Production of Bioactive Compounds in Grammatophyllum speciosum Blume Using Bioreactor Cultures Under Elicitation with Sodium Chloride
Abstract
1. Introduction
2. Results and Discussion
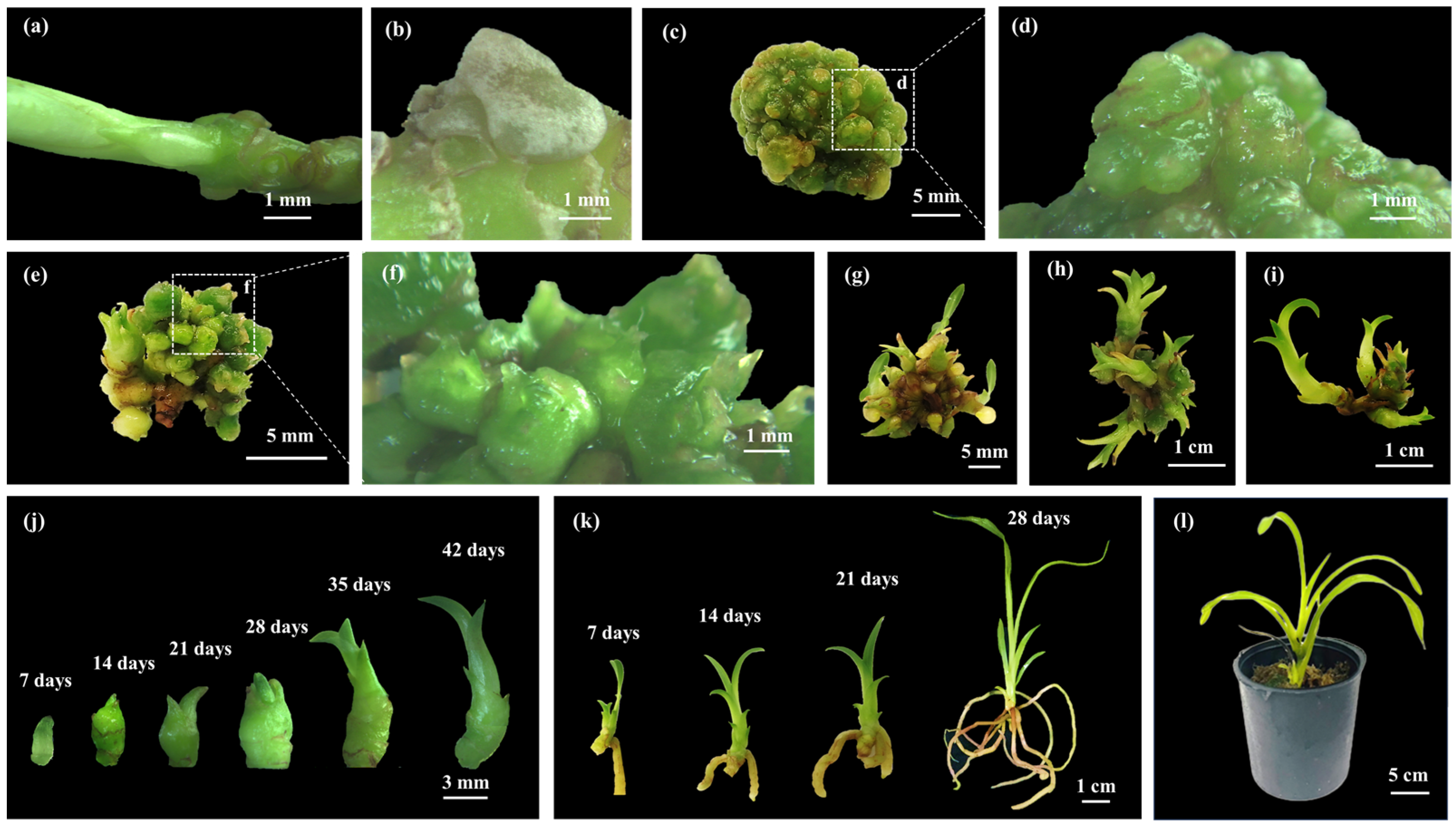
2.1. Induction of Protocorm-like Bodies
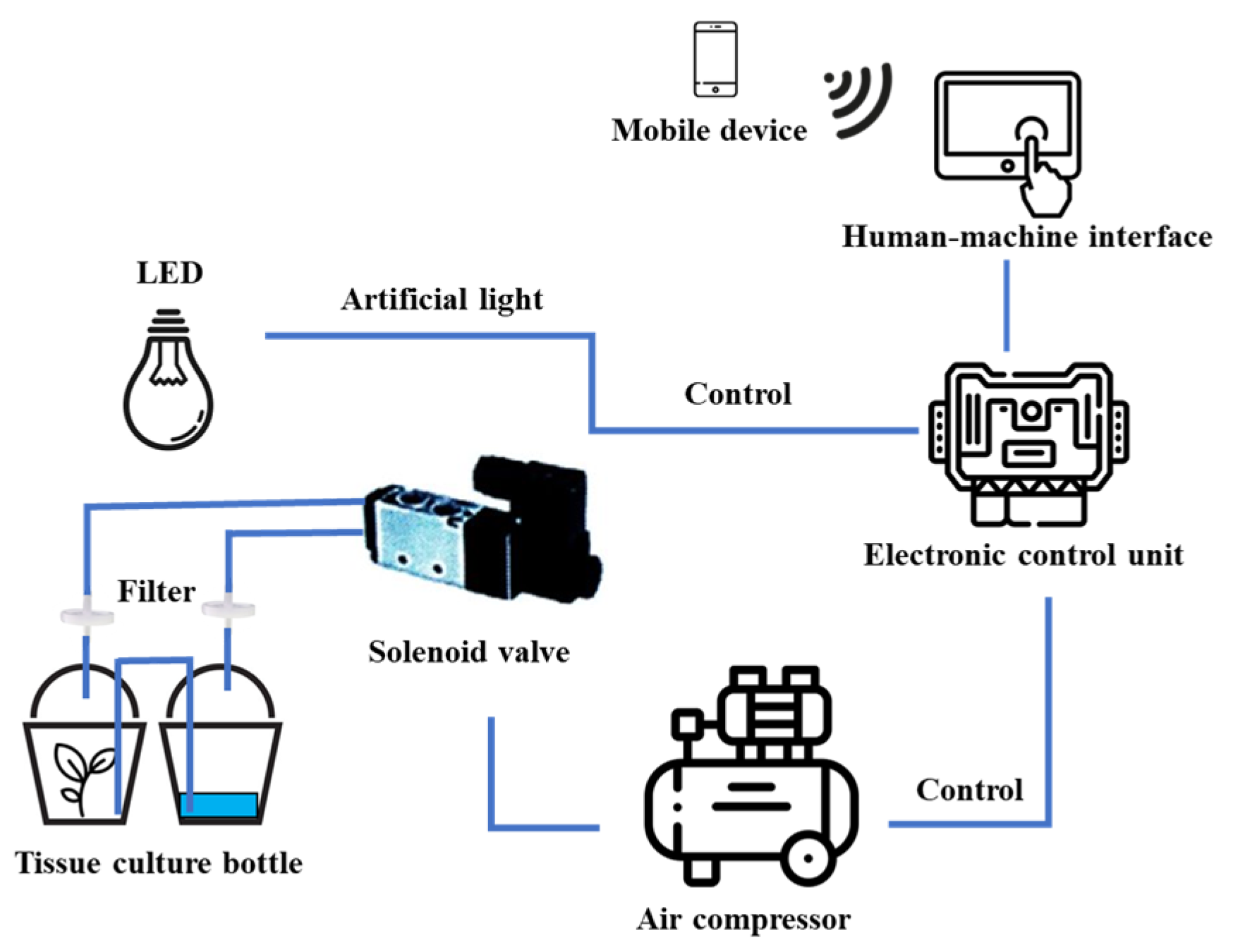
2.2. Effect of Different Immersion Times and Frequencies on Shoot Multiplication
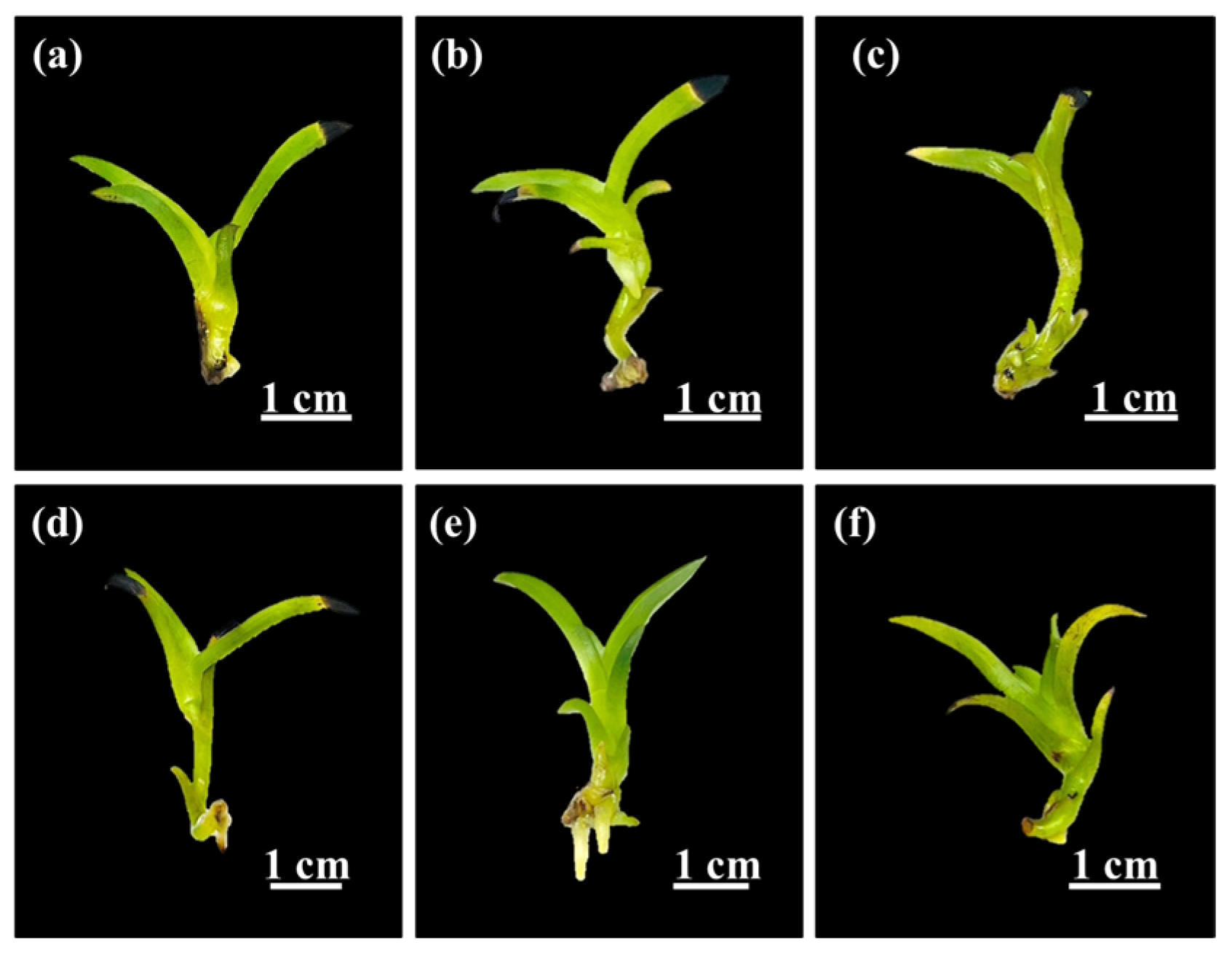
2.3. Root Induction
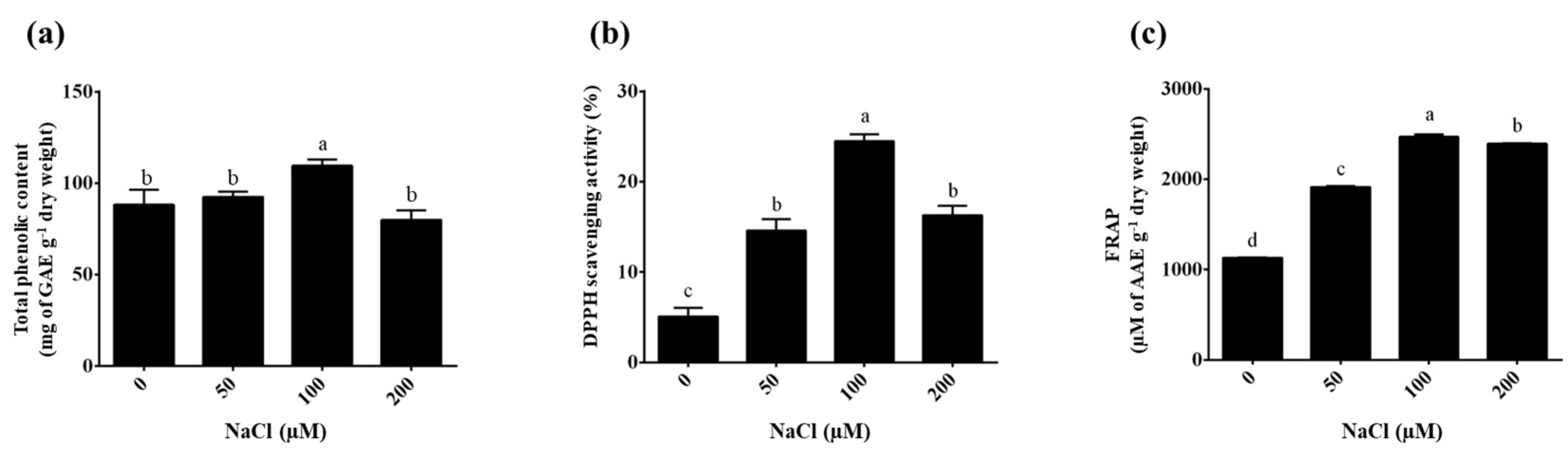
2.4. Effect of NaCl Stress on Total Phenolic Content
2.5. Antioxidant Activity
2.6. Antibacterial Activity
3. Materials and Methods
3.1. Plant Materials and Induction of Protocorm-like Bodies
3.2. Effect of Immersion Time and Frequency on Shoot Multiplication Efficiency
3.3. Root Induction Process
3.4. Morphological Evaluation
3.5. Sodium Chloride Stress Treatments
3.6. Preparation of the Crude Extract
3.7. Quantification of Total Phenolic Content
3.8. DPPH Radical Scavenging Assay
3.9. Ferric-Reducing Antioxidant Power Assay
3.10. Antibacterial Activity Testing
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yam, T.W.; Chua, J.; Tay, F.; Ang, P. Conservation of the native orchids through seedling culture and reintroduction—A Singapore experience. Bot. Rev. 2010, 76, 263–274. [Google Scholar] [CrossRef]
- Teoh, E.S. Medicinal Orchids of Asia; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Chowjarean, V.; Sadabpod, K. Antiproliferative effect of Grammatophyllum speciosum ethanolic extract and its bioactive compound on human breast cancer cells. Sci. World J. 2021, 2021, 3752169. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yingchutrakul, Y.; Sittisaree, W.; Mahatnirunkul, T.; Chomtong, T.; Tulyananda, T.; Krobthong, S. Cosmeceutical potentials of Grammatophyllum speciosum extracts: Anti-inflammations and anti-collagenase activities with phytochemical profile analysis using an untargeted metabolomics approach. Cosmetics 2021, 8, 116. [Google Scholar] [CrossRef]
- Chowjarean, V.; Phiboonchaiyanan, P.P.; Harikarnpakdee, S. Skin brightening efficacy of Grammatophyllum speciosum: A prospective, split-face, randomized placebo-controlled study. Sustainability 2022, 14, 16829. [Google Scholar] [CrossRef]
- Thamrongwatwongsa, J.; Chusrisom, J.; Katemala, K.; Tantasirin, S.; Jumnongjit, P.; Nateerom, P.; Sonjaroon, W.; Tongkok, P.; Pichaiyotinkul, P.; Paemanee, A. Determination of flavonoid content in Grammatophyllum speciosum and in vitro evaluation of their anti-skin cancer and antibacterial activities. Heliyon 2024, 10, e33330. [Google Scholar] [CrossRef]
- Etienne, H.; Berthouly, M. Temporary immersion systems in plant micropropagation. Plant Cell Tissue Organ Cult. 2002, 69, 215–231. [Google Scholar] [CrossRef]
