Investigating the Biocontrol and Plant Growth-Promoting Potential of Pseudomonas yamanorum for Sustainable Management of Tomato Early Blight (Alternaria alternata)
Abstract
1. Introduction
2. Results
2.1. Physiological and Biochemical Characterization of Pseudomonas yamanorum
2.2. Extracellular Enzymatic Activity and Plant-Growth-Promoting Traits of Pseudomonas yamanorum
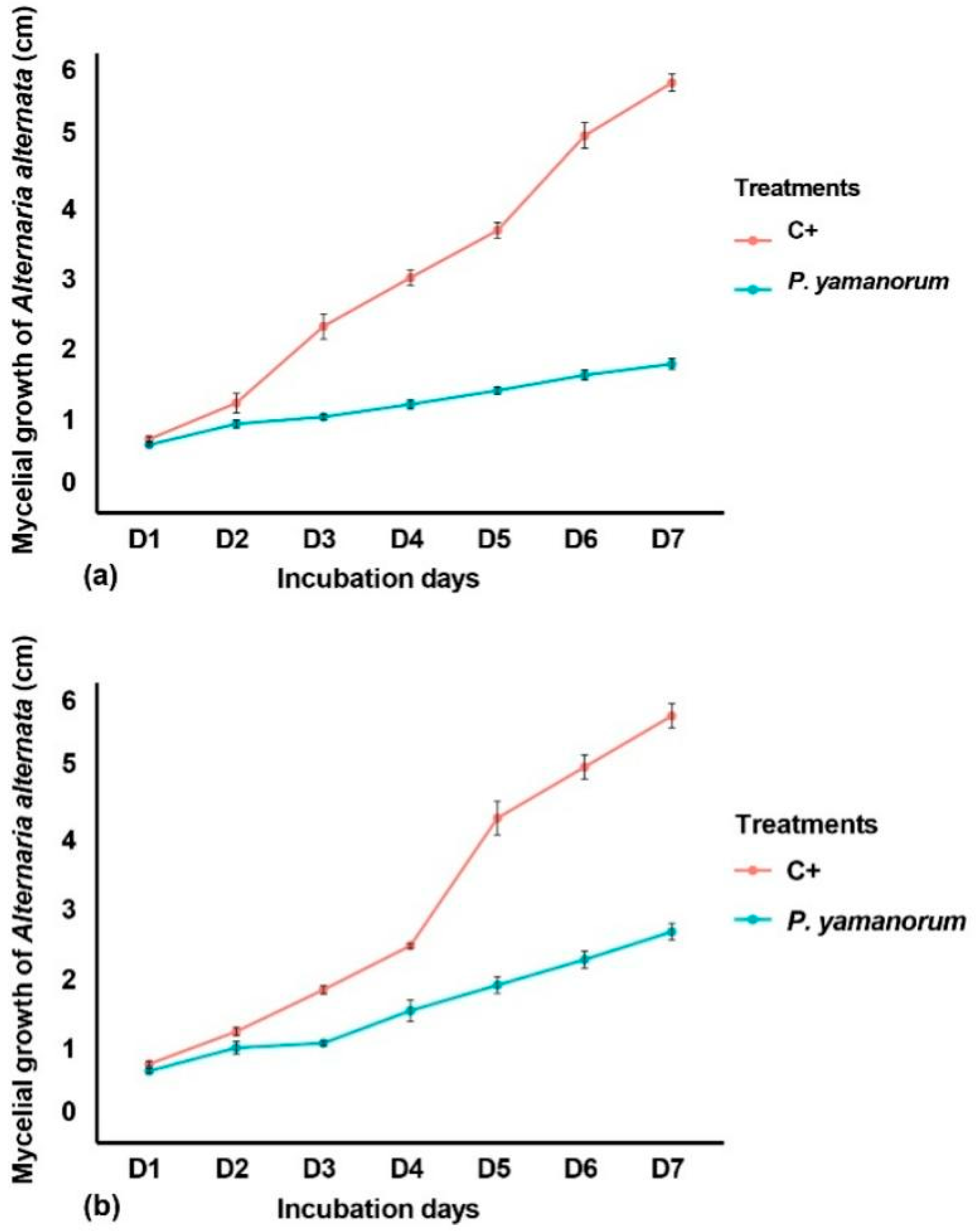
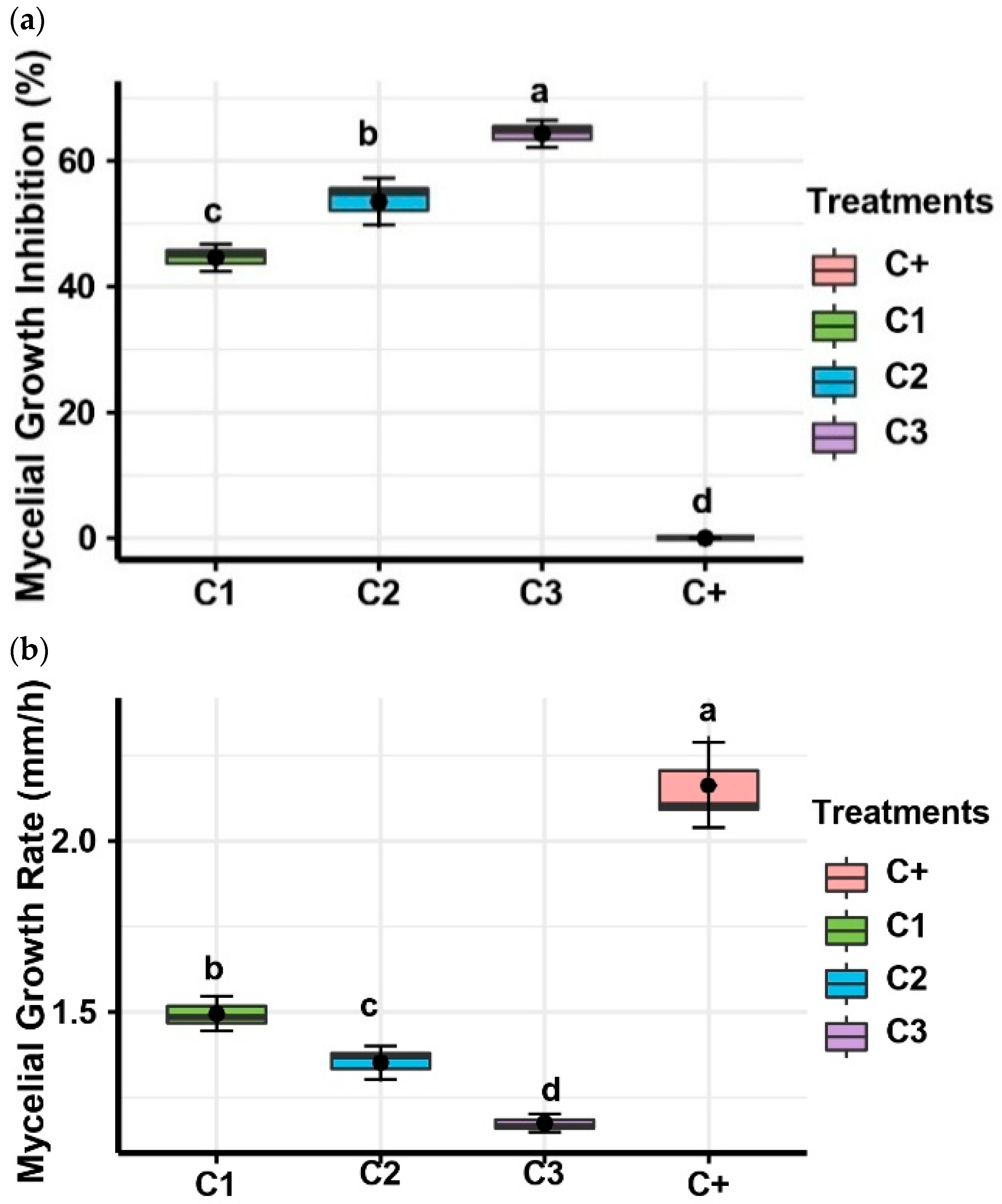
2.3. In Vitro, Antifungal Activities of Pseudomonas yamaranoum Against Alternaria alternata
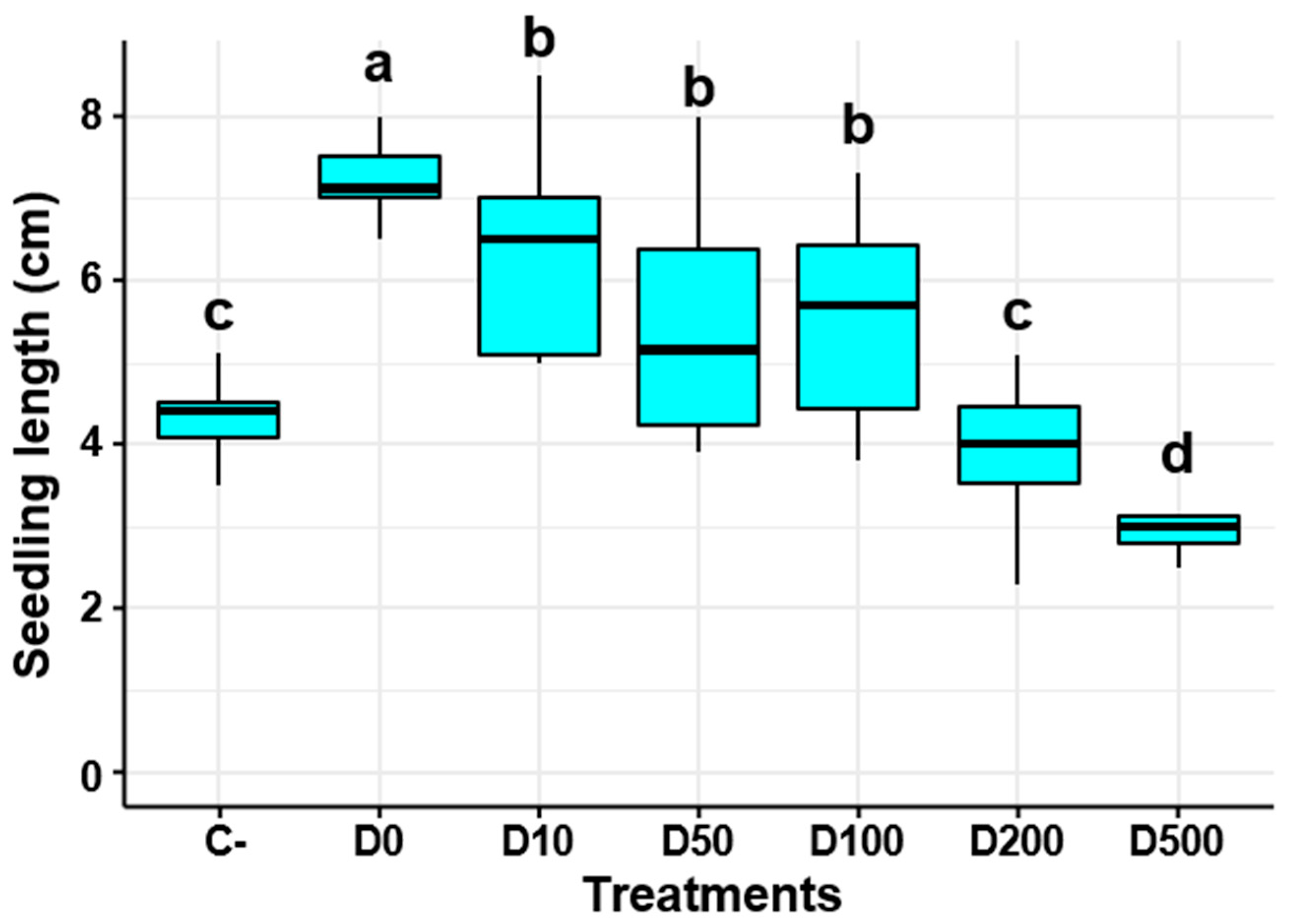
2.4. Evaluation of Pseudomonas yamanorum Filtrate Dilutions on Tomato Seedling Growth
2.5. In Planta, Biocontrol Effect of Pseudomonas yamaranoum and Salicylic Acid Against Alternaria alternata and Plant Growth Promoting Efficacy Under Greenhouse Conditions
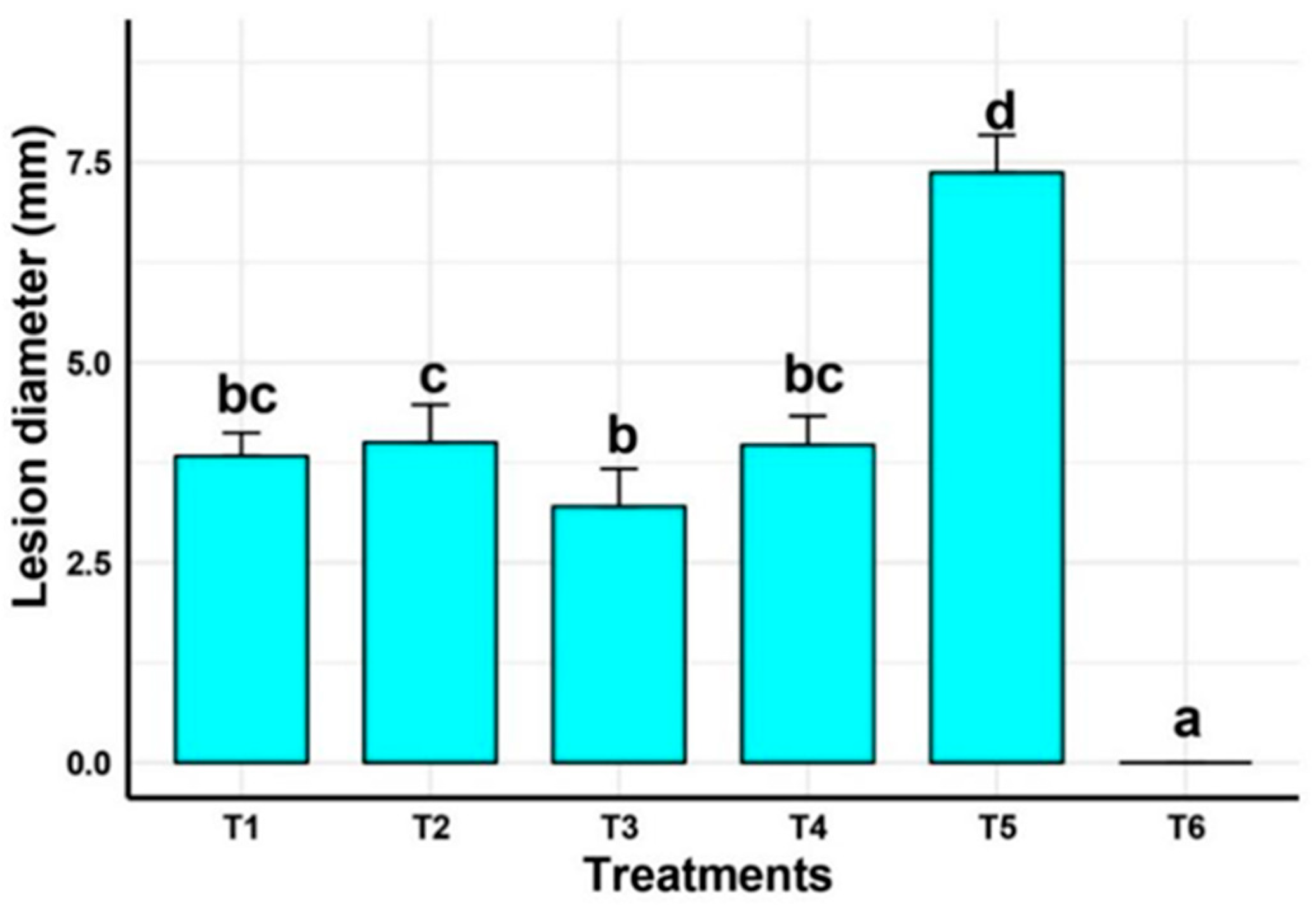
2.5.1. Disease Severity
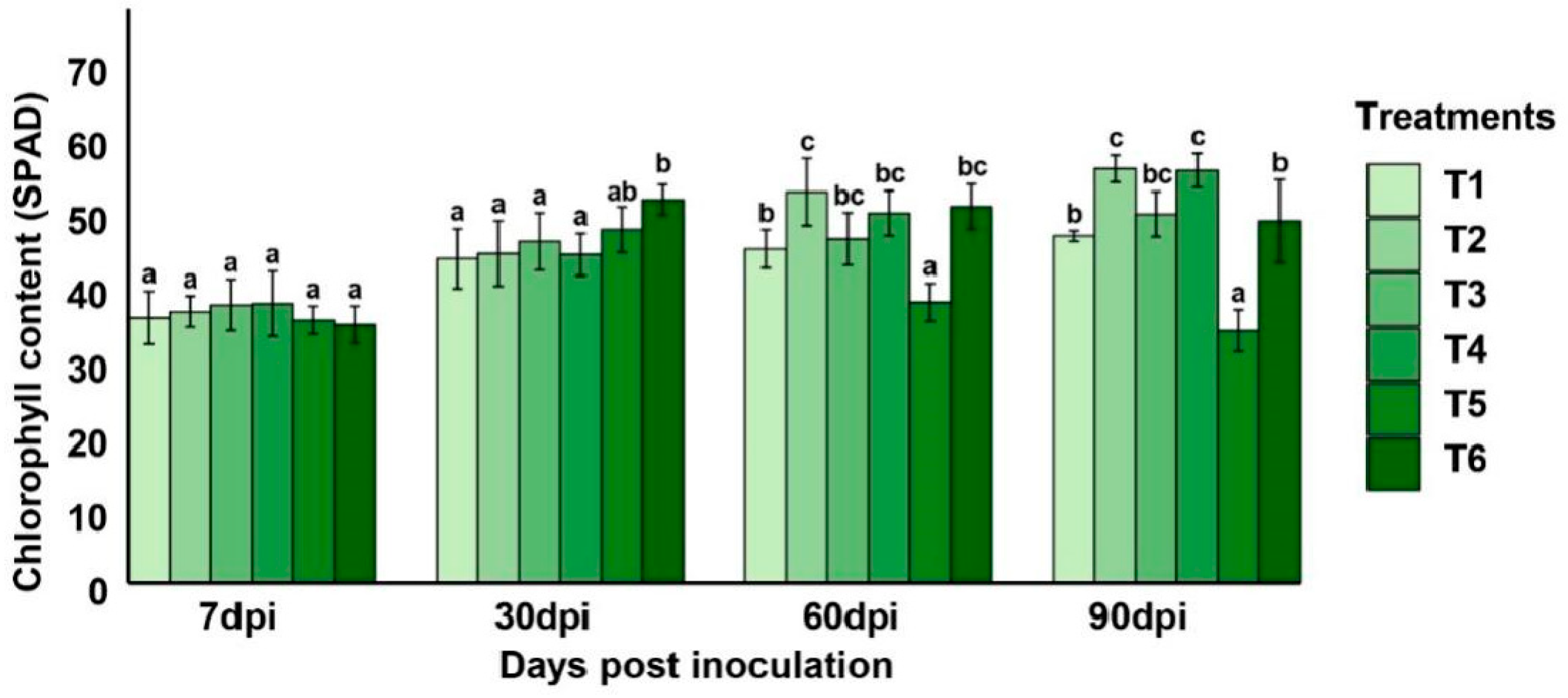
2.5.2. Impact of Pseudomonas yamanorum and Salicylic Acid on Plant Growth Promoting Parameters
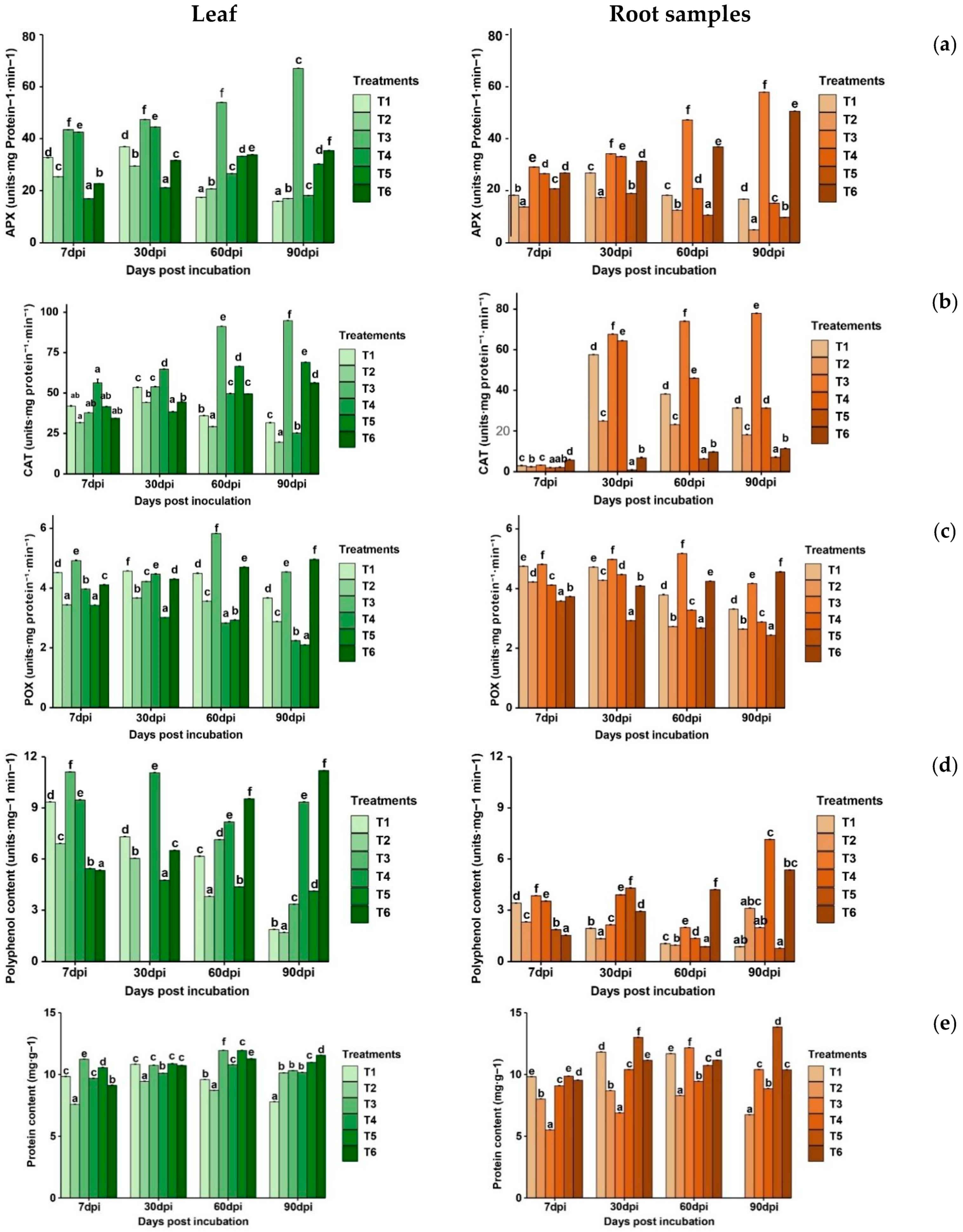
2.5.3. Enzymatic Activities
- Ascorbate peroxidase activity (APX)
- Catalase activity (CAT)
- Peroxidase activity
- Polyphenol activity
- Protein content
2.6. Effect of Pseudomonas yamanorum and Salicylic Acid on Tomato Fruit Quality
2.6.1. Efficacy of Treatments for Postharvest Control of Alternaria alternata in Tomato Fruits
2.6.2. Effect of Preventive Treatments on Biochemical and Physiological Parameters of Fruit Quality
3. Discussion
4. Materials and Methods
4.1. Fungal Pathogen
4.2. Bacterial Antagonist
4.3. Physiological Properties of P. yamanorum
4.4. Enzyme Production and Plant Growth Promotion Traits
4.5. In Vitro, the Antagonistic Effect of Pseudomonas yamanorum Against Alternaria alternata
4.5.1. Dual Culture Assays
4.5.2. Bacterial Culture Filtrates Effect on Pathogen Mycelial Growth and Spore Germination
4.6. Evaluation of Biocontrol Potential of P. yamanorum on Tomato Seed and Seedling
4.7. In Planta Evaluation of P. yamanorum and Salicylic Acid Treatments on Tomato Plants for A. alternata Control
4.7.1. Disease Severity Assessment
