The Dual Role of Bacillus sp. KKU-RE-018 Isolated from Medicinal Plants in Controlling Anthracnose Disease and Enhancing the Growth of Chili Plants
Abstract
1. Introduction
2. Results
2.1. Isolation of EPB
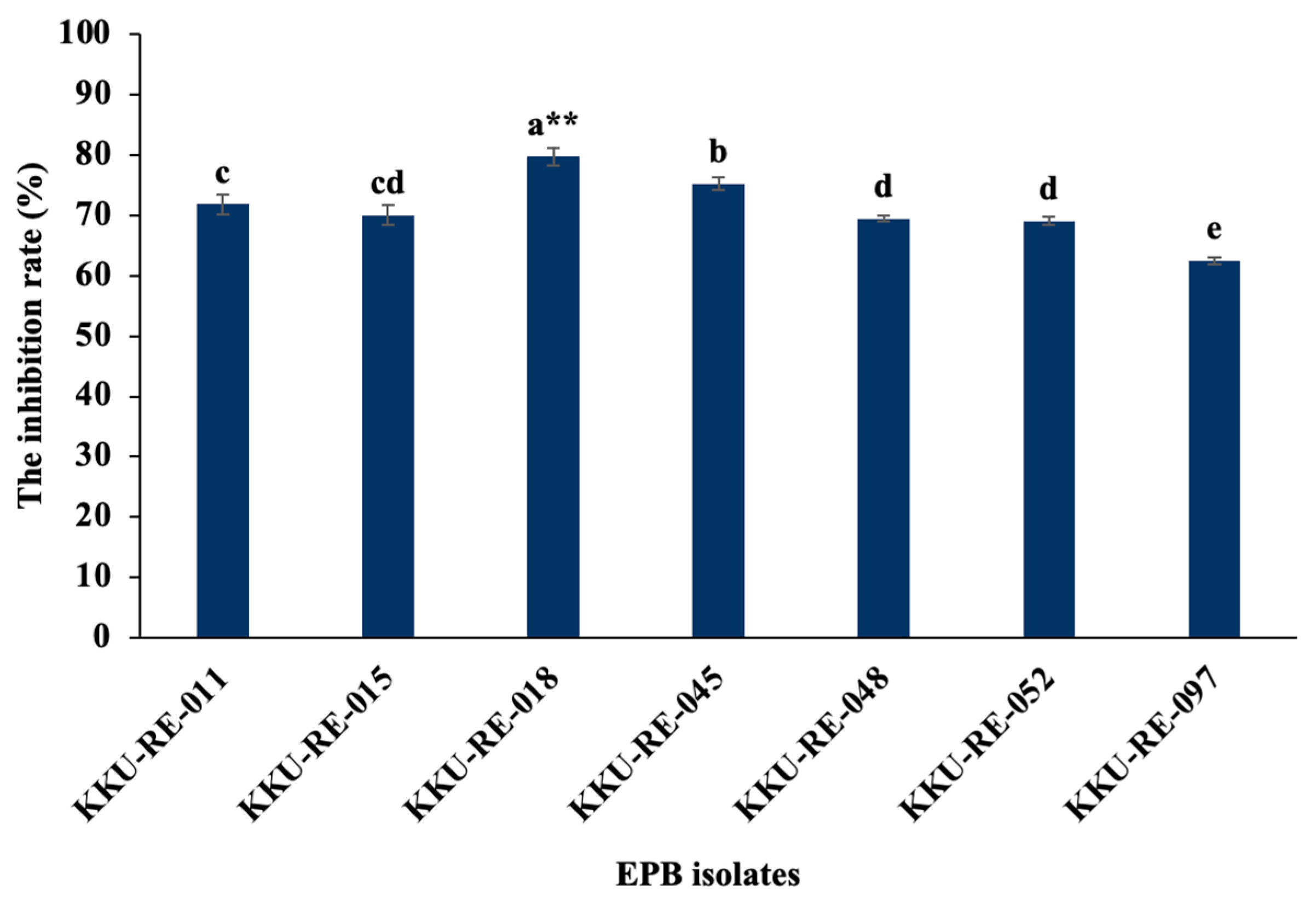
2.2. Screening EPB Strains for Antagonistic Activity In Vitro
2.3. In Vitro Screening of Endophytic Fungi for Enzyme Activity and PGP Properties
2.4. Molecular Identification of Bacterial Endophytes
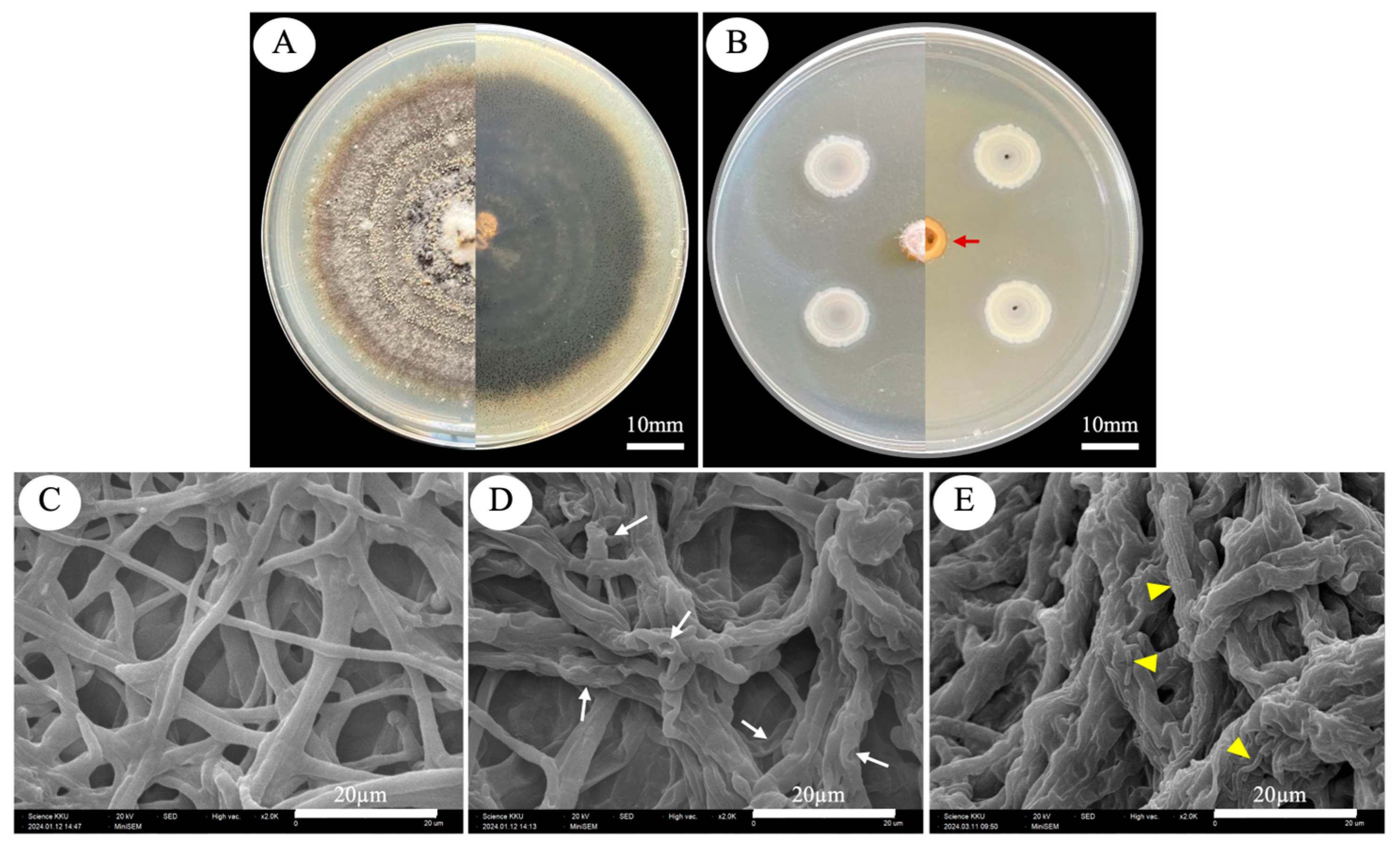
2.5. Scanning Electron Microscope Observation of C. capsici Changes in Mycelia Morphology
2.6. Biocontrol Potential of Bacillus sp. KKU-RE-018 on the Anthracnose Severity of Chili Fruit
2.7. Determination of Phenolic Compounds and Salicylic Acid in Chili Fruits
2.8. Effects of Bacillus sp. KKU-RE-018 on the Growth of Chili Plants
3. Discussion
4. Materials and Methods
4.1. Plant Sampling Site
4.2. Isolation of EPB
4.3. Sources of Pathogenic Fungi
4.4. Dual Culture Assay
4.5. Determination of Enzyme Activity Production
4.5.1. Chitinase Activity
4.5.2. β-1,3-Glucanase Activity
4.6. Determination of PGP Properties of EPB
4.6.1. Phosphate Solubilization of EPB
4.6.2. Indole-3-Acetic Acid (IAA) Production
4.6.3. Ammonia Production
4.6.4. Siderophore Production
4.6.5. Production of Hydrogen Cyanide (HCN)
4.7. Identification of EPB
4.8. Scanning Electron Microscopy Analysis of C. capsici Mycelia Under Dual Culture with EPB
4.9. Effect of EPB Against Anthracnose Disease on Chili Fruits
4.10. Determination of Phenolic Compounds and Salicylic Acid in Induced Chili Fruits
4.11. Determination of the PGP Parameter
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashwini, N.; Srividya, S. Potentiality of Bacillus subtilis as Biocontrol Agent for Management of Anthracnose Disease of Chilli Caused by Colletotrichum gloeosporioides OGC1. 3 Biotech. 2014, 4, 127–136. [Google Scholar] [CrossRef]
- Agnihotri, P.K.; Kumar, D.Y.; Singh, D.S. Bio-Control Ability of Trichoderma Isolates on Anthracnose Disease (Colletotrichum capsici) of Chilli (Capsicum annuum L.). Pharma Innov. 2023, 12, 13–17. [Google Scholar] [CrossRef]
- Yadav, M.; Dubey, M.K.; Upadhyay, R.S. Systemic Resistance in Chilli Pepper against Anthracnose (Caused by Colletotrichum truncatum) Induced by Trichoderma harzianum, Trichoderma asperellum and Paenibacillus dendritiformis. J. Fungi 2021, 7, 307. [Google Scholar] [CrossRef]
