PvPR10-3 Expression Confers Salt Stress Tolerance in Arabidopsis and Interferes with Jasmonic Acid and ABA Signaling
Abstract
1. Introduction
2. Results
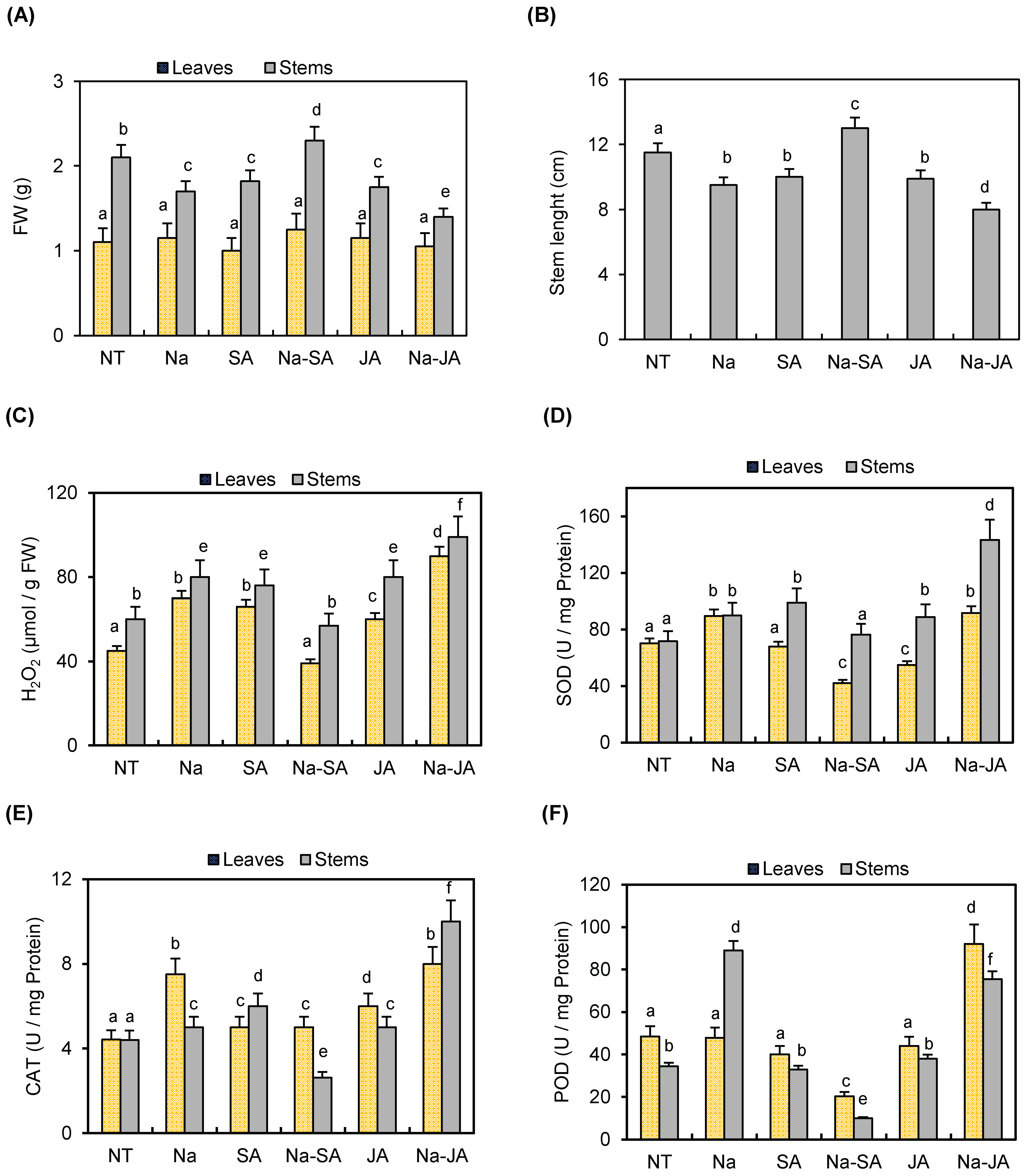
2.1. Response of Common Bean to Salt, Phytohormones, and Combined Salt–Phytohormone Stresses
2.2. Expression Profile Analysis of PvPR10 Genes Under Salt, Phytohormones, and Combined Salt–Phytohormone Stresses
2.3. Generation of PvPR10-3-Expressing Arabidopsis Lines
2.4. Enhanced JA-Mediated Salt Stress Tolerance in PvPR10-3 Arabidopsis Lines Is Linked to Reduced H2O2 Accumulation
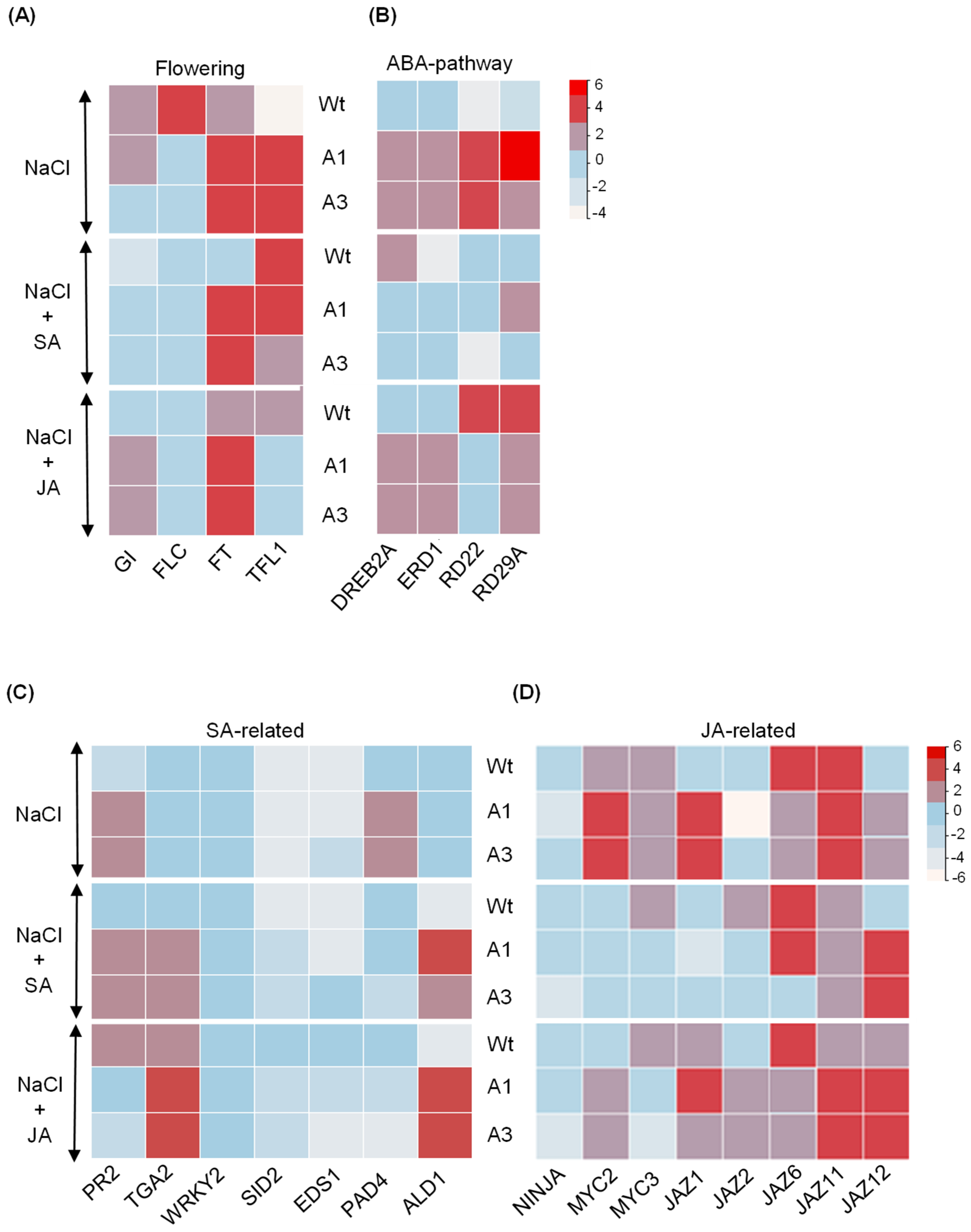
2.5. Enhanced Salt and JA Stress Resilience in PvPR10-3-Expressing Lines Involves ABA-Dependent and Independent Pathways and Phenolic Accumulation
2.6. Crosstalk Between JA and SA During Salt Stress Response of PvPR10-3-Expressing Arabidopsis Lines
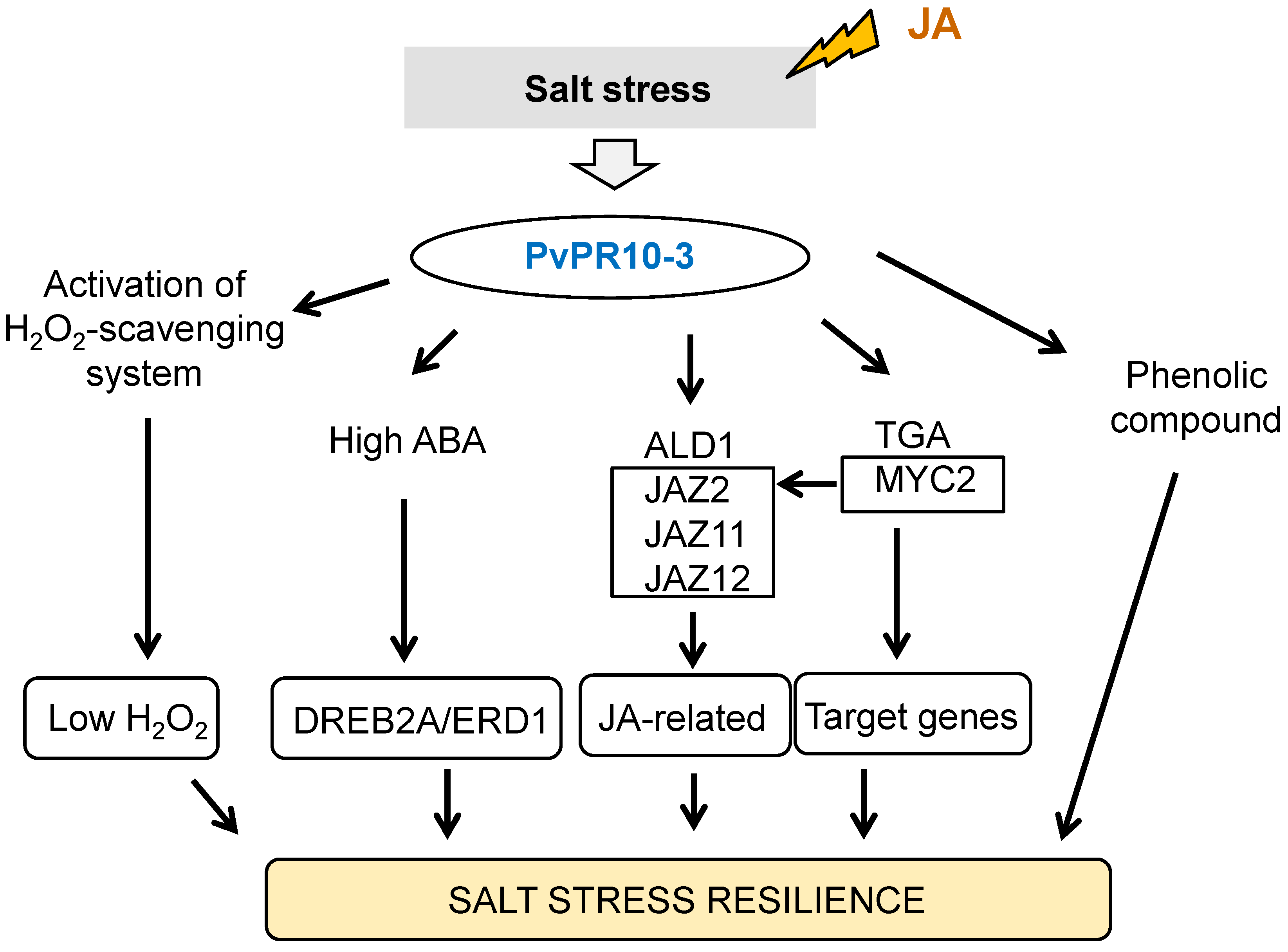
3. Discussion
4. Materials and Methods
4.1. Plant Material and Treatments
4.2. Isolation and Cloning of PvPR10-3 Gene
4.3. Arabidopsis Transformation and Selection of the Transgenic Lines
4.4. Response of the Transgenic Arabidopsis Plants to Salt and Combined Salt–Phytohormone Stresses
4.5. Analysis of Enzymatic ROS-Scavenging System
4.6. Determination of H2O2 and Chlorophyll Content
4.7. RNA Extraction and Gene Expression Profiles Analysis
4.8. Quantification of Phenolic Compounds and ABA Levels
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benitez-Alfonso, Y.; Soanes, B.K.; Zimba, S.; Sinanaj, B.; German, L.; Sharma, V.; Bohra, A.; Kolesnikova, A.; Dunn, J.A.; Martin, A.C.; et al. Enhancing climate change resilience in agricultural crops. Curr. Biol. 2023, 33, R1246–R1261. [Google Scholar] [CrossRef]
- Terán, F.; Vives-Peris, V.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Facing climate change: Plant stress mitigation strategies in agriculture. Physiol. Plant 2024, 176, e14484. [Google Scholar] [CrossRef]
- Xiao, F.; Zhou, H. Plant salt response: Perception, signaling, and tolerance. Front. Plant Sci. 2023, 13, 1053699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lang, Z.; Zhu, J.-K.; Wang, P. Tackling abiotic stress in plants: Recent insights and trends. Stress Biol. 2025, 5, 8. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Feki, K.; Tounsi, S.; Kamoun, H.; Al-Hashimi, A.; Brini, F. Decoding the role of durum wheat ascorbate peroxidase TdAPX7B-2 in abiotic stress response. Funct. Integr. Genom. 2024, 24, 223. [Google Scholar] [CrossRef] [PubMed]
- Ghorbel, M.; Feki, K.; Tounsi, S.; Bouali, N.; Besbes, M.; Brini, F. The Putative Auto-Inhibitory Domain of Durum Wheat Catalase (TdCAT1) Positively Regulates Bacteria Cells in Response to Different Stress Conditions. Antioxidants 2022, 11, 1820. [Google Scholar] [CrossRef]