- Rathore, J.S.; Rathore, V.; Shekhawat, N.S.; Singh, R.P.; Liler, G.; Phulwaria, M. Micropropagation of woody plants. In Plant Biotechnology and Molecular Markers; Springer: Dordrecht, The Netherlands, 2004; pp. 195–205. [Google Scholar]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In vitro plant tissue culture: Means for production of biological active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cardoso, J.C.; Zanello, C.A.; Chen, J.T. An Overview of Orchid Protocorm-Like Bodies: Mass Propagation, Biotechnology, Molecular Aspects, and Breeding. Int. J. Mol. Sci. 2020, 21, 985. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Esyanti, R.; Adhitama, N.; Manurung, R. Efficiency evaluation of vanda tricolor growth in temporary immerse system bioreactor and thin layer culture system. J. Adv. Agric. Technol. 2016, 3, 63–66. [Google Scholar] [CrossRef]
- Alvard, D.; Cote, F.; Teisson, C. Comparison of methods of liquid medium culture for banana micropropagation: Effects of temporary immersion of explants. Plant Cell Tissue Organ Cult. 1993, 32, 55–60. [Google Scholar] [CrossRef]
- Lorenzo, J.C.; Ojeda, E.; Espinosa, A.; Borroto, C. Field performance of temporary immersion bioreactor-derived sugarcane plants. Vitr. Cell. Dev. Biol. Plant 2001, 37, 803–806. [Google Scholar] [CrossRef]
- Reis, C.O.D.; Silva, A.B.D.; Landgraf, P.R.C.; Batista, J.A.; Jacome, G.A.R. Bioreactor in the micropropagation of ornamental pineapple. Ornam. Hortic. 2018, 24, 182–187. [Google Scholar] [CrossRef]
- Bello-Bello, J.J.; Schettino-Salomón, S.; Ortega-Espinoza, J.; Spinoso-Castillo, J.L. A temporary immersion system for mass micropropagation of pitahaya (Hylocereus undatus). 3 Biotech 2021, 11, 437. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Solis-Gracia, N.; Teale, M.K.; Mandadi, K.; Silva JAd Vales, M.I. Development of an in vitro microtuberization and temporary immersion bioreactor system to evaluate heat stress tolerance in potatoes (Solanum tuberosum L.). Front. Plant Sci. 2021, 12, 700328. [Google Scholar] [CrossRef]
- De Carlo, A.; Tarraf, W.; Lambardi, M.; Benelli, C. Temporary immersion system for production of biomass and bioactive compounds from medicinal plants. Agronomy 2021, 11, 2414. [Google Scholar] [CrossRef]
- Rout, G.R.; Samantaray, S.; Das, P. In vitro manipulation and propagation of medicinal plants. Biotechnol. Adv. 2000, 18, 91–120. [Google Scholar] [CrossRef]
- Le, K.-C.; Dedicova, B.; Johansson, S.; Lelu-Walter, M.-A.; Egertsdotter, U. Temporary immersion bioreactor system for propagation by somatic embryogenesis of hybrid larch (Larix × eurolepis Henry). Biotechnol. Rep. 2021, 32, e00684. [Google Scholar] [CrossRef]
