Population-Specific Salinity Tolerance in the Extremophile Colobanthus quitensis: Evidence of Adaptive Plasticity
Abstract
1. Introduction
2. Results
2.1. Morpho-Physiological Responses to Salinity Stress
2.2. Physiological Responses to Salinity Stress
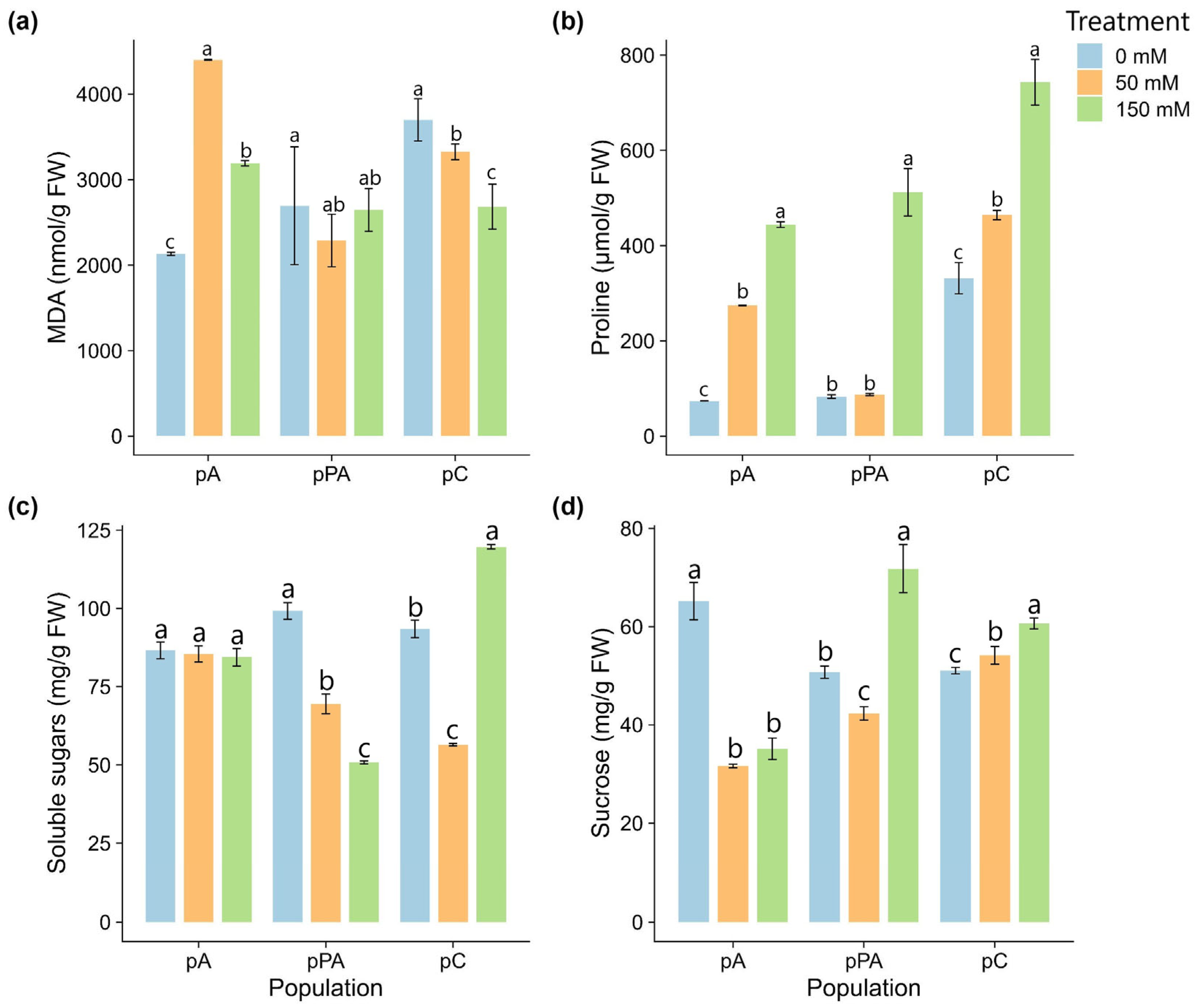
2.3. Osmoprotective and Oxidative Stress Responses
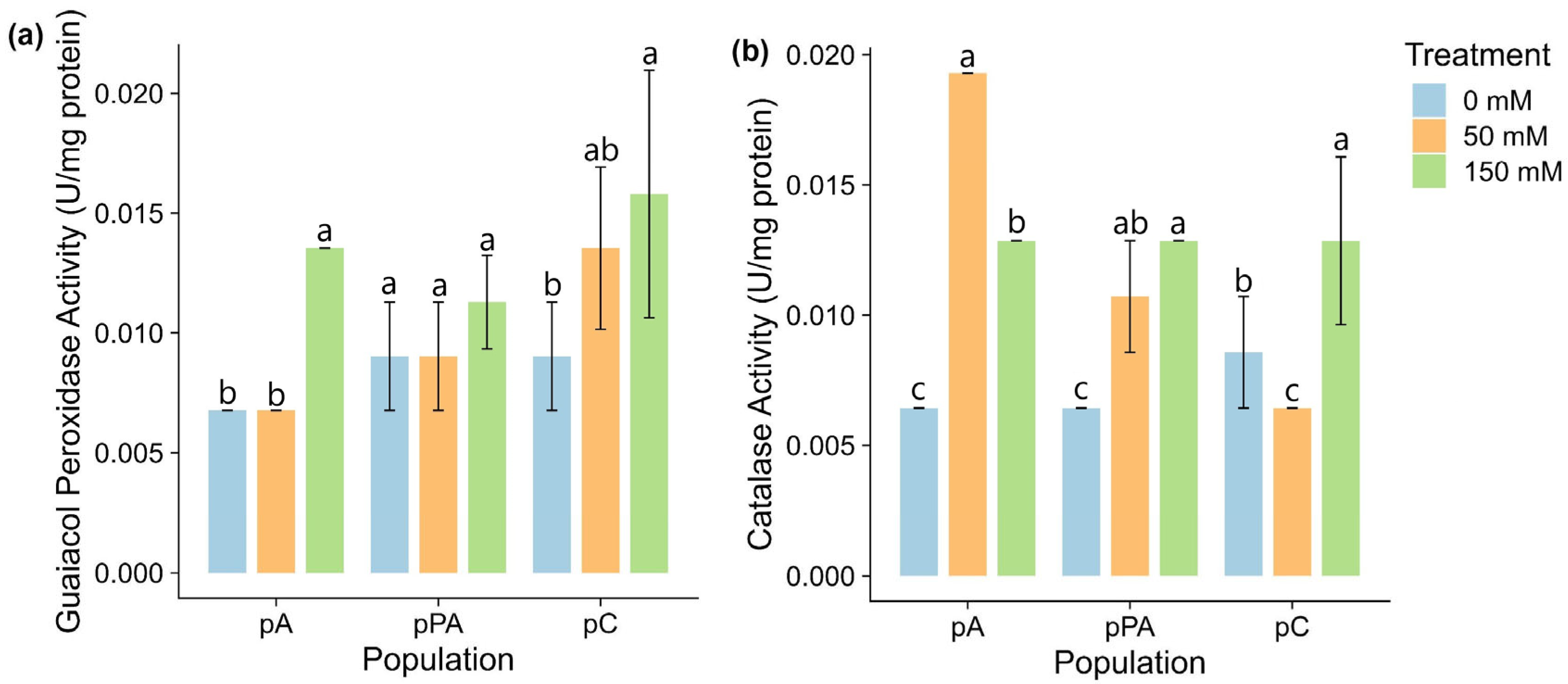
2.4. Antioxidant Enzyme Activity
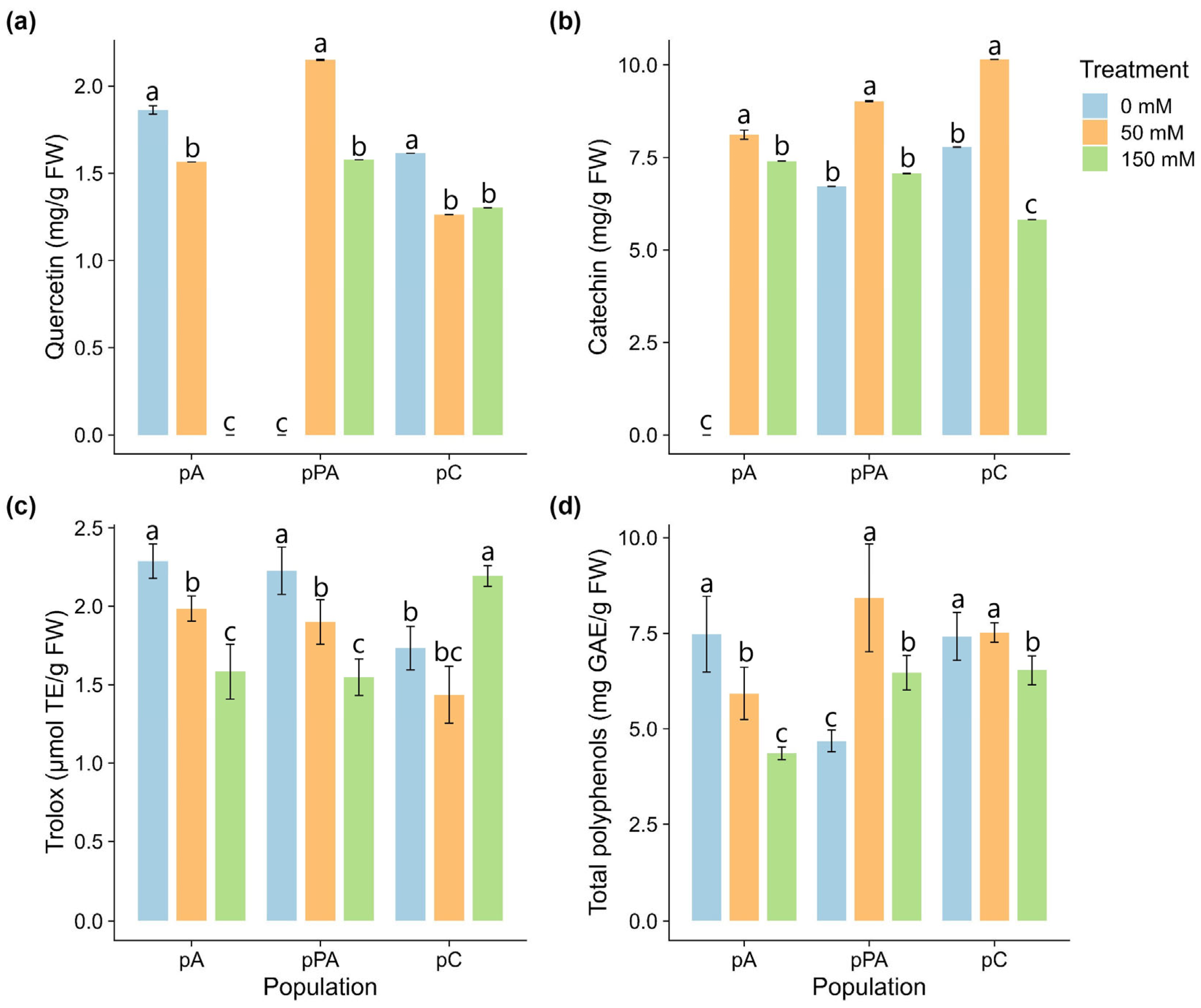
2.5. Non-Enzymatic Antioxidant Responses
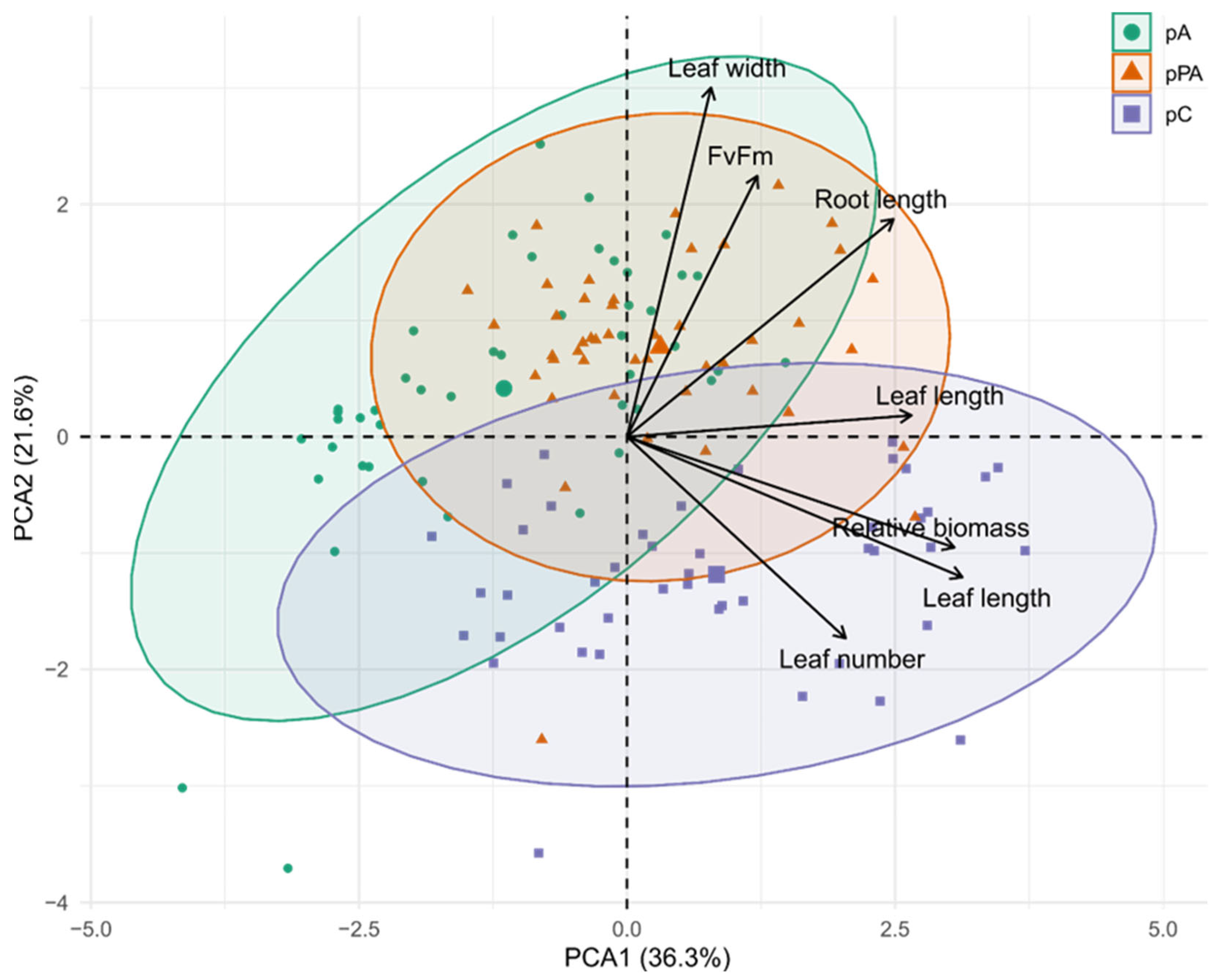
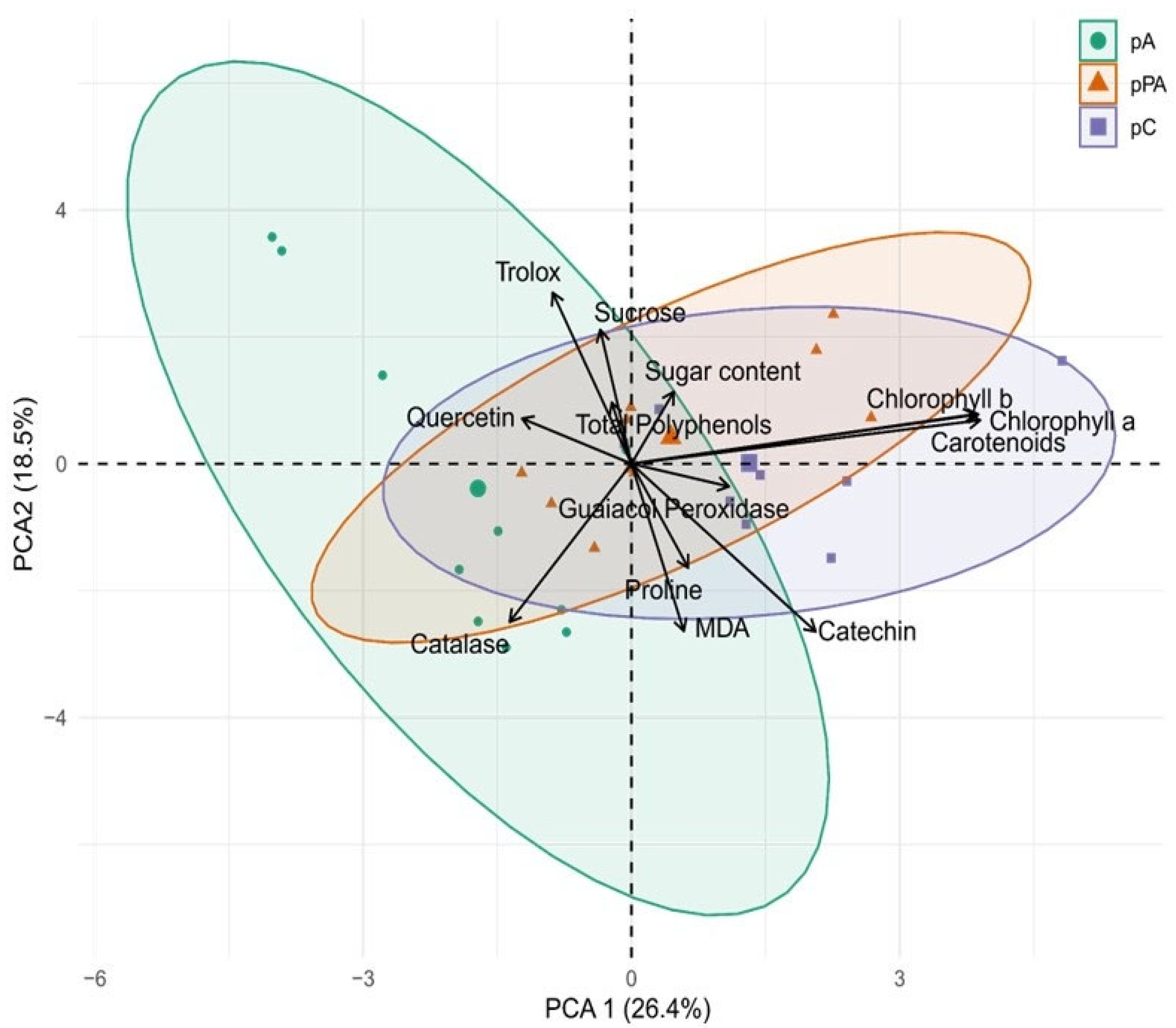
2.6. Integrative Multivariate Insights into Tolerance Mechanisms
3. Discussion
3.1. Morphological and Photosynthetic Adjustments
3.2. Osmotic Regulation and Membrane Protection
3.3. Antioxidant Defense: Enzymatic vs. Non-Enzymatic Strategies
3.4. Ecological and Functional Implications
3.5. Integrated Tolerance Profiles Among C. quitensis Populations
4. Materials and Methods
4.1. Experimental Design
4.1.1. Plant Material and Growth Conditions
4.1.2. Saline Treatments
4.2. Morpho-Physiological Variables
4.3. Physiological Variables
4.4. Biochemical Variables
4.4.1. Lipid Peroxidation
4.4.2. Proline Content
4.4.3. Total Soluble Sugar Content
4.4.4. Sucrose Content
4.5. Enzyme Activity Analysis
4.5.1. Protein Quantification
4.5.2. Guaiacol Peroxidase (GPx) Activity
4.5.3. Catalase (CAT) Activity
4.6. Antioxidant Activity and Phenolic Compounds
4.6.1. Total Polyphenols Content
4.6.2. Phenolic Compound Profile
4.6.3. Antioxidant Capacity Determination (FRAP Assay)
4.7. Statistical Analysis
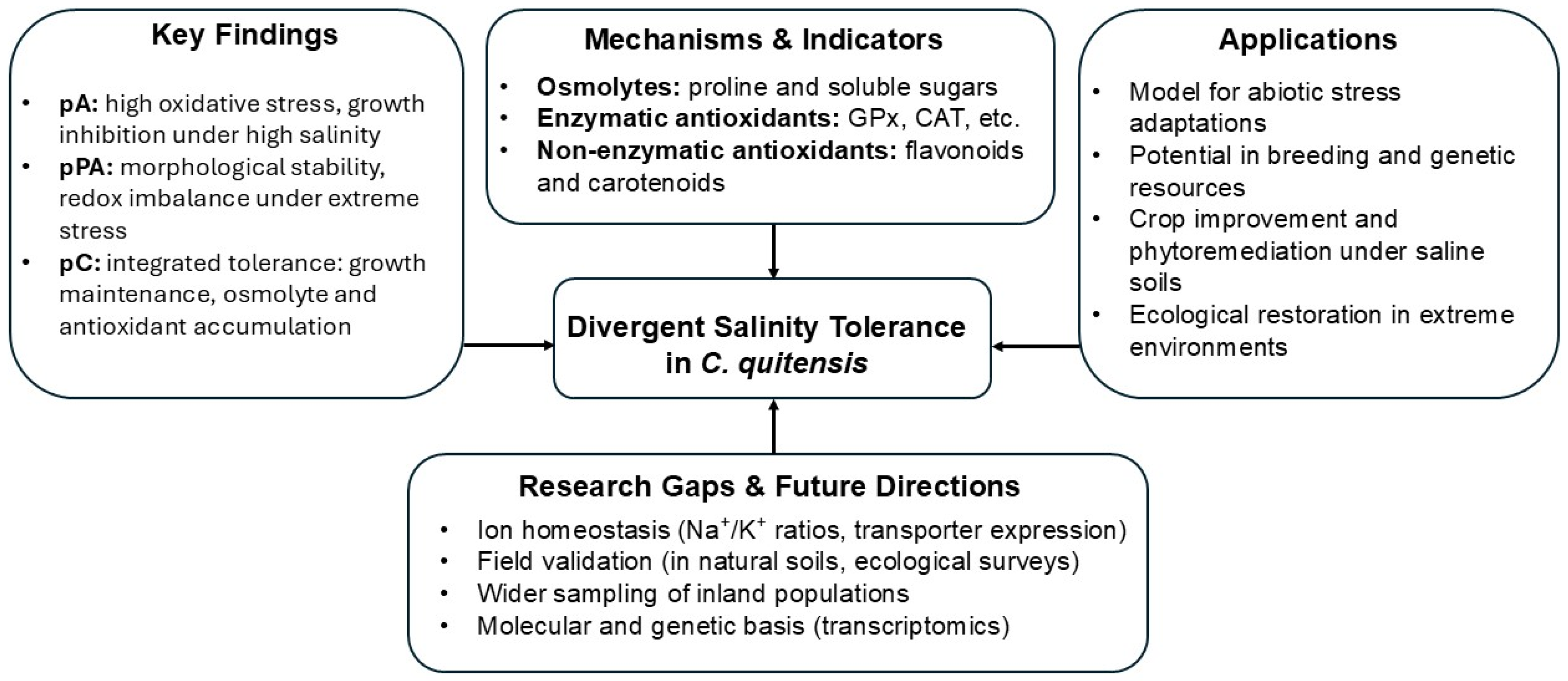
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ Response Mechanisms to Salinity Stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef]
- Pessarakli, M.; Szabolcs, I. Soil Salinity and Sodicity as Particular Plant/Crop Stress Factors. In Handbook of Plant and Crop Stress; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Junaid, M.D.; Gokce, A.F. Global Agricultural Losses and Their Causes. Bull. Biol. All. Sci. Res. 2024, 2024, 66. [Google Scholar] [CrossRef]
- Singh, P.; Dheri, G.S.; Nazir, G. Management of Saline and Sodic Soils for Carbon Sequestration. Commun. Soil Sci. Plant Anal. 2025, 56, 2618–2639. [Google Scholar] [CrossRef]
- Sebastián, M.; Bakka, K.; Challabathula, D.; Nikalje, G.C.; Shahnawaz, M.; Parihar, J.; Qazi, H.A. Metabolites and Their Regulation During Salinity Stress in Plants. In Plant Secondary Metabolites and Abiotic Stress; Zhu, D., Ed.; Wiley: Hoboken, NJ, USA, 2024; Volume 12, pp. 299–347. