Nanoparticles and Nanocarriers for Managing Plant Viral Diseases
Abstract
1. Introduction
2. Impact of Viral Infections
3. Emerging Tools for Managing Viruses in Plants
4. Nanoparticles with Antiviral Activity Against Plant Viruses
| Nanoparticle | Viral Species | Crop Host | Context | Reference |
|---|---|---|---|---|
| Silver (Ag) | P.phaseoluteum, BYMV (bean yellow mosaic virus (BYMV) | Broad bean (Vicia faba L.) | A total of 100 mg to 200 mg L−1 of AgNPs inactivated BYMV when applied 48 and 24 h before and after inoculation. NPs are bioreactive with viral particles, specifically toward the capsid protein, regulate the PR-1 gene related to pathogenesis, and induce the production of enzymes as a defense mechanism in plants. | [54] |
| Zinc oxide (ZnO) | B. capsicumhuastecoense, PHYVV (pepper huasteco yellow vein virus) | Pepper (Capsicum annum L.) | NPsZnO at 100 mM and 150 mM reduced disease severity and viral levels, inducing resistance mechanisms in plants through the activation of POD, SOD, and CAT. | [55] |
| Copper oxide (CuO) through biosynthesis with Haloxylon salicornicum | Alfamovirus AMV, AMV (alfalfa mosaic virus) | Tobacco (Nicotiana tabacum L.) | Foliar applications of CuO NPs (48 h before and after) improved tobacco plant growth, decreased viral symptoms, reduced AMV accumulation levels by 97%, increased the expression of antioxidant enzymes, and increased the expression of genes as an antiviral mechanism. CuO NPs showed high binding energy with viral replication protein 1a. | [56] |
| Carbon (C60), zinc (Zn) and iron (Fe) | Cucurbit chlorotic yellows virus (CCYV) | N. benthamiana | NPs of C60 at 100 mg L−1 delayed viral infection for up to 5 days after inoculation. Fe and Zn did not suppress viral progression, while C60 regulated the production of defense-related phytohormones (SA and JA). | [57] |
5. Mechanisms of Gene Silencing Against Plant Viruses
5.1. Endogenous and Virus-Derived Gene Silencing
5.2. Host-Induced and Spray-Induced Gene Silencing
6. Nanocarriers for dsRNA Delivery
6.1. Functionalization of Nanocarriers Against Insects Pests
6.2. Functionalization of Nanocarriers Against Pathogens
| Nanomaterial | Feature | Pathogen/Pest | Crops | Ref. |
|---|---|---|---|---|
| Chitosan | Cellulose structure; negative charges confer greater connection to dsRNA. | Spodoptera frugiperda | Cicer arietinum | [104,106,118] |
| Poly-l-arginine (PLR-polyplex), Au nanoparticles functionalized with poly-L-arginine (PLR/Au NPs) | Exposure of PLR-dsRNA in a stable S. frugiperda Sf9 cell line (Sf9_LUC) for 72 h inhibited the luciferase gene by 58%. | S. frugiperda | In vitro | [109] |
| Chitosan nanoparticles (CNPs) | dsRNAs against Helicoverpa armigera JHAMT and ACHE target genes loaded on cationic CNPS effectively protected from nuclease degradation and insect intestinal pH. CNP-ache-dsRNA at a low dose (0.028 g/ha) in chickpea showed a reduction in damage to the pods with high yields. | Helicoverpa armigera | Cicer arietinum | [100] |
| Chitosan polyplexes | dsRNAs impregnated in chitosan NPs regulate the key HCC gene and activate the CDE pathway, a component that improves the efficiency of RNAi. | Tetranychus cinnabarinus Boisduval | In vitro | [132] |
| Chitosan nanohydrogel | Knockdown potential of formulated dsRNA targeting ECR gene was evaluated through bioassay. A higher mortality rate of ≥80% was achieved through a low concentration of formulated dsRNA. | Bemisia tabaci | In vitro | [133] |
| Cationic nucleocap (nanodetergent) | Action on the chitinase gene of the midgut and cuticle target gene. | Aphis glycines | Glycine max | [134] |
| Star polycation nanocarrier (SPc) | Cationic dendrimer, which condenses random amino acids absorbed by endocytosis. | Chilo supperssalis | Glycine max | [108] |
| Not loaded onto nanocarriers. Topical application | The application of dsRNA targeting the EcR and USP did not promote the silencing of genes involved in growth and development. On the contrary, degradation of dsRNA was found in aphid salivary secretions, as well as in hemolymph from the hemocoel of the body. There was no expression of genes related to RNA dicer-2 argonaute-2, r2d2, and sid-1. | Acyrthosiphon pisum | In vitro | [135] |
| Chitosan-based polymer | Nanocomposites were tested by injection and orally to improve the stability of dsRNA targeting a gene encoding the third-instar larval protein (OnLgl). The combination of chitosan polymers and dsRNA improved the ECB silencing. | Ostrinia nubilalis | Ex vivo | [105] |
| Cellfectin II (CFII) transfection reagent | The formulation of dsRNA with CFII protected it from degradation by endonucleases. Exposure of dsRNA-CFII in S. frugiperda cells produced a decrease in endogenous genes (iap) and also had a negative effect on growth and mortality. | S. frugiperda | In vitro | [106] |
| Alginate-chitosan | The incubation of conidia in dsRNA deformed the germ tube of M. oryzae. Foliar spraying with alginate–chitosan NCs impregnated with dsRNA suppressed disease progression in the cereal. | Magnaporthe oryzae | Brachypodium distachyon | [136] |
| Cationic poly-aspartic acid-derived polymer (CPP6) | dsRNA was administered to Pratylenchus thornei and P. zeae stages by ingestion. The silencing targeted pat-10 and unc-87 genes, which caused paralysis and uncoordinated movements in both species, although to a greater extent in P. thornei. A greater reduction in gene transcription and reproduction was observed, indicating that P. thornei may be more susceptible to RNAi. | Xanthomonas oryzae pv. oryzae (Xoo) | Arabidopsis sp. and Oryza sativa | [137] |
| Gold nanoparticles functioned with fluorescent polyethyleneimine (PEI) (PEI-AuNPs) | siRNAs with PEI-AuNPs improved loading and delivery; fluorescence allowed siRNA traceability in cells. The silenced plants showed a higher resistance to P. syringae, showing a lower amount of bacteria and ROS. | Pseudomonas syringae | Arabidopsis thaliana (Col-0) | [126] |
| Was not used | dsRNA was administered to Pratylenchus thornei and P. zeae stages by ingestion. The silencing targeted pat-10 and unc-87 genes, which caused paralysis and uncoordinated movements in both species, although to a greater extent in P. thornei. A greater reduction in gene transcription and reproduction was observed, indicating greater susceptibility to RNAi. | Pratylenchus thornei and P. zeae | Tissue disks, carrot (Daucus carota) | [124] |
| Spherical protein nanoparticles (SNPs) | SNPs with diameters of 100–200 nm, formed by thermal annealing of TMGMV coat proteins, were impregnated with dsRNA. Topical application of dsRNA-SNP to a transgenic line of C. elegans triggers RNAi after ingestion and persists for 180 h. | Caenorhabditis elegans | In vitro | [138] |
| Was not used | dsRNA targeting lethal genes (Bxy1177, Bxy1239, Bxy1104, Bxy667 and BxyAK1) were synthesized. B. xylophilus nematodes were immersed on a dsRNA solution and fed with dsRNA-modified F. babinda. The genes were deleted using both methods. Nematodes that consumed fungi expressing dsL1177 and dsAK1 showed substantial decreases over time. | Bursaphelenchus xylophilus | In vitro | [125] |
| Double laminar hydroxide (LDH) | Nano sheets with clay-like anionic cations with layered structures similar to brucite, which facilitate dsRNA adhesion through an ion exchange mechanism. | P. phaseovulgaris, BCMV (bean common mosaic virus) | Vigna unguiculata | [9,10] |
| P. phaseovulgaris BCMV (bean common mosaic virus) | Nicotiana benthamiana | [91] | ||
| KH)9 péptide Bp100 | dsRNA delivery system based on the ionic complex of dsRNA and a peptide. The results showed that the complex was absorbed by leaf cells and induced rapid and efficient regulation of exogenous and endogenous genes. | N/E | A. thaliana | [130] |
| Single-walled carbon nanotubes | For use as an RNAi delivery system in plants. The system protects RNAi from degradation, resulting in mRNA elimination within one day with 95% efficiency. | Development of a platform for the administration of dsRNA using nanotubes | mGFP5 N. benthamiana | [12] |