- Pongyeela, A.; Pongwichai, S. A Study of Supply Chain of Chili Spur Pepper for Food Industry in Thailand. Kasem Bundit J. 2018, 19, 88–99. [Google Scholar]
- Puripunyavanich, V.; Na Nan, T.; Suwan, N.; Orpong, P.; Picha, R.; Maikaeo, L.; Tamman, A.; Bhasabuttra, T. Breeding for Anthracnose Disease Resistance in Chili Pepper (Capsicum annum L.) Using Gamma Irradiation. Trends Sci. 2024, 21, 7709. [Google Scholar] [CrossRef]
- Islam, T.; Danishuddin; Tamanna, N.T.; Matin, M.N.; Barai, H.R.; Haque, M.A. Resistance Mechanisms of Plant Pathogenic Fungi to Fungicide, Environmental Impacts of Fungicides, and Sustainable Solutions. Plants 2024, 13, 2737. [Google Scholar] [CrossRef]
- Khan, S.S.; Verma, V.; Rasool, S. Diversity and the Role of Endophytic Bacteria: A Review. Bot. Serb. 2020, 44, 103–120. [Google Scholar] [CrossRef]
- Ma, X.; Zou, D.; Ji, A.; Jiang, C.; Zhao, Z.; Ding, X.; Han, Z.; Bao, P.; Chen, K.; Ma, A.; et al. Identification of a Novel Chitinase from Bacillus paralicheniformis: Gene Mining, Sequence Analysis, and Enzymatic Characterization. Foods 2024, 13, 1777. [Google Scholar] [CrossRef]
- Senol, M.; Nadaroglu, H.; Dikbas, N.; Kotan, R. Purification of Chitinase Enzymes from Bacillus Subtilis Bacteria TV-125, Investigation of Kinetic Properties and Antifungal Activity against Fusarium culmorum. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Dewi, R.T.K.; Mubarik, N.R.; Suhartono, M.T. Medium Optimization of β-Glucanase Production by Bacillus subtilis SAHA 32.6 Used as Biological Control of Oil Palm Pathogen. Emir. J. Food Agric. 2016, 28, 116–125. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, D.; Liang, Z.; Huang, K.; Wu, X. Antagonistic Activity of Bacillus subtilis CW14 and Its β-Glucanase against Aspergillus Ochraceus. Food Cont. 2022, 131, 108475. [Google Scholar] [CrossRef]
- Samaniego-Gámez, B.Y.; Valle-Gough, R.E.; Garruña-Hernández, R.; Reyes-Ramírez, A.; Latournerie-Moreno, L.; Tun-Suárez, J.M.; Villanueva-Alonzo, H.d.J.; Nuñez-Ramírez, F.; Diaz, L.C.; Samaniego-Gámez, S.U.; et al. Induced Systemic Resistance in the Bacillus Spp.—Capsicum Chinense Jacq.—PepGMV Interaction, Elicited by Defense-Related Gene Expression. Plants 2023, 12, 2069. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Gui, Y.; Li, Z.; Jiang, C.; Guo, J.; Niu, D. Induced Systemic Resistance for Improving Plant Immunity by Beneficial Microbes. Plants 2022, 11, 386. [Google Scholar] [CrossRef]