4.7.2. Physiological Responses and Agronomic Growth Parameters
4.7.3. Antioxidant Enzymatic Activities
4.8. Fruit Quality Analysis
4.8.1. Efficacy of Post-Harvest Pathogenicity Assay
4.8.2. Fruit Quality Assessment
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Collins, E.J.; Bowyer, C.; Tsouza, A.; Chopra, M. Tomatoes: An Extensive Review of the Associated Health Impacts of Tomatoes and Factors That Can Affect Their Cultivation. Biology 2022, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, A.; Kaymak, H.C. Tomato production quantity estimates for 2023-2027 with ARIMA model: Evidence from leading producing countries including turkey. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural Dev. 2024, 24, 41–46. [Google Scholar]
- Food and Agriculture Organization of the United Nations. FAOSTAT Database. Available online: https://www.fao.org/faostat/en/#home (accessed on 16 July 2025).
- Salotti, I.; Giorni, P.; Battilani, P. Biology, ecology, and epidemiology of Alternaria species affecting tomato: Ground information for the development of a predictive model. Front. Plant Sci. 2024, 15, 1430965. [Google Scholar] [CrossRef] [PubMed]
- Yessimseitova, A.; Abdrakhmanova, A.; Tokbergenova, Z.; Abdullaeva, B.; Muranets, A.; Nurtaza, A.; Kakimzhanova, A. Identification and Characterization of Alternaria Species Causing Early Blight on Tomato in Kazakhstan. Agronomy 2025, 15, 1251. [Google Scholar] [CrossRef]
- Angelotti, F.; Hamada, E.; Bettiol, W. A Comprehensive Review of Climate Change and Plant Diseases in Brazil. Plants 2024, 13, 2447. [Google Scholar] [CrossRef]
- Nuwamanya, A.M.; Runo, S.; Mwangi, M. Farmers’ Perceptions on Tomato Early Blight, Fungicide Use Factors and Awareness of Fungicide Resistance: Insights from a Field Survey in Kenya. PLoS ONE 2023, 18, e0269035. [Google Scholar] [CrossRef]
- Fusar, P.E.; Fontefrancesco, M. Trends in the implementation of biopesticides in the Euro-Mediterranean region: A narrative literary review. Sustain. Earth Rev. 2024, 7, 14. [Google Scholar] [CrossRef]
