A Plant-Derived Antifungal Agent, Poacic Acid, Inhibits Germination and Tube Growth of Lily Pollen
Abstract
1. Introduction
2. Results
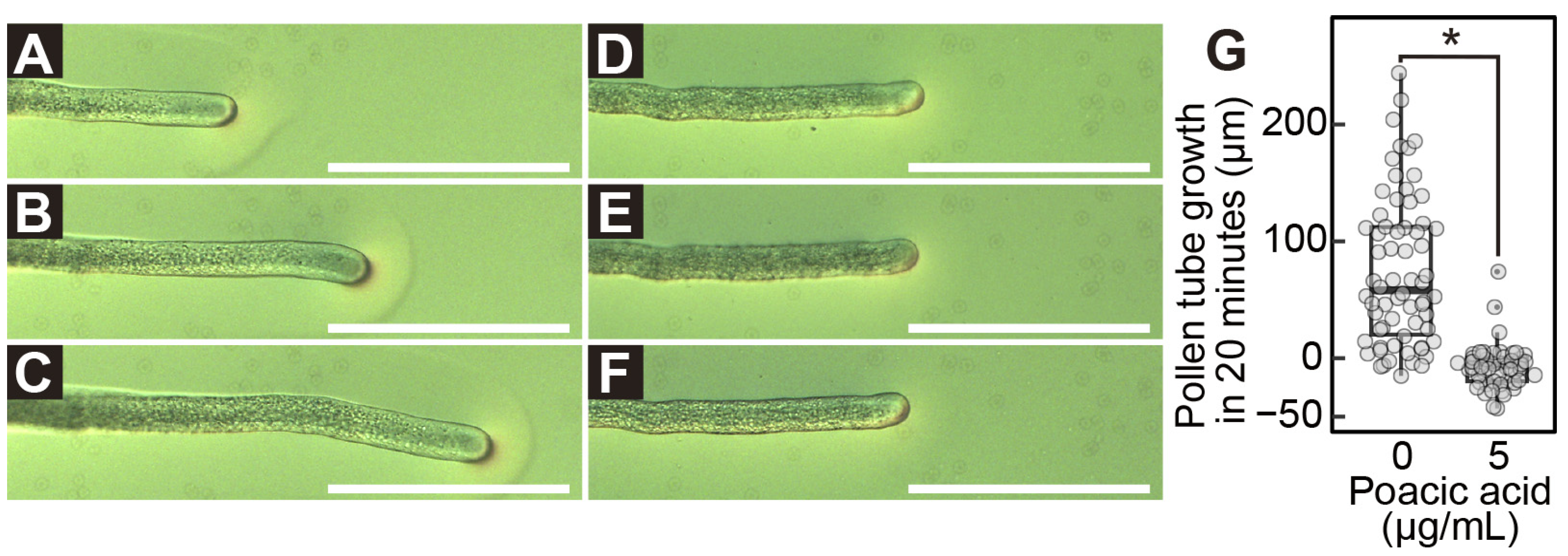
2.1. Poacic Acid Inhibits Lily Pollen Germination and Pollen Tube Growth
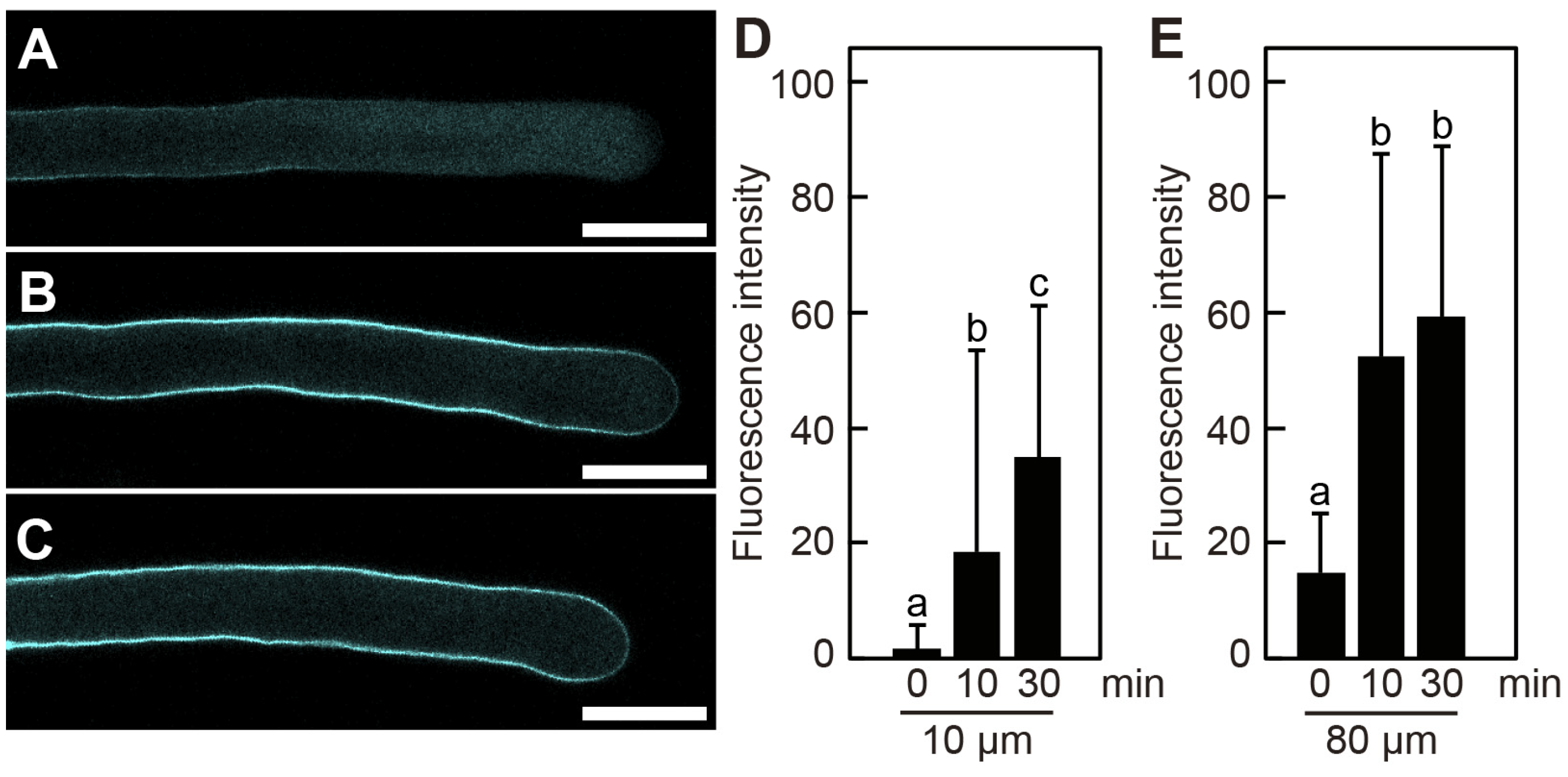
2.2. Poacic Acid Does Not Inhibit Callose Synthesis in Pollen Tubes
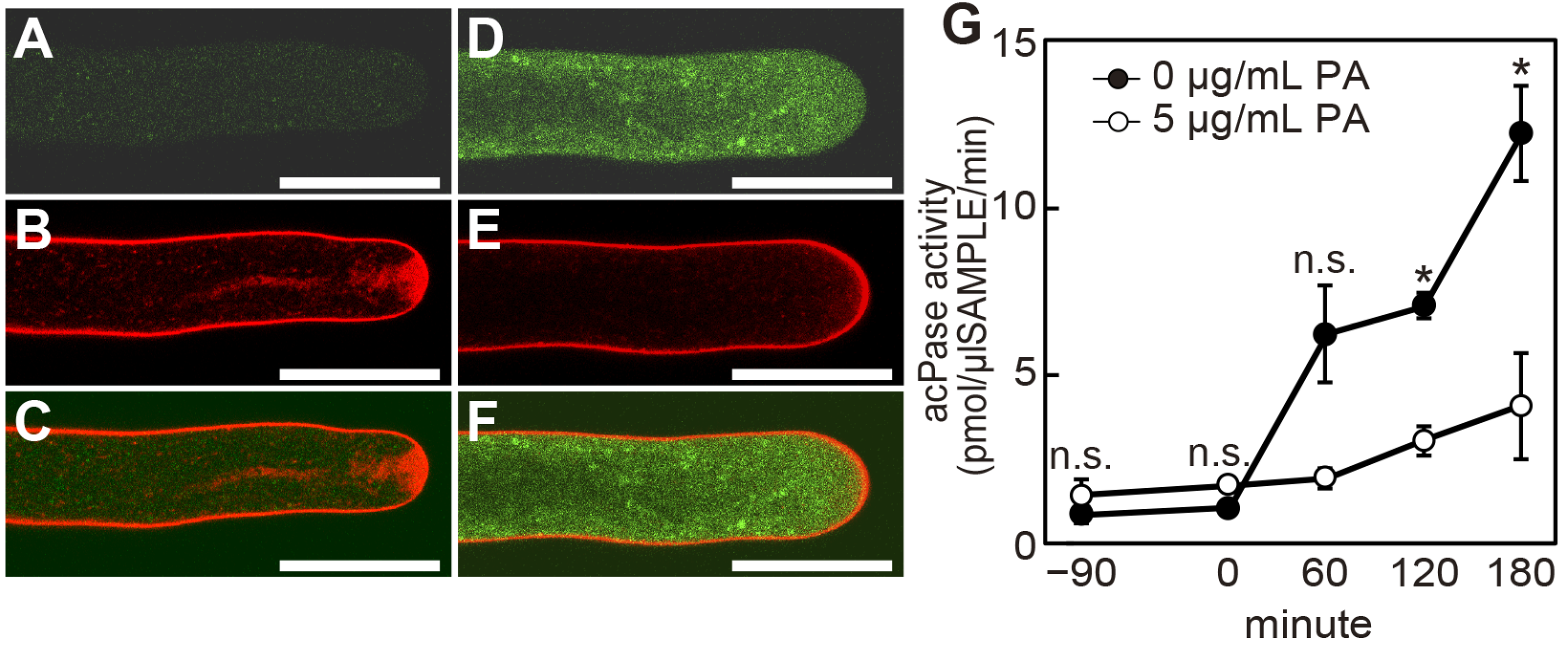
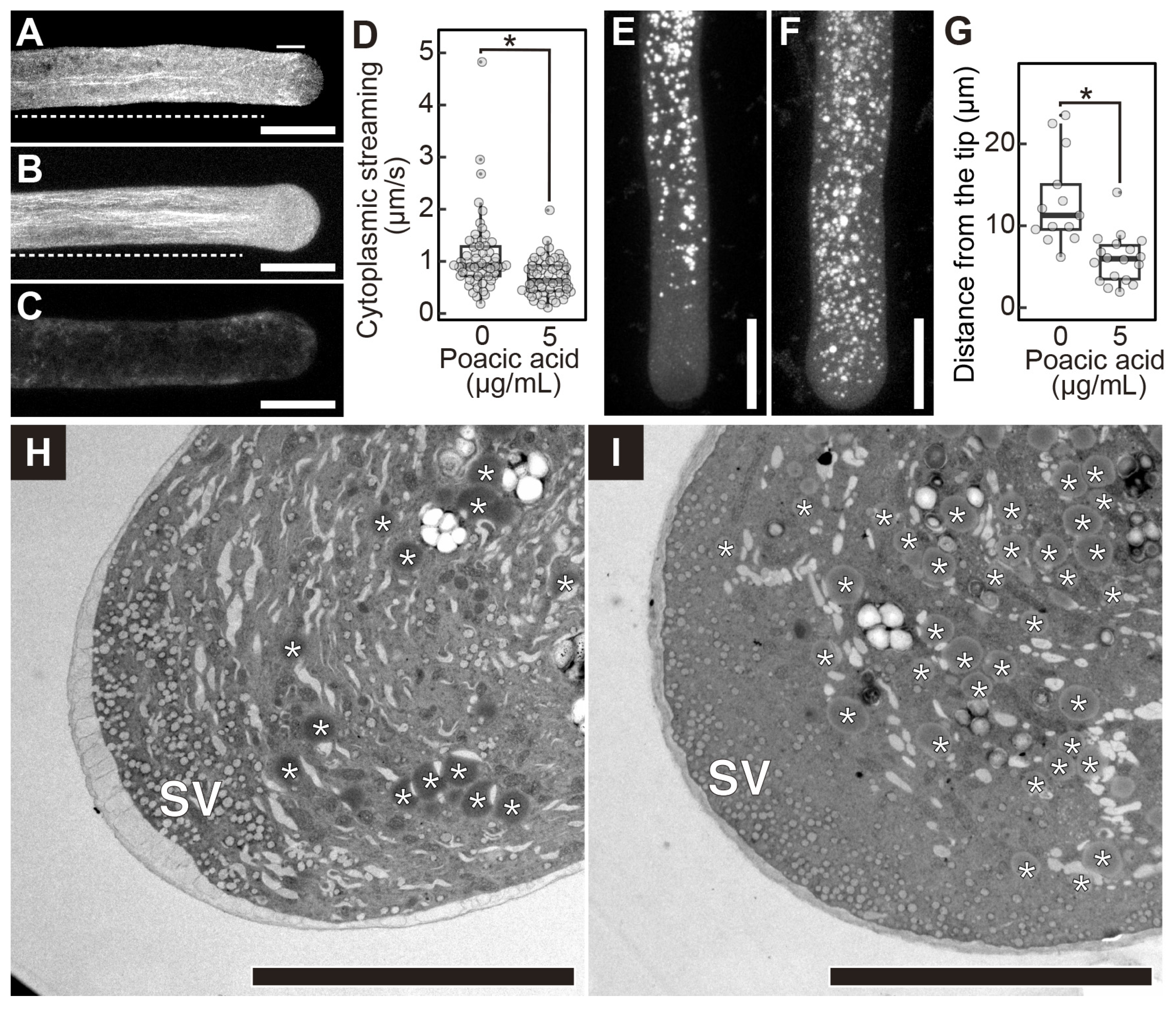
2.3. Poacic Acid Inhibits Endocytosis and Secretion and Disrupts Actin Filaments in Pollen Tubes
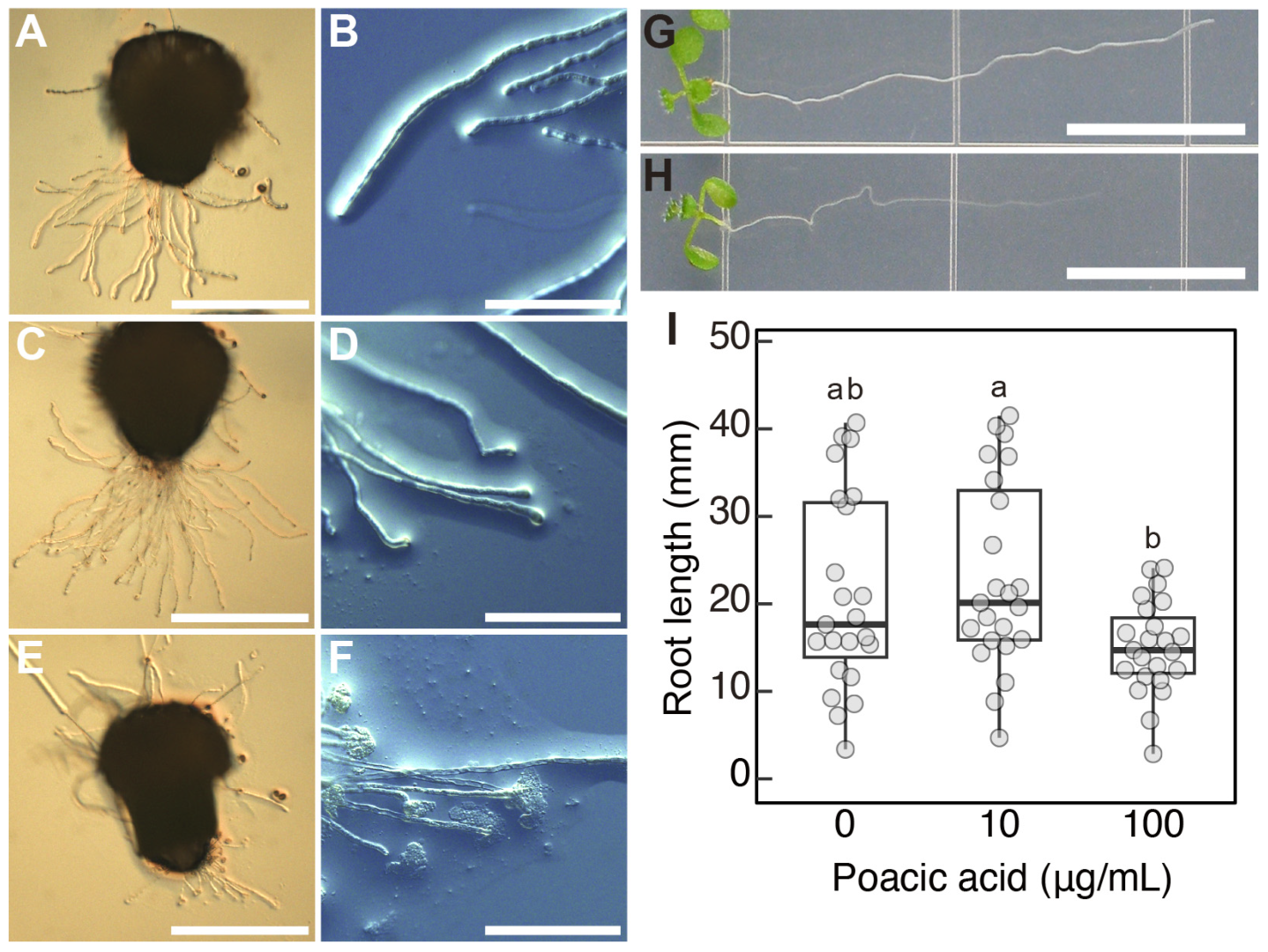
2.4. Poacic Acid Affects the Growth of Arabidopsis thaliana Pollen Tubes and Roots
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions and Chemical Material
4.2. Analysis of Pollen Growth
4.3. Pollen Staining
4.4. Acid Phosphatase Assay
4.5. Electron Microscopy
4.6. Quantification and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piotrowski, J.S.; Okada, H.; Lu, F.; Li, S.C.; Hinchman, L.; Ranjan, A.; Smith, D.L.; Higbee, A.J.; Ulbrich, A.; Coon, J.J.; et al. Plant-Derived Antifungal Agent Poacic Acid Targets β-1,3-Glucan. Proc. Natl. Acad. Sci. USA 2015, 112, E1490–E1497. [Google Scholar] [CrossRef]
- García, R.; Itto-Nakama, K.; Rodríguez-Peña, J.M.; Chen, X.; Sanz, A.B.; de Lorenzo, A.; Pavón-Vergés, M.; Kubo, K.; Ohnuki, S.; Nombela, C.; et al. Poacic Acid, a β-1,3-Glucan-Binding Antifungal Agent, Inhibits Cell-Wall Remodeling and Activates Transcriptional Responses Regulated by the Cell-Wall Integrity and High-Osmolarity Glycerol Pathways in Yeast. FASEB J. 2021, 35, e21778. [Google Scholar] [CrossRef]