- Gohil, R.B.; Raval, V.H.; Panchal, R.R.; Rajput, K.N. Plant Growth-Promoting Activity of Bacillus Sp. PG-8 Isolated from Fermented Panchagavya and Its Effect on the Growth of Arachis Hypogea. Front. Agron. 2022, 4, 805454. [Google Scholar] [CrossRef]
- Hao, K.; Ullah, H.; Qin, X.; Li, H.; Li, F.; Guo, P. Effectiveness of Bacillus pumilus PDSLzg-1, an Innovative Hydrocarbon-Degrading Bacterium Conferring Antifungal and Plant Growth-Promoting Function. 3 Biotech. 2019, 9, 305. [Google Scholar] [CrossRef]
- Abdullahi, S.; Muhammed, Y.G.; Muhammad, A.; Ahmed, J.M.; Shehu, D. Isolation and Characterization of Bacillus Spp. for Plant Growth Promoting Properties. Acta Biol. Marisiensis 2021, 5, 47–58. [Google Scholar] [CrossRef]
- Ghazy, N.; El-Nahrawy, S. Siderophore Production by Bacillus subtilis MF497446 and Pseudomonas koreensis MG209738 and Their Efficacy in Controlling Cephalosporium maydis in Maize Plant. Arch. Microbiol. 2021, 203, 1195–1209. [Google Scholar] [CrossRef]
- De Souza, A.E.S.; Filla, V.A.; da Silva, J.P.M.; Barbosa Júnior, M.R.; de Oliveira-Paiva, C.A.; Coelho, A.P.; Lemos, L.B. Application of Bacillus Spp. Phosphate-Solubilizing Bacteria Improves Common Bean Production Compared to Conventional Fertilization. Plants 2023, 12, 3827. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.; Lee, Y.; Balaraju, K.; Jeon, Y. Characterization and Evaluation of Bacillus subtilis GYUN-2311 as a Biocontrol Agent against Colletotrichum Spp. on Apple and Hot Pepper in Korea. Front. Microbiol. 2023, 14, 1322641. [Google Scholar] [CrossRef]
- Yanti, Y.; Hamid, H.; Reflin; Warnita; Habazar, T. The Ability of Indigenous Bacillus Spp. Consortia to Control the Anthracnose Disease (Colletotrichum capsici) and Increase the Growth of Chili Plants. Biodiversitas 2020, 21, 179–186. [Google Scholar] [CrossRef]
- Semenzato, G.; Fani, R. Endophytic Bacteria: A Sustainable Strategy for Enhancing Medicinal Plant Cultivation and Preserving Microbial Diversity. Front. Microbiol. 2024, 15, 1477465. [Google Scholar] [CrossRef]
- Lacava, P.T.; Bogas, A.C.; Cruz, F.d.P.N. Plant Growth Promotion and Biocontrol by Endophytic and Rhizospheric Microorganisms from the Tropics: A Review and Perspectives. Front. Sustain. Food Syst. 2022, 6, 796113. [Google Scholar] [CrossRef]
- Ramírez, C.; Cardozo, M.; López Gastón, M.; Galdeano, E.; Collavino, M.M. Plant Growth Promoting Activities of Endophytic Bacteria from Melia azedarach (Meliaceae) and Their Influence on Plant Growth under Gnotobiotic Conditions. Heliyon 2024, 10, e35814. [Google Scholar] [CrossRef] [PubMed]