- Tripathi, S.; Srivastava, P.; Devi, R.S.; Bhadouria, R. Influence of synthetic fertilizers and pesticides on soil health and soil microbiology. In Agrochemicals Detection, Treatment and Remediation; Butterworth-Heinemann: Oxford, UK, 2020; pp. 25–54. [Google Scholar]
- Zemmouri, B.; Lammoglia, S.K.; Bouras, F.Z.; Seghouani, M.; Rebouh, N.Y.; Latati, M. Modelling human health risks from pesticide use in innovative legume-cereal intercropping systems in Mediterranean conditions. Ecotoxicol. Environ. Saf. 2022, 238, 113590. [Google Scholar] [CrossRef]
- Burandt, Q.C.; Deising, H.B.; von Tiedemann, A. Further Limitations of Synthetic Fungicide Use and Expansion of Organic Agriculture in Europe Will Increase the Environmental and Health Risks of Chemical Crop Protection Caused by Copper-Containing Fungicides. Environ. Toxicol. Chem. 2024, 43, 19–30. [Google Scholar] [CrossRef]
- Islam, T.; Danishuddin; Tamanna, N.T.; Matin, M.N.; Barai, H.R.; Haque, M.A. Resistance Mechanisms of Plant Pathogenic Fungi to Fungicide, Environmental Impacts of Fungicides, and Sustainable Solutions. Plants 2024, 13, 2737. [Google Scholar] [CrossRef]
- Rebouh, N.Y.; Latati, M.; Polityko, P.; Kucher, D.; Hezla, L.; Norezzine, A.; Kalisa, L.; Utkina, A.; Vvedenskiy, V.; Gadzhikurbanov, A.; et al. Influence of three cultivation technologies to control Fusarium spp. in winter wheat (Triticum aestivum L.) production under Moscow conditions. Res. Crops 2020, 21, 17–25. [Google Scholar]
- Karačić, V.; Miljaković, D.; Marinković, J.; Ignjatov, M.; Milošević, D.; Tamindžić, G.; Ivanović, M. Bacillus Species: Excellent Biocontrol Agents against Tomato Diseases. Microorganisms 2024, 12, 457. [Google Scholar] [CrossRef]
- Rebouh, N.Y.; Polityko, P.M.; Pakina, E.; Plushikov, V.G.; Norezzine, A.; Gadzhikurbanov, A.; Vvedenskiy, V.; Duksi, F.; Iguer-Ouada, M. Impact of three integrated crop protection treatments on the varieties of winter wheat (Triticum aestivum L.) in Moscow area, Russia. Res. Crops 2019, 20, 161–168. [Google Scholar]
- Sun, W.; Shahrajabian, M.H.; Soleymani, A. The Roles of Plant-Growth-Promoting Rhizobacteria (PGPR)-Based Biostimulants for Agricultural Production Systems. Plants 2024, 13, 613. [Google Scholar] [CrossRef]
