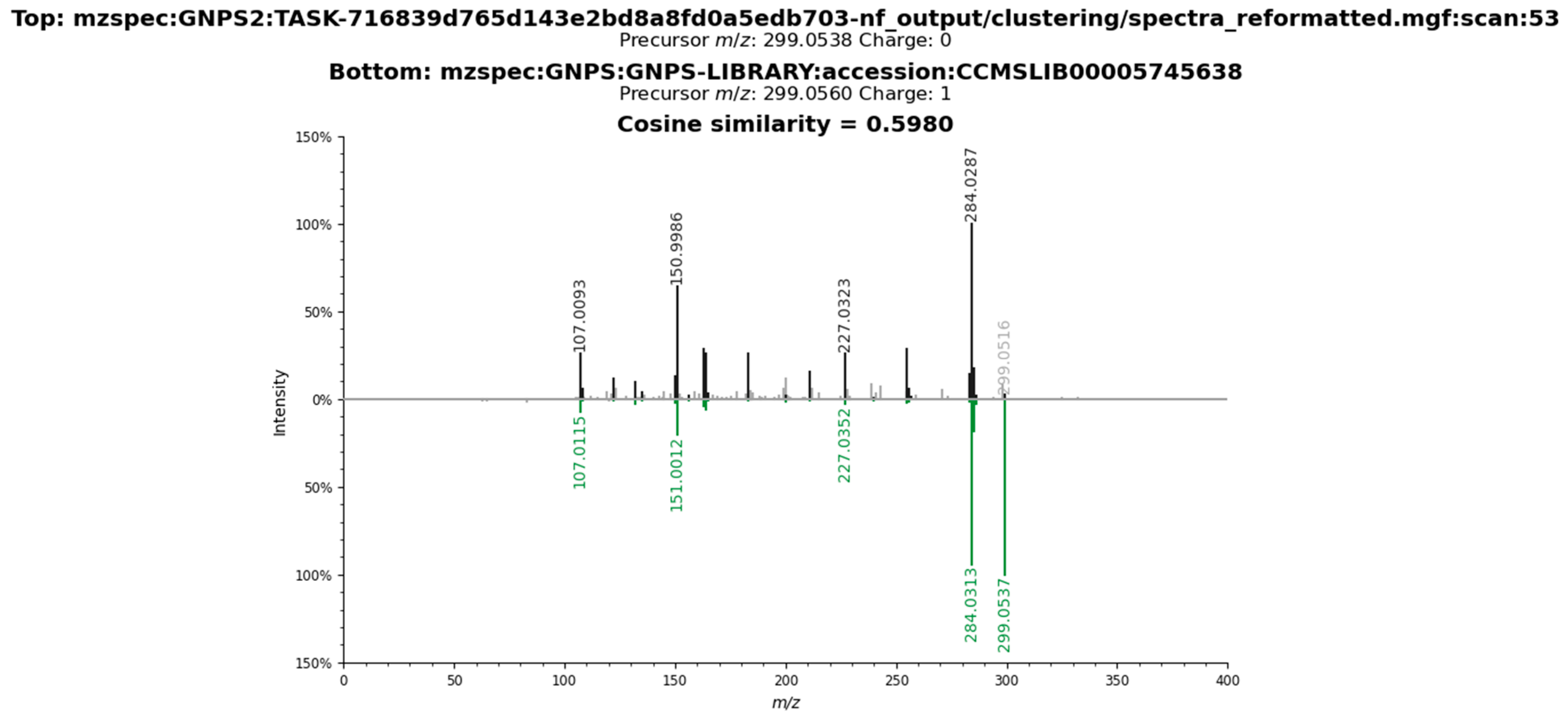
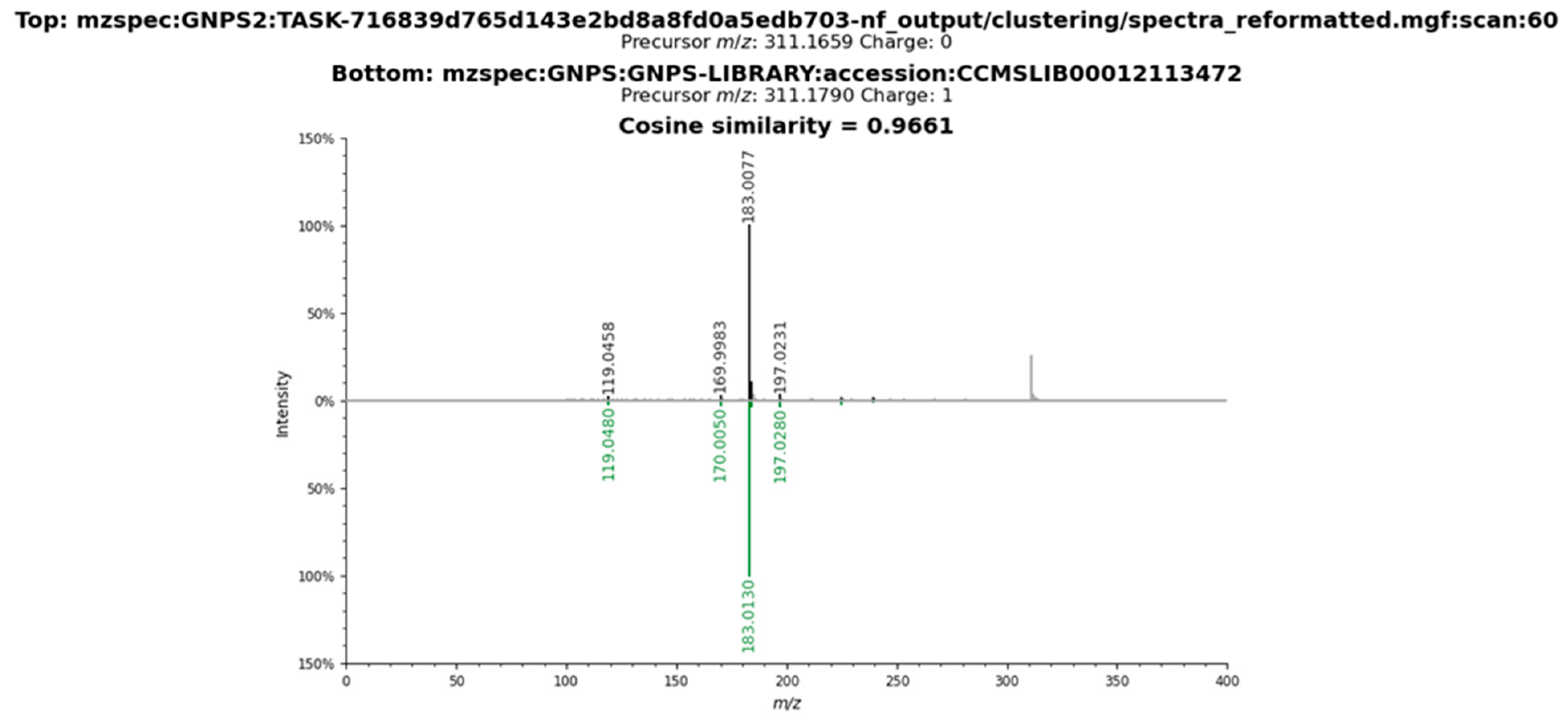
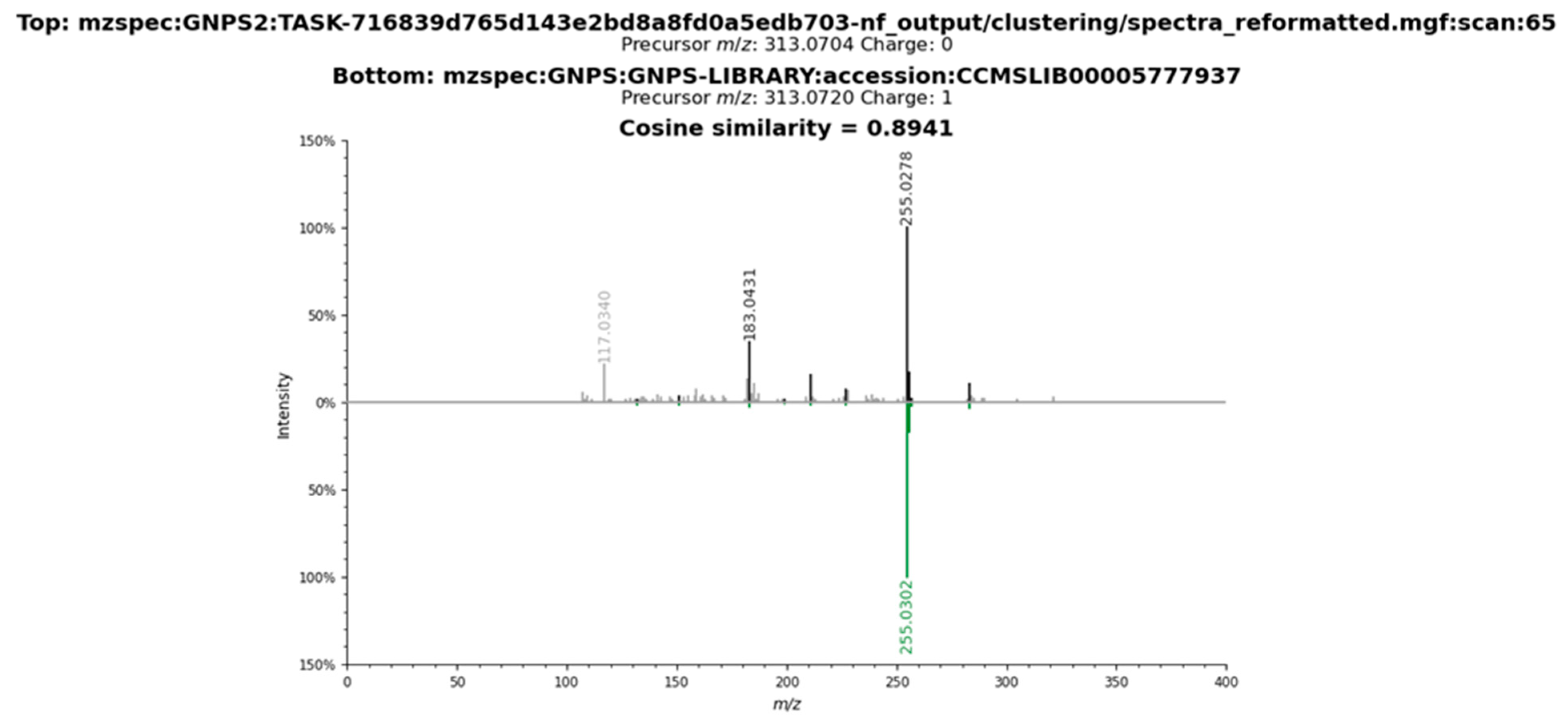
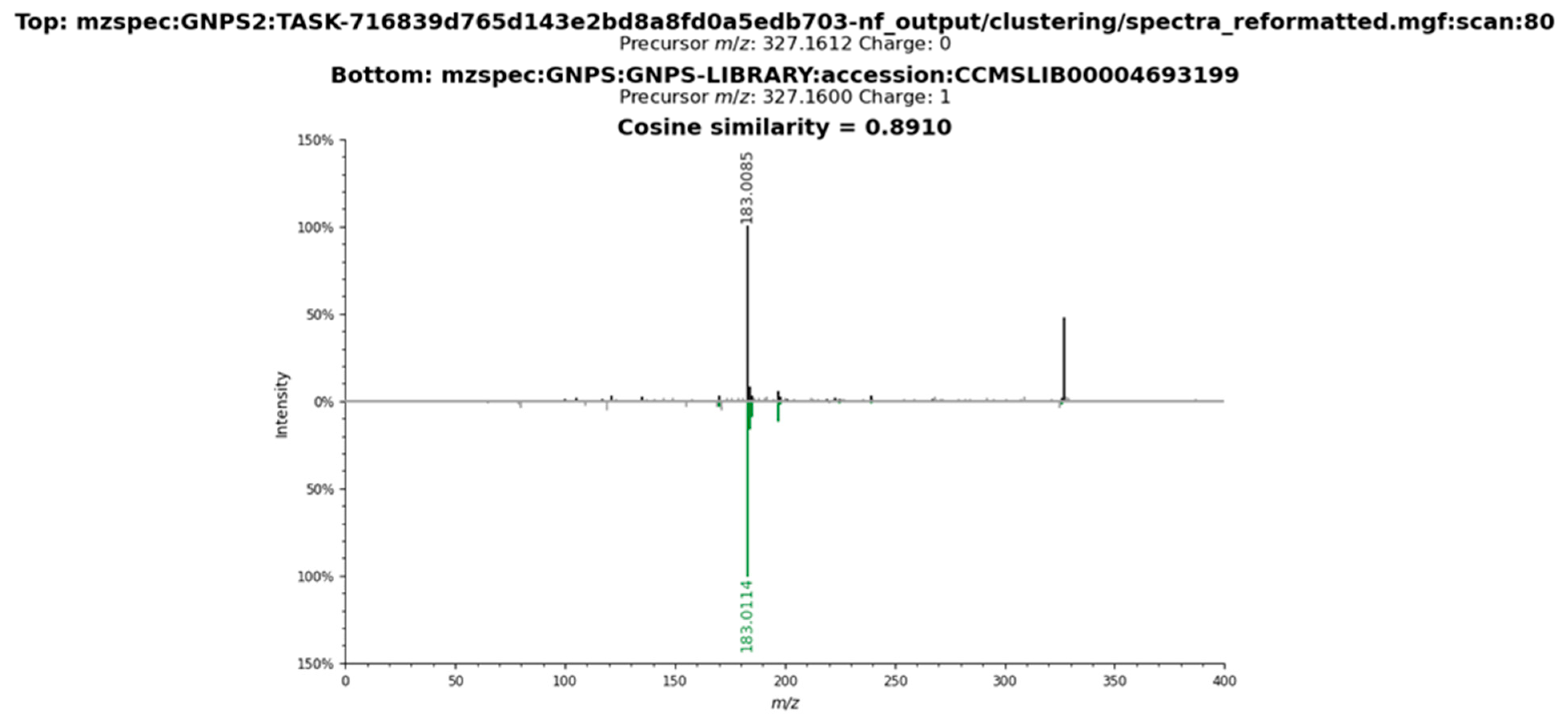
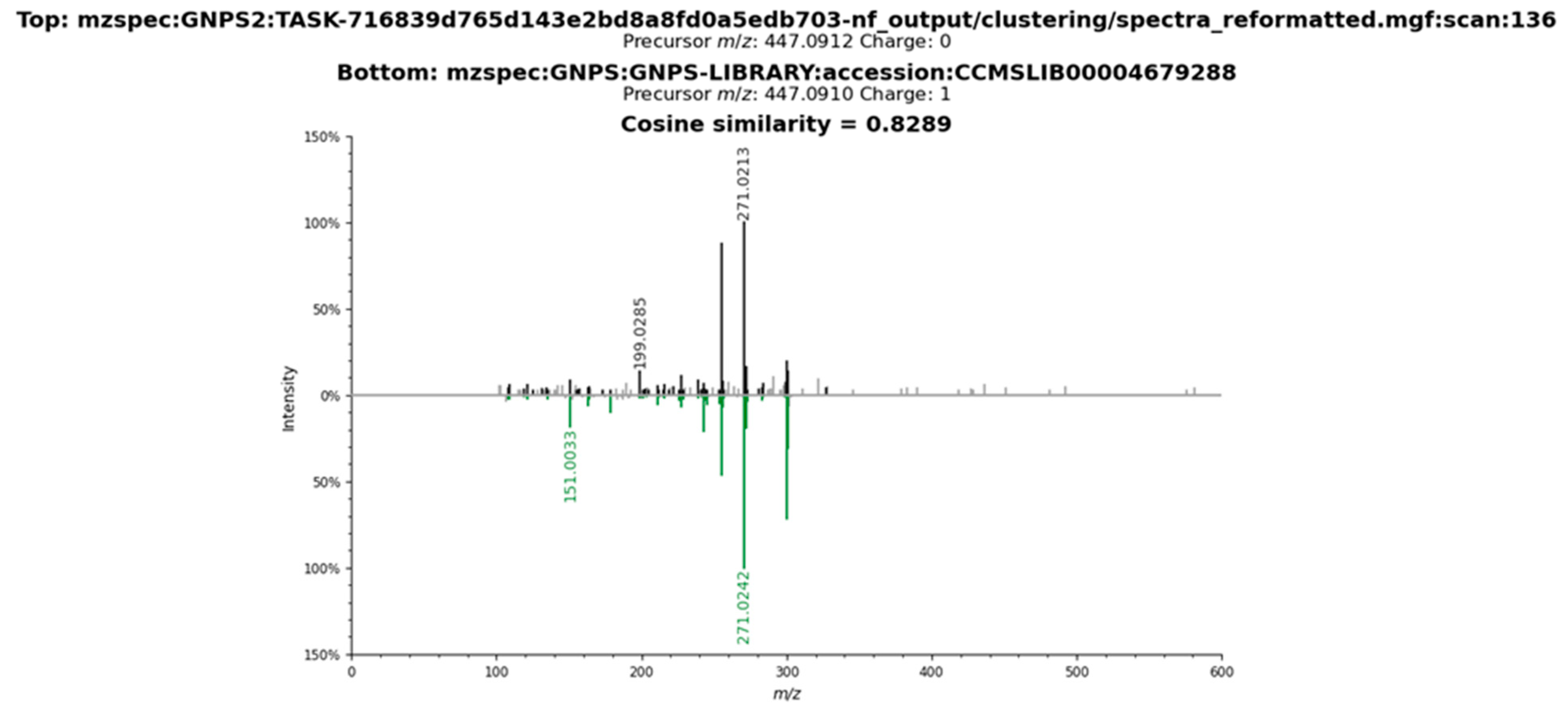
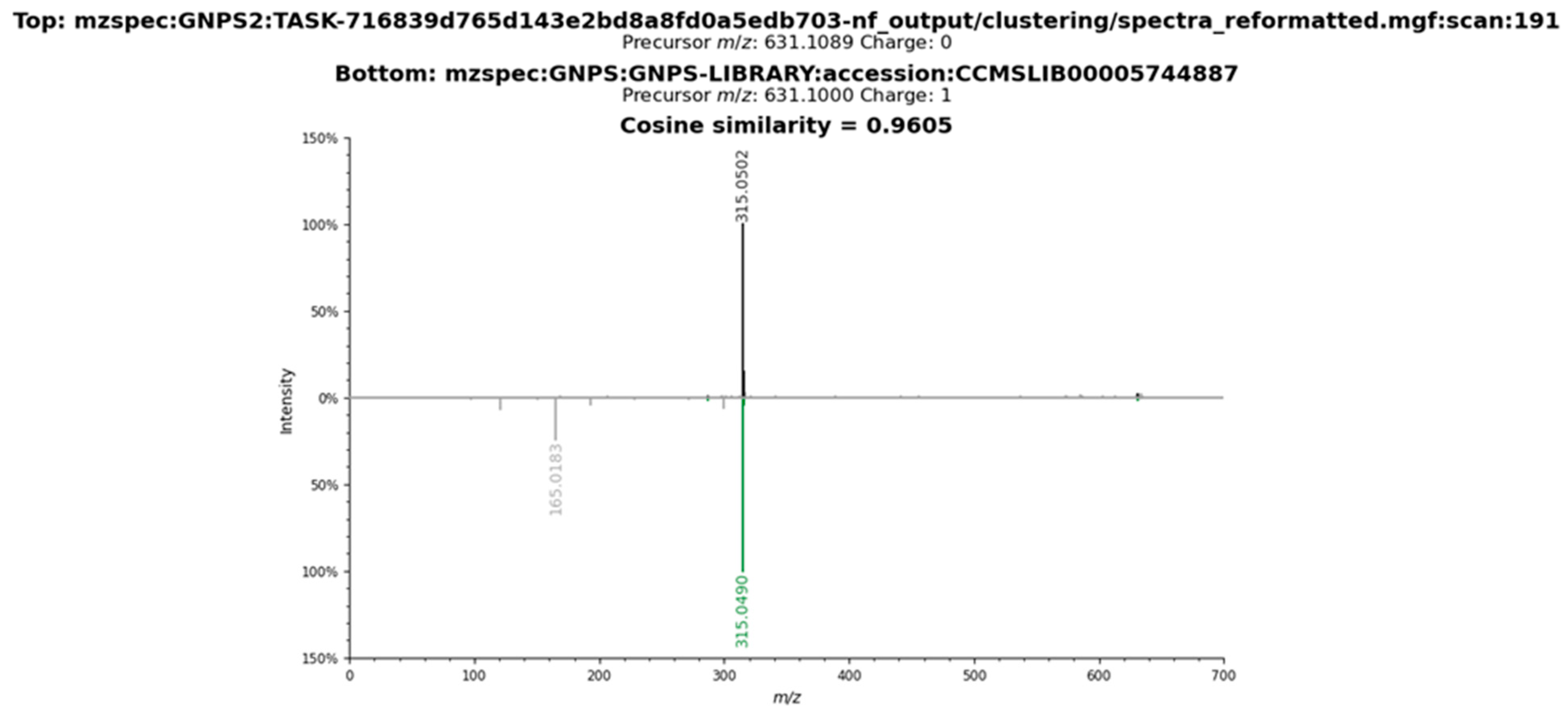
2.3. Metabolomic Profile of L. armeniaca Extracts
2.4. Cytotoxic Activity
3. Materials and Methods
3.1. Plant Material
3.2. Ultrasound-Assisted Extraction
3.3. Experimental Design and Optimization
3.4. Antioxidant Activity
3.5. Total Phenolics
3.6. Statistical Analysis and Experimental Validation
3.7. Calculation of Global Desirability of Harrington (d)
- Value (d) 0.8–1.00—Quite acceptable;
- Value (d) 0.63–0.79—Acceptable;
- Value (d) 0.37–0.62—Satisfactory;
- Value (d) 0.0–0.36—Unacceptable.
3.8. Liquid Chromatography–Tandem Mass Spectrometry Analysis
3.9. Spectrometric Data Processing
3.10. Cytotoxic Activity by MTT
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UAE | Ultrasound-Assisted Extraction |
| CCRD | Central Composite Rotational Design |
| RSM | Response Surface Methodology |
| LC-MS/MS | Liquid Chromatography coupled with Tandem Mass Spectrometry |