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a Multifaceted Signalling Molecule in Plant Responses to Abiotic Stress: Understanding the Physiological Mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive Oxygen Species, Abiotic Stress and Stress Combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of Reactive Oxygen Species and Antioxidant Defense in Plants Under Salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J. Improving Crop Salt Tolerance. J. Exp. Bot. 2004, 55, 307–319. [Google Scholar] [CrossRef]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant Salt-Tolerance Mechanism: A Review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.M. Studies in Colobanthus quitensis (Kunth) Bartl. and Deschampsia antarctica Desv. II. Taxonomy, Distribution and Relationships. Br. Antarct. Surv. Bull. 1970, 23, 63–80. [Google Scholar]
- Convey, P.; Hopkins, D.W.; Roberts, S.J.; Tyler, A.N. Global Southern Limit of Flowering Plants and Moss Peat Accumulation. Polar Res. 2011, 30, 8929. [Google Scholar] [CrossRef]
- Gianoli, E.; Inostroza, P.; Zúñiga-Feest, A.; Reyes-Díaz, M.; Cavieres, L.A.; Bravo, L.A.; Corcuera, L.J. Ecotypic Differentiation in Morphology and Cold Resistance in Populations of Colobanthus quitensis (Caryophyllaceae) from the Andes of Central Chile and the Maritime Antarctic. Arct. Antarct. Alp. Res. 2004, 36, 484–489. [Google Scholar] [CrossRef]
- Cuba-Díaz, M.; Klagges, M.; Fuentes-Lillo, E.; Cordero, C.M. Phenotypic variability and genetic differentiation in continental and island populations of Colobanthus quitensis (Caryophyllaceae: Antarctic pearlwort). Polar Biol. 2017, 40, 2397–2409. [Google Scholar] [CrossRef]
- Cuba-Díaz, M.; Acuña, D.; Klagges, M.; Dollenz, O.; Cordero, C. Colobanthus quitensis de La Marisma, Una Nueva Población Para La Colección Genética de La Especie. In Avances en Ciencia Antártica Latinoamericana. VII Congreso Latinoamericano de Ciencia Antártica; Leppe, M., Molina-Montenegro, M., González, M., MacDonell, S., Lavín, P., Oses, R., Gallardo, J., Rivadeneira, M., Arata, J., Canales, R., et al., Eds.; Instituto Antartico Chileno: Punta Arenas, Chile, 2013; pp. 436–439. [Google Scholar]
- Androsiuk, P.; Chwedorzewska, K.; Szandar, K.; Giełwanowska, I. Genetic Variability of Colobanthus quitensis from King George Island (Antarctica). Pol. Polar Res. 2015, 36, 281–295. [Google Scholar] [CrossRef]
- Cuba-Díaz, M.; Castel, K.; Acuña, D.; Machuca, Á.; Cid, I. Sodium Chloride Effect on Colobanthus quitensis Seedling Survival and In Vitro Propagation. Antarct. Sci. 2017, 29, 45–46. [Google Scholar] [CrossRef]
- Ontivero, Y.; Fuentes-Lillo, E.; Navarrete-Campos, D.; Vázquez-Villa, D.; Cabreras-Barjas, G.; Arroyo-Marín, F.B.; Cuba-Díaz, M. Preliminary Assessment of Seed Heteromorphism as an Adaptive Strategy of Colobanthus quitensis Under Saline Conditions. Sci. Rep. 2024, 14, 31120. [Google Scholar] [CrossRef]