- Farahani, F.; Majd, A. Comparison of liquid culture methods and effect of temporary immersion bioreactor on growth and multiplication of banana (Musa, cv. Dwarf Cavendish). Afr. J. Biotechnol. 2012, 11, 8302–8308. [Google Scholar] [CrossRef]
- Ahmadian, M.; Babaei, A.; Shokri, S.; Hessami, S. Micropropagation of carnation (Dianthus caryophyllus L.) in liquid medium by temporary immersion bioreactor in comparison with solid culture. J. Genet. Eng. Biotechnol. 2017, 15, 309–315. [Google Scholar] [CrossRef]
- Vendrame, W.A.; Xu, J.; Beleski, D.G. Micropropagation of Brassavola nodosa (L.) Lindl. using SETIS™ bioreactor. Plant Cell Tissue Organ Cult. (PCTOC) 2023, 153, 67–76. [Google Scholar] [CrossRef]
- Fritsche, Y.; Deola, F.; da Silva, D.A.; Holderbaum, D.F.; Guerra, M.P. Cattleya tigrina (Orchidaceae) in vitro regeneration: Main factors for optimal protocorm-like body induction and multiplication, plantlet regeneration, and cytogenetic stability. S. Afr. J. Bot. 2022, 149, 96–108. [Google Scholar] [CrossRef]
- Cui, H.-Y.; Murthy, H.N.; Moh, S.H.; Cui, Y.-Y.; Lee, E.-J.; Paek, K.-Y. Production of biomass and bioactive compounds in protocorm cultures of Dendrobium candidum Wall ex Lindl. using balloon type bubble bioreactors. Ind. Crops Prod. 2014, 53, 28–33. [Google Scholar] [CrossRef]
- Zhang, B.; Niu, Z.; Zhou, A.; Zhang, D.; Xue, Q.; Liu, W.; Chen, J.; Shen, J.; Ding, X. Micropropagation of Dendrobium nobile Lindl. plantlets by temporary immersion bioreactor. J. Biobased Mater. Bioenergy 2019, 13, 395–400. [Google Scholar] [CrossRef]
- Kang, F.; Hsu, S.; Shen, R. Virus elimination through meristem culture and rapid clonal propagation by temporary immersion system in Phalaenopsis. J. Taiwan Soc. Hortic. Sci. 2011, 57, 207–218. [Google Scholar]
- Spinoso-Castillo, J.; Chavez-Santoscoy, R.; Bogdanchikova, N.; Pérez-Sato, J.; Morales-Ramos, V.; Bello-Bello, J. Antimicrobial and hormetic effects of silver nanoparticles on in vitro regeneration of vanilla (Vanilla planifolia Jacks. ex Andrews) using a temporary immersion system. Plant Cell Tissue Organ Cult. (PCTOC) 2017, 129, 195–207. [Google Scholar] [CrossRef]
- Obchant, T. Orchids of Thailand. Baanlaesuan; Office of Environmental Policy and Planning: Bangkok, Thailand, 2003. [Google Scholar]
- Nitcha, L. Academic Documents “Grammatophyllum speciosum”; Department of Agriculture: Bangkok, Thailand, 2016. [Google Scholar]
- Samala, S.; Te-Chato, S.; Yenchon, S.; Thammasiri, K. Protocorm-like body proliferation of Grammatophyllum speciosum through asymbiotic seed germination. Sci. Asia 2014, 40, 379–383. [Google Scholar] [CrossRef][Green Version]
- Su, Y.-H.; Liu, Y.-B.; Zhang, X.-S. Auxin–cytokinin interaction regulates meristem development. Mol. Plant 2011, 4, 616–625. [Google Scholar] [CrossRef]