| GNPS | Global Natural Products Social Molecular Networking |
| TPC | Total phenolics |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| SLR | Solid-to-liquid ratio |
| EtOH | Ethanol |
| MAE | microwave-assisted extraction |
| MSI | Metabolomics Standards Initiative |
| IC50 | Inhibitory Concentration 50% |
| SI | Selective index |
| SiSGen | National System for Governance of Genetic Heritage and Associated Traditional Knowledge |
| ANOVA | Analysis of Variance |
| R2 | Regression coefficient |
| LOF | Fischer’s test and lack of fit |
Appendix A
Appendix A.1. CCRD Planning Matrix
| Level of Independent Variables | ||||
| Run | % EtOH | t (min) | RSL (% m/v) | |
| Leaves | Branches | |||
| 1 | 20.24 (−1) | 17.14 (−1) | 2.24 (−1) | 5.56 (−1) |
| 2 | 79.76 (1) | 17.14 (−1) | 2.24 (−1) | 5.56 (−1) |
| 3 | 20.24 (−1) | 52.86 (1) | 2.24 (−1) | 5.56 (−1) |
| 4 | 20.24 (−1) | 17.14 (−1) | 1.12 (1) | 16.35 (1) |
| 5 | 79.76 (1) | 52.86 (1) | 2.24 (−1) | 5.56 (−1) |
| 6 | 79.76 (1) | 17.14 (−1) | 6.54 (1) | 16.35 (1) |
| 7 | 20.24 (−1) | 52.86 (1) | 6.54 (1) | 16.35 (1) |
| 8 | 79.76 (1) | 52.86 (1) | 6.54 (1) | 16.35 (1) |
| 9 | 0 (−1.68) | 35 (0) | 4.4 (0) | 11 (0) |