- Tounsi, S.; Jemli, S.; Feki, K.; Brini, F.; Najib Saïdi, M. Superoxide dismutase (SOD) family in durum wheat: Promising candidates for improving crop resilience. Protoplasma 2023, 260, 145–158. [Google Scholar] [CrossRef]
- Tounsi, S.; Kamoun, Y.; Feki, K.; Jemli, S.; Saïdi, M.N.; Ziadi, H.; Alcon, C.; Brini, F. Localization and expression analysis of a novel catalase from Triticum monococcum TmCAT1 involved in response to different environmental stresses. Plant Physiol. Biochem. 2019, 139, 366–378. [Google Scholar] [CrossRef]
- Mishra, N.; Jiang, C.; Chen, L.; Paul, A.; Chatterjee, A.; Shen, G. Achieving abiotic stress tolerance in plants through antioxidative defense mechanisms. Front. Plant Sci. 2023, 14, 1110622. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Env. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Saibi, W.; Feki, K.; Yacoubi, I.; Brini, F. Bridging Between Proline Structure, Functions, Metabolism, and Involvement in Organism Physiology. App. Biochem. Biotech. 2015, 176, 2107–2119. [Google Scholar] [CrossRef]
- Renzetti, M.; Funck, D.; Trovato, M. Proline and ROS: A Unified Mechanism in Plant Development and Stress Response? Plants 2025, 14, 2. [Google Scholar] [CrossRef]
- Almeida, D.M.; Oliveira, M.M.; Saibo, N.J.M. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 2017, 40, 326–345. [Google Scholar] [CrossRef]
- Basu, S.; Kumar, A.; Benazir, I.; Kumar, G. Reassessing the role of ion homeostasis for improving salinity tolerance in crop plants. Physiol. Plant 2021, 171, 502–519. [Google Scholar] [CrossRef]
- Kamran, M.; Burdiak, P.; Karpiński, S. Crosstalk Between Abiotic and Biotic Stresses Responses and the Role of Chloroplast Retrograde Signaling in the Cross-Tolerance Phenomena in Plants. Cells 2025, 14, 176. [Google Scholar] [CrossRef]
- Liu, J.-J.; Ekramoddoullah, A.K.M. The family 10 of plant pathogenesis-related proteins: Their structure, regulation, and function in response to biotic and abiotic stresses. Physiol. Mol. Plant Pathol. 2006, 68, 3–13. [Google Scholar] [CrossRef]
- Zribi, I.; Ghorbel, M.; Brini, F. Pathogenesis Related Proteins (PRs): From Cellular Mechanisms to Plant Defense. Curr. Protein Peptide Sci. 2021, 22, 396–412. [Google Scholar] [CrossRef] [PubMed]
- Somssich, I.E.; Schmelzer, E.; Bollmann, J.; Hahlbrock, K. Rapid activation by fungal elicitor of genes encoding “pathogenesis-related” proteins in cultured parsley cells. Proc. Natl. Acad. Sci. USA 1986, 83, 2427–2430. [Google Scholar] [CrossRef] [PubMed]
- Khanfir, E.; Zribi, I.; Dhouib, H.; Ghorbel, M.; Hamdi, K.; Jrad, O.; Yacoubi, I.; Brini, F. Genome-Wide Identification of PR10 Family Members in Durum Wheat: Expression Profile and In Vitro Analyses of TdPR10.1 in Response to Various Stress Conditions. Plants 2024, 13, 3128. [Google Scholar] [CrossRef] [PubMed]