- Mangena, P. Benzyl adenine in plant tissue culture-succinct analysis of the overall influence in soybean [Glycine max (L.) Merrill.] seed and shoot culture establishment. J. Biotech Res. 2020, 11, 23–34. [Google Scholar]
- Moubayidin, L.; Di Mambro, R.; Sabatini, S. Cytokinin-auxin crosstalk. Trends Plant Sci. 2009, 14, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Madhulatha, P.; Anbalagan, M.; Jayachandran, S.; Sakthivel, N. Influence of liquid pulse treatment with growth regulators on in vitro propagation of banana (Musa spp. AAA). Plant Cell Tissue Organ Cult. 2004, 76, 189–192. [Google Scholar] [CrossRef]
- Jafari, N.; Othman, R.Y.; Khalid, N. Effect of benzylaminopurine (BAP) pulsing on in vitro shoot multiplication of Musa acuminata (banana) cv. Berangan. Afr. J. Biotechnol. 2011, 10, 2446–2450. [Google Scholar]
- Rahman, M.Z.; Nasiruddin, K.; Amin, M.A.; Islam, M.N. In vitro response and shoot multiplication of banana with BAP and NAA. Asian J. Plant Sci. 2004, 3, 406–409. [Google Scholar] [CrossRef]
- Zhang, B.; Song, L.; Bekele, L.D.; Shi, J.; Jia, Q.; Zhang, B.; Jin, L.; Duns, G.J.; Chen, J. Optimizing factors affecting development and propagation of Bletilla striata in a temporary immersion bioreactor system. Sci. Hortic. 2018, 232, 121–126. [Google Scholar] [CrossRef]
- Ekmekçigil, M.; Bayraktar, M.; Akkuş, Ö.; Gürel, A. High-frequency protocorm-like bodies and shoot regeneration through a combination of thin cell layer and RITA® temporary immersion bioreactor in Cattleya forbesii Lindl. Plant Cell Tissue Organ Cult. (PCTOC) 2019, 136, 451–464. [Google Scholar] [CrossRef]
- Reyes-Beristain, B.; Mancilla-Álvarez, E.; López-Buenfil, J.A.; Bello-Bello, J.J. Temporary Immersion Bioreactor for In Vitro Multiplication of Raspberry (Rubus idaeus L.). Horticulturae 2025, 11, 842. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Vacin, E.F.; Went, F.W. Some pH changes in nutrient solutions. Bot. Gaz. 1949, 110, 605–613. [Google Scholar] [CrossRef]
- Adelberg, J.W.; Delgado, M.P.; Tomkins, J.T. Spent medium analysis for liquid culture micropropagation of Hemerocallis on Murashige and Skoog medium. Vitr. Cell. Dev. Biol. Plant 2010, 46, 95–107. [Google Scholar] [CrossRef]
- Salsabila, S.; Fatimah, K.; Sidek, N.; Halimatun, T.; Aurifullah, M.; Zakaria, S. Effect of coconut water and peptone in micropropagation of Phalaenopsis amabilis (L.) Blume Orchid. IOP Conf. Ser. Earth Environ. Sci. 2022, 1102, 012002. [Google Scholar] [CrossRef]
- De Stefano, D.; Costa, B.N.S.; Downing, J.; Fallahi, E.; Khoddamzadeh, A.A. In-vitro micropropagation and acclimatization of an endangered native orchid using organic supplements. Am. J. Plant Sci. 2022, 13, 380–393. [Google Scholar] [CrossRef]