| 10 | 100 (1.68) | 35 (0) | 4.4 (0) | 11 (0) |
| 11 | 50 (0) | 5 (−1.68) | 4.4 (0) | 11 (0) |
| 12 | 50 (0) | 65 (1.68) | 4.4 (0) | 11 (0) |
| 13 | 50 (0) | 35 (0) | 0.8 (−1.68) | 2 (−1.68) |
| 14 | 50 (0) | 35 (0) | 8 (1.68) | 20 (1.68) |
| 15 | 50 (0) | 35 (0) | 4.4 (0) | 11 (0) |
| 16 | 50 (0) | 35 (0) | 4.4 (0) | 11 (0) |
| 17 | 50 (0) | 35 (0) | 4.4 (0) | 11 (0) |
Appendix A.2.


References
- Flora e Funga do Brasil. Jardim Botânico do Rio de Janeiro. Available online: http://floradobrasil.jbrj.gov.br/ (accessed on 22 July 2025).
- Fonseca, C.N.; Lisboa, P.L.B.; Urbinati, C.V. A Xiloteca (Coleção Walter A. Egler) do Museu Paraense Emílio Goeldi1. Bol. Mus. Para. Emílio Goeldi 2005, 1, 65–140. [Google Scholar]
- Barbosa, L.M.; Shirasuna, R.T.; De Lima, F.C.; Ortiz, P.R.T.; Barbosa, K.C.; Barbosa, T.C. Lista de espécies indicadas para restauração ecológica para diversas regiões do Estado de São Paulo/Luiz Mauro Barbosa-São Paulo: Instituto de Botânica, 2017; 344p. Available online: https://www.infraestruturameioambiente.sp.gov.br/institutodebotanica/wp-content/uploads/sites/235/2019/10/lista-especies-rad-2019.pdf (accessed on 12 July 2025).
