Erica spiculifolia Salisb. (Balkan Heath): A Focus on Metabolic Profiling and Antioxidant and Enzyme Inhibitory Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. LC-HRMS Profiling of Secondary Metabolites in Erica spiculifolia Methanol–Aqueous Extracts (ES1 and ES2)
- Acylquinic acids (ACAs)
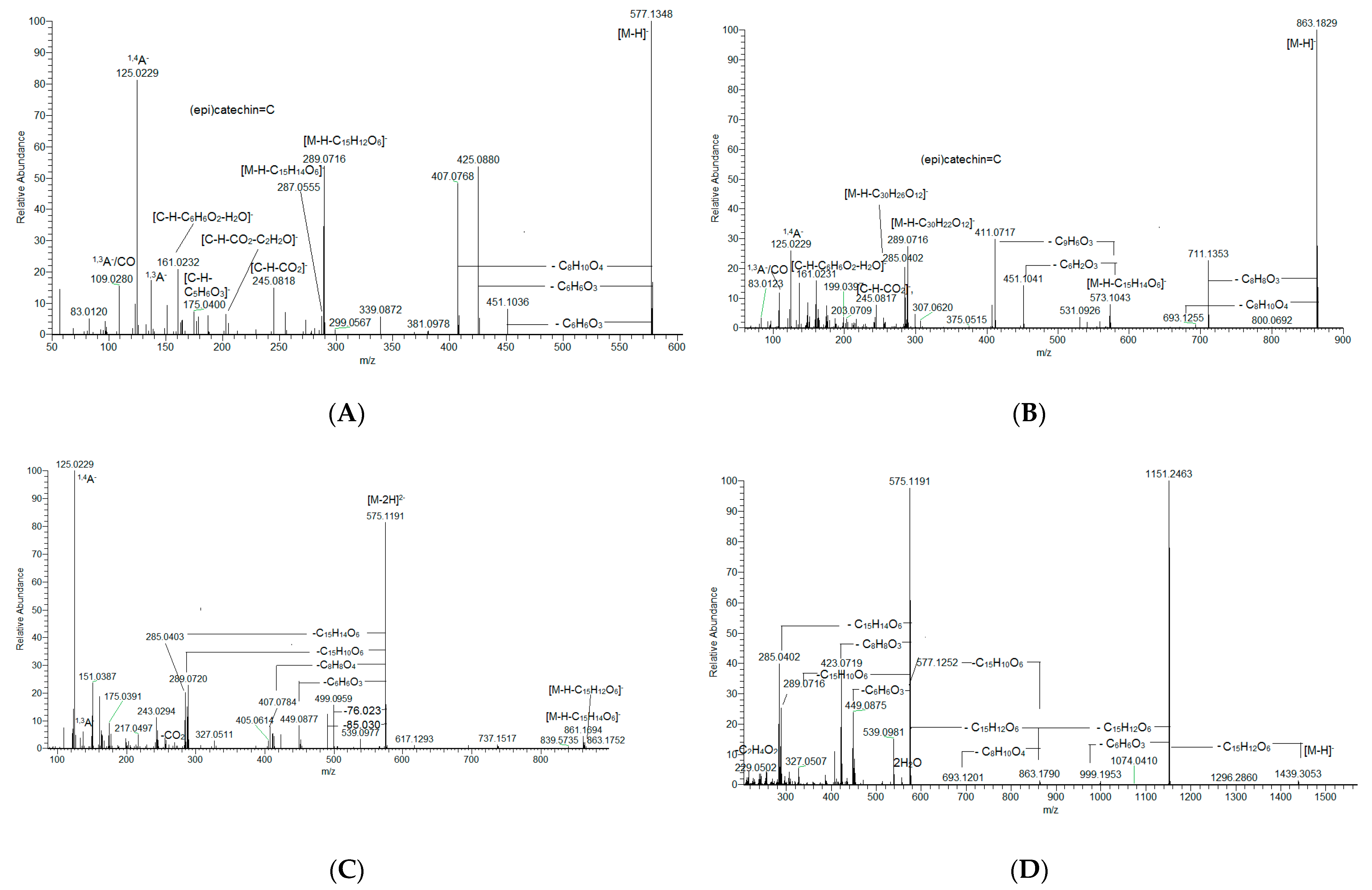
- Proanthocyanidins (PACs)
- Flavonoids
2.2. GC-MS Analysis
2.3. Total Bioactive Compounds and Antioxidant and Enzyme Inhibitory Properties of E. spiculifolia Methanol–Aqueous Extract (ES2)
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals
3.3. Sample Extraction
3.4. LC-HRMS
3.5. Assay for Total Phenolic and Flavonoid Contents
3.6. Assays for In Vitro Antioxidant Capacity
3.7. Inhibitory Effects Against Some Key Enzymes
3.8. Fractionation of Extracts by Thin-Layer Adsorption Chromatography (TLC)
3.9. Methylation of Triterpenoid Acids
3.10. Identification and Quantification of Steroids and Triterpenoids by Gas Chromatography–Mass Spectrometry (GC-MS)
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| ACAE | Acarbose equivalent |
| ACAs | Acylquinic acids |
| AChE | Acetylcholinesterase |
| BChE | Butyrylcholinesterase |
| C | (Epi)catechin moiety |
| CE | Catechin equivalent |
| CUPRAC | CUPric Reducing Antioxidant Capacity |
| diAQAs | Diacylquinic acids |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| EDTAE | Ethylenediaminetetraacetic acid equivalent |
| EGCG | Epigallocatechin galate |
| ES1 | Plant material from the beginning of flowering in July |
| ES2 | Plant material from the full flowering stage in August |
| FRAP | Ferric Reducing Antioxidant Power |
| GAE | Gallic acid equivalent |
| GALAE | Galanthamine equivalent |
| GC-MS | Gas chromatography–mass spectrometry |
| HRF | Heterocyclic ring fission |
| IC50 | The concentration required for 50% inhibition |
| KAE | Kojic acid equivalent |
| LC-HRMS | Liquid chromatography–high-resolution mass spectrometry |
| LC-MS | Liquid chromatography–mass spectrometry |
| monoAQAs | Monoacylquinic acids |
| Nrf2 | Nuclear factor erythroid 2-related factor |
| OE | Orlistat equivalent |
| PACs | Proanthocyanidins |
| QM | Quinine methane or interflavan fission |
| RDA | Retro–Diels–Alder fission |
| RE | Rutin equivalent |
| SD | Standard deviation |
| TE | Trolox equivalent |
| TLC | Thin-layer adsorption chromatography |
References
- Adu-Amankwaah, F.; Mpundu, H.V.; Nyambo, K.; Strauss, P.; Tapfuma, K.I.; Tshililo, N.; Badejo, M.V.; Mabasa, L.; Mavumengwana, V.; Baatjies, L. Phytochemical and Pharmacological Review of Erica Genus (L.) Ericaceae Plants. Phytomed. Plus 2025, 5, 100697. [Google Scholar] [CrossRef]
- Oliver, E.G.H. Systematics of Ericeae (Ericaceae-Ericoideae): Species with Indehiscent and Partially Dehiscent Fruits; Bolus Herbarium, University of Cape Town: Cape Town, South Africa, 2000; ISBN 978-0-7992-2020-9. [Google Scholar]
- Fagúndez, J.; Izco, J. Seed Morphology of Two Distinct European Species of Erica L. (Ericaceae). Acta Bot. Malacit. 2008, 33, 47–55. [Google Scholar] [CrossRef]