- Chowjarean, V.; Sucontphunt, A.; Vchirawongkwin, S.; Charoonratana, T.; Songsak, T.; Harikarnpakdee, S.; Tengamnuay, P. Validated RP-HPLC method for quantification of gastrodin in ethanolic extract from the pseudobulbs of Grammatophyllum speciosum blume. Malays. J. Anal. Sci. 2018, 22, 219–226. [Google Scholar] [CrossRef]
- Nag, S.; Kumaria, S. In silico characterization and transcriptional modulation of phenylalanine ammonia lyase (PAL) by abiotic stresses in the medicinal orchid Vanda coerulea Griff. ex Lindl. Phytochemistry 2018, 156, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Mohammed Lamine, K.; Boudjeniba, M.; Kameli, A. Effects of salt stress on micropropagation of potato (Solanum tuberosum L.). Afr. J. Biotechnol. 2011, 10, 7840–7845. [Google Scholar] [CrossRef]
- Joshi, M.; Mishra, A.; Jha, B. NaCl plays a key role for in vitro micropropagation of Salicornia brachiata, an extreme halophyte. Ind. Crops Prod. 2012, 35, 313–316. [Google Scholar] [CrossRef]
- Alturki, S. Effect of NaCl on growth and development of in vitro plants of date palm (Phoenix dactylifera L.) ’Khainazi’ cultivar. Asian J. Plant Sci. 2018, 17, 120–128. [Google Scholar] [CrossRef]
- Dogan, M. Effect of salt stress on in vitro organogenesis from nodal explant of Limnophila aromatica (Lamk.) Merr. and Bacopa monnieri (L.) Wettst. and their physio-morphological and biochemical responses. Physiol. Mol. Biol. Plants 2020, 26, 803–816. [Google Scholar] [CrossRef]
- Abdullakasim, S.; Kongpaisan, P.; Thongjang, P.; Saradhuldhat, P. Physiological responses of potted Dendrobium orchid to salinity stress. Hortic. Environ. Biotechnol. 2018, 59, 491–498. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Feng, F.; Liang, D.; Cheng, L.; Ma, F.; Shi, S. Overexpression of a Malus vacuolar Na+/H+ antiporter gene (MdNHX1) in apple rootstock M. 26 and its influence on salt tolerance. Plant Cell Tissue Organ Cult. (PCTOC) 2010, 102, 337–345. [Google Scholar] [CrossRef]
- Kumar, K.; Debnath, P.; Singh, S.; Kumar, N. An Overview of Plant phenolics and their involvement in abiotic stress tolerance. Stresses 2023, 3, 570–585. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fujita, M. Plant Responses and Tolerance to Salt Stress: Physiological and Molecular Interventions. Int. J. Mol. Sci. 2022, 23, 4810. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, R.B.; Rao, V.P.; Sengar, R.S. Study of salinity induced oxidative stress and antioxidant responses in callus cultures of sugarcane. Ecol. Genet. Genom. 2023, 26, 100164. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol. Adv. 2009, 27, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Kesawat, M.S.; Satheesh, N.; Kherawat, B.S.; Kumar, A.; Kim, H.-U.; Chung, S.