- Reddy, C.S.; Cho, M.; Kaul, T.; Joeng, J.T.; Kim, K.M. Pseudomonas fluorescens imparts cadmium stress tolerance in Arabidopsis thaliana via induction of AtPCR2 gene expression. J. Genet. Eng. Biotechnol. 2023, 21, 8. [Google Scholar] [CrossRef]
- Zhao, W.; Du, E.; Luo, R.; Chen, Y.; Sun, Z.; Gui, F. Arbuscular mycorrhizal fungus and Pseudomonas bacteria affect tomato response to Tuta absoluta (Lepidoptera: Gelechiidae) herbivory. BMC Plant Biol. 2024, 24, 1236. [Google Scholar] [CrossRef]
- Roo, V.D.; Verleysen, Y.; Kovács, B.; Matthias, V.; Girard, L.; Höfte, M.; De Mot, R.; Madder, A.; Geudens, N.; Martins, J.C. An NMR fingerprint matching approach for the identification and structural re-evaluation of Pseudomonas lipopeptides. Microbiol. Spectr. 2022, 10, e0126122. [Google Scholar] [CrossRef]
- Hajji-Hedfi, L.; Wannassi, T.; Abdel-Azeem, A.M. Harnessing a Microbial Consortium and Compost to Control Grapevine Pathogens: A Sustainable Viticulture Strategy for Disease Suppression and Quality Enhancement. Horticulturae 2025, 11, 769. [Google Scholar] [CrossRef]
- Hajji-Hedfi, L.; Rhouma, A.; Wannassi, T.; Utkina, A.O.; Rebouh, N.Y. Biocontrol assessment of Trichoderma species on tomato crops infested by Curvularia spicifera: Toward sustainable farming systems. Front. Sustain. Food Syst. 2025, 9, 1627903. [Google Scholar] [CrossRef]
- Ghadamgahi, F.; Tarighi, S.; Taheri, P.; Saripella, G.V.; Anzalone, A.; Kalyandurg, P.B.; Catara, V.; Ortiz, R.; Vetukuri, R.R. Plant Growth-Promoting Activity of Pseudomonas aeruginosa FG106 and Its Ability to Act as a Biocontrol Agent against Potato, Tomato and Taro Pathogens. Biology 2022, 11, 140. [Google Scholar] [CrossRef]
- Yi, Y.; Hou, Z.; Shi, Y.; Zhang, C.; Zhu, L.; Sun, X.; Zhang, R.; Wang, Z. Pseudomonas fluorescens RB5 as a Biocontrol Strain for Controlling Wheat Sheath Blight Caused by Rhizoctonia cerealis. Agronomy 2023, 13, 1986. [Google Scholar] [CrossRef]
- Pandey, S.; Gupta, S. Evaluation of Pseudomonas sp. for its multifarious plant growth promoting potential and its ability to alleviate biotic and abiotic stress in tomato (Solanum lycopersicum) plants. Sci. Rep. 2020, 10, 20951. [Google Scholar] [CrossRef]