- Mohammadizadeh-Heydari, N.; Tohidfar, M.; Maleki Zanjani, B.; Mohsenpour, M.; Ghanbari Moheb Seraj, R.; Esmaeilzadeh-Salestani, K. Co-Overexpression of Chitinase and β-1,3-Glucanase Significantly Enhanced the Resistance of Iranian Wheat Cultivars to Fusarium. BMC Biotechnol. 2024, 24, 35. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; He, K.; Song, X.; Yu, J.; Guo, Z.; Xu, M. Isolation and Identification of Bacillus subtilis LY-1 and Its Antifungal and Growth-Promoting Effects. Plants 2023, 12, 4158. [Google Scholar] [CrossRef]
- Tram, T.T.N.; Quang, H.T.; Vu, N.Q.H.; Nguyen, P.T.T.; Thi, T.N.M.; Phuong, T.T.B.; Thi, P.T.D. Isolation of Bacteria Displaying Potent Antagonistic Activity against Fungi Causes Anthracnose Disease in Chili. Biodiversitas 2023, 24, 4919–4926. [Google Scholar] [CrossRef]
- Mei, C.; Chretien, R.L.; Amaradasa, B.S.; He, Y.; Turner, A.; Lowman, S. Characterization of Phosphate Solubilizing Bacterial Endophytes and Plant Growth Promotion in Vitro and in Greenhouse. Microorganisms 2021, 9, 1935. [Google Scholar] [CrossRef]
- Kuang, W.; Chen, W.; Lei, C.; Dai, Y.; Tian, X.; Tang, S.; Qian, Q.; Zhang, C.; Fu, L.; Zhou, G.; et al. Diversity of Endophytic Bacterial Community in Rice Roots and Their Roles in Phosphate Solubilization and Plant Growth. Rhizosphere 2024, 30, 100877. [Google Scholar] [CrossRef]
- Iqbal, Z.; Ahmad, M.; Raza, M.A.; Hilger, T.; Rasche, F. Phosphate-Solubilizing Bacillus Sp. Modulate Soil Exoenzyme Activities and Improve Wheat Growth. Microb. Ecol. 2024, 87, 31. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Tian, B.; Xiong, J.; Lin, G.; Cheng, L.; Zhang, T.; Lin, B.; Ke, Z.; Li, X. Exploring IAA Biosynthesis and Plant Growth Promotion Mechanism for Tomato Root Endophytes with Incomplete IAA Synthesis Pathways. Chem. Biol. Technol. Agric. 2024, 11, 187. [Google Scholar] [CrossRef]
- Etesami, H.; Glick, B.R. Bacterial Indole-3-Acetic Acid: A Key Regulator for Plant Growth, Plant-Microbe Interactions, and Agricultural Adaptive Resilience. Microbiol. Res. 2024, 281, 127602. [Google Scholar] [CrossRef]
- Suliasih; Widawati, S. Isolation of Indole Acetic Acid (IAA) producing Bacillus siamensis from peat and optimization of the culture conditions for maximum IAA production. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Bogor, Indonesia, 28–29 October 2019; IOP Publishing: Bristol, UK, 2020; Volume 572, p. 012025. [Google Scholar] [CrossRef]