-M.; Kumar, M. Regulation of reactive oxygen species during salt stress in plants and their crosstalk with other signaling molecules—Current perspectives and future directions. Plants 2023, 12, 864. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Guo, B.; Wang, B.; Li, X.; Yang, T.; Li, N.; Wang, J.; Yu, Q. Integrated metabolomic and transcriptomic analysis reveals the role of phenylpropanoid biosynthesis pathway in tomato roots during salt stress. Front. Plant Sci. 2022, 13, 1023696. [Google Scholar] [CrossRef]
- Jia, J.; Wang, F.; Yuan, M.; Wang, Z.; Qin, Z.; Zhang, X.; Shao, Y.; Pei, H. Transcriptomic Analysis Reveals That the Photosynthesis and Carotenoid Metabolism Pathway Is Involved in the Salinity Stress Response in Brassica rapa L. ssp. Pekinensis. Plants 2025, 14, 566. [Google Scholar] [CrossRef]
- Li, C.; Qi, Y.; Zhao, C.; Wang, X.; Zhang, Q. Transcriptome Profiling of the Salt Stress Response in the Leaves and Roots of Halophytic Eutrema salsugineum. Front. Genet. 2021, 12, 770742. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, K.; Fan, G.; Yao, W.; Cheng, Z.; Zhou, B.; Jiang, T. PagMYB73 enhances salt stress tolerance by regulating reactive oxygen species scavenging and osmotic maintenance in poplar. Ind. Crops Prod. 2024, 208, 117893. [Google Scholar] [CrossRef]
- Cao, Y.H.; Lü, Z.L.; Li, Y.H.; Jiang, Y.; Zhang, J.L. Integrated metabolomic and transcriptomic analysis reveals the role of root phenylpropanoid biosynthesis pathway in the salt tolerance of perennial ryegrass. BMC Plant Biol. 2024, 24, 1225. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nagananda, G.; Rajath, S.; Shankar, P.A.; Rajani, M.L. Phytochemical evaluation and in vitro free radical scavenging activity of successive whole plant extract of orchid Cottonia peduncularis. Res. Art Biol. Sci. 2013, 3, 91. [Google Scholar]
- Athipornchai, A.; Jullapo, N. Tyrosinase inhibitory and antioxidant activities of Orchid (Dendrobium spp.). S. Afr. J. Bot. 2018, 119, 188–192. [Google Scholar] [CrossRef]
- Rashmi, K.; Shweta, S.; Sudeshna, C.; Vrushala, P.; Kekuda, T.P.; Raghavendra, H. Antibacterial and radical scavenging activity of selected orchids of Karnataka, India. Sci. Technol. Arts Res. J. 2015, 4, 160–164. [Google Scholar] [CrossRef]
- Chand, M.B.; Paudel, M.R.; Pant, B. The antioxidant activity of selected wild orchids of Nepal. J. Coast. Life Med. 2016, 4, 731–736. [Google Scholar] [CrossRef]
- Sahakitpichan, P.; Mahidol, C.; Disadee, W.; Chimnoi, N.; Ruchirawat, S.; Kanchanapoom, T. Glucopyranosyloxybenzyl derivatives of (R)-2-benzylmalic acid and (R)-eucomic acid, and an aromatic glucoside from the pseudobulbs of Grammatophyllum speciosum. Tetrahedron 2013, 69, 1031–1037. [Google Scholar] [CrossRef]