- Gupta, V.; Buch, A. Pseudomonas aeruginosa predominates as multifaceted rhizospheric bacteria with combined abilities of P-solubilization and biocontrol. J. Pure Appl. Microbiol. 2019, 13, 319–328. [Google Scholar] [CrossRef]
- Li, W.; Sun, L.; Wu, H.; Gu, W.; Lu, Y.; Liu, C.; Zhang, J.; Li, W.; Zhou, C.; Geng, H.; et al. Bacillus velezensis YXDHD1-7 Prevents Early Blight Disease by Promoting Growth and Enhancing Defense Enzyme Activities in Tomato Plants. Microorganisms 2024, 12, 921. [Google Scholar] [CrossRef]
- Kamle, M.; Borah, R.; Bora, H.; Jaiswal, A.K.; Singh, R.K.; Kumar, P. Systemic Acquired Resistance (SAR) and Induced Systemic Resistance (ISR): Role and Mechanism of Action Against Phytopathogens. In Fungal Biotechnology and Bioengineering; Hesham, A.L., Upadhyay, R., Sharma, G., Manoharachary, C., Gupta, V., Eds.; Springer: Cham, Switzerland, 2020; pp. 457–470. [Google Scholar] [CrossRef]
- Pane, C.; Zaccardelli, M. Evaluation of Bacillus Strains Isolated from Solanaceous Phylloplane for Biocontrol of Alternaria Early Blight of Tomato. Biol. Control 2015, 84, 11–18. [Google Scholar] [CrossRef]
- Solanki, M.K.; Yandigeri, M.S.; Kumar, S.; Singh, R.K.; Srivastava, A.K. Co-inoculation of different antagonists can enhance the biocontrol activity against Rhizoctonia solani in tomato. Antonie Van Leeuwenhoek 2019, 112, 1633–1644. [Google Scholar] [CrossRef]
- Zahoor, S.; Naz, R.; Keyani, R.; Roberts, T.H.; Hassan, M.N.; Yasmin, H.; Nosheen, A.; Farman, S. Rhizosphere bacteria associated with Chenopodium quinoa promote resistance to Alternaria alternata in tomato. Sci. Rep. 2022, 12, 19027. [Google Scholar] [CrossRef]
- Zandi, P.; Schnug, E. Reactive oxygen species, antioxidant responses and implications from a microbial modulation perspective. Biology 2022, 11, 155. [Google Scholar] [CrossRef]
- Tanveer, S.; Ilyas, N.; Akhtar, N.; Sayyed, R.Z.; Almalki, W.H. Induction of regulatory mechanisms by plant growth promoting rhizobacteria in crops facing drought stress. Crop Pasture Sci. 2023, 74, 856–870. [Google Scholar] [CrossRef]