- Ologundudu, F. Antioxidant Enzymes and Non-Enzymatic Antioxidants as Defense Mechanism of Salinity Stress in Cowpea (Vigna Unguiculata L. Walp)—Ife Brown and Ife Bpc. Bull. Natl. Res. Cent. 2021, 45, 152. [Google Scholar] [CrossRef]
- Muhammad, M.; Waheed, A.; Wahab, A.; Majeed, M.; Nazim, M.; Liu, Y.-H.; Li, L.; Li, W.-J. Soil Salinity and Drought Tolerance: An Evaluation of Plant Growth, Productivity, Microbial Diversity, and Amelioration Strategies. Plant Stress 2024, 11, 100319. [Google Scholar] [CrossRef]
- Ramírez, C.F.; Cavieres, L.A.; Sanhueza, C.; Vallejos, V.; Gómez-Espinoza, O.; Bravo, L.A.; Sáez, P.L. Ecophysiology of Antarctic Vascular Plants: An Update on the Extreme Environment Resistance Mechanisms and Their Importance in Facing Climate Change. Plants 2024, 13, 449. [Google Scholar] [CrossRef] [PubMed]
- Al-Ashkar, I.; Alderfasi, A.; Ben Romdhane, W.; Seleiman, M.F.; El-Said, R.A.; Al-Doss, A. Morphological and Genetic Diversity Within Salt Tolerance Detection in Eighteen Wheat Genotypes. Plants 2020, 9, 287. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan, M.; Lu, M.; Sehar, S.; Holford, P.; Wu, F. Comparison of Biochemical, Anatomical, Morphological, and Physiological Responses to Salinity Stress in Wheat and Barley Genotypes Deferring in Salinity Tolerance. Agronomy 2020, 10, 127. [Google Scholar] [CrossRef]
- Sakinah, A.I.; Musa, Y.; Farid, M.; Hairmansis, A.; Anshori, M.F.; Nasaruddin, N. Rice Selection Criteria Based on Morphological and Image-Based Phenotyping Under Drought- and Salinity-Stress Conditions. SABRAO J. Breed. Genet. 2022, 54, 686–699. [Google Scholar] [CrossRef]
- Pathak, J.; Ahmed, H.; Kumari, N.; Pandey, A.; Sinha, R.P. Role of Calcium and Potassium in Amelioration of Environmental Stress in Plants. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress: Biochemical and Molecular Perspectives; Roychoudhury, A., Tripathi, D.K., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2020. [Google Scholar]
- Ramegowda, V.; Da Costa, M.V.J.; Harihar, S.; Karaba, N.N.; Sreeman, S.M. Abiotic and Biotic Stress Interactions in Plants: A Cross-Tolerance Perspective. In Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 267–302. [Google Scholar]
- Majeed, A.; Muhammad, Z. Salinity: A Major Agricultural Problem—Causes, Impacts on Crop Productivity and Management Strategies. In Plant Abiotic Stress Tolerance; Springer International Publishing: Cham, Switzerland, 2019; pp. 83–99. [Google Scholar]