- Feki, K.; Tounsi, S.; Jemli, S.; Boubakri, H.; Saidi, M.N.; Mrabet, M.; Brini, F.; Mhadhbi, H. Genome-wide identification of PR10 family members and expression profile analysis of PvPR10 in common bean (Phaseolus vulgaris L.) in response to hormones and several abiotic stress conditions. Plant Growth Reg. 2024, 102, 279–295. [Google Scholar] [CrossRef]
- Xu, P.; Jiang, L.; Wu, J.; Li, W.; Fan, S.; Zhang, S. Isolation and characterization of a pathogenesis-related protein 10 gene (GmPR10) with induced expression in soybean (Glycine max) during infection with Phytophthora sojae. Mol. Biol. Rep. 2014, 41, 4899–4909. [Google Scholar] [CrossRef]
- Longsaward, R.; Viboonjun, U. Genome-wide identification of rubber tree pathogenesis-related 10 (PR-10) proteins with biological relevance to plant defense. Sci. Rep. 2024, 14, 1072. [Google Scholar] [CrossRef] [PubMed]
- Viboonjun, U.; Longsaward, R. Genome-wide identification and data mining reveals major-latex protein (MLP) from the PR-10 protein family played defense-related roles against phytopathogenic challenges in cassava (Manihot esculenta Crantz). Genetica 2024, 152, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, H.; Michalska, K.; Sikorski, M.; Jaskolski, M. Structural and functional aspects of PR-10 proteins. FEBS J. 2013, 280, 1169–1199. [Google Scholar] [CrossRef] [PubMed]
- Zandvakili, N.; Zamani, M.; Motallebi, M.; Moghaddassi Jahromi, Z. Cloning, Overexpression and in vitro Antifungal Activity of Zea Mays PR10 Protein. Iranian J. Biotech. 2017, 15, 42–49. [Google Scholar] [CrossRef][Green Version]
- Fernandes, H.; Pasternak, O.; Bujacz, G.; Bujacz, A.; Sikorski, M.M.; Jaskolski, M. Lupinus luteus pathogenesis-related protein as a reservoir for cytokinin. J. Mol. Biol. 2008, 378, 1040–1051. [Google Scholar] [CrossRef]
- Lopes, N.D.S.; Santos, A.S.; de Novais, D.P.S.; Pirovani, C.P.; Micheli, F. Pathogenesis-related protein 10 in resistance to biotic stress: Progress in elucidating functions, regulation and modes of action. Front. Plant Sci. 2023, 14, 1193873. [Google Scholar] [CrossRef]
- Pasternak, O.; Bujacz, G.D.; Fujimoto, Y.; Hashimoto, Y.; Jelen, F.; Otlewski, J.; Sikorski, M.M.; Jaskolski, M. Crystal structure of Vigna radiata cytokinin-specific binding protein in complex with zeatin. Plant Cell 2006, 18, 2622–2634. [Google Scholar] [CrossRef]
- Sliwiak, J.; Sikorski, M.; Jaskolski, M. PR-10 proteins as potential mediators of melatonin-cytokinin cross-talk in plants: Crystallographic studies of LlPR-10.2B isoform from yellow lupine. FEBS J. 2018, 285, 1907–1922. [Google Scholar] [CrossRef]