- Hajji-Hedfi, L.; Wannassi, T.; Tawfeeq Al-Ani, L.K.; Balbool, B.A.; Tissaoui, S.; Mougou-Hamdane, A.; Hamdi, W.; Azeem, A.M.A.; Rebouh, N.Y. Investigating the potential role of beneficial rhizobacteria for protecting grapevine health and promoting growth. Front. Sustain. Food Syst. 2025, 9, 1619801. [Google Scholar] [CrossRef]
- Latha, P.; Anand, T.; Ragupathi, N.; Prakasam, V.; Samiyappan, R. Antimicrobial activity of plant extracts and induction of systemic resistance in tomato plants by mixtures of PGPR strains and Zimmu leaf extract against Alternaria solani. Biol. Control 2009, 50, 85–93. [Google Scholar] [CrossRef]
- García-Latorre, C.; Rodrigo, S.; Santamaria, O. Biological Control of Pseudomonas syringae in Tomato Using Filtrates and Extracts Produced by Alternaria leptinellae. Horticulturae 2024, 10, 334. [Google Scholar] [CrossRef]
- Hahn, L.; Sá, E.D.; Machado, R.G.; Silva, W.D.; Oldra, S.; Damasceno, R.G.; Schönhofen, A. Growth promotion in maize with diazotrophic bacteria in succession with ryegrass and white clover. Am.-Eurasian J. Agric. Environ. Sci. 2014, 14, 11–16. [Google Scholar]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Madden, T.L.; Tatusov, R.L.; Zhang, J. Applications of network BLAST server. Methods Enzymol. 1996, 266, 131–141. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Pikovskaya, R.I. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 1948, 17, 362–370. [Google Scholar]
- Hajji-Hedfi, L.; Rhouma, A.; Hajlaoui, H.; Hajlaoui, F.; Rebouh, N.Y. Understanding the Influence of Applying Two Culture Filtrates to Control Gray Mold Disease (Botrytis cinerea) in Tomato. Agronomy 2023, 13, 1774. [Google Scholar] [CrossRef]
- Milagres, A.M.; Machuca, A.; Napoleão, D. Detection of siderophore production from several fungi and bacteria by a modification of chrome azurol S (CAS) agar plate assay. J. Microbiol. Methods 1999, 37, 1–6. [Google Scholar] [CrossRef]
- Pranaw, K.; Pidlisnyuk, V.; Trögl, J.; Malinská, H. Bioprospecting of a Novel Plant Growth-Promoting Bacterium Bacillus altitudinis KP-14 for Enhancing Miscanthus × giganteus Growth in Metals Contaminated Soil. Biology 2020, 9, 305. [Google Scholar] [CrossRef]