| Quaternary ammonium salt of chitosan (HACC) | HACC, complexed with selected siRNA, targeted genes of the CP of the TMV and the TMV RdRP1 to form siRNA-HACC. | T. tabaci, TMV (tobacco mosaic virus) | N. benthamiana | [127] |
| Chitosan quaternary ammonium salt (CQAS), amine functionalized silica nano powder (ASNP), and carbon quantum dots (CQD) | The application of ASNP in root immersion effectively silenced genes in plants and provided 14 days of protection against PVY. | P. yitiburosum (PVY). | Solanum tubersoum | [128] |
| Lipid-modified polyethyleneimine (lmPEI) | The application of the lmPEI NCs for dsRNA (250bp) silenced RNA polymerase and CP genes of GLRaV-3. The structure protected the dsRNA from degradation by ribonucleases. A single foliar application of lmPEI reduced the viral titer, and multiple applications maintained the basal viral load in the vine and berries. | Ampelovirus trivitis, GLRaV-3 (grapevine leafroll-associated virus 3) | Vitis vinifera L. | [139] |
| Quercetin nanoliposomes | The field application of nanoliposomes (Nbhsp70er-1 and Nbhsp70c-A) released quercetin and inhibited the expression of the hsp70 gene by 42%. The efficiency of the TMV control under field conditions was 38%. | T. tabaci, TMV (tobacco mosaic virus) | N. benthamiana | [129] |
| Double-laminar hydroxide (LDH) | RNAds were loaded into monodisperse, biodegradable hexagonal LDH layers, which provided high stability. Topical spraying was performed on cells, leaves, petiole adsorption, and tomato root immersion. It provided protection against crown and root rot for 60 days. | F. oxysporum f. sp. radicis-lycopersici (FORL) | Solanum lycopersicum L. | [140] |
| Functionalized carbon points (CDs) | The dual treatment with dsRNA-CDs showed a 90% protective effect in plants. The elution of CDs enhances the internalization of dsR-NA into recipient cells. First application of a nano-administration system to improve the effect of the SIGS. | Phytophthora infestans, P. sojae and P. capsici | N. benthamiana | [119] |
| Chitosan (CS), polyethyleneimine (PEI), protamine, carbon quantum dot (CQD), polyamidoamine (PAMAM) | Spraying of CS, PEI, CQD, PAMAM and CSC with dsRNA protected rice pods for up to 20 days against R. solani. The NCs improved the carrying capacity and stability of dsRNA. | Rhizoctonia solani | Rice sheaths (Oryza sativa L.) | [120] |
| Small-layered double hydroxide (sLDH) | The spraying of in vitro synthesized dsRNA molecules (BcBmp1, BcBmp3 and BcPls1) loaded in LDHs, decreased the symptoms of the disease, and showed greater protection in inoculated plants at 27 days later. | Botrytis cinerea | Lettuce (Lactuca sativa) | [122] |
| Chitosan polyplex/dsRNA nanoparticles | Chitosan can transport dsRNA, and successfully silence the RiABCG6.3 gene in R. irregularis compared to naked RNAds. | Rhizophagus irregularis | Astragalus sinicus | [121] |
| Nanoparticles formed by ε-poly-L-lysine (ε-PL) and carboxymethylchitosan (CMCS) | RsGH1 was identified as a potential siRNA target for controlling R. solani. Self-assembled nanoparticles ε-PL and CMCS can load dsRNA efficiently. ε-PL@CMCS effectively protects RNase A from dsRNA degradation and markedly improves RNAi efficiency. ds RsGH1 exhibited broad-spectrum activity against R. solani AG1-IA in rice and maize plants. | R. solani | Nicotiana tabacum (N. tabacum) | [123] |
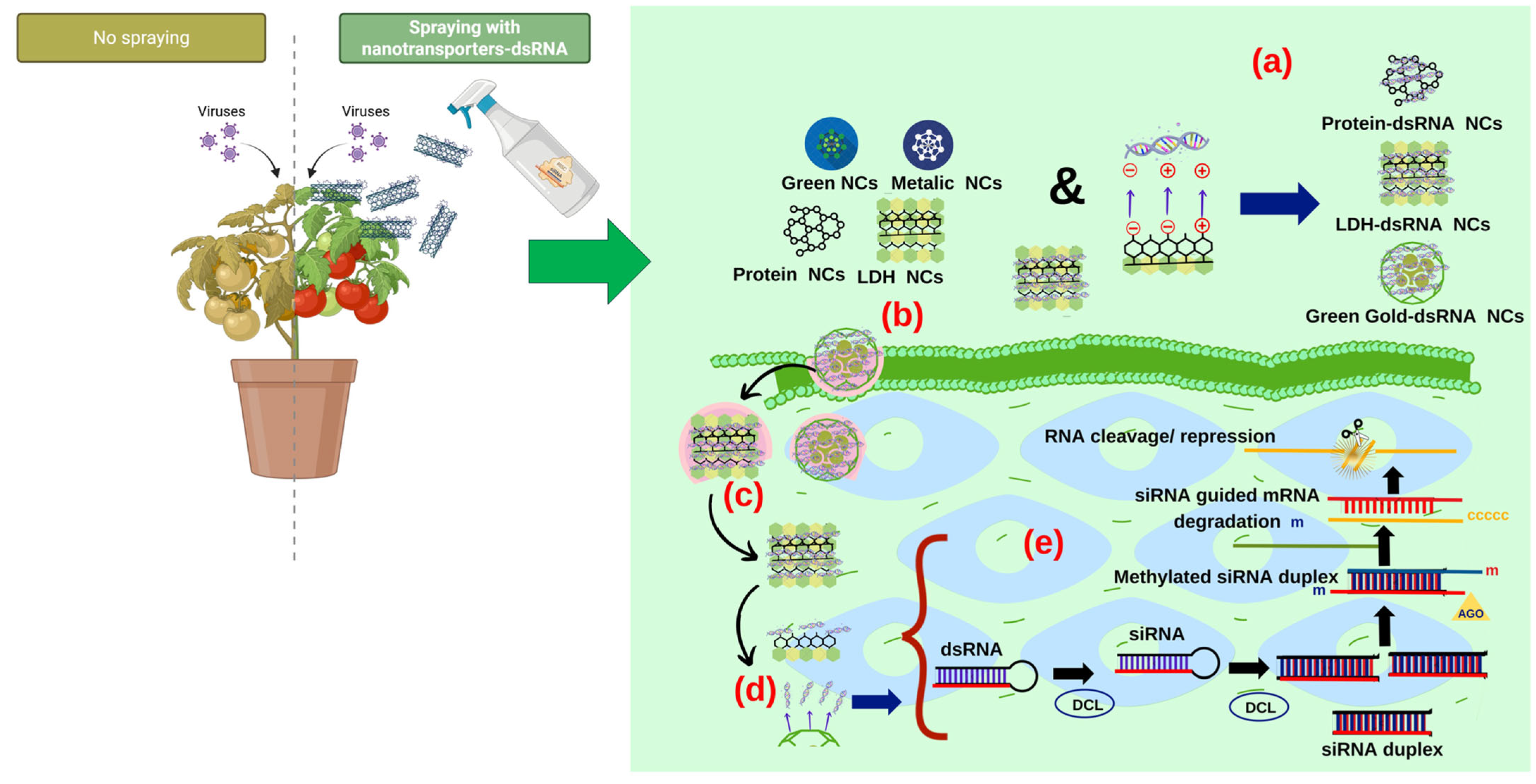
7. Entry and Mode of Action of dsRNA Nanocarriers
- (a)
- Binding or encapsulation of dsRNA: double-stranded RNA (dsRNA) is loaded into nanoparticles using techniques such as electrostatic interactions (non-covalent bonds), covalent bonds, or physical encapsulation to improve stability and protect against enzymatic degradation [73]. Covalent interactions occur when two non-metallic atoms share electrons. These bonds are strong and stable and form the basis of organic molecules. This occurs with biogenic and green synthetic nanoparticles [143]. Non-covalent interactions, on the other hand, are attractive forces between atoms that do not share electrons but are attracted by opposite charges. They are weaker than covalent interactions, but the effect of several non-covalent interactions achieves the stabilization of a dsRNA molecule [144]. Some types of non-covalent interactions involved in dsRNA binding with nanomaterials are ion–ion interactions (ion groups with opposite charges), dipole–dipole interactions (alignment of molecules with positive and negative poles), ion–dipole interactions (an ion with a polar molecule), Van der Waals forces (electrostatic density in molecules), and hydrogen bonds (interaction of H+ bound to an electronegative heteroatom) [145]. Nanoparticles, which are composed of lipids, polymers, or inorganic materials, serve as carriers to improve the bioavailability of dsRNA [146]. In most cases, positively charged NCs can self-assemble with negatively charged dsRNA through electrostatic interaction, forming dsRNA/nanocarrier complexes with hydrogen bonds and Van der Waals forces, which contribute to the self-assembly process of complexes [141]. An example of this could be chitosan, which has a large number of positively charged amino groups under acidic conditions that interact electrostatically with negatively charged dsRNA, forming dsRNA/chitosan complexes [147].
- (b)
- Cellular uptake: dsRNA-loaded nanoparticles are internalized into target cells via endocytosis, with surface modifications such as the selection of ligands that recognize specific cell receptors [148]. This improves cellular uptake, ensuring delivery to the desired intracellular locations [149]. Positively charged dsRNA complexes facilitate interaction with the membrane, allowing entry through receptor-mediated endocytosis [150]. After cellular uptake, dsRNA complexes are coated by vesicles called endosomes in the membranes. In the case of cationic polymers, this leads to endosome lysis [151].
- (c)
- Endosomal escape: once internalized, nanoparticles escape from endosomal compartments to avoid degradation; this is achieved through the proton sponge effect, membrane fusion, or endosomal disruption [145]. Efficient endosomal escape is crucial to ensure that dsRNA reaches the cytoplasm for its biological activity, gene silencing [152].
- (d)
- Release of nucleic acids or degradation of nano-complexes: after escaping, dsRNA is released through cellular environmental stimuli, e.g., pH, redox conditions, and degradation of the nanoparticle matrix (phytochemical compounds) [153]. Controlled release allows dsRNA to interact with RNA interference pathways or other cellular targets for antiviral action [154]. Subsequently, the dsRNA/nanocarrier complex delivered by late endosomes is dispersed, after which the dsRNA is released from the nanocarrier to activate RNAi and exert biological effects on viral infections [92]. The mechanism of dsRNA release has not yet been confirmed. One mechanism of dsRNA release from the nanotransporter is the slow displacement process, while the second mechanism is based on the response to intracellular stimuli such as acidic pH and cytosolic reductants [92]. For example, poly(β-aminoester) nanocarriers respond to changes in environmental pH, whereas those containing disulfide (SS) are stimulated by intracellular glutathione redox reactions, and these changes aid in the release of dsRNA/siRNA [155,156]. Release of dsRNA in the cell activates gene silencing and results in the formation of small interfering siRNAs [83]. The mechanism of gene silencing occurs as explained in Section 5.1 (Figure 2e).
8. Prospects for Nanoparticles and dsRNA Nanocarriers Against Viruses
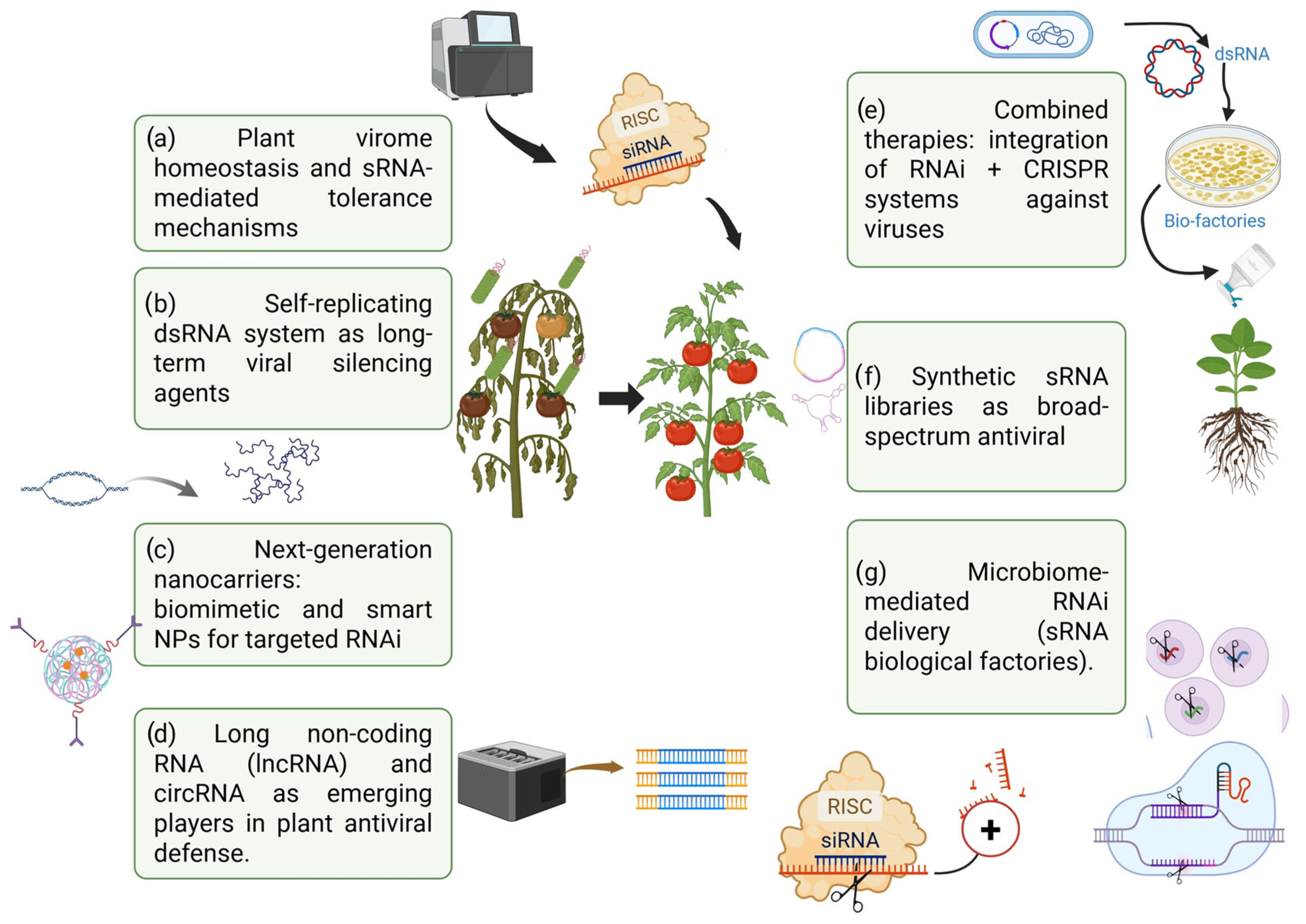
9. Future Directions for the Management of Plant Viruses
- (a)
- Plant virome homeostasis and viral tolerance mechanisms through RNA. Management should not only focus on resistance (virus elimination) but also explore natural “tolerance” mechanisms where mRNA pathways stop viral infections without causing severe damage, maintaining “virome homeostasis.” RNAi can shift from being a resistance strategy to a tool that induces tolerance against mixed viral infections.