- Jamily, A.S.; Koyama, Y.; Win, T.A.; Toyota, K.; Chikamatsu, S.; Shirai, T.; Uesugi, T.; Murakami, H.; Ishida, T.; Yasuhara, T. Effects of Inoculation with a Commercial Microbial Inoculant Bacillus subtilis C-3102 Mixture on Rice and Barley Growth and Its Possible Mechanism in the Plant Growth Stimulatory Effect. J. Plant Prot. Res. 2019, 59, 193–205. [Google Scholar] [CrossRef]
- Hassan, S.E.D. Plant Growth-Promoting Activities for Bacterial and Fungal Endophytes Isolated from Medicinal Plant of Teucrium polium L. J. Adv. Res. 2017, 8, 687–695. [Google Scholar] [CrossRef]
- Yadav, A.N.; Kumar, V.; Dhaliwal, H.S.; Prasad, R.; Saxena, A.K. Microbiome in Crops: Diversity, Distribution, and Potential Role in Crop Improvement. In Crop Improvement Through Microbial Biotechnology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 305–332. [Google Scholar]
- Baard, V.; Bakare, O.O.; Daniel, A.I.; Nkomo, M.; Gokul, A.; Keyster, M.; Klein, A. Biocontrol Potential of Bacillus subtilis and Bacillus tequilensis against Four Fusarium Species. Pathogens 2023, 12, 254. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, X.; Jiang, H.; Yao, Q.; Liang, S.; Chen, W.; Shi, G.; Tian, B.; Hegazy, A.; Ding, S. Mechanism of a Novel Bacillus subtilis JNF2 in Suppressing Fusarium oxysporum f. Sp. Cucumerium and Enhancing Cucumber Growth. Front. Microbiol. 2024, 15, 1459906. [Google Scholar] [CrossRef]
- Yi, Y.; Luan, P.; Wang, K.; Li, G.; Yin, Y.; Yang, Y.; Zhang, Q.; Liu, Y. Antifungal Activity and Plant Growth-Promoting Properties of Bacillus mojovensis B1302 against Rhizoctonia cerealis. Microorganisms 2022, 10, 1682. [Google Scholar] [CrossRef] [PubMed]
- Le Thanh, T.; Cong, V.K.; Sangpueak, R.; Numparditsub, P.; Papathoti, N.K.; Machikowa, T.; Buensanteai, K. Efficacy of Bacillus subtilis for Controlling Anthracnose in Chilli. Agric. Nat. Resour. 2023, 57, 223–232. [Google Scholar] [CrossRef]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic Acids Act as Signaling Molecules in Plant-Microbe Symbioses. Plant Signal Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef]
- Xu, L.; Wang, X. A Comprehensive Review of Phenolic Compounds in Horticultural Plants. Int. J. Mol. Sci. 2025, 26, 5767. [Google Scholar] [CrossRef]