- Lesage, G.; Bussey, H. Cell Wall Assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2006, 70, 317–343. [Google Scholar] [CrossRef]
- Douglas, C. Fungal Beta(1,3)-D-Glucan Synthesis. Mikol. Lek. 2001, 39 (Suppl. S1), 55–66. [Google Scholar]
- Hu, X.; Yang, P.; Chai, C.; Liu, J.; Sun, H.; Wu, Y.; Zhang, M.; Zhang, M.; Liu, X.; Yu, H. Structural and Mechanistic Insights into Fungal β-1,3-Glucan Synthase FKS1. Nature 2023, 616, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.K.; Kubo, K.; Abdelaziz, J.A.; Cunningham, I.; de Silva Dantas, A.; Chen, X.; Okada, H.; Ohya, Y.; Gow, N.A.R. Yeast Species-Specific, Differential Inhibition of β-1,3-Glucan Synthesis by Poacic Acid and Caspofungin. Cell Surf. 2018, 3, 12–25. [Google Scholar] [CrossRef]
- Adhikari, P.B.; Liu, X.; Kasahara, R.D. Mechanics of Pollen Tube Elongation: A Perspective. Front. Plant Sci. 2020, 11, 589712. [Google Scholar] [CrossRef] [PubMed]
- Chebli, Y.; Kroeger, J.; Geitmann, A. Transport Logistics in Pollen Tubes. Mol. Plant 2013, 6, 1037–1052. [Google Scholar] [CrossRef]