- (b)
- Self-replicating dsRNA systems as long-term viral silencing agents. The administration of SIGS and dsRNA in plants is important, but the use of self-replicating RNA replicons (such as the minimal replicons of alphavirus) to produce dsRNA within the plant after a single treatment remains to be explored. This could prolong protection without the use of genetically modified organisms and reduce the frequency of application. Some preliminary studies suggest this idea, but very few relate it directly to virus control in plants.
- (c)
- Next-generation nanocarriers: biomimetic and intelligent nanoparticles for targeted RNAi. The idea of using biomimetic nanoparticles that mimic viruses or target ligands to recognize infected cells, release dsRNA specifically in infected tissues, and reduce off-target effects. The emergence of smart reactive delivery systems (activated by pH, ROS, and viral protease activity) could have antiviral uses in plants, although these have not yet been described.
- (d)
- RNA long non-coding RNAs (lncRNAs) and circRNAs as emerging players in plant antiviral defense. In recent years, long non-coding RNAs (lncRNAs) and circular RNAs (circRNAs) have emerged as new layers of regulation in the antiviral response of plants. Although attention has traditionally focused on small RNAs (sRNAs), recent re-search suggests that lncRNAs and circRNAs play critical roles in modulating gene expression, acting as miRNA sponges, epigenetic regulators, or mRNA stability modulators, thereby altering the outcomes of viral infection [160,161]. Stability, especially in the case of circRNAs, confers an additional advantage against viral degradation mechanisms. These findings open up new possibilities for the design of bio-technological viral resistance strategies based on the silencing, tolerance, or modulation of non-coding RNA regulatory networks [142]. Looking ahead, targeted manipulation of lncRNA and circRNA could offer complementary approaches to traditional RNAi methods, providing more durable and specific alternatives for protecting crops against viral infections [162]. This emerging area of research promises to transform the current understanding of the plant virome and the engineering of antiviral resistance.
- (e)
- Combination therapies: integration of RNAi + CRISPR systems against viruses. The combination of RNAi-based strategies and CRISPR-Cas13 systems represents a promising avenue for strengthening antiviral resistance in plants, using combination therapies that integrate RNA interference with CRISPR-Cas-based gene editing technologies [163]. RNAi has proven to be an effective tool for the targeted degradation of viral RNA using siRNA and miRNA, while the CRISPR-Cas13 system offers the ability to recognize and cut viral RNA sequences in a specific manner. The combination of both platforms would allow for a double antiviral blockade: RNAi would decrease initial viral accumulation, and CRISPR-Cas13 would effectively eliminate residual viral RNAs, reducing the likelihood of mutational escape. In addition, the use of multiplexed systems would allow multiple viruses or variants to be attacked simultaneously, a critical aspect in the face of mixed infections or complex viromes [164]. Although still in the experimental stages, this combined approach promises to revolutionize antiviral resistance strategies in agricultural crops, providing more robust, durable, and specific solutions [89].
- (f)
- Engineering synthetic sRNA libraries (artificial sRNA groups) for broad-spectrum viral protection. Is dsRNA necessary for a virus? We should aim to develop “cocktail libraries” of artificial siRNA or miRNA targeting multiple virus families. This would allow us to predict virus mutations (quasispecies) and design groups of small RNAs that “cover” the future evolution of the virus (preventing viral escape).
- (g)
- RNAi administration mediated by biological siRNA factories. Use endophytic bacteria or fungi to produce and distribute dsRNA within the plant. Emerging studies explore genetically modified symbionts to express antiviral RNA [113]. Bacteria have been engineered for effective RNAi-mediated control of mosquito larvae [165]. This highlights the innovative use of genetically modified microorganisms as biofactories for sRNA production and delivery.
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Organización Organización de las Naciones Unidas para la Alimentación y la Agricultura (FAO). FAOSTAT: Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 27 May 2025).
- Jones, R.A.C. Global Plant Virus Disease Pandemics and Epidemics. Plants 2021, 10, 233. [Google Scholar] [CrossRef]
- Rao, G.P.; Reddy, M.G. Overview of yield losses due to plant viruses. In Applied Plant Virology; Academic Press: Cambridge, MA, USA, 2020; pp. 531–562. [Google Scholar] [CrossRef]
- Vasquez-Gutierrez, U.; Frías-Treviño, G.A.; Delgado-Ortiz, J.C.; Aguirre-Uribe, L.A.; Flores-Olivas, A. Severity of Tomato brown rugose fruit virus in tomato (Solanum lycopersicum L.) from a region of Coahuila, México. Int. J. Hortic. Agric. Food Sci. 2023, 7, 1–6. [Google Scholar] [CrossRef]
- Vásquez-Gutiérrez, U.; Frías-Treviño, G.A.; López-López, H.; Delgado-Ortiz, J.C.; Aguirre-Uribe, L.A.; Flores-Olivas, A. Evaluation of the Pathogenicity of Three Isolates of Tomato brown rugose fruit virus in tomato plants (Solanum lycopersicum L.) from Coahuila, Mexico. Rev. Bio Cienc. 2024, 11, e1576. [Google Scholar] [CrossRef]
- Gutiérrez, U.V.; Treviño, G.A.F.; Ortiz, J.C.D.; Uribe, L.A.A.; Olivas, A.F.; Beache, M.B.; Castillo, F.D.H. Chlorine Dioxide: Antiviral That Reduces the Spread of ToBRFV in Tomato (Solanum lycopersicum L.) Plants. Viruses 2024, 16, 1510. [Google Scholar] [CrossRef]
- Vásquez, G.U.; Delgado-Ortiz, J.C.; Frías-Treviño, G.A.; Aguirre-Uribe, L.A.; Flores-Olivas, A. Tobamovirus fructirugosum an emerging disease: Review and current situation in Mexico. Mex. J. Phytopathol. 2025, 43, 34. [Google Scholar] [CrossRef]
- Akbar, S.; Wei, Y.; Zhang, M.-Q. RNA Interference: Promising Approach to Combat Plant Viruses. Int. J. Mol. Sci. 2022, 23, 5312. [Google Scholar] [CrossRef] [PubMed]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Li, P.; Jain, R.G.; Taochy, C.; Fletcher, S.J.; Carroll, B.J.; Lu, G.Q.; Xu, Z.P. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants 2017, 3, 16207. [Google Scholar] [CrossRef] [PubMed]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Xu, Z.P.; Carroll, B.J. Induction of virus resistance by exogenous application of double-stranded RNA. Curr. Opin. Virol. 2017, 26, 49–55. [Google Scholar] [CrossRef]
- Eid, N.A.; Ibrahim, A.M.H.; Elsharawy, A.A.; Salem, K.F.M. Nanofertilizers for plant viral disease management. In Nanofertilizers for Sustainable Agriculture; Kumar, P., Dubey, R.C., Eds.; Springer: Cham, Switzerland, 2025. [Google Scholar] [CrossRef]
- Demirer, G.S.; Zhang, H.; Goh, N.S.; Pinals, R.L.; Chang, R.; Landry, M.P. Carbon nanocarriers deliver siRNA to intact plant cells for efficient gene knockdown. Sci. Adv. 2020, 6, eaaz0495. [Google Scholar] [CrossRef]
- Rani, S.; Kumar, P.; Dahiya, P.; Dang, A.S.; Suneja, P. Biogenic synthesis of zinc nanoparticles, their applications, and toxicity prospects. Front. Microbiol. 2022, 13, 824427. [Google Scholar] [CrossRef]
- Garcia-Ruiz, H.; Szurek, B.; Van den Ackerveken, G. Stop Helping Pathogens: Engineering Plant Susceptibility Genes for Durable Resistance. Curr. Opin. Biotechnol. 2021, 70, 187–195. [Google Scholar] [CrossRef]
- Alabi, O.J.; Kumar, P.L.; Naidu, R.A. Cassava Mosaic Disease: A Curse to Food Security in Sub-Saharan Africa. APSnet Features 2011. [Google Scholar] [CrossRef]
- Eni, A.O.; Efekemo, O.P.; Onile-ere, O.A.; Pita, J.S. South West and North Central Nigeria: Assessment of Cassava Mosaic Disease and Field Status of African Cassava Mosaic Virus and East African Cassava Mosaic Virus. Ann. Appl. Biol. 2021, 178, 466–479. [Google Scholar] [CrossRef]
- Patil, B.L.; Legg, J.P.; Kanju, E.; Fauquet, C.M. Cassava Brown Streak Disease: A Threat to Food Security in Africa. J. Gen. Virol. 2015, 96, 956–968. [Google Scholar] [CrossRef] [PubMed]
- Munguti, F.M.; Nyaboga, E.N.; Kilalo, D.C.; Yegon, H.K.; Macharia, I.; Mwango’mbe, A.W. Survey of cassava brown streak disease and association of factors influencing its epidemics in smallholder cassava cropping systems of coastal Kenya. Front. Sustain. Food Syst. 2023, 6, 1015315. [Google Scholar] [CrossRef]
- Kannan, M.; Ismail, I.; Bunawan, H. Maize Dwarf Mosaic Virus: From Genome to Disease Management. Viruses 2018, 10, 492. [Google Scholar] [CrossRef] [PubMed]
- Mahuku, G.; Lockhart, B.E.; Wanjala, B.; Jones, M.W.; Kimunye, J.N.; Stewart, L.R.; Cassone, B.L.; Sevgan, S.; Nyasani, J.O.; Kusia, E.; et al. Maize lethal necrosis (MLN), an emerging threat to maize-based food security in sub-Saharan Africa. Phytopathology 2015, 105, 956–965. [Google Scholar] [CrossRef]
- Gray, S.; De Boer, S.; Lorenzen, J.; Karasev, A.; Whitworth, J.; Nolte, P.; Singh, R.; Boucher, A.; Xu, H. Potato virus Y: An evolving concern for potato crops in the United States and Canada. Plant Dis. 2010, 94, 1384–1397. [Google Scholar] [CrossRef]
- Karasev, A.V.; Gray, S.M. Continuous and emerging challenges of potato virus Y in potato. Annu. Rev. Phytopathol. 2013, 51, 571–586. [Google Scholar] [CrossRef]