- Aiba, C.J.; Gottlieb, O.R.; Maia JGSPagliosa, F.M.; Yoshida, M. Benzofuranoid neolignans from Licaria armeniaca. Phytochemistry 1978, 1, 2038–2039. [Google Scholar] [CrossRef]
- Alegrio, L.V.; Fo, R.B.; Gottlieb, O.R.; Maia, J.G.S. Lignans and Neolignans from Licaria armeniaca. Phytochemistry 1981, 20, 1963–1965. [Google Scholar] [CrossRef]
- Abdel-Hafiz, M.A.; Slatkin, D.J.; Schiff, P. Neolignans and alkaloids from Licaria arminiaca (Nees) Kosterm. Part 1. Bull. Pharm. Sci. Assiut Univ. 1985, 8, 28–40. [Google Scholar] [CrossRef]
- Barbosa-Filho, J.M.; Yoshida, M.; Gottlieb, O.R. Neolignans from the Fruits of Licaria armeniaca. Phytochemistry 1986, 26, 319–321. [Google Scholar] [CrossRef]
- Bhambhani, S.; Kondhare, K.R.; Giri, A.P. Diversity in chemical structures and biological properties of plant alkaloids. Molecules 2021, 26, 3374. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.Y.; Kim, M.Y.; Cho, J.Y. Antioxidant, anti-inflammatory, anti-menopausal, and anti-cancer effects of lignans and their metabolites. Int. J. Mol. Sci. 2022, 23, 15482. [Google Scholar] [CrossRef]
- Osmakov, D.I.; Kalinovskii, A.P.; Belozerova, A.O.; Andreev, Y.A.; Kozlov, S.A. Lignans as pharmacological agents in disorders related to oxidative stress and inflammation: Chemical synthesis approaches and biological activities. Int. J. Mol. Sci. 2022, 23, 6031. [Google Scholar] [CrossRef]
- Saadati, F.; Modarresi Chahardehi, A.; Jamshidi, N.; Jamshidi, N.; Ghasemi, D. Coumarin: A natural solution for alleviating inflammatory disorders. CRPHAR 2024, 25, 100202. [Google Scholar] [CrossRef]
- Mahajan, M.; Kuiry, R.; Pal, P.K. Understanding the consequence of environmental stress for accumulation of secondary metabolites in medicinal and aromatic plants. J. Appl. Res. Med. Aromat. Plants 2020, 18, 100255. [Google Scholar] [CrossRef]
- Oubannin, S.; Bijla, L.; Ahmed, M.N.; Ibourki, M.; El Kharrassi, Y.; Devkota, K.; Bouyahya, A.; Maggi, F.; Caprioli, G.; Sakar, E.H.; et al. Recent advances in the extraction of bioactive compounds from plant matrices and their use as potential antioxidants for vegetable oils enrichment. J. Food Compos. Anal. 2024, 128, 105995. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; Prakash, P.; Singha, P.; Prasad, K.; Singh, S. Modeling and optimization of ultrasound-assisted extraction of bioactive compounds from Allium sativum leaves using response surface methodology and artificial neural network coupled with genetic algorithm. Foods 2023, 12, 1925. [Google Scholar] [CrossRef]
- Da Silva, D.T.; Herrera, R.; Heinzmann, B.M.; Calvo, J.; Labidi, J. Nectandra grandiflora by-products obtained by alternative extraction methods as a source of phytochemicals with antioxidant and antifungal properties. Molecules 2018, 23, 372. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.; Barbosa, A.; Advinha, B.; Sales, H.; Pontes, R.; Nunes, J. Green Extraction Techniques of Bioactive Compounds: A State-of-the-Art Review. Processes 2023, 11, 2255. [Google Scholar] [CrossRef]
- Cannavacciuolo, C.; Pagliari, S.; Celano, R.; Campone, L.; Rastrelli, L. Critical analysis of green extraction techniques used for botanicals: Trends, priorities, and optimization strategies—A review. TrAC Trends Anal. Chem. 2024, 173, 117627. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Aurora-Vigo, E.F.; Villagrán, Z.; Rodríguez-Lafitte, E.; Ruvalcaba-Gómez, J.M.; Solano-Cornejo, M.Á.; Zamora-Gasga, V.M.; Montalvo-González, E.; Gómez-Rodríguez, H.; Aceves-Aldrete, C.E.; et al. Design of experiments for optimizing ultrasound-assisted extraction of bioactive compounds from plant-based sources. Molecules 2023, 28, 7752. [Google Scholar] [CrossRef]
- Albani, S.M.; Borges, A.P.; Martins, M.C.; Restello, R.M.; Camera, F.D.; Paroul, N.; Mielniczki-Pereira, A.A. Padronização da quantificação de glutationa redutase em aegla singularis (anomura, crustacea) utilizando planejamento experimental dccr. Quím. Nova 2020, 43, 607–612. [Google Scholar] [CrossRef]
- Custodio, T.N.; Morais, A.D.; Muniz, J.A. Superfície de resposta em experimento com parcelas subdivididas. Ciência Agrotecnologia 2000, 24, 1008–1023. [Google Scholar]
- Harrington, E.C. The desirability function. Ind. Qual. Control 1965, 21, 494–498. [Google Scholar]
- Islam, M.A.; Sakkas, V.; Albanis, T.A. Application of statistical design of experiment with desirability function for the removal of organophosphorus pesticide from aqueous solution by low-cost material. J. Hazard. Mater. 2009, 170, 230–238. [Google Scholar] [CrossRef]