- Aloisi, I.; Cai, G.; Faleri, C.; Navazio, L.; Serafini-Fracassini, D.; Del Duca, S. Spermine Regulates Pollen Tube Growth by Modulating Ca2+-Dependent Actin Organization and Cell Wall Structure. Front. Plant Sci. 2017, 8, 1701. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Teng, N.; Wu, X.; Wang, Y.; Tang, W.; Samaj, J.; Baluska, F.; Lin, J. Disruption of Actin Filaments by Latrunculin B Affects Cell Wall Construction in Picea Meyeri Pollen Tube by Disturbing Vesicle Trafficking. Plant Cell Physiol. 2007, 48, 19–30. [Google Scholar] [CrossRef]
- Gibbon, B.C.; Kovar, D.R.; Staiger, C.J. Latrunculin B Has Different Effects on Pollen Germination and Tube Growth. Plant Cell 1999, 11, 2349–2363. [Google Scholar] [CrossRef]
- Laggoun, F.; Dardelle, F.; Dehors, J.; Falconet, D.; Driouich, A.; Rochais, C.; Dallemagne, P.; Lehner, A.; Mollet, J.-C. A Chemical Screen Identifies Two Novel Small Compounds That Alter Arabidopsis thaliana Pollen Tube Growth. BMC Plant Biol. 2019, 19, 152. [Google Scholar] [CrossRef]
- Ueda, T.; Tsukaya, H.; Anai, T.; Hirata, A.; Hasegawa, K.; Uchimiya, H. Brefeldin A Induces the Accumulation of Unusual Membrane Structures in Elongating Pollen Tubes of Nicotiana Tabacum L. J. Plant Physiol. 1996, 149, 683–689. [Google Scholar] [CrossRef]
- Wang, Q.; Kong, L.; Hao, H.; Wang, X.; Lin, J.; Samaj, J.; Baluska, F. Effects of Brefeldin A on Pollen Germination and Tube Growth. Antagonistic Effects on Endocytosis and Secretion. Plant Physiol. 2005, 139, 1692–1703. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lin, Z.; Yu, P.; Zeng, Y.; Du, S.; Huang, L.-J. The Multifarious Role of Callose and Callose Synthase in Plant Development and Environment Interactions. Front. Plant Sci. 2023, 14, 1183402. [Google Scholar] [CrossRef] [PubMed]