- Casañal, A.; Zander, U.; Muñoz, C.; Dupeux, F.; Luque, I.; Botella, M.A.; Schwab, W.; Valpuesta, V.; Marquez, J.A. The strawberry pathogenesis-related 10 (PR-10) Fra a proteins control flavonoid biosynthesis by binding to metabolic intermediates. J. Biol. Chem. 2013, 288, 35322–35332. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Facchini, P. Norcoclaurine synthase is a member of the pathogenesis-related 10/Bet v1 protein family. Plant Cell 2010, 22, 3489–3503. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.S.; Hwang, I.S.; Hwang, B.K. Requirement of the cytosolic interaction between PATHOGENESIS-RELATED PROTEIN10 and LEUCINE-RICH REPEAT PROTEIN1 for cell death and defense signaling in pepper. Plant Cell 2012, 24, 1675–1690. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Diaz, J.C.; Laugé, R.; Delannoy, E.; Huguet, S.; Paysant-Le Roux, C.; Gratias, A.; Geffroy, V. Genome-Wide Transcriptomic Analysis of the Effects of Infection with the Hemibiotrophic Fungus Colletotrichum lindemuthianum on Common Bean. Plants 2022, 11, 1995. [Google Scholar] [CrossRef]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How Plant Hormones Mediate Salt Stress Responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef]
- Jiang, L.; Xiao, M.; Huang, R.; Wang, J. The Regulation of ROS and Phytohormones in Balancing Crop Yield and Salt Tolerance. Antioxidants 2025, 14, 63. [Google Scholar] [CrossRef]
- Kumar, P.; Pandey, S.; Pati, P.K. Interaction between pathogenesis-related (PR) proteins and phytohormone signaling pathways in conferring disease tolerance in plants. Physiol. Plant 2025, 177, e70174. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, F. Involvement of Pathogenesis-Related Proteins and Their Roles in Abiotic Stress Responses in Plants. Biomolecules 2025, 15, 1103. [Google Scholar] [CrossRef]
- Kumar, K.; Debnath, P.; Singh, S.; Kumar, N. An Overview of Plant Phenolics and Their Involvement in Abiotic Stress Tolerance. Stresses 2023, 3, 570–585. [Google Scholar] [CrossRef]
- Pauwels, L.; Goossens, A. The JAZ proteins: A crucial interface in the jasmonate signaling cascade. Plant Cell 2011, 23, 3089–3100. [Google Scholar] [CrossRef]
- Broughton, W.J.; Hernández, G.; Blair, M.; Beebe, S.; Gepts, P.; Vanderleyden, J. Beans (Phaseolus spp.)—model food legumes. Plant Soil 2003, 252, 55–128. [Google Scholar] [CrossRef]
- Lone, A.A.; Khan, M.N.; Gul, A.; Dar, Z.A.; Iqbal, A.M.; Lone, B.A.; Ahangar, A.; Rasool, F.U.; Khan, M.H.; Ali, G.; et al. Common Beans and Abiotic Stress Challenges. Curr. J. App. Sci. Technol. 2021, 40, 41–53. [Google Scholar] [CrossRef]