- Rufián, J.S.; Rueda-Blanco, J.; Beuzón, C.R.; Ruiz-Albert, J. Protocol: An Improved Method to Quantify Activation of Systemic Acquired Resistance (SAR). Plant Methods 2019, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Gowtham, H.G.; Murali, M.; Singh, S.B.; Lakshmeesha, T.R.; Narasimha Murthy, K.; Amruthesh, K.N.; Niranjana, S.R. Plant Growth Promoting Rhizobacteria- Bacillus Amyloliquefaciens Improves Plant Growth and Induces Resistance in Chilli against Anthracnose Disease. Biol. Control 2018, 126, 209–217. [Google Scholar] [CrossRef]
- Jabborova, D.; Enakiev, Y.; Sulaymanov, K.; Kadirova, D.; Ali, A.; Annapurna, K. Plant Growth Promoting Bacteria Bacillus subtilis Promote Growth and Physiological Parameters of Zingiber officinale Roscoe. Plant Sci. Today 2021, 8, 66–71. [Google Scholar] [CrossRef]
- Nxumalo, C.I.; Ngidi, L.S.; Shandu, J.S.E.; Maliehe, T.S. Isolation of Endophytic Bacteria from the Leaves of Anredera cordifolia CIX1 for Metabolites and Their Biological Activities. BMC Complement. Med. Ther. 2020, 20, 300. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Hoes, J.A. Penetration and Infection of Sclerotinia sclerotiorum by Coniothyrium minitans. Can. J. Bot. 1976, 54, 5–6. [Google Scholar] [CrossRef]
- Vincent, J.M. Distortion of Fungal Hyphae in the Presence of Certain Inhibitors. Nature 1947, 159, 850. [Google Scholar] [CrossRef] [PubMed]
- Khairah, M.; Mubarik, N.R.; Manaf, L.A. Bacterial Selection and Characterization of Chitinase Enzyme from Bacteria Controlling Fusarium proliferatum. Biodiversitas 2023, 24, 1926–1933. [Google Scholar] [CrossRef]
- Lorenz Miller, G. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. J. Anal. Chem. 1959, 531, 426–428. [Google Scholar] [CrossRef]
- Suryadi, Y.; Susilowati, D.; Lestari, P.; Priyatno, T.; Samudra, I.; Hikmawati, N.; Mubarik, N. Characterization of bacterial isolates producing chitinase and glucanase for biocontrol of plant fungal pathogens. J. Agric. Technol. 2014, 10, 983–999. [Google Scholar]
- Cheng, S.; Jiang, J.W.; Tan, L.T.; Deng, J.X.; Liang, P.Y.; Su, H.; Sun, Z.X.; Zhou, Y. Plant Growth-Promoting Ability of Mycorrhizal Fusarium Strain KB-3 Enhanced by Its IAA Producing Endohyphal Bacterium, Klebsiella aerogenes. Front. Microbiol. 2022, 13, 855399. [Google Scholar] [CrossRef]