- Derringer, G.; Suich, R. Simultaneous optimization of several response variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Muñiz-Márquez, D.B.; Martínez-Ávila, G.C.; Wong-Paz, J.E.; Belmares-Cerda, R.; Rodríguez-Herrera, R.; Aguilar, C.N. Ultrasound-assisted extraction of phenolic compounds from Laurus nobilis L. and their antioxidant activity. Ultrason. Sonochem. 2013, 20, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Marzouk, M.M.; Hegazi, N.M.; El Shabrawy, M.O.A.; Farid, M.M.; Kawashty, S.A.; Hussein, S.R.; Saleh, N.A.M. Discriminative metabolomics analysis and cytotoxic evaluation of flowers, leaves, and roots extracts of Matthiola longipetala subsp. livida. Metabolites 2023, 13, 909. [Google Scholar] [CrossRef] [PubMed]
- Allwood, J.W.; Goodacre, R. An introduction to liquid chromatography—Mass spectrometry instrumentation applied to plant metabolomics analysis. Phytochem. Anal. 2010, 21, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Karakas, E.; Bulut, M.; Fernie, A. Metabolome guided treasure hunt—Learning from metabolic diversity. J. Plant Physiol. 2025, 309, 154494. [Google Scholar] [CrossRef]
- Loneman, D.M.; Peddicord, L.; Al-Rashid, A.; Nikolau, B.J.; Lauter, N.; Yandeau-Nelson, M.D. A robust and efficient method for the extraction of plant extracellular surface lipids as applied to the analysis of silks and seedling leaves of maize. PLoS ONE 2017, 12, e0180850. [Google Scholar] [CrossRef]
- Carreira-Casais, A.; Carpena, M.; Pereira, A.G.; Chamorro, F.; Soria-Lopez, A.; Perez, P.G.; Prieto, M.A. Critical varia-bles influencing the ultrasound-assisted extraction of bioactive compounds—A review. Chem. Proc. 2021, 5, 50. [Google Scholar]
- Da Silva, R.F.; Carneiro, C.N.; De Sousa, C.B.C.; Gomez, F.J.V.; Espino, M.; Boiteux, J.; Fernández, M.A.; Silva, M.F.; Dias, F.S. Sustainable extraction bioactive compounds procedures in medicinal plants based on the principles of green analytical chemistry: A review. Microchem. J. 2022, 175, 107184. [Google Scholar] [CrossRef]
- Sevindik, M.; Gürgen, A.; Krupodorova, T.; Uysal, İ.; Koçer, O. A hybrid artificial neural network and multi-objective genetic algorithm approach to optimize extraction conditions of Mentha longifolia and biological activities. Sci. Rep. 2024, 14, 31403. [Google Scholar] [CrossRef]
- Gürgen, A.; Sevindik, M.; Krupodorova, T.; Uysal, I.; Unal, O. Biological activities of Hypericum spectabile extract optimized using artificial neural network combined with genetic algorithm application. BMC Biotechnol. 2024, 24, 83. [Google Scholar] [CrossRef]
- Antonio, A.S.; Veiga-Junior, V.F.; Silveira, L.; Wiedemann, M. Phytochemistry Ocotea complex: A metabolomic analysis of a Lauraceae genus. Phytochemistry 2020, 173, 112314. [Google Scholar] [CrossRef]
- Custódio, D.L.; Veiga-Junior, V.F. Lauraceae alkaloids. RSC Adv. 2014, 4, 21864–21890. [Google Scholar] [CrossRef]
- Silva Teles, M.M.R.; Vieira Pinheiro, A.A.; Da Silva Dias, C.; Fechine Tavares, J.; Barbosa Filho, J.M.; Leitão Da Cunha, E.V. Alkaloids of the Lauraceae. In The Alkaloids: Chemistry and Biology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 82, pp. 147–304. [Google Scholar] [CrossRef]
- Salleh, W.M.N.H.W.; Ahmad, F.; Khong, H.Y.; Mohamed Zulkifli, R. Comparative Study of the Essential Oils of Three Beilschmiedia Species and Their Biological Activities. Int. J. Food Sci. Tech. 2016, 51, 240–249. [Google Scholar] [CrossRef]
- Dey, P.; Kundu, A.; Kumar, A.; Gupta, M.; Lee, B.M.; Bhakta, T.; Dash, S.; Kim, H.S. Analysis of Alkaloids (Indole Alkaloids, Isoquinoline Alkaloids, Tropane Alkaloids). In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 505–567. [Google Scholar] [CrossRef]
- Hayati, E.K.; Madjid, A.D.R.; Yuliani, D.; Safitri, E.W.; Rahmah, F.T.; Qoriati, Y. Optimization of ultrasound-assisted extraction of alkaloids from Acalypha indica: Solvent and extraction time variation. AIP Conf. Proc. 2019, 2120, 080026. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, T.H.; Ong, P.Y.; Wong, S.L.; Hamdan, N.; Elgharbawy, A.A.M.; Azmi, N.A. Integrated ultrasound-mechanical stirrer technique for extraction of total alkaloid content from Annona muricata. Process Biochem. 2021, 109, 104–116. [Google Scholar] [CrossRef]
- Teng, H.; Choi, Y.H. Optimization of Ultrasonic-Assisted Extraction of Bioactive Alkaloid Compounds from Rhizoma coptidis (Coptis Chinensis Franch.) Using Response Surface Methodology. Food Chem. 2014, 142, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Dary, C.; Baghdikian, B.; Kim, S.; Mabrouki, F.; Hul, S.; Jabbour, F.; Ollivier, E.; Bun, S.-S. Optimization of Ultrasound-Assisted Extraction of Bioactive Alkaloids from Stephania Cambodica Using Response Surface Methodology. Comptes Rendus. Chim. 2017, 20, 996–1005. [Google Scholar] [CrossRef]