- Ajijah, N.; Fiodor, A.; Dziurzynski, M.; Stasiuk, R.; Pawlowska, J.; Dziewit, L.; Pranaw, K. Biocontrol potential of Pseudomonas protegens ML15 against Botrytis cinerea causing gray mold on postharvest tomato (Solanum lycopersicum var. Cerasiforme). Front. Plant Sci. 2023, 14, 1288408. [Google Scholar] [CrossRef]
- Dennis, C.; Webster, J. Antagonistic properties of species-groups of Trichoderma: II. Production of volatile antibiotics. Trans. Br. Mycol. Soc. 1971, 57, 363–369. [Google Scholar] [CrossRef]
- Pulla Reddy, A.C.; Lokesh, B.R. Studies on spice principles as antioxidants in the inhibition of lipid peroxidation of rat liver microsomes. Mol. Cell. Biochem. 1992, 111, 117–124. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Geasa, M.M.M.; Hassan, M.H.A. Effect of Mechanical Damage on Tomato Fruits under Storage Conditions. J. Soil Sci. Agric. Eng. 2022, 31, 93–98. [Google Scholar] [CrossRef]
- Haraguchi, H.; Saito, T.; Okamura, N.; Yagi, A. Inhibition of lipid peroxidation and superoxide generation by diterpenoids from Rosmarinus officinalis. Planta Med. 1995, 61, 333–336. [Google Scholar] [CrossRef]




| Treatments | 30 dpi | 60 dpi | 90 dpi |
|---|---|---|---|
| T1 | 0.66 ± 0.51 ab | 1.66 ± 0.51 b | 2.5 ± 0.54 b |
| T2 | 0.66 ± 0.51 ab | 2 ± 0.63 b | 3.33 ± 0.18 bc |
| T3 | 0.5 ± 0.4 ab | 2.16 ± 0.40 b | 3.16 ± 2.61 bc |
| T4 | 0.66 ± 0.51 ab | 2.33 ± 0.51 b | 4 ± 1.09 c |
| T5 | 1.33 ± 0.51 b | 3.5 ± 1.22 c | 6 ± 0.89 d |
| T6 | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a |
| p-value | p < 0.01 | p < 0.01 | p < 0.01 |
| Treatments | Firmness | pH | EC (mS/cm) | TA (g/10 mL Juice) | Sugar Content (°Brix) | Nitrate (mg/L) | WC (%) | Juice Yield (%) | MDA (µmol/ g DW) | Protein (mg/g) | Polyphenol |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | 2.17 ± 0.25 b | 4.44 ± 0.04 ab | 4.54 ± 0.36 c | 7.13 ± 0.15 a | 5.17 ± 0.75 d | 1366.67 ± 152.75 c | 92.01 ± 1 ab | 40.34 ± 0.29 c | 2.02 ± 0.02 b | 1.6 ± 0.33 c | 0.7 ± 0.08 c |
| T2 | 1.23 ± 0.15 c | 4.84 ± 0.05 a | 3.73 ± 0.05 d | 5.73 ± 0.21 b | 5.77 ± 0.15 c | 1366.67 ± 57.74 c | 91.34 ± 0.29 bc | 35.44 ± 0.51 e | 2.25 ± 0.06 a | 3.22 ± 0.32 ab | 0.4 ± 0.04 d |