- Fouda, A.; Eid, A.M.; Elsaied, A.; El-Belely, E.F.; Barghoth, M.G.; Azab, E.; Gobouri, A.A.; Hassan, S.E.D. Plant Growth-Promoting Endophytic Bacterial Community Inhabiting the Leaves of Pulicaria incisa (LAM.) DC Inherent to Arid Regions. Plants 2021, 10, 76. [Google Scholar] [CrossRef]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Dubey, A.; Saiyam, D.; Kumar, A.; Hashem, A.; Abduallah, E.F.; Khan, M.L. Bacterial Root Endophytes: Characterization of Their Competence and Plant Growth Promotion in Soybean (Glycine max (L.) Merr.) under Drought Stress. Int. J. Environ. Res. Public. Health 2021, 18, 931. [Google Scholar] [CrossRef]
- Salo, E.N.; Novero, A. Identification and Characterisation of Endophytic Bacteria from Coconut (Cocos nucifera) Tissue Culture. Trop. Life Sci. Res. 2020, 31, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Liotti, R.G.; da Silva Figueiredo, M.I.; Soares, M.A. Streptomyces griseocarneus R132 Controls Phytopathogens and Promotes Growth of Pepper (Capsicum annuum). Biol. Control 2019, 138, 104065. [Google Scholar] [CrossRef]
- Thumanu, K.; Wongchalee, D.; Sompong, M.; Phansak, P.; Le Thanh, T.; Namanusart, W.; Vechklang, K.; Kaewnum, S.; Buensanteai, N. Synchrotron-Based Ftir Microspectroscopy of Chili Resistance Induced by Bacillus subtilis Strain D604 against Anthracnose Disease. J. Plant Interact. 2017, 12, 255–263. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; De Mello, J.C.P. Application and Analysis of the Folin Ciocalteu Method for the Determination of the Total Phenolic Content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef]
- Warrier, R.R.; Paul, M.; Vineetha, M.V. Estimation of salicylic acid in Eucalyptus leaves using spectrophotometric methods. Genet. Plant Physiol. 2013, 3, 90–97. [Google Scholar]
- Cabra Cendales, T.; Rodríguez González, C.A.; Villota Cuásquer, C.P.; Tapasco Alzate, O.A.; Hernández Rodríguez, A. Bacillus effect on the germination and growth of tomato seedlings (Solanum lycopersicum L.). Acta Biolo Colomb. 2017, 22, 37–44. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]




| EPB Isolates | Enzyme Activity (U·mL−1) | |
|---|---|---|
| Chitinase | β-1,3-Glucanase | |
| KKU-RE-011 | 1.05 ± 0.07 e | 1.09 ± 0.01 c |
| KKU-RE-015 | 1.59 ± 0.02 c | 1.10 ± 0.03 c |
| KKU-RE-018 | 1.98 ± 0.05 a ** | 1.78 ± 0.07 a ** |
| KKU-RE-045 | 1.47 ± 0.03 d | 0.98 ± 0.00 d |
| KKU-RE-048 | 1.87 ± 0.06 b | 0.92 ± 0.01 e |
| KKU-RE-052 | 0.42 ± 0.01 f | 1.18 ± 0.01 b |
| KKU-RE-097 | 0.22 ± 0.01 g | 0.60 ± 0.01 f |