- Gupta, S.; Kaur, R.; Upadhyay, A.; Chauhan, A.; Tripathi, V. Unveiling the secrets of abiotic stress tolerance in plants through molecular and hormonal insights. 3 Biotech. 2024, 14, 252. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Nehra, A.; Kalwan, G.; Bhardwaj, D.; Yasheshwar; Rani, V.; Agarwala, N.; Tuteja, N.; Gill, R.; Ansari, M.W.; et al. Harnessing Jasmonate, Salicylate, and Microbe Synergy for Abiotic Stress Resilience in Crop Plants. J. Plant Growth Reg. 2025, 44, 40–61. [Google Scholar] [CrossRef]
- El-Taher, A.M.; Abd El-Raouf, H.S.; Osman, N.A.; Azoz, S.N.; Omar, M.A.; Elkelish, A.; Abd El-Hady, M.A.M. Effect of Salt Stress and Foliar Application of Salicylic Acid on Morphological, Biochemical, Anatomical, and Productivity Characteristics of Cowpea (Vigna unguiculata L.) Plants. Plants 2022, 11, 115. [Google Scholar] [CrossRef]
- Jini, D.; Joseph, B. Physiological Mechanism of Salicylic Acid for Alleviation of Salt Stress in Rice. Rice Sci. 2017, 24, 97–108. [Google Scholar] [CrossRef]
- da Silva, B.R.S.; Lobato, E.M.S.G.; dos Santos, L.A.; Pereira, R.M.; Batista, B.L.; Alyemeni, M.N.; Ahmad, P.; Lobato, A.K.d.S. How Different Na+ Concentrations Affect Anatomical, Nutritional Physiological, Biochemical, and Morphological Aspects in Soybean Plants: A Multidisciplinary and Comparative Approach. Agronomy 2023, 13, 232. [Google Scholar] [CrossRef]
- Ahmadi, F.I.; Karimi, K.; Struik, P.C. Effect of exogenous application of methyl jasmonate on physiological and biochemical characteristics of Brassica napus L. cv. Talaye under salinity stress. S. Afr. J. Bot. 2018, 115, 5–11. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, Y.; Cai, P.; Duan, X.; Sang, S.; Qiu, Z. Jasmonic acid pretreatment improves salt tolerance of wheat by regulating hormones biosynthesis and antioxidant capacity. Front. Plant Sci. 2022, 13, 968477. [Google Scholar] [CrossRef]
- Delgado, C.; Mora-Poblete, F.; Ahmar, S.; Chen, J.-T.; Figueroa, C.R. Jasmonates and Plant Salt Stress: Molecular Players, Physiological Effects, and Improving Tolerance by Using Genome-Associated Tools. Inter. J. Mol. Sci. 2021, 22, 3082. [Google Scholar] [CrossRef]
- Yerlikaya, B.A.; Yerlikaya, S.; Aydin, A.; Yilmaz, N.N.; Bahadır, S.; Abdulla, M.F.; Mostafa, K.; Kavas, M. Enhanced drought and salt stress tolerance in Arabidopsis via ectopic expression of the PvMLP19 gene. Plant Cell Rep. 2025, 44, 130. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, M.S.; Desouky, A.F.; Asker, M.S.; Zaki, E.R. Impact of homologous overexpression of PR10a gene on improving salt stress tolerance in transgenic Solanum tuberosum. J. Genet. Eng. Biotech. 2024, 22, 100437. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Z.; Sui, N. Sensitivity and responses of chloroplasts to salt stress in plants. Front. Plant Sci. 2024, 15, 1374086. [Google Scholar] [CrossRef] [PubMed]