- Nihad, S.A.I.; Manidas, A.C.; Hasan, K.; Hasan, M.A.I.; Honey, O.; Latif, M.A. Genetic variability, heritability, genetic advance and phylogenetic relationship between rice tungro virus resistant and susceptible genotypes revealed by morphological traits and SSR markers. Curr. Plant Biol. 2021, 25, 100194. [Google Scholar] [CrossRef]
- Pinel-Galzi, A.; Traoré, O.; Séré, Y.; Hébrard, E.; Fargette, D. The biogeography of viral emergence: Rice yellow mottle virus as a case study. Curr. Opin. Virol. 2015, 10, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Singhal, P.; Nabi, S.U.; Yadav, M.K.; Dubey, A. Mixed infection of plant viruses: Diagnostics, interactions and impact on host. J. Plant Dis. Prot. 2021, 128, 353–368. [Google Scholar] [CrossRef]
- Tatineni, S.; Hein, G.L. Plant viruses of agricultural importance: Current and future perspectives of virus disease management strategies. Phytopathology 2023, 113, 117–141. [Google Scholar] [CrossRef]
- Nancarrow, N.; Aftab, M.; Hollaway, G.; Rodoni, B.; Trębicki, P. Yield Losses Caused by Barley Yellow Dwarf Virus-PAV Infection in Wheat and Barley: A Three-Year Field Study in South-Eastern Australia. Microorganisms 2021, 9, 645. [Google Scholar] [CrossRef]
- Ashwathappa, K.V.; Krishna Reddy, M.; Venkataravanappa, V.; Madhavi Reddy, K.; Hemachandra Reddy, P.; Lakshminarayana Reddy, C.N. Genome characterization and host range studies of Cucumber mosaic virus belonging to the Subgroup IB infecting chilli in India and screening of chilli genotypes for identification of resistance. Virus Dis. 2021, 32, 535–547. [Google Scholar] [CrossRef]
- Sevik, M.A.; Arli-Sokmen, M. Estimation of the effect of Tomato spotted wilt virus (TSWV) infection on some yield components of tomato. Phytoparasitica 2012, 40, 87–93. [Google Scholar] [CrossRef]
- Walls, J.; Rajotte, E.; Rosa, C. The past, present, and future of barley yellow dwarf management. Agriculture 2019, 9, 23. [Google Scholar] [CrossRef]
- Hanssen, I.M.; Thomma, B.P.H.J. Pepino mosaic virus: A successful pathogen that rapidly evolved from emerging to endemic in tomato crops. Mol. Plant Pathol. 2010, 11, 179–189. [Google Scholar] [CrossRef]
- Caruso, A.G.; Tortorici, S.; Davino, S.; Bertacca, S.; Ragona, A.; Lo Verde, G.; Panno, S. The invasive tomato pest Tuta absoluta can transmit the emergent tomato brown rugose fruit virus. Entomol. Gen. 2024, 44, 289–296. [Google Scholar] [CrossRef]
- Ciuffo, M.; Kinoti, W.M.; Tiberini, A.; Forgia, M.; Tomassoli, L.; Constable, F.E.; Turina, M. A new blunervirus infects tomato crops in Italy and Australia. Arch. Virol. 2020, 165, 2379–2384. [Google Scholar] [CrossRef]
- Nakasu, E.Y.; Nagata, T.; Inoue-Nagata, A.K. First report of tomato fruit blotch virus infecting tomatoes in Brazil. Plant Dis. 2022, 106, 2271. [Google Scholar] [CrossRef]
- Beris, D.; Galeou, A.; Kektsidou, O.; Varveri, C. First report of Tomato fruit blotch fruit virus infecting tomato in Greece. New Dis. Rep. 2023, 48, 2. [Google Scholar] [CrossRef]
- Dombrovsky, A.; Tran-Nguyen, L.T.T.; Jones, R.A.C. Cucumber green mottle mosaic virus: Rapidly Increasing Global Distribution, Etiology, Epidemiology, and Management. Annu. Rev. Phytopathol. 2017, 55, 231–256. [Google Scholar] [CrossRef]
- Giesler, L.J.; Ghabrial, S.A.; Hunt, T.E.; Hill, J.H. Bean pod mottle virus: A threat to US soybean production. Plant Dis. 2002, 86, 1280–1289. [Google Scholar] [CrossRef]
- Hill, J.H.; Whitham, S.A. Control of virus diseases in soybeans. In Advances in Virus Research; Academic Press: Cambridge, MA, USA, 2014; Volume 90, pp. 355–390. [Google Scholar] [CrossRef]
- Werker, A.R.; Dewar, A.; Harrington, R. Modelling the incidence of virus yellows in sugar beet in the UK in relation to numbers of migrating Myzus persicae. J. Appl. Ecol. 1998, 35, 811–818. [Google Scholar] [CrossRef]
- Hossain, R.; Menzel, W.; Lachmann, C.; Varrelmann, M. New insights into virus yellows distribution in Europe and effects of beet yellows virus, beet mild yellowing virus, and beet chlorosis virus on sugar beet yield following field inoculation. Plant Pathol. 2021, 70, 584–593. [Google Scholar] [CrossRef]
- Putra, L.K.; Kristini, A.; Jati, W.W. Viral diseases of sugarcane in Indonesia: Occurrence notes, pathogenic characteristics and management strategies. In Proceedings of the 5th International Conference on Agriculture and Life Science 2021 (ICALS 2021), Jember, Indonesia, 3–4 November 2021; AIP Publishing: Melville, NY, USA, 2023; Volume 2583. No. 1. [Google Scholar] [CrossRef]
- Lopez-Gomollon, S.; Baulcombe, D.C. Roles of RNA Silencing in Viral and Non-Viral Plant Immunity and in the Crosstalk between Disease Resistance Systems. Nat. Rev. Mol. Cell Biol. 2022, 23, 645–662. [Google Scholar] [CrossRef]
- Mujtaba, M.; Wang, D.; Carvalho, L.B.; Oliveira, J.L.; Espirito Santo Pereira, A.D.; Sharif, R.; Fraceto, L.F. Nanocarrier-Mediated Delivery of miRNA, RNAi, and CRISPR-Cas for Plant Protection: Current Trends and Future Directions. ACS Agric. Sci. Technol. 2021, 1, 417–435. [Google Scholar] [CrossRef]
- Thanjavur, N.; Ankireddy, S.R.; Rayi, R. Nanotechnology Advancements in Detecting Pathogenic Human RNA Viruses. In Recent Developments in Nanomaterial-Based Sensing of Human Pathogens; Academic Press: Cambridge, MA, USA, 2024; pp. 131–152. [Google Scholar]
- Zhao, Y.; Zhou, Y.; Xu, J.; Fan, S.; Zhu, N.; Meng, Q.; Dai, S.; Yuan, X. Cross-Kingdom RNA Transport Based on Extracellular Vesicles Provides Innovative Tools for Plant Protection. Plants 2024, 13, 2712. [Google Scholar] [CrossRef]
- Cai, L.; Liu, C.; Fan, G.; Liu, C.; Sun, X. Preventing Viral Disease by ZnONPs through Directly Deactivating TMV and Activating Plant Immunity in Nicotiana benthamiana. Environ. Sci. Nano 2019, 6, 3653–3669. [Google Scholar] [CrossRef]
- Dutta, P.; Kumari, A.; Mahanta, M.; Biswas, K.K.; Dudkiewicz, A.; Thakuria, D.; Abdelrhim, A.S.; Singh, S.B.; Muthukrishnan, G.; Sabarinathan, K.G.; et al. Advances in Nanotechnology as a Potential Alternative for Plant Viral Disease Management. Front. Microbiol. 2022, 13, 935193. [Google Scholar] [CrossRef]
- Kumari, S.; Raturi, S.; Kulshrestha, S.; Chauhan, K.; Dhingra, S.; András, K.; Singh, T. A Comprehensive Review on Various Techniques Used for Synthesizing Nanoparticles. J. Mater. Res. Technol. 2023, 21, 3456–3478. [Google Scholar] [CrossRef]
- Gowtham, H.G.; Shilpa, N.; Singh, S.B.; Aiyaz, M.; Abhilash, M.R.; Nataraj, K.; Murali, M. Toxicological Effects of Nanoparticles in Plants: Mechanisms Involved at Morphological, Physiological, Biochemical and Molecular Levels. Plant Physiol. Biochem. 2024, 210, 108604. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Shariq, M.; Asif, M.; Siddiqui, M.A.; Malan, P.; Ahmad, F. Green Nanotechnology: Plant-Mediated Nanoparticle Synthesis and Application. Nanomaterials 2022, 12, 673. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Adeel, M.; Shakoor, N.; Ali, I.; Ishfaq, M.; Haider, F.U.; Deng, X. Unraveling the Roles of Modified Nanomaterials in Nano-Enabled Agriculture. Plant Physiol. Biochem. 2023, 202, 107944. [Google Scholar] [CrossRef]
- Paramo, L.A.; Feregrino-Pérez, A.A.; Guevara, R.; Mendoza, S.; Esquivel, K. Nanoparticles in Agroindustry: Applications, Toxicity, Challenges, and Trends. Nanomaterials 2020, 10, 1654. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver Nanoparticles: Various Methods of Synthesis, Size Affecting Factors and Their Potential Applications—A Review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- El Gamal, A.Y.; Tohamy, M.R.; I Abou-Zaid, M.; Atia, M.M.; El Sayed, T.; Farroh, K.Y. Silver Nanoparticles as a Viricidal Agent to Inhibit Plant-Infecting Viruses and Disrupt Their Acquisition and Transmission by Their Aphid Vector. Arch. Virol. 2022, 167, 85–97. [Google Scholar] [CrossRef] [PubMed]
- De Jesús Rivero-Montejo, S.; Rivera-Bustamante, R.F.; Saavedra-Trejo, D.L.; Vargas-Hernandez, M.; Palos-Barba, V.; Macias-Bobadilla, I.; Torres-Pacheco, I. Inhibition of Pepper Huasteco Yellow Veins Virus by Foliar Application of ZnO Nanoparticles in Capsicum annuum L. Plant Physiol. Biochem. 2023, 203, 108074. [Google Scholar] [CrossRef]
- Aseel, D.G.; Rabie, M.; El-Far, A.; Abdelkhalek, A. Antiviral Properties and Molecular Docking Studies of Eco-Friendly Biosynthesized Copper Oxide Nanoparticles against Alfalfa Mosaic Virus. BMC Plant Biol. 2024, 24, 1089. [Google Scholar] [CrossRef]
- Al-Zaban, M.I.; Alhag, S.K.; Dablool, A.S.; Ahmed, A.E.; Alghamdi, S.; Ali, B.; Al-Saeed, F.A.; Saleem, M.H.; Poczai, P. Manufactured Nano-Objects Confer Viral Protection against Cucurbit Chlorotic Yellows Virus (CCYV) Infecting Nicotiana benthamiana. Microorganisms 2022, 10, 1837. [Google Scholar] [CrossRef]
- Mascia, T.; Nigro, F.; Abdallah, A.; Ferrara, M.; De Stradis, R.; Faedda, P.; Palukaitis, P.; Gallitelli, D. Gene Silencing and Gene Expression in Phytopathogenic Fungi Using a Plant Virus Vector. Proc. Natl. Acad. Sci. USA 2014, 111, 4291–4296. [Google Scholar] [CrossRef]
- Garcia-Ruiz, H.; Carbonell, A.; Hoyer, J.S.; Fahlgren, N.; Gilbert, K.B.; Takeda, A.; Giampetruzzi, A.; Ruiz, M.T.G.; McGinn, M.G.; Lowery, N.; et al. Roles and Programming of Arabidopsis ARGONAUTE Proteins during Turnip Mosaic Virus Infection. PLoS Pathog. 2015, 11, e1004755. [Google Scholar] [CrossRef]