- Kauss, H. Callose Synthesis, Membranes: Specialized Functions in Plants; Smallwood, M., Knox, J.P., Bowles, D.J., Eds.; Bios Scientific Publishers: Guildford, UK, 1996; pp. 77–92. [Google Scholar]
- Ellinger, D.; Voigt, C.A. Callose Biosynthesis in Arabidopsis with a Focus on Pathogen Response: What We Have Learned within the Last Decade. Ann. Bot. 2014, 114, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Heslop-Harrison, J.; Heslop-Harrison, Y. Evaluation of Pollen Viability by Enzymatically Induced Fluorescence; Intracellular Hydrolysis of Fluorescein Diacetate. Stain. Technol. 1970, 45, 115–120. [Google Scholar] [CrossRef]
- Zhao, L.; Rehmani, M.S.; Wang, H. Exocytosis and Endocytosis: Yin-Yang Crosstalk for Sculpting a Dynamic Growing Pollen Tube Tip. Front. Plant Sci. 2020, 11, 572848. [Google Scholar] [CrossRef]
- Parton, R.M.; Fischer-Parton, S.; Watahiki, M.K.; Trewavas, A.J. Dynamics of the Apical Vesicle Accumulation and the Rate of Growth Are Related in Individual Pollen Tubes. J. Cell Sci. 2001, 114, 2685–2695. [Google Scholar] [CrossRef]
- Ibrahimu, H.; Heidi, P.I.; Pittertschatscher, K.; Fadl-Allah, E.; El-Shahed, A.; Bentrup, F.-W.; Obermeyer, G. Release of an Add Phosphatase Activity during Lily Pollen Tube Growth Involves Components of the Secretory Pathway. Protoplasma 2002, 219, 176–183. [Google Scholar] [CrossRef]