- Navarrete-Gallegos, A.A.; Bravo, L.A.; Molina-Montenegro, M.A.; Corcuera, L.J. Respuestas Antioxidantes En Dos Ecotipos de Colobanthus quitensis (Caryophyllaceae) Expuestos a Alta Radiación UV-B y Baja Temperatura. Rev. Chil. Hist. Nat. 2012, 85, 419–433. [Google Scholar] [CrossRef]
- Min, K.; Sulaiman, S.; Jeong, J.; Lee, H.; Lee, J.; Lee, J.H.; Lee, H. Exploring the Mechanisms Underlying Recovery from Freeze-Thaw Injury in Colobanthus quitensis: Mechanistic Insights via Transcriptome Profiling. Physiol. Plant. 2024, 176, e14642. [Google Scholar] [CrossRef]
- Hereme, R.; Galleguillos, C.; Molina-Montenegro, M.A. Climate Change and Epigenetics: Unraveling the Role of Methylation in Response to Thermal Instability in the Antarctic Plant Colobanthus quitensis. Physiol. Plant. 2025, 177, e70043. [Google Scholar] [CrossRef]
- Basu, P.S.; Sharma, A.; Sukumaran, N.P. Changes in Net Photosynthetic Rate and Chlorophyll Fluorescence in Potato Leaves Induced by Water Stress. Photosynthetica 1998, 35, 13–19. [Google Scholar] [CrossRef]
- Gori, A.; Brunetti, C.; Dos Santos Nascimento, L.B.; Marino, G.; Guidi, L.; Ferrini, F.; Centritto, M.; Fini, A.; Tattini, M. Photoprotective Role of Photosynthetic and Non-Photosynthetic Pigments in Phillyrea Latifolia: Is Their “Antioxidant” Function Prominent in Leaves Exposed to Severe Summer Drought? Int. J. Mol. Sci. 2021, 22, 8303. [Google Scholar] [CrossRef] [PubMed]
- Bose, J.; Munns, R.; Shabala, S.; Gilliham, M.; Pogson, B.; Tyerman, S.D. Chloroplast Function and Ion Regulation in Plants Growing on Saline Soils: Lessons from Halophytes. J. Exp. Bot. 2017, 68, 3129–3143. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Du, G.; Li, X.; Zhang, C.; Guo, J. A Major Locus Controlling Malondialdehyde Content Under Water Stress Is Associated with Fusarium Crown Rot Resistance in Wheat. Mol. Genet. Genomics 2015, 290, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Contreras, R.A.; Pizarro, M.; Köhler, H.; Sáez, C.A.; Zúñiga, G.E. Copper Stress Induces Antioxidant Responses and Accumulation of Sugars and Phytochelatins in Antarctic Colobanthus quitensis (Kunth) Bartl. Biol. Res. 2018, 51, 48. [Google Scholar] [CrossRef] [PubMed]
- Hnilickova, H.; Kraus, K.; Vachova, P.; Hnilicka, F. Salinity Stress Affects Photosynthesis, Malondialdehyde Formation, and Proline Content in Portulaca oleracea L. Plants 2021, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Sanwal, S.K.; Kumar, A.; Pundir, R.K.; Yadav, M.; Sehrawat, N. Role of Osmolytes Dynamics in Plant Metabolism to Cope with Salinity Induced Osmotic Stress. Discov. Agric. 2024, 2, 59. [Google Scholar] [CrossRef]
- Salinas, R.; Sánchez, E.; Ruíz, J.M.; Lao, M.T.; Romero, L. Proline, Betaine, and Choline Responses to Different Phosphorus Levels in Green Bean. Commun. Soil Sci. Plant Anal. 2013, 44, 465–472. [Google Scholar] [CrossRef]