- Parperides, E.; El Mounadi, K.; Garcia-Ruiz, H. Induction and Suppression of Gene Silencing in Plants by Nonviral Microbes. Mol. Plant Pathol. 2023, 24, 1347–1356. [Google Scholar] [CrossRef]
- Voinnet, O. Induction and Suppression of RNA Silencing: Insights from Viral Infections. Nat. Rev. Genet. 2005, 6, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Kocken, J.M.M. Molecular Directors: Non-Coding RNA and Extracellular Vehicle in Right Ventricle Remodeling. J. Mol. Med. 2024, 102, 211–223. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, S.; Xu, X.; He, Y.; Shou, W.; Munaiz, E.D.; Yu, C.; Shen, J. Application and Expansion of Virus-Induced Gene Silencing for Functional Studies in Vegetables. Horticulturae 2023, 9, 934. [Google Scholar] [CrossRef]
- Tomari, Y.; Zamore, P.D. Perspective: Machines for RNAi. Genes Dev. 2005, 19, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Cullen, B.R. RNA Interference in Human Cells Is Restricted to the Cytoplasm. RNA 2002, 8, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, T.A.; Howell, M.D.; Cuperus, J.T.; Li, D.; Hansen, J.E.; Alexander, A.L.; Chapman, E.J.; Fahlgren, N.; Allen, E.; Carrington, J.C. Specificity of ARGONAUTE7-miR390 Interaction and Dual Functionality in TAS3 Trans-Acting siRNA Formation. Cell 2008, 133, 128–141. [Google Scholar] [CrossRef]
- Cuperus, J.T.; Carbonell, A.; Fahlgren, N.; Garcia-Ruiz, H.; Burke, R.T.; Takeda, A.; Sullivan, C.M.; Gilbert, S.D.; Montgomery, T.A.; Carrington, J.C. Unique Functionality of 22-nt miRNAs in Triggering RDR6-Dependent siRNA Biogenesis from Target Transcripts in Arabidopsis. Nat. Struct. Mol. Biol. 2010, 17, 997–1003. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Farooq, M.A.; Zhao, T.; Wang, P.; Tabusam, J.; Wang, Y.; Xuan, S.; Zhao, J.; Chen, X.; Shen, S.; et al. Virus-Induced Gene Silencing (VIGS): A Powerful Tool for Crop Improvement and Its Advancement towards Epigenetics. Int. J. Mol. Sci. 2023, 24, 5608. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Qian, Y.; Li, Z.; Zhou, X. Virus-Induced Gene Silencing and Its Application in Plant Functional Genomics. Sci. China Life Sci. 2012, 55, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yan, S.; Li, M.; Sun, L.; Dong, M.; Yin, M.; Shen, J.; Zhao, Z. NPFR Regulates the Synthesis and Metabolism of Lipids and Glycogen via AMPK: Novel Targets for Efficient Corn Borer Management. Int. J. Biol. Macromol. 2023, 247, 125816. [Google Scholar] [CrossRef]
- Venu, E.; Ramya, A.; Babu, P.L.; Srinivas, B.; Kumar, S.; Reddy, N.K.; Babu, Y.M.; Majumdar, A.; Manik, S. Exogenous dsRNA-Mediated RNAi: Mechanisms, Applications, Delivery Methods and Challenges in the Induction of Viral Disease Resistance in Plants. Viruses 2025, 17, 49. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.T.; Voinnet, O.; Baulcombe, D.C. Initiation and Maintenance of Virus-Induced Gene Silencing. Plant Cell 1998, 10, 937–946. [Google Scholar] [CrossRef][Green Version]
- Shi, J.; Liu, W.; Fu, Y.; Yin, N.; Zhang, H.; Chang, J.; Zhang, Z. “US-Detonated Nano Bombs” Facilitate Targeting Treatment of Resistant Breast Cancer. J. Control. Release 2018, 274, 9–23. [Google Scholar] [CrossRef]
- Valentine, T.; Shaw, J.; Blok, V.C.; Phillips, M.S.; Oparka, K.J.; Lacomme, C. Efficient Virus-Induced Gene Silencing in Roots Using a Modified Tobacco Rattle Virus Vector. Plant Physiol. 2004, 136, 3999–4009. [Google Scholar] [CrossRef]
- Yin, Y.; Zhao, P.; Sun, Y.; Han, T.; Wang, M.; Yan, M.; Wu, J.; Zhou, H.; Ye, J. Development and Application of Sugarcane Yellow Leaf Virus Vectors in Sugarcane. Phytopathol. Res. 2025, 7, 6. [Google Scholar] [CrossRef]
- Gaafar, Y.Z.A.; Ziebell, H. Novel Targets for Engineering Physostegia Chlorotic Mottle and Tomato Brown Rugose Fruit Virus-Resistant Tomatoes: In Silico Prediction of Tomato MicroRNA Targets. PeerJ 2020, 8, e10096. [Google Scholar] [CrossRef]
- Ameres, S.L.; Martinez, J.; Schroeder, R. Molecular Basis for Target RNA Recognition and Cleavage by Human RISC. Cell 2007, 130, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Akbar, S.; Tahir, M.; Wang, M.B.; Liu, Q. Expression Analysis of Hairpin RNA Carrying Sugarcane Mosaic Virus (SCMV) Derived Sequences and Transgenic Resistance Development in a Model Rice Plant. Biomed. Res. Int. 2017, 1, 10, 1646140. [Google Scholar] [CrossRef]
- Qi, T.; Guo, J.; Peng, H.; Liu, P.; Kang, Z.; Guo, J. Host-Induced Gene Silencing: A Powerful Strategy to Control Diseases of Wheat and Barley. Int. J. Mol. Sci. 2019, 20, 206. [Google Scholar] [CrossRef]
- Harvey, J.J.; Lewsey, M.G.; Patel, K.; Westwood, J.; Heimstädt, S.; Carr, J.P.; Baulcombe, D.C. An Antiviral Defense Role of AGO2 in Plants. PLoS ONE 2011, 6, e14639. [Google Scholar] [CrossRef]
- Fahim, M.; Millar, A.A.; Wood, C.C.; Larkin, P.J. Resistance to Wheat Streak Mosaic Virus Generated by Expression of an Artificial Polycistronic MicroRNA in Wheat. Plant Biotechnol. J. 2012, 10, 150–163. [Google Scholar] [CrossRef]
- Wassenegger, M.; Krczal, G. Nomenclature and Functions of RNA-Directed RNA Polymerases. Trends Plant Sci. 2006, 11, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Boutet, S.; Vazquez, F.; Liu, J.; Béclin, C.; Fagard, M.; Gratias, A.; Morel, J.B.; Crété, P.; Chen, X.; Vaucheret, H. Arabidopsis HEN1: A Genetic Link between Endogenous miRNA Controlling Development and siRNA Controlling Transgene Silencing and Virus Resistance. Curr. Biol. 2003, 13, 843–848. [Google Scholar] [CrossRef]
- Garcia Ruiz, M.T.; Knapp, A.N.; Garcia-Ruiz, H. Profile of Genetically Modified Plants Authorized in Mexico. GM Crops Food 2018, 9, 152–168. [Google Scholar] [CrossRef]
- Kwon, J.; Kasai, A.; Maoka, T.; Masuta, C.; Sano, T.; Nakahara, K.S. RNA Silencing-Related Genes Contribute to Tolerance of Infection with Potato Virus X and Y in a Susceptible Tomato Plant. Virol. J. 2020, 17, 149. [Google Scholar] [CrossRef]
- Cisneros, A.; Martín-García, T.; Primc, A.; Orlando, F.; Gomez, J.J.L.; Kuziuta, W.; Espadas, A.P. Transgene-Free, Virus-Based Gene Silencing in Plants by Artificial Small RNAs Derived from Minimal Precursors. Nucleic Acids Res. 2023, 51, gkad747. [Google Scholar] [CrossRef]
- Vetukuri, R.R.; Dubey, M.; Kalyandurg, P.B.; Carlsson, A.S.; Whisson, S.C.; Ortiz, R. Spray-Induced Gene Silencing: An Innovative Strategy for Plant Trait Improvement and Disease Control. Crop Breed. Appl. Biotechnol. 2021, 21, e387921S11. [Google Scholar] [CrossRef]
- Dubrovina, A.S.; Kiselev, K.V. Exogenous RNAs for Gene Regulation and Plant Resistance. Int. J. Mol. Sci. 2019, 20, 2282. [Google Scholar] [CrossRef]
- Kasi Viswanath, K.; Hamid, A.; Ateka, E.; Pappu, H.R. CRISPR/Cas, Multiomics, and RNA Interference in Virus Disease Management. Phytopathology® 2023, 113, 1661–1676. [Google Scholar] [CrossRef] [PubMed]
- Vaddoriya, H.K.; Vaddoriya, H.K.; Shelar, V.B.; Pampaniya, A.; Panwala, A. “BioClay™”: One Step toward the Sustainable and Novel Plant Protection Method. Int. J. Econ. Plants 2024, 11, 142–146. [Google Scholar] [CrossRef]
- Worrall, E.A.; Bravo-Cazar, A.; Nilon, A.T.; Fletcher, S.J.; Robinson, K.E.; Carr, J.P.; Mitter, N. Exogenous Application of RNAi-Inducing Double-Stranded RNA Inhibits Aphid-Mediated Transmission of a Plant Virus. Front. Plant Sci. 2019, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Chen, J.; Dong, M.; Shen, J.; Yan, S. Nanocarrier-Based Eco-Friendly RNA Pesticides for Sustainable Management of Plant Pathogens and Pests. Nanomaterials 2024, 14, 1874. [Google Scholar] [CrossRef]
- Chariou, P.L.; Ortega-Rivera, O.A.; Steinmetz, N.F. Nanocarriers for the Delivery of Medical, Veterinary, and Agricultural Active Ingredients. ACS Nano 2020, 14, 2678–2701. [Google Scholar] [CrossRef]
- Vogel, E.; Santos, D.; Mingels, L.; Verdonckt, T.-W.; Broeck, J.V. RNA Interference in Insects: Protecting Beneficials and Controlling Pests. Front. Physiol. 2019, 9, 1912. [Google Scholar] [CrossRef]
- Sangwan, A.; Gupta, D.; Singh, O.W.; Roy, A.; Mukherjee, S.K.; Mandal, B.; Singh, N. Size Variations of Mesoporous Silica Nanoparticle Control Uptake Efficiency and Delivery of AC2-Derived dsRNA for Protection against Tomato Leaf Curl New Delhi Virus. Plant Cell Rep. 2023, 42, 1571–1587. [Google Scholar] [CrossRef]
- Miyamoto, T.; Numata, K. Advancing Biomolecule Delivery in Plants: Harnessing Synthetic Nanocarriers to Overcome Multiscale Barriers for Cutting-Edge Plant Bioengineering. Bull. Chem. Soc. Jpn. 2023, 96, 1026–1044. [Google Scholar] [CrossRef]
- Yoon, J.-S.; Gurusamy, D.; Pali, S.R. Accumulation of dsRNA in Endosomes Contributes to Inefficient RNA Interference in the Fall Armyworm, Spodoptera frugiperda. Insect Biochem. Mol. Biol. 2017, 90, 53–60. [Google Scholar] [CrossRef]
- Fu, Y.; Li, L.; Wang, H.; Jiang, Y.; Liu, H.; Cui, X.; Wang, P.; Lü, C. Silica Nanoparticles-Mediated Stable Genetic Transformation in Nicotiana tabacum. Chem. Res. Chin. Univ. 2015, 31, 976–981. [Google Scholar] [CrossRef]
- Karimi, M.; Ghasemi, A.; Sahandi Zangabad, P.; Rahighi, R.; Moosavi Basri, S.M.; Mirshekari, H.; Amiri, M.; Shafaei Pishabad, Z.; Aslani, A.; Bozorgomid, M. Smart Micro/Nanoparticles in Stimulus-Responsive Drug/Gene Delivery Systems. Chem. Soc. Rev. 2016, 45, 1457–1501. [Google Scholar] [CrossRef]