- Qu, X.; Jiang, Y.; Chang, M.; Liu, X.; Zhang, R.; Huang, S. Organization and Regulation of the Actin Cytoskeleton in the Pollen Tube. Front. Plant Sci. 2014, 5, 786. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, S. Control of the Actin Cytoskeleton Within Apical and Subapical Regions of Pollen Tubes. Front. Cell Dev. Biol. 2020, 8, 614821. [Google Scholar] [CrossRef] [PubMed]
- Lovy-Wheeler, A.; Wilsen, K.L.; Baskin, T.I.; Hepler, P.K. Enhanced Fixation Reveals the Apical Cortical Fringe of Actin Filaments as a Consistent Feature of the Pollen Tube. Planta 2005, 221, 95–104. [Google Scholar] [CrossRef]
- Lovy-Wheeler, A.; Kunkel, J.G.; Allwood, E.G.; Hussey, P.J.; Hepler, P.K. Oscillatory Increases in Alkalinity Anticipate Growth and May Regulate Actin Dynamics in Pollen Tubes of Lily. Plant Cell 2006, 18, 2182–2193. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Parrotta, L.; Cresti, M. Organelle Trafficking, the Cytoskeleton, and Pollen Tube Growth. J. Integr. Plant Biol. 2015, 57, 63–78. [Google Scholar] [CrossRef]
- Cai, G.; Cresti, M. Organelle Motility in the Pollen Tube: A Tale of 20 Years. J. Exp. Bot. 2009, 60, 495–508. [Google Scholar] [CrossRef]