- Reddy, P.; Jogeswar, G.; Rasineni, G.K.; Maheswari, M.; Reddy, A.R.; Varshney, R.K.; Kavi Kishor, P.B. Proline Over-Accumulation Alleviates Salt Stress and Protects Photosynthetic and Antioxidant Enzyme Activities in Transgenic Sorghum [Sorghum bicolor (L.) Moench]. Plant Physiol. Biochem. 2015, 94, 104–113. [Google Scholar] [CrossRef]
- Inayat, H.; Mehmood, H.; Danish, S.; Alharbi, S.A.; Ansari, M.J.; Datta, R. Impact of Cobalt and Proline Foliar Application for Alleviation of Salinity Stress in Radish. BMC Plant Biol. 2024, 24, 287. [Google Scholar] [CrossRef] [PubMed]
- Stefanov, M.; Yotsova, E.; Gesheva, E.; Dimitrova, V.; Markovska, Y.; Doncheva, S.; Apostolova, E.L. Role of Flavonoids and Proline in the Protection of Photosynthetic Apparatus in Paulownia Under Salt Stress. S. Afr. J. Bot. 2021, 139, 246–253. [Google Scholar] [CrossRef]
- Dubrovna, O.V.; Mykhalska, S.I.; Komisarenko, A.G. Using Proline Metabolism Genes in Plant Genetic Engineering. Cytol. Genet. 2022, 56, 361–378. [Google Scholar] [CrossRef]
- Mohammadkhani, N.; Heidari, R. Drought-Induced Accumulation of Soluble Sugars and Proline in Two Maize Varieties. World Appl. Sci. J. 2008, 3, 448–453. [Google Scholar]
- Nemati, I.; Moradi, F.; Gholizadeh, S.; Esmaeili, M.A.; Bihamta, M.R. The Effect of Salinity Stress on Ions and Soluble Sugars Distribution in Leaves, Leaf Sheaths and Roots of Rice (Oryza sativa L.) Seedlings. Plant Soil Environ. 2011, 57, 26–33. [Google Scholar] [CrossRef]
- Clemente-Moreno, M.J.; Omranian, N.; Sáez, P.; Figueroa, C.M.; Del-Saz, N.; Elso, M.; Poblete, L.; Orf, I.; Cuadros-Inostroza, A.; Cavieres, L.; et al. Cytochrome Respiration Pathway and Sulphur Metabolism Sustain Stress Tolerance to Low Temperature in the Antarctic Species Colobanthus quitensis. New Phytol. 2020, 225, 754–768. [Google Scholar] [CrossRef]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of Sugars Under Abiotic Stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef]
- Espasandin, F.D.; Calzadilla, P.I.; Maiale, S.J.; Ruiz, O.A.; Sansberro, P.A. Overexpression of the Arginine Decarboxylase Gene Improves Tolerance to Salt Stress in Lotus tenuis Plants. J. Plant Growth Regul. 2018, 37, 156–165. [Google Scholar] [CrossRef]
- Mushtaq, N.U.; Saleem, S.; Rasool, A.; Shah, W.H.; Tahir, I.; Hakeem, K.R.; Rehman, R.U. Functional Characterization of the Antioxidant Enzymes in Plants Exposed to Environmental Stresses. In Antioxidant Defense in Plants; Springer Nature: Singapore, 2022; pp. 15–30. [Google Scholar]