- Kolge, H.; Kadam, K.; Ghormade, V. Chitosan Nanocarriers Mediated dsRNA Delivery in Gene Silencing for Helicoverpa armigera Biocontrol. Pestic. Biochem. Physiol. 2023, 189, 105292. [Google Scholar] [CrossRef]
- Ma, Y.F.; Liu, T.T.; Zhao, Y.Q.; Luo, J.; Feng, H.Y.; Zhou, Y.Y.; He, P. RNA Interference-Screening of Potentially Lethal Gene Targets in the White-Backed Planthopper Sogatella furcifera via a Spray-Induced and Nanocarrier-Delivered Gene Silencing System. J. Agric. Food Chem. 2024, 72, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Xu, X.; Huang, X.; Peng, J.; Ma, W.; Hull, J.J.; Hua, H.; Chen, L. Spray-Induced and Nanocarrier-Delivered Gene Silencing System Targeting Juvenile Hormone Receptor Components: Potential Application as Fertility Inhibitors for Adelphocoris suturalis Management. Pest Manag. Sci. 2024, 80, 3743–3751. [Google Scholar] [CrossRef]
- Lv, H.; Li, X.; Li, J.; Yu, C.; Zeng, Q.; Ning, G.; He, S. Overcoming Resistance in Insect Pest with a Nanoparticle-Mediated dsRNA and Insecticide Co-Delivery System. Chem. Eng. J. 2023, 475, 146239. [Google Scholar] [CrossRef]
- Wang, K.; Peng, Y.; Chen, J.; Peng, Y.; Wang, X.; Shen, Z.; Han, Z. Comparison of Efficacy of RNAi Mediated by Various Nanoparticles in the Rice Striped Stem Borer (Chilo suppressalis). Pestic. Biochem. Physiol. 2020, 165, 104467. [Google Scholar] [CrossRef]
- Cooper, A.M.; Song, H.; Yu, Z.; Biondi, M.; Bai, J.; Shi, X.; Ren, Z.; Weerasekara, S.M.; Hua, D.H.; Silver, K.; et al. Comparison of Strategies for Enhancing RNA Interference Efficiency in Ostrinia nubilalis. Pest Manag. Sci. 2021, 77, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Gurusamy, D.; Mogilicherla, K.; Shukla, J.N.; Palli, S.R. Lipids Help Double-Stranded RNA in Endosomal Escape and Improve RNA Interference in the Fall Armyworm, Spodoptera frugiperda. Arch. Insect Biochem. Physiol. 2020, 104, e21678. [Google Scholar] [CrossRef]
- Yan, S.; Qian, J.; Cai, C.; Ma, Z.; Li, J.; Yin, M.; Ren, B.; Shen, J. Spray Method Application of Transdermal dsRNA Delivery System for Efficient Gene Silencing and Pest Control on Soybean Aphid Aphis glycines. J. Pest Sci. 2019, 93, 449–459. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, P.; Abouzaid, M.; Zhou, H.; Liu, H.; Yang, P.; Lin, Y.; Hull, J.J.; Ma, W. Nanomaterial-Wrapped dsCYP15C1, a Potential RNAi-Based Strategy for Pest Control against Chilo suppressalis. Pest Manag. Sci. 2020, 76, 2483–2489. [Google Scholar] [CrossRef]
- Laisney, J.; Gurusamy, D.; Baddar, Z.E.; Palli, S.R.; Unrine, J.M. RNAi in Spodoptera frugiperda Sf9 Cells via Nanomaterial-Mediated Delivery of dsRNA: A Comparison of Poly-l-Arginine Polyplexes and Poly-l-Arginine-Functionalized Au Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 25645–25657. [Google Scholar] [CrossRef] [PubMed]
- Martin-Ortigosa, S.; Peterson, D.J.; Valenstein, J.S.; Lin, V.S.-Y.; Trewyn, B.G.; Lyznik, L.A.; Wang, K. Mesoporous Silica Nanoparticle-Mediated Intracellular Cre Protein Delivery for Maize Genome Editing via loxP Site Excision. Plant Physiol. 2014, 164, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Feng, X.; Sun, Z.; Al Omari, H.; Zhang, G.; Zhu, J.; Aldayel, M.F.; Li, C. Nanotechnology-Driven Gene Silencing: Advancements in SIGS–dsRNA Technology for Sustainable Disease Management. Chem. Biol. Technol. Agric. 2025, 12, 31. [Google Scholar] [CrossRef]
- Park, M.G.; Choi, J.Y.; Park, D.H.; Wang, M.; Kim, H.J.; Je, Y.H. Simultaneous Control of Sacbrood Virus (SBV) and Galleria mellonella Using a Bt Strain Transformed to Produce dsRNA Targeting the SBV vp1 Gene. Entomol. Gen. 2021, 41, 233–242. [Google Scholar] [CrossRef]
- Guan, R.; Chu, D.; Han, X.; Miao, X.; Li, H. Advances in the Development of Microbial Double-Stranded RNA Production Systems for Application of RNA Interference in Agricultural Pest Control. Front. Bioeng. Biotechnol. 2021, 9, 753790. [Google Scholar] [CrossRef]
- Low, Y.C.; Lawton, M.A.; Di, R. Ethylene Insensitive 2 (EIN2) as a Potential Target Gene to Enhance Fusarium Head Blight Disease Resistance. Plant Sci. 2022, 322, 111361. [Google Scholar] [CrossRef]
- Lu, Y.; Deng, X.; Zhu, Q.; Wu, D.; Zhong, J.; Wen, L.; Yu, X. The dsRNA Delivery, Targeting and Application in Pest Control. Agronomy 2023, 13, 714. [Google Scholar] [CrossRef]
- Yang, P.; Yi, S.Y.; Nian, J.N.; Yuan, Q.S.; He, W.J.; Zhang, J.B.; Liao, Y.C. Application of Double-Strand RNAs Targeting Chitin Synthase, Glucan Synthase, and Protein Kinase Reduces Fusarium graminearum Spreading in Wheat. Front. Microbiol. 2021, 12, 660976. [Google Scholar] [CrossRef] [PubMed]
- Scarpin, D.; Nerva, L.; Chitarra, W.; Moffa, L.; D’Este, F.; Vuerich, M.; Filippi, A.; Braidot, E.; Petrussa, E. Characterisation and Functionalisation of Chitosan Nanoparticles as Carriers for Double-Stranded RNA (dsRNA) Molecules towards Sustainable Crop Protection. Biosci. Rep. 2023, 43, BSR20230817. [Google Scholar] [CrossRef]
- Wytinck, N.; Manchur, C.L.; Li, V.H.; Whyard, S.; Belmonte, M.F. dsRNA Uptake in Plant Pests and Pathogens: Insights into RNAi-Based Insect and Fungal Control Technology. Plants 2020, 9, 1780. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Zhang, B.; Gao, X.; Shi, M.; Zhang, S.; Zhong, S.; Zheng, Y.; Liu, X. Functionalized Carbon Dot-Delivered RNA Nano Fungicides as Superior Tools to Control Phytophthora Pathogens through Plant RdRP1 Mediated Spray-Induced Gene Silencing. Adv. Funct. Mater. 2023, 33, 2213143. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, Q.; Lan, C.; Tang, T.; Wang, K.; Shen, J.; Niu, D. Nanoparticle Carriers Enhance RNA Stability and Uptake Efficiency and Prolong the Protection against Rhizoctonia solani. Phytopathol. Res. 2023, 5, 2. [Google Scholar] [CrossRef]
- Yan, C.; Wang, Y.; Guo, Q.; Huan, H.; Wang, S.; Fan, X.; Xie, X. Silencing Arbuscular Mycorrhizal Fungal Gene Using Chitosan Nanoparticle-Mediated dsRNA Delivery System. Bio-Protocol 2025, 15, e5326. [Google Scholar] [CrossRef] [PubMed]
- Spada, M.; Pugliesi, C.; Fambrini, M.; Palpacelli, D.; Caneo, A.; Pecchia, S. Spray-Induced Gene Silencing (SIGS): Nanocarrier-Mediated dsRNA Delivery Improves RNAi Efficiency in the Management of Lettuce Gray Mold Caused by Botrytis cinerea. Agronomy 2025, 15, 194. [Google Scholar] [CrossRef]
- Ding, X.; Li, X.; Li, Y.; Guo, H.; Cao, K.; An, M.; Zhang, C.; Wu, Y.; Liu, H.; Zhou, R. A Novel Self-Assembled Nanocarrier-Mediated dsRNA Fungicide for Broad-Spectrum Management of Rhizoctonia solani. Mater. Today Bio 2025, 34, 102099. [Google Scholar] [CrossRef]
- Tan, J.A.; Jones, M.G.; Fosu-Nyarko, J. Gene Silencing in Root Lesion Nematodes (Pratylenchus spp.) Significantly Reduces Reproduction in a Plant Host. Exp. Parasitol. 2013, 133, 166–178. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, R.; Li, Y.; Li, D.; Wang, X.; Wen, X.; Feng, Y.; Liu, Z.; Ma, S.; Zhang, X. Engineered Pine Endophytic Fungus Expressing Double-Stranded RNA Targeting Lethal Genes to Control the Plant-Parasitic Nematode Bursaphelenchus xylophilus. Phytopathology 2025, 115, 224–233. [Google Scholar] [CrossRef]
- Qi, J.; Li, Y.; Yao, X.; Li, G.; Xu, W.; Chen, L.; Xie, Z.; Gu, J.; Wu, H.; Li, Z. Rational Design of ROS Scavenging and Fluorescent Gold Nanoparticles to Deliver siRNA to Improve Plant Resistance to Pseudomonas syringae. J. Nanobiotechnol. 2024, 22, 446. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jiao, Y.; Shen, L.; Li, Y.; Mei, Y.; Yang, W.; Li, C.; Cao, Y.; Chen, F.; Li, B.; et al. Nanoparticle-dsRNA Treatment of Pollen and Root Systems of Diseased Plants Effectively Reduces the Rate of Tobacco Mosaic Virus in Contemporary Seeds. ACS Appl. Mater. Interfaces 2023, 15, 29052–29063. [Google Scholar] [CrossRef]
- Xu, X.; Yu, T.; Zhang, D.; Song, H.; Huang, K.; Wang, Y.; Shen, L.; Li, Y.; Wang, F.; Zhang, S.; et al. Evaluation of the Anti-Viral Efficacy of Three Different dsRNA Nanoparticles against Potato Virus Y Using Various Delivery Methods. Ecotoxicol. Environ. Saf. 2023, 255, 114775. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hao, K.; Yu, F.; Shen, L.; Wang, F.; Yang, J.; Su, C. Field Application of Nanoliposomes Delivered Quercetin by Inhibiting Specific hsp70 Gene Expression against Plant Virus Disease. J. Nanobiotechnol. 2022, 20, 16. [Google Scholar] [CrossRef]
- Numata, K.; Ohtani, M.; Yoshizumi, T.; Demura, T.; Kodama, Y. Local Gene Silencing in Plants via Synthetic dsRNA and Carrier Peptide. Plant Biotechnol. J. 2014, 12, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Kolge, H.; Kadam, K.; Galande, S.; Lanjekar, V.; Ghormade, V. New Frontiers in Pest Control: Chitosan Nanoparticles-Shielded dsRNA as an Effective Topical RNAi Spray for Gram Podborer Biocontrol. ACS Appl. Bio Mater. 2021, 4, 5145–5157. [Google Scholar] [CrossRef]
- Zhou, H.; Wan, F.; Jian, Y.; Guo, F.; Zhang, M.; Shi, S.; Yang, L.; Li, S.; Liu, Y.; Ding, W. Chitosan/dsRNA Polyplex Nanoparticles Advance Environmental RNA Interference Efficiency through Activating Clathrin-Dependent Endocytosis. Int. J. Biol. Macromol. 2023, 253, 127021. [Google Scholar] [CrossRef] [PubMed]
- Keppanan, R.; Karuppannasamy, A.; Nagaraja, B.C.; Thiruvengadam, V.; Kesavan, S.; Dhawane, Y.A.; Ramasamy, A. Effectiveness of Chitosan Nanohydrogel Mediated Encapsulation of EcR dsRNA against the Whitefly, Bemisia tabaci Asia-I (Gennedius) (Hemiptera: Aleyrodidae). Pestic. Biochem. Physiol. 2024, 198, 105712. [Google Scholar] [CrossRef]