- Sousa, V.Q.; Messias, W.F.S.; Pereira, Y.C.; da Silva, B.R.S.; Lobato, E.M.S.G.; Alyemeni, M.N.; Ahmad, P.; Lobato, A.K.d.S. Pretreatment with 24-Epibrassinolide Synergistically Protects Root Structures and Chloroplastic Pigments and Upregulates Antioxidant Enzymes and Biomass in Na+-Stressed Tomato Plants. J. Plant Growth Reg. 2022, 41, 2869–2885. [Google Scholar] [CrossRef]
- Qiu, Z.; Guo, J.; Zhu, A.; Zhang, L.; Zhang, M. Exogenous jasmonic acid can enhance tolerance of wheat seedlings to salt stress. Ecotoxicol. Env. Safety 2014, 104, 202–208. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Zahid, Z.; Mubarik, M.S.; Javed, R.; Siddiqui, M.H.; Hasanuzzaman, M. Jasmonic acid: A key frontier in conferring abiotic stress tolerance in plants. Plant Cell Rep. 2021, 40, 1513–1541. [Google Scholar] [CrossRef]
- Seckin-Dinler, B.; Tasci, E.; Sarisoy, U.; Gul, V. The cooperation between methyl jasmonate and salicylic acid to protect soybean (Glycin max L.) from salinity. Fresenius Environ. Bull. 2018, 27, 1618–1626. [Google Scholar]
- Li, N.; Han, X.; Feng, D.; Yuan, D.; Huang, L.J. Signaling Crosstalk between Salicylic Acid and Ethylene/Jasmonate in Plant Defense: Do We Understand What They Are Whispering? Inter. J. Mol. Sci. 2019, 20, 671. [Google Scholar] [CrossRef]
- Seo, H.S.; Kim, S.K.; Jang, S.W.; Choo, Y.S.; Sohn, E.Y.; Lee, I.J. Effect of jasmonic acid on endogenous gibberellins and abscisic acid in rice under NaCl stress. Biol. Plant 2005, 49, 447–450. [Google Scholar] [CrossRef]
- Kim, S.K.; Sohn, E.Y.; Joo, G.J.; Lee, I.J. Influence of jasmonic acid on endogenous gibberellin and abscisic acid in salt-stressed chard plant. J. Env. Biol. 2009, 30, 333–338. [Google Scholar]
- Rai, G.K.; Khanday, D.M.; Choudhary, S.M.; Kumar, P.; Kumari, S.; Martínez-Andújar, C.; Martínez-Melgarejo, P.A.; Rai, P.K.; Pérez-Alfocea, F. Unlocking nature’s stress buster: Abscisic acid’s crucial role in defending plants against abiotic stress. Plant Stress 2024, 11, 100359. [Google Scholar] [CrossRef]
- Chen, G.; Zheng, D.; Feng, N.; Zhou, H.; Mu, D.; Zhao, L.; Shen, X.; Rao, G.; Meng, F.; Huang, A. Physiological mechanisms of ABA-induced salinity tolerance in leaves and roots of rice. Sci. Rep. 2022, 12, 8228. [Google Scholar] [CrossRef]
- Brossa, R.; López-Carbonell, M.; Jubany-Marí, T.; Alegre, L. Interplay Between Abscisic Acid and Jasmonic Acid and its Role in Water-oxidative Stress in Wild-type, ABA-deficient, JA-deficient, and Ascorbate-deficient Arabidopsis Plants. J. Plant Growth Reg. 2011, 30, 322–333. [Google Scholar] [CrossRef]
- Wu, G.; Tian, N.; She, F.; Cao, A.; Wu, W.; Zheng, S.; Yang, N. Characteristics analysis of Early Responsive to Dehydration genes in Arabidopsis thaliana (AtERD). Plant Signal. Behav. 2023, 18, 2105021. [Google Scholar] [CrossRef]