- Pérez-Torres, E.; Bravo, L.A.; Corcuera, L.J.; Dinamarca, J. Responses of Colobanthus quitensis (Kunth) Bartl. to High Light and Low Temperature. Polar Biol. 2004, 27, 183–189. [Google Scholar] [CrossRef]
- Bravo, L.A.; Saavedra-Mella, F.A.; Vera, F.; Guerra, A.; Cavieres, L.A.; Ivanov, A.G.; Huner, N.P.A.; Corcuera, L.J. Effect of Cold Acclimation on the Photosynthetic Performance of Two Ecotypes of Colobanthus quitensis (Kunth) Bartl. J. Exp. Bot. 2007, 58, 3581–3590. [Google Scholar] [CrossRef]
- Bascuñán-Godoy, L.; García-Plazaola, J.I.; Bravo, L.A.; Corcuera, L.J. Leaf Functional and Micro-Morphological Photoprotective Attributes in Two Ecotypes of Colobanthus quitensis from the Andes and Maritime Antarctic. Polar Biol. 2010, 33, 885–896. [Google Scholar] [CrossRef]
- Cuba-Díaz, M.; Marín, C.; Castel, K.; Machuca, Á.; Rifo, S. Effect of Copper (II) Ions on Morpho-Physiological and Biochemical Variables in Colobanthus quitensis. J. Soil Sci. Plant Nutr. 2017. [Google Scholar] [CrossRef]
- Bertini, L.; Proietti, S.; Focaracci, F.; Canini, F.; Bravo, L.A.; Rabert, C.; Caruso, C. Identification and Validation of New Reference Genes for Accurate Quantitative Reverse Transcriptase-PCR Normalization in the Antarctic Plant Colobanthus quitensis Under Abiotic Stress Conditions. Polar Biol. 2021, 44, 389–405. [Google Scholar] [CrossRef]
- Bertini, L.; Proietti, S.; Fongaro, B.; Holfeld, A.; Picotti, P.; Falconieri, G.S.; Bizzarri, E.; Capaldi, G.; Polverino de Laureto, P.; Caruso, C. Environmental Signals Act as a Driving Force for Metabolic and Defense Responses in the Antarctic Plant Colobanthus quitensis. Plants 2022, 11, 3176. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants Under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef] [PubMed]
- Contreras, R.A.; Pizarro, M.; Köhler, H.; Zamora, P.; Zúñiga, G.E. UV-B Shock Induces Photoprotective Flavonoids but Not Antioxidant Activity in Antarctic Colobanthus quitensis (Kunth) Bartl. Environ. Exp. Bot. 2019, 159, 179–190. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Singh, P.; Arif, Y.; Bajguz, A.; Hayat, S. The Role of Quercetin in Plants. Plant Physiol. Biochem. 2021, 166, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Farago, M.E.; Mullen, W.A. Plants Which Accumulate Metals. Part IV. A Possible Copper-Proline Complex from the Roots of Armeria Maritima. Inorganica Chim. Acta 1979, 32, L93–L94. [Google Scholar] [CrossRef]
- Kumar, D.; Tarafdar, A.; Dass, S.L.; Pareek, S.; Badgujar, P.C. Antioxidant Potential and Amino Acid Profile of Ultrafiltration Derived Peptide Fractions of Spent Hen Meat Protein Hydrolysate. J. Food Sci. Technol. 2023, 60, 1195–1201. [Google Scholar] [CrossRef]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The Role of Na+ and K+ Transporters in Salt Stress Adaptation in Glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef]