- Rounds, C.M.; Hepler, P.K.; Winship, L.J. The Apical Actin Fringe Contributes to Localized Cell Wall Deposition and Polarized Growth in the Lily Pollen Tube. Plant Physiol. 2014, 166, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Bouchnak, I.; Coulon, D.; Salis, V.; D’Andréa, S.; Bréhélin, C. Lipid Droplets Are Versatile Organelles Involved in Plant Development and Plant Response to Environmental Changes. Front. Plant Sci. 2023, 14, 1193905. [Google Scholar] [CrossRef]
- Yang, L.; Liu, J.; Wong, C.-K.; Lim, B.L. Movement of Lipid Droplets in the Arabidopsis Pollen Tube Is Dependent on the Actomyosin System. Plants 2023, 12, 2489. [Google Scholar] [CrossRef]
- Fang, K.; Gao, S.; Zhang, W.; Xing, Y.; Cao, Q.; Qin, L. Addition of Phenylboronic Acid to Malus Domestica Pollen Tubes Alters Calcium Dynamics, Disrupts Actin Filaments and Affects Cell Wall Architecture. PLoS ONE 2016, 11, e0149232. [Google Scholar] [CrossRef]
- Nishikawa, S.-I.; Zinkl, G.M.; Swanson, R.J.; Maruyama, D.; Preuss, D. Callose (Beta-1,3 Glucan) Is Essential for Arabidopsis Pollen Wall Patterning, but Not Tube Growth. BMC Plant Biol. 2005, 5, 22. [Google Scholar] [CrossRef]
- Gu, F.; Nielsen, E. Targeting and Regulation of Cell Wall Synthesis during Tip Growth in Plants. J. Integr. Plant Biol. 2013, 55, 835–846. [Google Scholar] [CrossRef]
- Guo, J.; Yang, Z. Exocytosis and Endocytosis: Coordinating and Fine-Tuning the Polar Tip Growth Domain in Pollen Tubes. J. Exp. Bot. 2020, 71, 2428–2438. [Google Scholar] [CrossRef]