| EPB Isolates | Phosphate Solubilization (µg·mL−1) | IAA Production (µg·mL−1) | NH3 Production (µg·mL−1) | Siderophore Production (µg·mL−1) | HCN Production (µg·mL−1) | |
|---|---|---|---|---|---|---|
| +L-tryp | −L-tryp | |||||
| KKU-RE-011 | 714.81 ± 12.90 f | 5.21 ± 0.07 d | 9.93 ± 1.69 bc | 37.51 ± 0.98 b | + | − |
| KKU-RE-015 | 1067.13 ± 9.86 a ** | 4.35 ± 0.35 d | 11.81 ± 0.04 bc | 33.02 ± 0.51 cd | + | − |
| KKU-RE-018 | 918.52 ± 14.85 b | 7.36 ± 0.55 c | 15.10 ± 1.53 a ** | 31.59 ± 1.00 d | + | − |
| KKU-RE-045 | 766.67 ± 13.39 d | 8.52 ± 1.89 c | 9.21 ± 2.26 c | 42.15 ± 1.23 a ** | + | − |
| KKU-RE-048 | 776.85 ± 10.52 d | 10.69 ± 0.60 b | 10.16 ± 2.41 bc | 38.70 ± 0.57 b | − | − |
| KKU-RE-052 | 861.11 ± 9.10 c | 3.82 ± 0.66 d | 9.10 ± 1.11 c | 33.54 ± 0.52 c | + | − |
| KKU-RE-097 | 743.52 ± 14.72 e | 19.35 ± 1.10 a ** | 13.13 ± 1.72 ab | 25.33 ± 1.00 e | − | − |
| Treatments | Phenolic (GEA·g−1 FW) | Salicylic Acid (µg·g−1 FW) |
|---|---|---|
| Pathogen-inoculated (control) | 16.56 | 178.11 |
| Pathogen-inoculated + Bacillus sp. KKU-RE-018 | 21.83 ** | 218.00 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gateta, T.; Seemakram, W.; Suebrasri, T.; Chantawong, S.; Klinsukon, C.; Ekprasert, J.; Boonlue, S. The Dual Role of Bacillus sp. KKU-RE-018 Isolated from Medicinal Plants in Controlling Anthracnose Disease and Enhancing the Growth of Chili Plants. Plants 2025, 14, 3010. https://doi.org/10.3390/plants14193010
Gateta T, Seemakram W, Suebrasri T, Chantawong S, Klinsukon C, Ekprasert J, Boonlue S. The Dual Role of Bacillus sp. KKU-RE-018 Isolated from Medicinal Plants in Controlling Anthracnose Disease and Enhancing the Growth of Chili Plants. Plants. 2025; 14(19):3010. https://doi.org/10.3390/plants14193010
Chicago/Turabian StyleGateta, Thanawan, Wasan Seemakram, Thanapat Suebrasri, Saranya Chantawong, Chaiya Klinsukon, Jindarat Ekprasert, and Sophon Boonlue. 2025. "The Dual Role of Bacillus sp. KKU-RE-018 Isolated from Medicinal Plants in Controlling Anthracnose Disease and Enhancing the Growth of Chili Plants" Plants 14, no. 19: 3010. https://doi.org/10.3390/plants14193010
APA StyleGateta, T., Seemakram, W., Suebrasri, T., Chantawong, S., Klinsukon, C., Ekprasert, J., & Boonlue, S. (2025). The Dual Role of Bacillus sp. KKU-RE-018 Isolated from Medicinal Plants in Controlling Anthracnose Disease and Enhancing the Growth of Chili Plants. Plants, 14(19), 3010. https://doi.org/10.3390/plants14193010