- Cuba-Díaz, M.; Cerda, G.; Rivera, C.; Gómez, A. Genome Size Comparison in Colobanthus quitensis Populations Show Differences in Species Ploidy. Polar Biol. 2017, 40, 1475–1480. [Google Scholar] [CrossRef]
- Cuba-Díaz, M.; Acuña, D.; Fuentes-Lillo, E. Antarctic Pearlwort (Colobanthus quitensis) Populations Respond Differently to Pre-Germination Treatments. Polar Biol. 2019, 42, 1209–1215. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf Senescence: Correlated with Increased Levels of Membrane Permeability and Lipid Peroxidation, and Decreased Levels of Superoxide Dismutase and Catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Bates, L.; Waldren, P.P.; Teare, J.D. Rapid Determination of the Free Proline of Water Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Roe, J.H. A Colorimetric Method for the Determination of Fructose in Blood and Urine. J. Biol. Chem. 1934, 107, 15–22. [Google Scholar] [CrossRef]
- Bravo, L.A.; Ulloa, N.; Zuñiga, G.E.; Casanova, A.; Corcuera, L.J.; Alberdi, M. Cold Resistance in Antarctic Angiosperms. Physiol. Plant. 2001, 111, 55–65. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Mazhoudi, S.; Chaoui, A.; Habib Ghorbal, M.; El Ferjani, E. Response of Antioxidant Enzymes to Excess Copper in Tomato (Lycopersicon esculentum, Mill.). Plant Sci. 1997, 127, 129–137. [Google Scholar] [CrossRef]
- Frary, A.; Göl, D.; Keleş, D.; Okmen, B.; Pinar, H.; Siğva, H.O.; Yemenicioğlu, A.; Doğanlar, S. Salt Tolerance in Solanum pennellii: Antioxidant Response and Related QTL. BMC Plant Biol. 2010, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- van de Staaij, J.; de Bakker, N.V.J.; Oosthoek, A.; Broekman, R.; van Beem, A.; Stroetenga, M.; Aerts, R.; Rozema, J. Flavonoid Concentrations in Three Grass Species and a Sedge Grown in the Field and Under Controlled Environment Conditions in Response to Enhanced UV-B Radiation. J. Photochem. Photobiol. B 2002, 66, 21–29. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 10 July 2025).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019; Available online: https://www.john-fox.ca/Companion/ (accessed on 15 July 2025).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Wilke, C.O. Cowplot: Streamlined Plot Theme and Plot Annotations for ‘ggplot2′. R Package Version 1.1.1. 2020. Available online: https://CRAN.R-project.org/package=cowplot (accessed on 16 July 2025).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: AnRPackage for Multivariate Analysis. J. Stat. Softw. 2008, 25. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 16 July 2025).