- Ražná, K.; Nôžková, J.; Vargaová, A.; Harenčár, Ľ.; Bjelková, M. Biological functions of lignans in plants. Agric. (Pol’nohospodárstvo) 2021, 67, 155–165. [Google Scholar] [CrossRef]
- Teponno, R.B.; Kusari, S.; Spiteller, M. Recent advances in research on lignans and neolignans. Nat. Prod. Rep. 2016, 33, 1044–1092. [Google Scholar] [CrossRef] [PubMed]
- Plaha, N.S.; Awasthi, S.; Sharma, A.; Kaushik, N. Distribution, biosynthesis and therapeutic potential of lignans. Biotech 2022, 12, 255. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed Minimum Reporting Standards for Chemical Analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Dührkop, K.; Nothias, L.-F.; Fleischauer, M.; Reher, R.; Ludwig, M.; Hoffmann, M.A.; Petras, D.; Gerwick, W.H.; Rousu, J.; Dorrestein, P.C.; et al. Systematic classification of unknown metabolites using high-resolution fragmentation mass spectra. Nat. Biotechnol. 2021, 39, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A rapid tool for turning tandem mass spectra into metabolite structure information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef]
- Liga, S.; Paul, C.; Péter, F. Flavonoids: Overview of Biosynthesis, Biological Activity, and Current Extraction Techniques. Plants 2023, 12, 2732. [Google Scholar] [CrossRef]
- Chen, J.; Li, G.; Sun, C.; Peng, F.; Yu, L.; Chen, Y.; Tan, Y.; Cao, X.; Tang, Y.; Xie, X.; et al. Chemistry, pharmacoki-netics, pharmacological activities, and toxicity of quercitrin. Phytother. Res. 2022, 36, 1545–1575. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Xia, C.; Wu, H.; Gu, Q.; Li, P. Metabolism of quercitrin in the colon and its beneficial regulatory effects on gut microbiota J. Sci. Food Agric. 2024, 104, 9255–9264. [Google Scholar] [CrossRef]
- Raha, S.; Paidi, R.; Dutta, D.; Pahan, K. Cinnamic acid, a natural plant compound, exhibits neuroprotection in a mouse model of Sandhoff disease via PPARα. NIPT 2024, 3, 17–32. [Google Scholar] [CrossRef]
- Thong-Asa, W.; Wassana, C.; Sukkasem, K.; Innoi, P.; Dechakul, M.; Timda, P. Neuroprotective Effect of Gallic Acid in Mice with Rotenone-Induced Neurodegeneration. Exp. Anim. 2024, 73, 23–0165. [Google Scholar] [CrossRef]
- Guo, J.; Yan, L.; Yang, Q.; Li, H.; Tian, Y.; Yang, J.; Xie, J.; Zhang, F.; Hao, E. Multiple Strategies Confirm the Anti Hepatocellular Carcinoma Effect of Cinnamic Acid Based on the PI3k-AKT Pathway. Pharmaceuticals 2025, 18, 1205. [Google Scholar] [CrossRef]
- Tumilaar, S.G.; Hardianto, A.; Dohi, H.; Kurnia, D. Density Functional Theory, Molecular Docking Study, and In Vitro Antioxidant Activity of Cinnamic Acid Isolated from Piper betle Leaves. Biochem. Res. Int. 2025, 2025, 1691257. [Google Scholar] [CrossRef]
- Zawiła, T.; Swolana, D.; Rok, J.; Rzepka, Z.; Wojtyczka, R.D. Evaluation of the Antibacterial Activity of Cinnamic Acid and Its Derivatives: Synergistic Effects with Cloxacillin. Molecules 2025, 30, 660. [Google Scholar] [CrossRef]
- Bai, Y.; Tan, D.; Deng, Q.; Miao, L.; Wang, Y.; Zhou, Y.; Yang, Y.; Wang, S.; Vong, C.T.; Cheang, W.S. Cinnamic acid alleviates endothelial dysfunction and oxidative stress by targeting PPARδ in obesity and diabetes. Chin. Med. 2025, 20, 13. [Google Scholar] [CrossRef]
- Gangadharan, G.; Gupta, S.; Kudipady, M.L.; Puttaiahgowda, Y.M. Gallic Acid Based Polymers for Food Preservation: A Review. ACS Omega 2024, 9, 37530–37547. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Ma, Y.; Yan, C.; Wei, X.; Zhang, L.; Jiang, H.; Ma, Y.; Zhang, S.; Xing, M.; Gao, Y. Mechanism, Formulation, and Efficacy Evaluation of Natural Products for Skin Pigmentation Treatment. Pharmaceutics 2024, 16, 1022. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, T.; Seo, J.; Yan, L. Fukunda, Junji. Cinnamic acid promotes elongation of hair peg-like sprouting in hair follicle organoids via oxytocin receptor activation. Sci. Rep. 2024, 14, 4709. [Google Scholar] [CrossRef]
- Apaza Ticona, L.; Bermejo, P.; Guerra, J.A.; Abad, M.J.; Beltrán, M.; Martín Lázaro, R.; Alcamí, J.; Bedoya, L.M. Ethanolic extract of Artemisia campestris subsp. glutinosa (Besser) Batt. inhibits HIV-1 replication in vitro through the activity of terpenes and flavonoids on viral entry and NF-κB pathway. J. Ethnopharmacol. 2020, 263, 113163. [Google Scholar] [CrossRef]