- Picton, J.M.; Steer, M.W. Membrane Recycling and the Control of Secretory Activity in Pollen Tubes. J. Cell Sci. 1983, 63, 303–310. [Google Scholar] [CrossRef]
- Cárdenas, L.; Lovy-Wheeler, A.; Kunkel, J.G.; Hepler, P.K. Pollen Tube Growth Oscillations and Intracellular Calcium Levels Are Reversibly Modulated by Actin Polymerization. Plant Physiol. 2008, 146, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.F.; Muday, G.K. Reactive oxygen species are signaling molecules that modulate plant reproduction. Plant Cell Environ. 2024, 47, 1592–1605. [Google Scholar] [CrossRef]
- Hao, G.-J.; Ying, J.; Li, L.-S.; Yu, F.; Dun, S.-S.; Su, L.-Y.; Zhao, X.-Y.; Li, S.; Zhang, Y. Two Functionally Interchangeable Vps9 Isoforms Mediate Pollen Tube Penetration of Style. New Phytol. 2024, 244, 840–854. [Google Scholar] [CrossRef]
- Hao, G.-J.; Li, L.-S.; Zhao, X.-Y.; Ying, J.; Zhang, M.-M.; Cui, X.-C.; Sun, T.; Li, E.; Su, L.-Y.; Shen, J.; et al. Canonical Rab5 GTPases Are Essential for Pollen Tube Growth through Style in Arabidopsis. New Phytol. 2023, 239, 1740–1753. [Google Scholar] [CrossRef] [PubMed]
- Szumlanski, A.L.; Nielsen, E. The Rab GTPase RabA4d Regulates Pollen Tube Tip Growth in Arabidopsis Thaliana. Plant Cell 2009, 21, 526–544. [Google Scholar] [CrossRef]