| Variable | Treatment | pA | pPA | pC |
|---|---|---|---|---|
| Number of leaves | 0 mM | 18.67 ± 0.93 a | 12.67 ± 1.16 a | 16.53 ± 0.97 ab |
| 50 mM | 11.47 ± 1.02 b | 10.4 ± 0.89 a | 20.27 ± 1.99 a | |
| 150 mM | 8.87 ± 0.50 b | 9.67 ± 0.57 a | 14.07 ± 0.66 b | |
| Leaf width (mm) | 0 mM | 1.42 ± 0.52 a | 1.19 ± 0.04 a | 0.76 ± 0.03 a |
| 50 mM | 1.14 ± 0.06 b | 1.32 ± 0.05 a | 0.86 ± 0.03 a | |
| 150 mM | 1.01 ± 0.06 b | 1.28 ± 0.04 a | 0.81 ± 0.04 a | |
| Leaf length (mm) | 0 mM | 13.02 ± 0.63 a | 17.9 ± 0.93 ab | 23.87 ± 1.32 a |
| 50 mM | 10.36 ± 0.75 b | 19.9 ± 1.79 a | 25.11 ± 1.58 a | |
| 150 mM | 8.86 ± 0.38 b | 14.31 ± 1.33 b | 24.10 ± 1.36 a | |
| Root length (mm) | 0 mM | 45.90 ± 2.35 a | 60.89 ± 3.86 a | 57.88 ± 3.68 a |
| 50 mM | 40.39 ± 2.93 a | 61.04 ± 2.93 a | 62.81 ± 7.70 a | |
| 150 mM | 31.15 ± 1.49 b | 62.24 ± 3.82 a | 49.96 ± 4.67 a | |
| Biomass (g) | 0 mM | 1.02 ± 0.10 a | 1 ± 0.15 a | 0.98 ± 0.18 a |
| 50 mM | 0.62 ± 0.09 b | 1.05 ± 0.08 a | 1.24 ± 0.13 a | |
| 150 mM | 0.43 ± 0.07 b | 0.87 ± 0.09 a | 1.28 ± 0.14 a | |
| Water content (%) | 0 mM | 81.16 ± 1.67 a | 81.94 ± 1.15 a | 82.40 ± 1.20 a |
| 50 mM | 70.65 ± 3.63 b | 81.93 ± 1.83 a | 80.38 ± 1.21 a | |
| 150 mM | 73.26 ± 2.81 ab | 81.77 ± 1.31 a | 71.41 ± 2.65 b |
| Population | Origin | Geographical Location | Altitude (m.a.s.l.) | Marine Influence |
|---|---|---|---|---|
| pA | Polish Antarctic Station H. Arctowski, Admiralty Bay, King George Island | 62°09′ S; 58°28′ W | 3–23 | Regularly exposed to sea spray due to strong coastal winds |
| pPA | Punta Santa María Sector, South of Punta Arenas, Chile | 53°22′ S; 70°58′ W | 1–3 | Subject to seawater flooding during high tides |
| pC | Conguillío National Park, La Araucanía, Chile | 38°36′ S; 71°36 W | 2575 | No marine influence |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuba-Díaz, M.; Ontivero, Y.; Fuentes-Lillo, E.; Klagges, M.; Arriagada, P.; Cabrera-Barja, G.; Sepúlveda, B. Population-Specific Salinity Tolerance in the Extremophile Colobanthus quitensis: Evidence of Adaptive Plasticity. Plants 2025, 14, 3116. https://doi.org/10.3390/plants14203116
Cuba-Díaz M, Ontivero Y, Fuentes-Lillo E, Klagges M, Arriagada P, Cabrera-Barja G, Sepúlveda B. Population-Specific Salinity Tolerance in the Extremophile Colobanthus quitensis: Evidence of Adaptive Plasticity. Plants. 2025; 14(20):3116. https://doi.org/10.3390/plants14203116
Chicago/Turabian StyleCuba-Díaz, Marely, Yadiana Ontivero, Eduardo Fuentes-Lillo, Macarena Klagges, Paulina Arriagada, Gustavo Cabrera-Barja, and Benjamín Sepúlveda. 2025. "Population-Specific Salinity Tolerance in the Extremophile Colobanthus quitensis: Evidence of Adaptive Plasticity" Plants 14, no. 20: 3116. https://doi.org/10.3390/plants14203116
APA StyleCuba-Díaz, M., Ontivero, Y., Fuentes-Lillo, E., Klagges, M., Arriagada, P., Cabrera-Barja, G., & Sepúlveda, B. (2025). Population-Specific Salinity Tolerance in the Extremophile Colobanthus quitensis: Evidence of Adaptive Plasticity. Plants, 14(20), 3116. https://doi.org/10.3390/plants14203116