- Zheng, Y.; Hu, Y.; Yan, S.; Zhou, H.; Song, D.; Yin, M. A Polymer/Detergent Formulation Improves dsRNA Penetration through the Body Wall and RNAi-Induced Mortality in the Soybean Aphid Aphis glycines. Pest Manag. Sci. 2019, 75, 1993–1999. [Google Scholar] [CrossRef]
- Christiaens, O.; Swevers, L.; Smagghe, G. dsRNA Degradation in the Pea Aphid (Acyrthosiphon pisum) Associated with Lack of Response in RNAi Feeding and Injection Assay. Peptides 2014, 53, 307–314. [Google Scholar] [CrossRef]
- Zheng, Y.; Moorlach, B.; Jakobs-Schönwandt, D.; Patel, A.; Pastacaldi, C.; Jacob, S.; Sede, A.R.; Heinlein, M.; Poranen, M.M.; Kogel, K.-H.; et al. Exogenous dsRNA Triggers Sequence-Specific RNAi and Fungal Stress Responses to Control Magnaporthe oryzae in Brachypodium distachyon. Commun. Biol. 2025, 8, 121. [Google Scholar] [CrossRef]
- Pal, G.; Ingole, K.D.; Yavvari, P.S.; Verma, P.; Kumari, A.; Chauhan, C.; Chaudhary, D.; Srivastava, A.; Bajaj, A.; Vemanna, R.S. Exogenous Application of Nanocarrier-Mediated Double-Stranded RNA Manipulates Physiological Traits and Defence Response against Bacterial Diseases. Mol. Plant Pathol. 2024, 25, e13417. [Google Scholar] [CrossRef]
- Opdensteinen, P.; Caparco, A.A.; Steinmetz, N.F. Protein-Based Spherical Nanoparticles for dsRNA Delivery to Nematodes—A Platform Technology for RNA Silencing. Mater. Today 2025, 88, 117–128. [Google Scholar] [CrossRef]
- Avital, A.; Muzika, N.S.; Persky, Z.; Bar, G.; Michaeli, Y.; Fridman, Y.; Karny, A.; Shklover, J.; Shainsky, J.; Savaldi-Goldstein, S.; et al. Foliar Delivery of siRNA Particles for Treating Viral Infections in Agricultural Grapevines. Adv. Funct. Mater. 2021, 31, 2101003. [Google Scholar] [CrossRef] [PubMed]
- Mosa, M.A.; Youssef, K. Topical Delivery of Host Induced RNAi Silencing by Layered Double Hydroxide Nanosheets: An Efficient Tool to Decipher Pathogenicity Gene Function of Fusarium Crown and Root Rot in Tomato. Physiol. Mol. Plant Pathol. 2021, 115, 101684. [Google Scholar] [CrossRef]
- Ma, Z.; Zheng, Y.; Chao, Z.; Chen, H.; Zhang, Y.; Yin, M.; Shen, J.; Yan, S. Visualization of the Process of a Nanocarrier-Mediated Gene Delivery: Stabilization, Endocytosis and Endosomal Escape of Genes for Intracellular Spreading. J. Nanobiotechnol. 2022, 20, 124. [Google Scholar] [CrossRef]
- Shidore, T.; Zuverza-Mena, N.; White, J.C.; da Silva, W. Nanoenabled Delivery of RNA Molecules for Prolonged Antiviral Protection in Crop Plants: A Review. ACS Appl. Nano Mater. 2021, 4, 12891–12904. [Google Scholar] [CrossRef]
- Whitfield, R.; Anastasaki, A.; Truong, N.P.; Cook, A.B.; Omedes-Pujol, M.; Loczenski Rose, V.; Haddleton, D.M. Efficient Binding, Protection, and Self-Release of dsRNA in Soil by Linear and Star Cationic Polymers. ACS Macro Lett. 2018, 7, 909–915. [Google Scholar] [CrossRef]
- Borowski, L.S.; Dziembowski, A.; Hejnowicz, M.S.; Stepien, P.P.; Szczesny, R.J. Human Mitochondrial RNA Decay Mediated by PNPase–hSuv3 Complex Takes Place in Distinct Foci. Nucleic Acids Res. 2013, 41, 1223–1240. [Google Scholar] [CrossRef]
- Merino, S.; Martín, C.; Kostarelos, K.; Prato, M.; Vázquez, E. Nanocomposite Hydrogels: 3D Polymer-Nanoparticle Synergies for On-Demand Drug Delivery. ACS Nano 2015, 9, 4686–4697. [Google Scholar] [CrossRef]
- Deng, L.; Albertazzi, L.; Palmans, A.R.A. Elucidating the Stability of Single-Chain Polymeric Nanoparticles in Biological Media and Living Cells. Biomacromolecules 2022, 23, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, J.; Zhu, K. Chitosan/Double-Stranded RNA Nanoparticle-Mediated RNA Interference to Silence Chitin Synthase Genes through Larval Feeding in the African Malaria Mosquito (Anopheles gambiae). Insect Mol. Biol. 2010, 19, 683–693. [Google Scholar] [CrossRef]
- Benny, O.; Fainaru, O.; Adini, A.; Cassiola, F.; Bazinet, L.; Adini, I.; Pravda, E.; Nahmias, Y.; Koirala, S.; Corfas, G.; et al. An Orally Delivered Small-Molecule Formulation with Antiangiogenic and Anticancer Activity. Nat. Biotechnol. 2008, 26, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Okuda, T.; Lu, X.Y.; Chan, H.K. Amorphous Powders for Inhalation Drug Delivery. Adv. Drug Deliv. Rev. 2016, 100, 102–115. [Google Scholar] [CrossRef]
- Zhou, J.; Shum, K.T.; Burnett, J.C.; Rossi, J.J. Nanoparticle-Based Delivery of RNAi Therapeutics: Progress and Challenges. Pharmaceuticals 2013, 6, 85–107. [Google Scholar] [CrossRef] [PubMed]
- Benjaminsen, R.V.; Mattebjerg, M.A.; Henriksen, J.R.; Moghimi, S.M.; Andresen, T.L. The Possible “Proton Sponge” Effect of Polyethylenimine (PEI) Does Not Include Change in Lysosomal pH. Mol. Ther. 2013, 21, 149–157. [Google Scholar] [CrossRef]
- Sandonas, L.M.; Sevinçli, H.; Gutierrez, R.; Cuniberti, G. First-Principle-Based Phonon Transport Properties of Nanoscale Graphene Grain Boundaries. Adv. Sci. 2018, 5, 1700365. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Li, L.; Hu, P.; Yang, Y.; Wei, W.; Deng, X.; Wang, L.; Tay, F.R.; Ma, J. Recent Advances in Stimulus-Responsive Nanocarriers for Gene Therapy. Adv. Sci. 2021, 8, 2100540. [Google Scholar] [CrossRef]
- Ke, X.; Yang, C.; Cheng, W.; Yang, Y.Y. Delivery of NF-κB shRNA Using Carbamate-Mannose Modified PEI for Eliminating Cancer Stem Cells. Nanomedicine 2018, 14, 405–414. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, G.; Zheng, H.; Chen, X. Rigid Nanoparticle-Based Delivery of Anti-Cancer siRNA: Challenges and Opportunities. Biotechnol. Adv. 2014, 32, 831–843. [Google Scholar] [CrossRef]
- Tzeng, S.Y.; Green, J.J. Subtle Changes to Polymer Structure and Degradation Mechanism Enable Highly Effective Nanoparticles for siRNA and DNA Delivery to Human Brain Cancer. Adv. Healthc. Mater. 2013, 2, 468–480. [Google Scholar] [CrossRef]
- Naimi-Shamel, N.; Pourali, P.; Dolatabadi, S. Green Synthesis of Gold Nanoparticles Using Fusarium oxysporum and Antibacterial Activity of Its Tetracycline Conjugant. J. Mycol. Med. 2019, 29, 7–13. [Google Scholar] [CrossRef]
- Soliman, M.K.Y.; Abu-Elghait, M.; Salem, S.S.; Azab, M.S. Multifunctional Properties of Silver and Gold Nanoparticles Synthesis by Fusarium pseudonygamai. Biomass Conv. Bioref. 2024, 14, 28253–28270. [Google Scholar] [CrossRef]
- Neira-Vielma, A.A.; Meléndez-Ortiz, H.I.; García-López, J.I.; Sanchez-Valdes, S.; Cruz-Hernández, M.A.; Rodríguez-González, J.G.; Ramírez-Barrón, S.N. Green Synthesis of Silver Nanoparticles Using Pecan Nut (Carya illinoinensis) Shell Extracts and Evaluation of Their Antimicrobial Activity. Antibiotics 2022, 11, 1150. [Google Scholar] [CrossRef]
- Yadav, A.; Mathan, J.; Dubey, A.K.; Singh, A. The Emerging Role of Non-Coding RNAs (ncRNAs) in Plant Growth, Development, and Stress Response Signaling. Non-Coding RNA 2024, 10, 13. [Google Scholar] [CrossRef]
- Taliansky, M.; Samarskaya, V.; Zavriev, S.K.; Fesenko, I.; Kalinina, N.O.; Love, A.J. RNA-Based Technologies for Engineering Plant Virus Resistance. Plants 2021, 10, 82. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, H.; Hu, W.; Ji, W. The Emerging Role of Long Non-Coding RNAs in Plant Defense Against Fungal Stress. Int. J. Mol. Sci. 2020, 21, 2659. [Google Scholar] [CrossRef] [PubMed]
- Khoo, Y.W.; Wang, Q.; Liu, S.; Zhan, B.; Xu, T.; Lv, W.; Liu, G.; Li, S.; Zhang, Z. Resistance of the CRISPR-Cas13a Gene-Editing System to Potato Spindle Tuber Viroid Infection in Tomato and Nicotiana benthamiana. Viruses 2024, 16, 1401. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Zhang, F.; Li, N.; Xu, K.; Wang, X.; Gao, S.; Yin, Y.; Yuan, W.; Chen, W.; Ren, Z.; et al. CRISPR/Cas: An Emerging Toolbox for Engineering Virus Resistance in Plants. Plants 2024, 13, 3313. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Cui, C.; Wang, G.; Wei, G.; Bai, L.; Li, Y.; Sun, P.; Dong, L.; Liu, Z.; Yun, J.; et al. Engineered Gut Symbiotic Bacterium-Mediated RNAi for Effective Control of Anopheles Mosquito Larvae. Microbiol. Spectr. 2023, 11, e01666-23. [Google Scholar] [CrossRef] [PubMed]

| Disease | Host | Symptoms | Common Name and Viral Species | Distribution | Transmission Type | Annual Impact | Reference |
|---|---|---|---|---|---|---|---|
| Cassava mosaic disease a | Cassava (Manihot esculenta) | Yellowing, mosaics, mottling, distortion of leaves and tubers | Begomovirus manihotis, ACMV (African cassava mosaic virus); B. manihotisafricaense, EACMV/MW (East African cassava mosaic virus); B. manihotiscameroonense, EACMCMV (East African cassava mosaic Cameroon virus. B. manihotisindianense, ICMV (Indian cassava mosaic virus); B. manihotiskenyaense, EACMKV (East African cassava mosaic Kenya virus); B. manihotismadagascarense CMMGV (cassava mosaic Madagascar virus); B. manihotismalawiense, EACMMV (East African cassava mosaic Malawi virus); B. manihotiszanzibarense, EACMZV (East African cassava mosaic Zanzibar vírus); B. stanleyi, SLCMV/LK (Sri Lankan cassava mosaic virus); B. warburgi, SACMV (South African cassava mosaic virus) | African continent and Indian subcontinent | Bemisia tabaci and infected cuttings | USD 1.9–2.7 billion | [15,16] |