- Gebert, M.; Dresselhaus, T.; Sprunck, S. F-Actin Organization and Pollen Tube Tip Growth in Arabidopsis Are Dependent on the Gametophyte-Specific Armadillo Repeat Protein ARO1. Plant Cell 2008, 20, 2798–2814. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, Y.; Higashiyama, T. Antisense Gene Inhibition by Phosphorothioate Antisense Oligonucleotide in Arabidopsis Pollen Tubes. Plant J. 2014, 78, 516–526. [Google Scholar] [CrossRef]
- Pfeiffer, W. Auxin Induces Exocytosis of Acid Phosphatase in Coleoptiles from Zea Mays. Physiol. Plant. 1996, 98, 773–779. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, N.; Ohya, Y.; Hayashi, Y.; Nishikawa, S.-i. A Plant-Derived Antifungal Agent, Poacic Acid, Inhibits Germination and Tube Growth of Lily Pollen. Plants 2025, 14, 3093. https://doi.org/10.3390/plants14193093
Kobayashi N, Ohya Y, Hayashi Y, Nishikawa S-i. A Plant-Derived Antifungal Agent, Poacic Acid, Inhibits Germination and Tube Growth of Lily Pollen. Plants. 2025; 14(19):3093. https://doi.org/10.3390/plants14193093
Chicago/Turabian StyleKobayashi, Nanami, Yoshikazu Ohya, Yasuko Hayashi, and Shuh-ichi Nishikawa. 2025. "A Plant-Derived Antifungal Agent, Poacic Acid, Inhibits Germination and Tube Growth of Lily Pollen" Plants 14, no. 19: 3093. https://doi.org/10.3390/plants14193093
APA StyleKobayashi, N., Ohya, Y., Hayashi, Y., & Nishikawa, S.-i. (2025). A Plant-Derived Antifungal Agent, Poacic Acid, Inhibits Germination and Tube Growth of Lily Pollen. Plants, 14(19), 3093. https://doi.org/10.3390/plants14193093