- Chao, C.L.; Huang, H.C.; Ding, H.Y.; Lai, J.H.; Lin, H.C.; Wen-Liang Chang, W.L. A new macrocyclic diterpenoid from Anisomeles indica. Nat. Prod. Res. 2019, 34, 2737–2745. [Google Scholar] [CrossRef]
- Su, Y.K.; Bamodu, O.A.; Tzeng, Y.M.; Hsiao, M.; Yeh, C.T.; Lin, C.M. Ovatodiolide inhibits the oncogenicity and cancer stem cell-like phenotype of glioblastoma cells, as well as potentiate the anticancer effect of temozolomide. Phytomedicine 2019, 61, 152840. [Google Scholar] [CrossRef] [PubMed]
- Bich-Loan, N.T.; Kien, K.T.; Thanh, N.L.; Kim-Thanh, N.T.; Huy, N.; The-Hai, P.; Muller, M.; Nachtergael, A.; Duez, P.; Thang, N.D. Toxicity and Anti-Proliferative Properties of Anisomeles indica Ethanol Extract on Cervical Cancer HeLa Cells and Zebrafish Embryos. Life 2021, 11, 257. [Google Scholar] [CrossRef]
- Lien, H.M.; Huang, S.H.; Chang, C.H.; Huang, C.L.; Chen, C.C.; Chyau, C.C. Innovative Purification Method of Ovatodiolide from Anisomeles indica to Induce Apoptosis in Human Gastric Cancer Cells. Molecules 2022, 27, 587. [Google Scholar] [CrossRef]
- Lien, H.M.; Wu, H.Y.; Hung, C.L.; Chen, C.J.; Wu, C.L.; Chen, K.W.; Huang, C.L.; Chang, S.J.; Chen, C.C.; Lin, H.J.; et al. Antibacterial activity of ovatodiolide isolated from Anisomeles indica against Helicobacter pylori. Sci. Rep. 2019, 9, 4205. [Google Scholar] [CrossRef]
- Delshad, N.; Paul, P.; Silva, M.S.; Yatkin, E.; Voipio, M.; Rajendran, S.K.; Eriksson, J.E. Pharmacokinetics, Metabolism, and Toxicity of Anisomelic Acid and Ovatodiolide: Guiding Route of Administration in Preclinical Studies. Eur. J. Pharm. Sci. 2025, 213, 107235. [Google Scholar] [CrossRef]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef]
- Badisa, R.B.; Darling-Reed, S.F.; Joseph, P.; Cooperwood, J.S.; Latinwo, L.M.; Goodman, C.B. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer Res. 2009, 29, 2993–2996. [Google Scholar] [PubMed] [PubMed Central]
- Olofinsan, K.; Abrahamse, H.; George, B.P. Therapeutic Role of Alkaloids and Alkaloid Derivatives in Cancer Management. Mol. 2023, 28, 5578. [Google Scholar] [CrossRef] [PubMed]
- Ożarowski, M.; Karpiński, T.M.; Czerny, B.; Kamiński, A.; Seremak-Mrozikiewicz, A. Plant Alkaloids as Promising Anticancer Compounds with Blood–Brain Barrier Penetration in the Treatment of Glioblastoma: In Vitro and In Vivo Models. Molecules 2025, 30, 1561. [Google Scholar] [CrossRef]
- Seidel, E.C.; Birkemeyer, C.; Baran-Schmidt, R.; Meixensberger, J.; Oppermann, H.; Gaunitz, F. Viability of glioblastoma cells and fibroblasts in the presence of imidazole-containing compounds. Int. J. Mol. Sci. 2022, 23, 5834. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Khan, A.; Song, D.; Dai, Z.; Liu, Y.P.; Yu, H.F.; Wang, B.; Zhu, P.F.; Ding, C.F.; Zhao, X.D.; et al. Three New Pyridine Alkaloids from Vinca major Cultivated in Pakistan. Nat. Prod. Bioprospect. 2017, 7, 323–327. [Google Scholar] [CrossRef]
- Riaz, A.; Rasul, A.; Batool, R.; Kanwal, L.; Hussain, G.; Sarfraz, I.; Shah, M.A.; Rao, F.; Ucak, I.; Adem, S.; et al. Alkaloids in the Treatment of Gastrointestinal Tract Cancer. In Phytonutrients in the Treatment of Gastrointestinal Cancer; Khan, H., Ed.; Bentham Science Publishers: Singapore, 2023; pp. 182–208. [Google Scholar] [CrossRef]
- Zakaria, F.; Tan, J.K.; Mohd Faudzi, S.M.; Abdul Rahman, M.B.; Ashari, S.E. Ultrasound-assisted extraction conditions optimisation using response surface methodology from Mitragyna speciosa (Korth.) Havil leaves. Ultrason. Sonochem. 2021, 81, 105851. [Google Scholar] [CrossRef]
- Rodrigues, M.I.; Iemma, A.F. Planejamento de Experimental e Otimização de Processos, 3a ed.; Cárita Ed.: São Paulo, Brazil, 2009. [Google Scholar]
- Carvalho, N.C.C.; Monteiro, O.S.; Da Rocha, C.Q.; Da Silva, J.K.R.; Maia, J.G.S. Phenolic Compounds and Antioxidant Properties of Puruí (Alibertia edulis, Rubiaceae), an Edible Dark Purple Fruit from the Brazilian Amazon. Nutraceuticals 2023, 3, 529–539. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphotungstic phosphomolybdic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- UC San Diego. MassIVE MSV000099256: GNPS—Optimized Extracts from Licaria armeniaca [Dataset]. MassIVE. 2025. Available online: https://massive.ucsd.edu/ProteoSAFe/dataset.jsp?accession=MSV000099256 (accessed on 27 July 2025).
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef] [PubMed]