- Duan, X.-H.; Li, Z.-L.; Yang, D.-S.; Zhang, F.-L.; Lin, Q.; Dai, R. Study on the chemical constituents of Gastrodia elata. J. Chin. Med. Mater. 2013, 36, 1608–1611. [Google Scholar]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial properties of polyphenols: Characterization and qsar (quantitative structure-activity relationship) models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- González-Cortazar, M.; López-Gayou, V.; Tortoriello, J.; Domínguez-Mendoza, B.E.; Ríos-Cortes, A.M.; Delgado-Macuil, R.; Hernández-Beteta, E.E.; Blé-González, E.A.; Zamilpa, A. Antimicrobial gastrodin derivatives isolated from Bacopa procumbens. Phytochem. Lett. 2019, 31, 33–38. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J. Clin. Med. 2019, 9, 109. [Google Scholar] [CrossRef]
- Irimescu, L.S.; Digută, C.F.; Encea, R.Ş.; Matei, F. Preliminary study on the antimicrobial potential of phalaenopsis orchids methanolic extracts. Sci. Bull. Ser. F Biotechnol. 2020, 24, 149–153. [Google Scholar]
- Olivares, C.G. Studies on the Inhibitory Potential of Orchid Extracts Against Acne Associated Bacteria. Bachelor’s Thesis, Valencia Polytechnic University, Valencia, Italy, 2020. [Google Scholar]
- Attard, E. A rapid microtitre plate Folin-Ciocalteu method for the assessment of polyphenols. Cent. Eur. J. Biol. 2013, 8, 48–53. [Google Scholar] [CrossRef]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Basri, D.; Fan, S. The potential of aqueous and acetone extracts of galls of Quercus infectoria as antibacterial agents. Indian J. Pharmacol. 2005, 37, 26–29. [Google Scholar] [CrossRef]




| Treatment | Hormone (mg L−1) | No. Shoots/Explant | Fresh Growth Index | |||||
|---|---|---|---|---|---|---|---|---|
| BAP | NAA | 28 Days | 35 Days | 42 Days | 28 Days | 35 Days | 42 Days | |
| T1 | - | - | 9.00 ± 1.73 b | 18.33 ± 1.53 b | 19.33 ± 1.15 b | 0.63 ± 0.11 ns | 5.44 ± 0.46 b | 6.69 ± 0.40 b |
| T2 | 1.0 | 0.5 | 16.00 ± 1.73 a | 32.33 ± 2.52 a | 35.00 ± 3.00 a | 1.00 ± 0.34 ns | 8.78 ± 2.09 a | 9.92 ± 1.31 a |
| T3 | 1.0 | 1.0 | 7.67 ± 0.58 b c | 18.00 ± 2.00 b | 18.67 ± 2.08 b | 0.55 ± 0.15 ns | 5.14 ± 0.77 b | 6.41 ± 0.77 bc |
| T4 | 2.0 | 0.5 | 4.33 ± 2.31 c | 10.67 ± 1.15 c | 12.33 ± 1.53 c | 0.23 ± 0.07 ns | 4.44 ± 1.07 bc | 4.88 ± 0.60 cd |
| T5 | 2.0 | 1.0 | 8.00 ± 1.00 bc | 12.67 ± 2.08 c | 13.67 ± 1.53 c | 0.66 ± 0.12 ns | 4.89 ± 0.19 bc | 5.83 ± 0.06 bc |
| T6 | 2.0 | 2.0 | 5.67 ± 1.15 bc | 6.33 ± 1.15 d | 6.67 ±0.58 d | 0.38 ± 0.06 ns | 3.17 ± 1.11 c | 3.82 ± 0.77 d |
| Treatment | Immersion Frequency | No. Shoots/Explant | Shoot Height (cm) | Fresh Growth Index | |
|---|---|---|---|---|---|
| Frequency/Day | Immersion | ||||
| IF1 | 8 | 5 min, every 3 h | 20.00 ± 0.82 d | 2.77 ± 0.31 bc | 0.23 ± 0.02 c |