- Reis, R.R.; Andrade Dias Brito da Cunha, B.; Martins, P.K.; Martins, M.T.B.; Alekcevetch, J.C.; Chalfun-Júnior, A.; Andrade, A.C.; Ribeiro, A.P.; Qin, F.; Mizoi, J.; et al. Induced over-expression of AtDREB2A CA improves drought tolerance in sugarcane. Plant Sci. 2014, 221–222, 59–68. [Google Scholar] [CrossRef]
- Liu, L.; Xie, Y.; Yahaya, B.S.; Wu, F. GIGANTEA Unveiled: Exploring Its Diverse Roles and Mechanisms. Genes 2024, 15, 94. [Google Scholar] [CrossRef] [PubMed]
- Riboni, M.; Galbiati, M.; Tonelli, C.; Conti, L. GIGANTEA enables drought escape response via abscisic acid-dependent activation of the florigens and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS. Plant Physiol. 2013, 162, 1706–1719. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Rong, X.; Huang, X.; Cheng, S. Recent advances of flowering locus T gene in higher plants. Inter. J. Mol. Sci. 2012, 13, 3773–3781. [Google Scholar] [CrossRef]
- Lu, C.; Liu, X.; Tang, Y.; Fu, Y.; Zhang, J.; Yang, L.; Li, P.; Zhu, Z.; Dong, P. A comprehensive review of TGA transcription factors in plant growth, stress responses, and beyond. Inter. J. Biol. Macromol. 2024, 258, 128880. [Google Scholar] [CrossRef]
- Luo, L.; Wang, Y.; Qiu, L.; Han, X.; Zhu, Y.; Liu, L.; Man, M.; Li, F.; Ren, M.; Xing, Y. MYC2: A Master Switch for Plant Physiological Processes and Specialized Metabolite Synthesis. Inter. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef]
- Rao, M.J.; Zheng, B. The Role of Polyphenols in Abiotic Stress Tolerance and Their Antioxidant Properties to Scavenge Reactive Oxygen Species and Free Radicals. Antioxidants 2025, 14, 74. [Google Scholar] [CrossRef]
- Sun, M.; Shi, M.; Wang, Y.; Huang, Q.; Yuan, T.; Wang, Q.; Wang, C.; Zhou, W.; Kai, G. The biosynthesis of phenolic acids is positively regulated by the JA-responsive transcription factor ERF115 in Salvia miltiorrhiza. J. Exp. Bot. 2018, 70, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Wu, Q.; Li, J.; Huang, S.; Cai, L.; Mao, L.; Luo, Z.; Li, L.; Ying, T. Exogenous methyl jasmonate regulates phenolic compounds biosynthesis during postharvest tomato ripening. Postharvest Biol. Technol. 2022, 184, 111760. [Google Scholar] [CrossRef]
- Xu, L.; Wang, X. A Comprehensive Review of Phenolic Compounds in Horticultural Plants. Inter. J. Mol. Sci. 2025, 26, 5767. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, A.K.; Dubey, S.K.; Shah, S.; Kumar, A. A Short Review on Genes Regulating Biosynthesis of Major Secondary Metabolites. In Phytochemical Genomics: Plant Metabolomics and Medicinal Plant Genomics; Swamy, M.K., Kumar, A., Eds.; Springer Nature: Singapore, 2022; pp. 501–519. [Google Scholar]
- Feki, K.; Brini, F.; Ben Amar, S.; Saibi, W.; Masmoudi, K. Comparative functional analysis of two wheat Na+/H+ antiporter SOS1 promoters in Arabidopsis thaliana under various stress conditions. J. Appl. Genet. 2015, 56, 15–26. [Google Scholar] [CrossRef]