- Schmid, R.; Heuckeroth, S.; Korf, A.; Smirnov, A.; Myers, O.; Dyrlund, T.S.; Bushuiev, R.; Murray, K.J.; Hoffmann, N.; Lu, M.; et al. Integrative analysis of multimodal mass spectrometry data in MZmine 3. Nat. Biotechnol. 2023, 41, 447–449. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.; Phelan, V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Bittremieux, W.; Avalon, N.E.; Thomas, S.P.; Kakhkhorov, S.A.; Aksenov, A.A.; Gomes, P.W.P.; Aceves, C.M.; Caraballo-Rodríguez, A.M.; Gauglitz, J.M.; Gerwick, W.H.; et al. Open access repository-scale propagated nearest neighbor suspect spectral library for untargeted metabolomics. Nat. Commun. 2023, 14, 8488. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Nunes, J.A.; Teixeira, L.L.; Nascimento, W.M.O.; Lopes, D.C.F.; Silva, J.K.R.; Pinto, L.C.; Santos, P.V.L.; Figueiredo, P.L.B. seasonal variation on chemical composition and in vitro cytotoxic and anti-inflammatory activities of Myrciaria dubia (kunth) mcvaugh essential oil from Amazon. Biochem. Syst. Ecol. 2025, 121, 105001. [Google Scholar] [CrossRef]

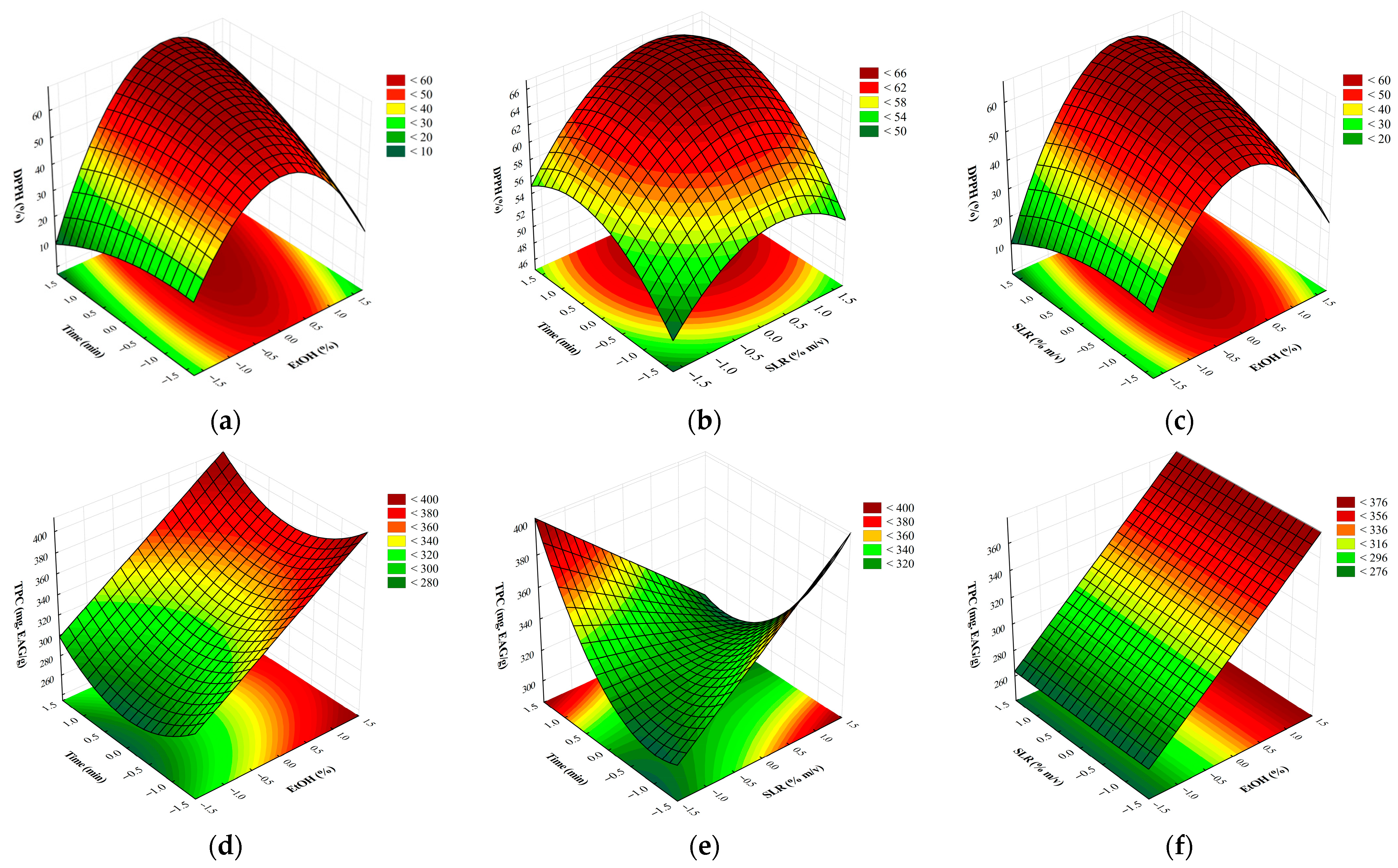
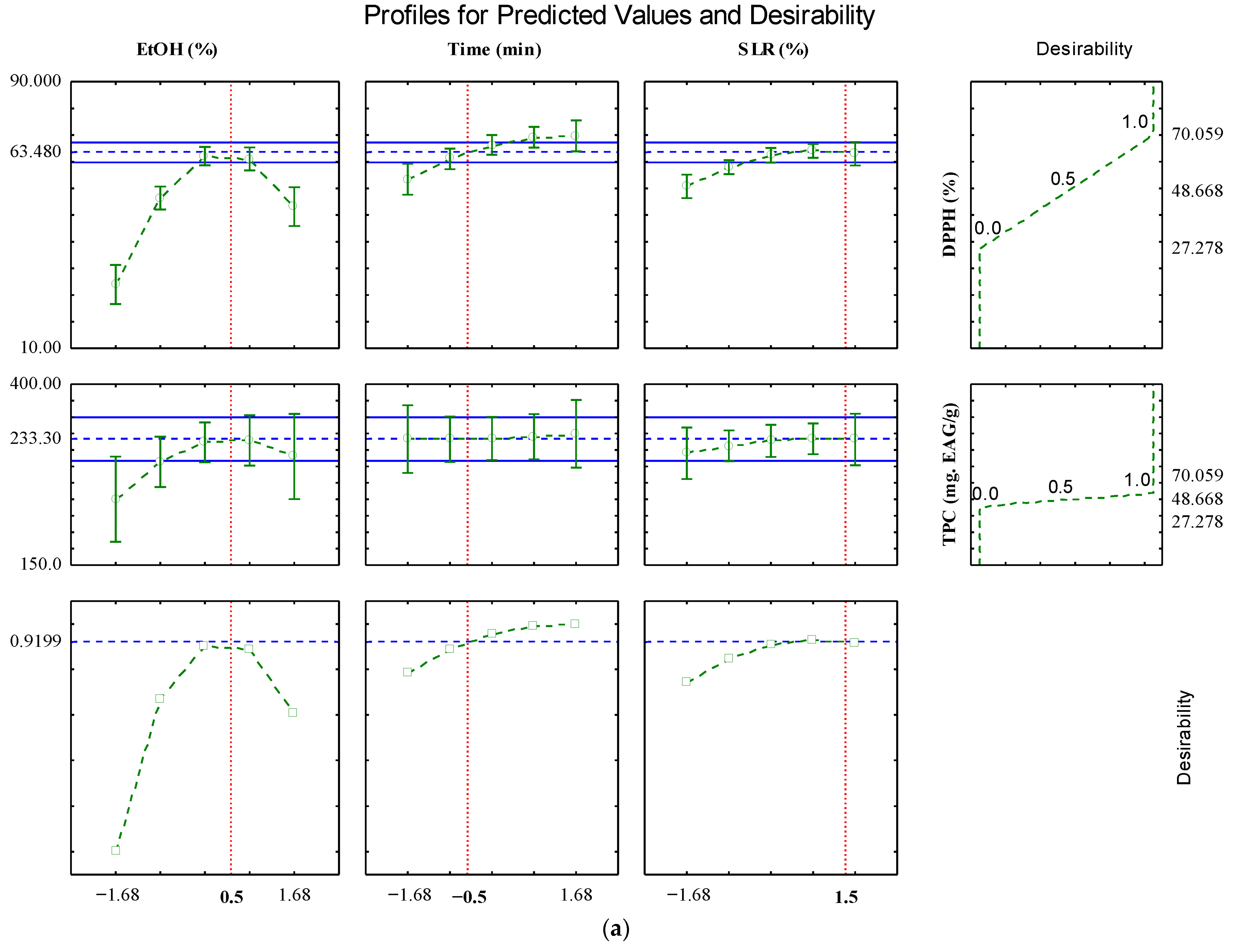
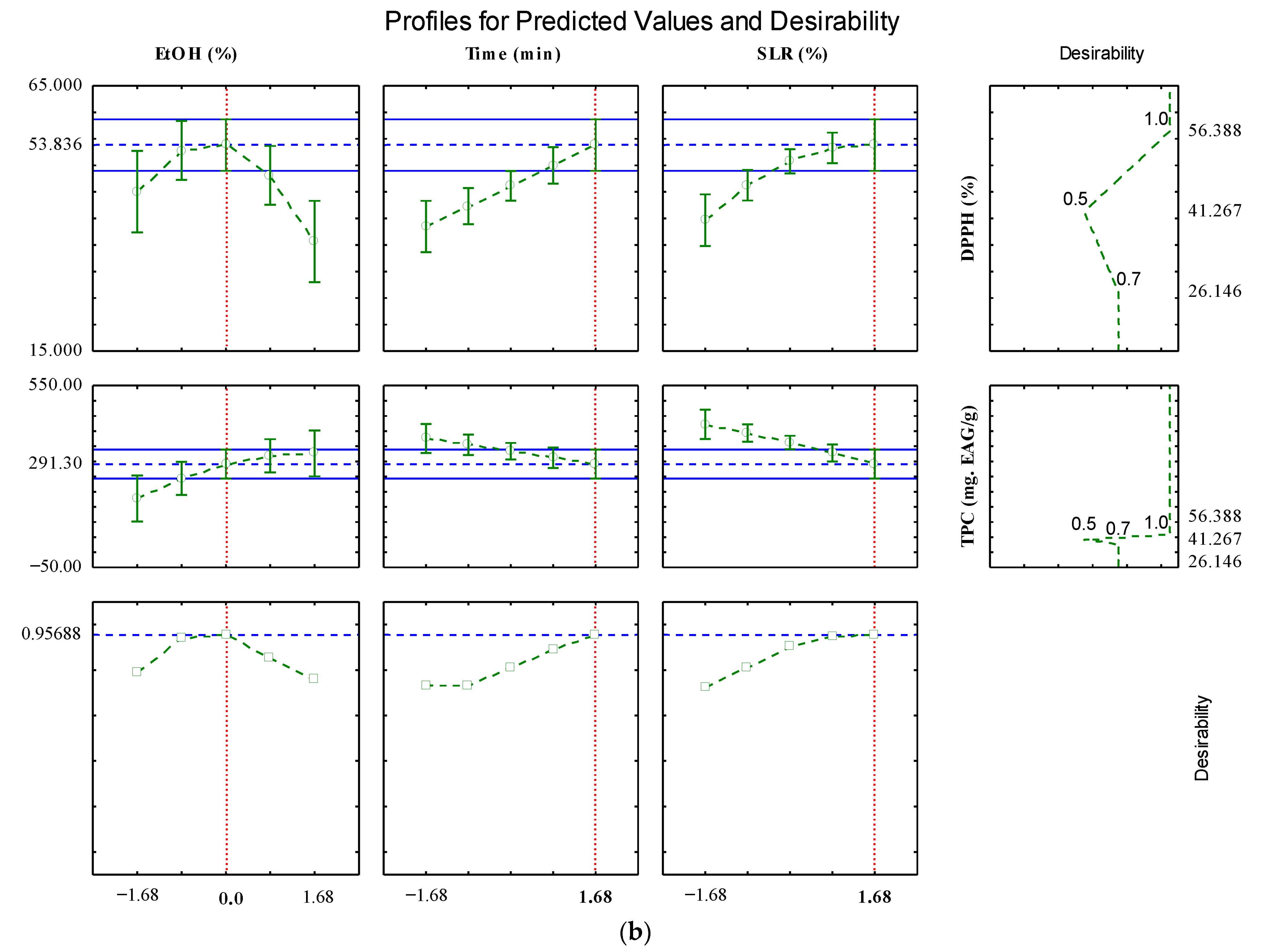

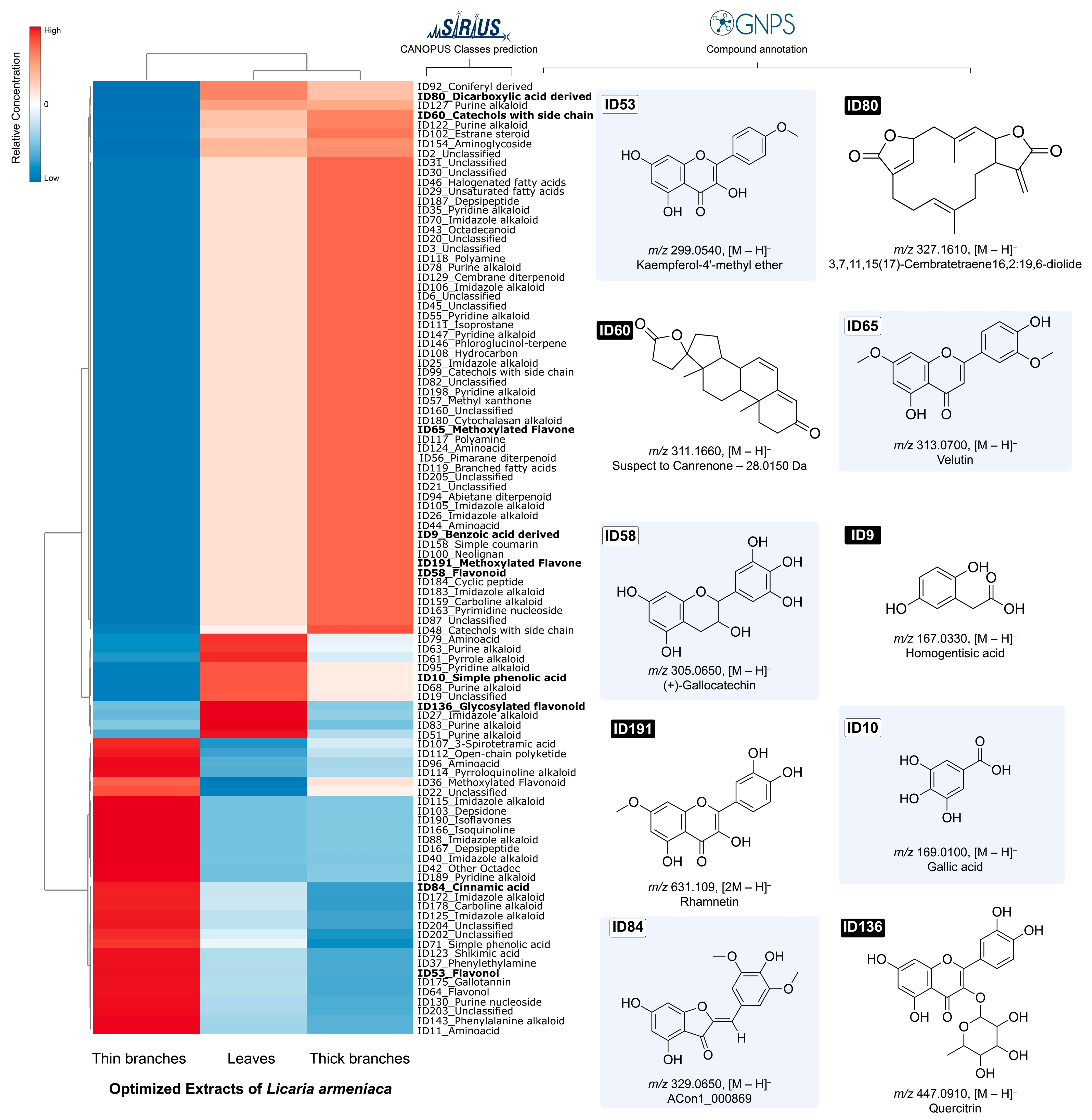
| Source of Variation | QS | Df | MQ | Fcalc. | Ftab. (p ≤ 0.1) |
|---|---|---|---|---|---|
| Leaves—DPPH | |||||
| Regression | 2515.1 | 8 | 314.4 | 14.20 | 2.59 |
| Residue | 177.1 | 8 | 22.1 | ||
| Lack of fit | 170.710 | 6 | 28.452 | 8.92 | 9.33 |
| Pure error | 6.383 | 2 | 3.191 | ||
| Total | 2692.234 | 16 | |||
| Adjusted R2 | 0.9342 | ||||
| R2 | 0.9976 | ||||
| Thin Branches—TPC | |||||
| Regression | 26343.4 | 3 | 8781.1 | 12.23 | 2.56 |
| Residue | 9335.7 | 13 | 718.1 | ||
| Lack of fit | 9034.92 | 11 | 821.36 | 5.46 | 9.4 |
| Pure error | 300.81 | 2 | 105.40 | ||
| Total | 35,679.12 | 16 | |||
| Adjusted R2 | 0.7383 | ||||
| R2 | 0.9916 | ||||
| Thin Branches—DPPH | |||||
| Regression | 582.0 | 8 | 72.8 | 1.47 | 2.59 |
| Residue | 397.1 | 8 | 49.6 | ||
| Lack of fit | 394.0452 | 6 | 65.6742 | 42.61 | 9.33 |
| Pure error | 3.0824 | 2 | 1.5412 | ||
| Total | 979.1334 | 16 | |||
| Adjusted R2 | 0.5944 | ||||
| R2 | 0.9969 |
| Assays | Y1F (%) | Y2GF (mgGAE/g) |
|---|---|---|
| Predicted | 63.48 | 291.30 |
| Observed | 61.16–64.02 a | 275.4–315.9 a |
| MAE (%) | 1.78 | 3.33 |
| Extract | Cell lines IC50 (µg/mL) | |||
|---|---|---|---|---|
| AGP-01 Gastric Ascite | AHOL Human Glioblastoma | A549 Lung Cancer | RAW 264.7 Murine Macrophages | |
| Leaves | 27.63 (20.82–36.67) SI: 1.04 | 11.52 (8.054–16.49) SI: 2.5 | 45.97 (14.87–142.1) SI: 0.63 | 28.81 (23.17–35.83) |
| Thin branches | 15.71 (12.22–20.22) SI: 0.94 | 18.89 (13.55–26.36) SI: 0.78 | 31.5 (19.67–50.46) SI: 0.47 | 14.72 (10.93–19.84) |
| Thick branches | 13.59 (10.29–17.96) SI: 3.74 | 26.52 (23.24–30.26) SI: 1.92 | 16.95 (12.92–22.24) SI: 3.0 | 50.86 (43.24–59.83) |
| Variable | Variable Level | |||||
|---|---|---|---|---|---|---|
| −1.68 | −1 | 0 | 1 | 1.68 | ||
| X1 | EtOH percentage (%) | 0 | 20.24 | 50 | 79.76 | 100 |
| X2 | Extraction time (min) | 5 | 17.14 | 35 | 52.86 | 65 |
| X3 | Solid-to-liquid ratio to leaves (% m/v) | 0.8 | 2.24 | 4.4 | 6.54 | 8.0 |
| X3 | Solid-to-liquid ratio for branches (% m/v) | 2 | 5.56 | 11 | 16.35 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, L.R.; Abelém, B.R.; Reis, J.D.E.; Herman, C.A.N.P.; Figueiredo, P.L.B.; Pinto, L.C.; Martins, L.H.; Silva, M.N.d.; Gomes, P.W.P.; Silva, J.K.R.d. Optimization of the Extraction of Bioactive Compounds and Metabolomic Profile of Licaria armeniaca. Plants 2025, 14, 3158. https://doi.org/10.3390/plants14203158
Ferreira LR, Abelém BR, Reis JDE, Herman CANP, Figueiredo PLB, Pinto LC, Martins LH, Silva MNd, Gomes PWP, Silva JKRd. Optimization of the Extraction of Bioactive Compounds and Metabolomic Profile of Licaria armeniaca. Plants. 2025; 14(20):3158. https://doi.org/10.3390/plants14203158
Chicago/Turabian StyleFerreira, Lanalice R., Bianca R. Abelém, José Diogo E. Reis, Christelle Anne N. P. Herman, Pablo Luis B. Figueiredo, Laine Celestino Pinto, Luiza Helena Martins, Milton Nascimento da Silva, Paulo Wender P. Gomes, and Joyce Kelly R. da Silva. 2025. "Optimization of the Extraction of Bioactive Compounds and Metabolomic Profile of Licaria armeniaca" Plants 14, no. 20: 3158. https://doi.org/10.3390/plants14203158
APA StyleFerreira, L. R., Abelém, B. R., Reis, J. D. E., Herman, C. A. N. P., Figueiredo, P. L. B., Pinto, L. C., Martins, L. H., Silva, M. N. d., Gomes, P. W. P., & Silva, J. K. R. d. (2025). Optimization of the Extraction of Bioactive Compounds and Metabolomic Profile of Licaria armeniaca. Plants, 14(20), 3158. https://doi.org/10.3390/plants14203158