| T3 | 3.13 ± 0.25 a | 4.02 ± 0.04 b | 0.59 ± 0.03 f | 5.37 ± 0.55 b | 6.43 ± 0.15 b | 1566.67 ± 115.47 b | 93.06 ± 0.08 a | 55.88 ± 0.21 a | 2.02 ± 0.04 b | 1.47 ± 0.26 c | 0.81 ± 0.04 b |
| T4 | 3.13 ± 0.25 a | 4.64 ± 0.26 a | 5.95 ± 0.07 a | 5.33 ± 0.06 b | 5.83 ± 0.12 c | 1500 ± 100 bc | 90.34 ± 0.09 c | 37.05 ± 1 d | 2.12 ± 0.04 b | 3.58 ± 0.35 a | 0.71 ± 0.03 c |
| T5 | 0.5 ± 0.17 d | 4.71 ± 0.48 a | 1.4 ± 0.11 e | 4.7 ± 0.2 c | 6.53 ± 0.06 b | 690 ± 20 d | 83.3 ± 1.54 d | 16.99 ± 0.02 f | 2.13 ± 0.04 ab | 2.79 ± 0.16 b | 0.39 ± 0.03 d |
| T6 | 3.43 ± 0.47 a | 4.06 ± 0.04 b | 5.48 ± 0.1 b | 7.53 ± 0.25 a | 7.5 ± 0.1 a | 1966.67 ± 57.74 a | 91.9 ± 0.17 ab | 44.7 ± 0.2 b | 2.04 ± 0.15 b | 3.57 ± 0.45 a | 1.04 ± 0.06 a |
| p-value | <0.01 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajji-Hedfi, L.; Wannassi, T.; Khlif, A.; Kavhiza, N.J.; Rebouh, N.Y. Investigating the Biocontrol and Plant Growth-Promoting Potential of Pseudomonas yamanorum for Sustainable Management of Tomato Early Blight (Alternaria alternata). Plants 2025, 14, 3117. https://doi.org/10.3390/plants14203117
Hajji-Hedfi L, Wannassi T, Khlif A, Kavhiza NJ, Rebouh NY. Investigating the Biocontrol and Plant Growth-Promoting Potential of Pseudomonas yamanorum for Sustainable Management of Tomato Early Blight (Alternaria alternata). Plants. 2025; 14(20):3117. https://doi.org/10.3390/plants14203117
Chicago/Turabian StyleHajji-Hedfi, Lobna, Takwa Wannassi, Amira Khlif, Nyasha J. Kavhiza, and Nazih Y. Rebouh. 2025. "Investigating the Biocontrol and Plant Growth-Promoting Potential of Pseudomonas yamanorum for Sustainable Management of Tomato Early Blight (Alternaria alternata)" Plants 14, no. 20: 3117. https://doi.org/10.3390/plants14203117
APA StyleHajji-Hedfi, L., Wannassi, T., Khlif, A., Kavhiza, N. J., & Rebouh, N. Y. (2025). Investigating the Biocontrol and Plant Growth-Promoting Potential of Pseudomonas yamanorum for Sustainable Management of Tomato Early Blight (Alternaria alternata). Plants, 14(20), 3117. https://doi.org/10.3390/plants14203117