| Cassava brown streak disease a | Cassava (Manihot esculenta) | Chlorosis in leaves, brown streaks on stems, and dry, hard rot in roots | Ipomovirus brunusmanihotis, CBSV (cassava brown streak vírus); I. manihotis, UCBSV (Ugandan cassava brown streak virus) | East, Central, and Southern Africa | Bemisia tabaci and possibly by aphids. | Losses are estimated at USD 750 million annually | [17,18] |
| Dwarf corn mosaic a | Corn (Zea mays L.) | Mosaic and streaks on leaves and reduction in size | Potyvirus zeananus, MDMV (maize dwarf mosaic vírus) | All over the world | Aphid species: Rhopalosiphum maidis (corn leaf aphid), Myzus persicae (green peach aphid), Brevicoryne brassicae (cabbage aphid), Aphis fabae (black bean aphid), Acyrthosiphon pisum, Aphis gossypii (Cotton aphid). | Up to 70% loss in production, producing USD 30 billion | [19] |
| Maize lethal necrosis diseases a | Corn (Zea mays L.) | Stunted growth, chlorotic streaks and spots on leaves, and necrosis (tissue death) | Machlomovirus zeae, MCMV (maize chlorotic mottle virus); P. sacchari, SCMV (sugarcane mosaic vírus; Tritimovirus tritici, WSMV (wheat streak mosaic virus); | Southeast Asia and South America, Eastern Sub-Saharan Africa | Aphid species: MCMV; mite (Aceria tosichella); SCMV: Hysteroneura setariae; WSMV: Aceria tosichella Keifer | USD 52 million in Kenya alone. Losses in yield due to co-infection of these viral species of up to 90%. | [20] |
| Potato necrosis and discoloration syndrome 1, a | Potato (Solanum tuberosum L.) | Spotting, mosaics, necrosis in leaf veins, reduced growth, and defoliation | P. duobatatae, SPV 2 (sweet potato virus 2); P. yituberosi, PVY (potato virus and serotypes C, N, NTN, O, strain C and Chinese isolate) | Potato-producing regions around the world | Aphid: Myzus persicae. | Estimated yield losses of 40 to 70% | [21,22] |
| Rice blast disease a | Arroz (Oryza sativa L.) | Stunted growth, yellow discoloration of leaves, reduced branching, and poor grain development | Waikavirus oryzae RTSV (rice tungro spherical virus); Tungrovirus oryzae) RTBV (rice tungro bacilliform virus) | Southwest and East Asia | Cicadellidae: Nephotettix virescens | USD 1 billion in annual losses | [23] |
| Yellow mottle disease of rice 1, d | Rice (Oryza sativa L.) | Yellowing, spotting, stunted growth, reduced tillering, and spikelet sterility | Sobemovirus RYMV (rice yellow mottle virus) | East, West, and Southern African countries | Beetles (Chrysomelidae) mechanically, through contact between infected plant roots | Up to 70% loss in yield | [24] |
| Wheat streak mosaic disease 1, a, d | Wheat (T. aestivum L., H. vulgare L.) | Mosaic patterns, growth retardation. | (Tritimovirus tritici, WSMV (wheat streak mosaic virus); Poacevirus tritici, TriMV (Triticum mosaic virus); Emaravirus tritici, HPWMoV (high plains wheat mosaic virus); | Eastern Europe, the Middle East, Mexico, Argentina, Australia, and Canada | Wheat mite (Aceria tosichella), seeds, and wind | Yield losses that can reach up to 100% | [25,26] |
| Wheat yellow dwarf disease 1, d | Wheat (Triticum aestivum) | Yellowing, reddening and growth retardation | Luteovirus sp., BYD (barley yellow dwarf virus-kerII, kerIII, MAV, PAS, PAV, SGV, RPV, RMV, GPV). Polerovirus sp. strains: RPS and RPV | Europe, Middle East, Asia, Asia, Oceania, North, Central and South America, North Africa, Sub-Saharan Africa | Aphids, especially Rhopalosiphum padi (PAV, CYDV), R. maidis (RMV), Sitobion avenae (MAV) and Schizaphis graminium (SGV). | Yield losses of up to 84%, with annual costs of approximately USD 220 billion | [27] |
| Cucumber mosaic disease b | Tomato (Solanum lycopersicum L.) Chile (Capsicum spp.) | Mosaics, stunted growth, leaf curling, distorted or reduced fruit. | (Cucumovirus CMV (cucumber mosaic virus) | Tropical and subtropical areas with favorable conditions for growing these species | By aphids. Around 75 species and by seeds of plant species | Reduces production yield by 10 to 40% | [28] |
| Tomato spotted wilt disease 1, a | Tomato (Solanum lycopersicum L.) Chile (Capsicum spp.) | Tanning, necrosis (tissue death), rings, or concentric spots | Orthotospovirus tomatomaculae, TSWV (tomato spotted wilt virus) | Tropical and subtropical areas of the world that are conducive to cultivation | Thrips (Frankiniella occidentalis). | Tomato crops have suffered losses ranging from 50 to 90%, amounting to an economic cost of USD 1 billion per year. | [29] |
| Tomato yellow leaf curl disease 1, a | Tomato (Solanum lycopersicum L.) Chile (Capsicum spp.) | Delayed growth, yellowing and curling of leaves, reduced fruit production | B. coheni, TYLCV (tomato yellow leaf curl virus) | Asia, the Middle East, North and South America, North Africa, and Sub-Saharan Africa | Bemisia tabaci | Yield losses of 11 to 33%, representing USD 247,000 per hectare | [30] |
| Pepino mosaic disease 1, a | Tomato (Solanum lycopersicum L.) Chile (Capsicum spp.) | Stunted growth, distorted or curled leaves with yellow or brown spots and mosaics | Potexvirus pepini, PepMV (Pepino mosaic vírus). | North and South America, Europe, the Middle East, and South Africa | Mechanical, through contact between infected and healthy plants, grafts, slight transmission by seeds | Up to 38% loss in crop production | [31] |
| Tomato rugose virus 1, b, c | Tomato (Solanum lycopersicum L.), (Capsicum spp.) and Solanum melongena. | Mosaics, yellowing, blistering on leaves, and brown roughness on fruits | Tobamovirus fructirugosum, ToBRFV (tomato brown rugose fruit virus) | Worldwide distribution in arable areas | Mechanical, due to contact between plants, machinery, and work equipment. Seeds and occasional reports of Tuta absoluta. Contaminated soil and substrate | Yield losses of 40 to 90% | [5,6,32] |
| Tomato fruit spot disease, d | Tomato (Solanum lycopersicum L.) | Irregular ripening, spots on fruit, hollows, and dark spots | Blunervirus solani, TFBV (tomato fruit blotch virus) | Italy, Europe (Greece, Portugal, Slovenia, Spain, Switzerland) South America (Brazil) and Oceania (Australia) | Red mite (Aculops lycopersici) | Not estimated | [33,34,35] |
| Mosaic disease a, 1, b | Tomato (Solanum lycopersicum L.) y (Capsicum spp.) | Mosaic patterns, mottling, and discoloration on leaves and fruits | T. viridimaculae, CGMMV (cucumber green mottle mosaic virus) | Worldwide distribution in arable areas | Seeds, contaminated soil, and mechanics through contact between plants and tools | Production losses of 5 to 40% | [36] |
| Bean pod mottling disease 1, c, d | Soybean (Glycine max L.) | Spots on bean pods, distortion, green and light spots, reduction in pod size and number | Comovirus siliquae, BPMV (bean pod mottle virus) | North America: Southern and Southeastern United States | Beetle (Cerotoma trifurcata), low transmission by seed and infectious sap | Losses of 3 to 52% in global production | [37] |
| Tobacco ring spot disease a, c, d | Soybean (Glycine max L.) | Chlorotic and necrotic rings, which often resemble an oak leaf | Nepovirus nicotianae, TRSV (tobacco ringspot virus) | Global distribution. Strong presence in North America, the US Midwest, Ontario, Canada, Australia, China, and Russia | Nematodes: Xiphinema americanum; mechanical transmission; infected seeds | 30 to 48% loss in yield | [38] |
| Viral yellowing disease 30 to 48% yield losses a, c, d | Sugar beet (Beta vulgaris) | Yellowing, discoloration, thickening, brittleness, and possible necrosis | Closterovirus flavibetae, BYV (beet yellows virus) | Europe, North and South America, Asia, and Australia | Aphid: Myzus persicae | Yield losses of 28 to 50% in production | [39,40] |
| Sugarcane mosaic disease a, c, d | Sugarcane (Saccharum officinarum) | Yellowing of central veins, dark green mosaic patterns on leaves. Leaf drying and stunted growth | P. sacchari, SMV (sugarcane mosaic virus); Poacevirus sacchari, MSSMV (sugarcane streak mosaic virus); Polerovirus SCYLV, (sugarcane yellow leaf virus) | Asia, Africa, the Middle East, and Central America | Aphid: Melanaphis sacchari, Dactynotus ambrosiae, Hysteroneura setariae, Longiunguis sacchari, Rhopalosiphum maidis and Toxoptera graminum | Yield losses of 10 to 50%, in some cases up to 80% | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasquez-Gutierrez, U.; Frias-Treviño, G.A.; Aguirre-Uribe, L.A.; Ramírez-Barrón, S.N.; Mendez-Lozano, J.; Hernández-Juárez, A.; García-Ruíz, H. Nanoparticles and Nanocarriers for Managing Plant Viral Diseases. Plants 2025, 14, 3118. https://doi.org/10.3390/plants14203118
Vasquez-Gutierrez U, Frias-Treviño GA, Aguirre-Uribe LA, Ramírez-Barrón SN, Mendez-Lozano J, Hernández-Juárez A, García-Ruíz H. Nanoparticles and Nanocarriers for Managing Plant Viral Diseases. Plants. 2025; 14(20):3118. https://doi.org/10.3390/plants14203118
Chicago/Turabian StyleVasquez-Gutierrez, Ubilfrido, Gustavo Alberto Frias-Treviño, Luis Alberto Aguirre-Uribe, Sonia Noemí Ramírez-Barrón, Jesús Mendez-Lozano, Agustín Hernández-Juárez, and Hernán García-Ruíz. 2025. "Nanoparticles and Nanocarriers for Managing Plant Viral Diseases" Plants 14, no. 20: 3118. https://doi.org/10.3390/plants14203118
APA StyleVasquez-Gutierrez, U., Frias-Treviño, G. A., Aguirre-Uribe, L. A., Ramírez-Barrón, S. N., Mendez-Lozano, J., Hernández-Juárez, A., & García-Ruíz, H. (2025). Nanoparticles and Nanocarriers for Managing Plant Viral Diseases. Plants, 14(20), 3118. https://doi.org/10.3390/plants14203118