- Pasternak, T.; Groot, E.P.; Kazantsev, F.V.; Teale, W.; Omelyanchuk, N.; Kovrizhnykh, V.; Palme, K.; Mironova, V.V. Salicylic Acid Affects Root Meristem Patterning via Auxin Distribution in a Concentration-Dependent Manner. Plant Physiol. 2019, 180, 1725–1739. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, S.; Wang, C.; Ding, G.; Cai, H.; Shi, L.; Xu, F. Induction of jasmonic acid biosynthetic genes inhibits Arabidopsis growth in response to low boron. J. Integr. Plant Biol. 2021, 63, 937–948. [Google Scholar] [CrossRef]
- Feki, K.; Tounsi, S.; Brini, F. Comparison of an antioxidant system in tolerant and susceptible wheat seedlings in response to salt stress. Spanish J. Agr. Res. 2017, 15, e0805. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Maehly, A.C.; Chance, B. The assay of catalases and peroxidases. Methods Biochem. Anal. 1954, 1, 357–424. [Google Scholar] [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Rio, D.C.; Ares, M., Jr.; Hannon, G.J.; Nilsen, T.W. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb. Protoc. 2010, 2010, pdb.prot5439. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agr. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Sweetser, P.B.; Vatvars, A. High-performance liquid chromatographic analysis of abscisic acid in plant extracts. Anal. Biochem. 1976, 71, 68–78. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feki, K.; Kamoun, H.; Ben Romdhane, A.; Tounsi, S.; Harrabi, W.; Salhi, S.; Mhadhbi, H.; Trovato, M.; Brini, F. PvPR10-3 Expression Confers Salt Stress Tolerance in Arabidopsis and Interferes with Jasmonic Acid and ABA Signaling. Plants 2025, 14, 3092. https://doi.org/10.3390/plants14193092
Feki K, Kamoun H, Ben Romdhane A, Tounsi S, Harrabi W, Salhi S, Mhadhbi H, Trovato M, Brini F. PvPR10-3 Expression Confers Salt Stress Tolerance in Arabidopsis and Interferes with Jasmonic Acid and ABA Signaling. Plants. 2025; 14(19):3092. https://doi.org/10.3390/plants14193092
Chicago/Turabian StyleFeki, Kaouthar, Hanen Kamoun, Amal Ben Romdhane, Sana Tounsi, Wissal Harrabi, Sirine Salhi, Haythem Mhadhbi, Maurizio Trovato, and Faiçal Brini. 2025. "PvPR10-3 Expression Confers Salt Stress Tolerance in Arabidopsis and Interferes with Jasmonic Acid and ABA Signaling" Plants 14, no. 19: 3092. https://doi.org/10.3390/plants14193092
APA StyleFeki, K., Kamoun, H., Ben Romdhane, A., Tounsi, S., Harrabi, W., Salhi, S., Mhadhbi, H., Trovato, M., & Brini, F. (2025). PvPR10-3 Expression Confers Salt Stress Tolerance in Arabidopsis and Interferes with Jasmonic Acid and ABA Signaling. Plants, 14(19), 3092. https://doi.org/10.3390/plants14193092