| IF2 | 4 | 5 min, every 6 h | 38.00 ± 2.94 c | 1.93 ± 0.12 bc | 2.12 ± 0.63 b |
| IF3 | 2 | 5 min, every 12 h | 33.00 ± 3.27 cd | 1.10 ± 0.33 cd | 2.32 ± 0.50 b |
| IF4 | 8 | 10 min, every 3 h | 127.00 ± 2.16 a | 5.00 ± 0.51 a | 4.26 ± 0.52 a |
| IF5 | 4 | 10 min, every 6 h | 95.33 ± 7.59 b | 1.27 ± 0.12 d | 3.21 ± 0.59 ab |
| IF6 | 2 | 10 min, every 12 h | 47.33 ± 5.56 c | 3.70 ± 0.45 b | 3.05 ± 0.50 ab |
| Treatment | Medium | Hormone (mg L−1) | No. Roots /Explant | Length of Roots (cm) | Fresh Growth Index | Shoot Height (cm) | No. Leaves /Explant | Length of Leaves (cm) | |
|---|---|---|---|---|---|---|---|---|---|
| ½ MS | BAP | NAA | |||||||
| RT1 | ½ MS ½ MS | 1.0 | 0.5 | 1.00 ± 0.00 | 0.40 ± 0.10 b | 1.17 ± 0.50 c | 2.35 ± 0.15 ns | 4.50 ± 0.50 ns | 1.25 ± 0.05 ns |
| RT2 | - | 0.5 | 0.50 ± 0.50 b | 0.20 ± 0.20 b | 2.42 ± 0.08 | 3.45 ± 0.35 ns | 3.50 ± 0.50 ns | 2.20 ± 0.30 ns | |
| RT3 | MS | 1.0 | 0.5 | nd | nd | 1.75 ± 0.08 b | 2.65 ± 0.15 ns | 4.50 ± 0.50 ns | 0.95 ± 0.15 ns |
| RT4 | MS | - | 0.5 | nd | nd | 1.83 ± 0.17 b | 2.75 ± 0.25 ns | 3.50 ± 0.50 ns | 1.75 ± 0.25 ns |
| RT5 | VW * | - | - | 2.50 ± 0.50 a | 1.35 ± 0.15 a | 3.92 ± 0.25 a | 2.85 ± 0.05 ns | 4.50 ± 0.50 ns | 2.25 ± 0.15 ns |
| RT6 | VW * | - | - | 0.50 ± 0.50 b | 0.50 ± 0.50 b | 3.00 ± 0.33 ab | 3.35 ± 0.15 ns | 3.50 ± 0.50 ns | 0.95 ± 0.25 ns |
| Bacterium | MIC (mg mL−1) | MBC (mg mL−1) |
|---|---|---|
| Staphylococcus aureus | 12.8 | 25.6 |
| Staphylococcus epidermidis | 25.6 | 51.2 |
| Propionibacterium acnes | 6.4 | 12.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chusrisom, J.; Matmarurat, G.; T-Thienprasert, N.P.; Phonphoem, W.; Tongkok, P. Production of Bioactive Compounds in Grammatophyllum speciosum Blume Using Bioreactor Cultures Under Elicitation with Sodium Chloride. Plants 2025, 14, 3083. https://doi.org/10.3390/plants14193083
Chusrisom J, Matmarurat G, T-Thienprasert NP, Phonphoem W, Tongkok P. Production of Bioactive Compounds in Grammatophyllum speciosum Blume Using Bioreactor Cultures Under Elicitation with Sodium Chloride. Plants. 2025; 14(19):3083. https://doi.org/10.3390/plants14193083
Chicago/Turabian StyleChusrisom, Jittraporn, Gadewara Matmarurat, Nattanan Panjaworayan T-Thienprasert, Wannarat Phonphoem, and Pattama Tongkok. 2025. "Production of Bioactive Compounds in Grammatophyllum speciosum Blume Using Bioreactor Cultures Under Elicitation with Sodium Chloride" Plants 14, no. 19: 3083. https://doi.org/10.3390/plants14193083
APA StyleChusrisom, J., Matmarurat, G., T-Thienprasert, N. P., Phonphoem, W., & Tongkok, P. (2025). Production of Bioactive Compounds in Grammatophyllum speciosum Blume Using Bioreactor Cultures Under Elicitation with Sodium Chloride. Plants, 14(19), 3083. https://doi.org/10.3390/plants14193083