- Pavlović, R.D.; Kovacevic, N.; Lakusic, B.; Kovacević, N. Arbutin Content and Antioxidant Activity of Some Ericaceae Species. Pharmazie 2009, 64, 656–659. [Google Scholar] [CrossRef]
- Dragićević, A.; Matejić, J.; Kovačević, N.; Dobrić, S.; Pavlović, D. Antioxidative and Anti-Inflammatory Study on the Ethanolic Extract of the Root of Bruckentalia Spiculifolia (Salisb.) Reichb. Biol. Nyssana 2024, 15, 37–45. [Google Scholar] [CrossRef]
- Pavlović, D.R.; Tasić-Kostov, M.; Marčetić, M.; Lakušić, B.; Kitić, D.; Savić, S.; Kovačević, N. Evaluation of in Vivo Effects on Surfactant-Irritated Human Skin, Antioxidant Properties and Phenolic Composition of Five Ericaceae Species Extracts. La Riv. Ital. Delle Sostanze Grasse 2013, 90, 255–264. [Google Scholar]
- Mitic, V.D.; Ilic, M.D.; Stankov-Jovanovic, V.P.; Stojanovic, G.S.; Dimitrijevic, M.V. Essential Oil Composition of Erica Spiculifolia Salisb-First Report. Nat. Prod. Res. 2018, 32, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Kuş, Ç.; Duru, M.E.; Küçükaydın, S. Anticholinesterase Activities from Aqueous Extract of Different Plant Parts of Erica Manipuliflora. Int. J. Second. Metab. 2017, 4, 372–375. [Google Scholar] [CrossRef][Green Version]
- Jenab, A.; Roghanian, R.; Emtiazi, G. Bacterial Natural Compounds with Anti-Inflammatory and Immunomodulatory Properties (Mini Review). Drug Des. Dev. Ther. 2020, 14, 3787–3801. [Google Scholar] [CrossRef]
- Pavlović, D.R.; Dobrić, S.; Stojanović, N.; Zlatković, B.; Matejić, J.; Kovačević, N. Antioxidative and Anti-Inflammatory Activities of Erica Spiculifolia Extracts and Fractions. Nat. Prod. Res. 2025, 39, 1502–1511. [Google Scholar] [CrossRef]
- Pavlović, D.; Stojanović, N.; Zlatković, B.; Radulović, N.; Kovačević, N. In Vitro Cytotoxic and Anti-Inflammatory Activity of Bruckenthalia Spiculifolia Extracts and Fractions. Arh. Za Farm. 2022, 72, 453–454. [Google Scholar]
- Symma, N.; Hensel, A. Advanced Analysis of Oligomeric Proanthocyanidins: Latest Approaches in Liquid Chromatography and Mass Spectrometry Based Analysis. Phytochem. Rev. 2022, 21, 809–833. [Google Scholar] [CrossRef]
- Adu-Amankwaah, F.; Tapfuma, K.I.; Hussan, R.H.; Tshililo, N.; Baatjies, L.; Masiphephethu, M.V.; Mabasa, L.; Mavumengwana, V. Cytotoxic Activity of Cape Fynbos against Triple-Negative Breast Cancer Cell Line. S. Afr. J. Bot. 2022, 150, 702–710. [Google Scholar] [CrossRef]
- Bijttebier, S.; Van Der Auwera, A.; Voorspoels, S.; Noten, B.; Hermans, N.; Pieters, L.; Apers, S. A First Step in the Quest for the Active Constituents in Filipendula Ulmaria (Meadowsweet): Comprehensive Phytochemical Identification by Liquid Chromatography Coupled to Quadrupole-Orbitrap Mass Spectrometry. Planta Med. 2016, 82, 559–572. [Google Scholar] [CrossRef]
- Rush, M.D.; Rue, E.A.; Wong, A.; Kowalski, P.; Glinski, J.A.; Van Breemen, R.B. Rapid Determination of Proanthocyanidins Using MALDI-ToF/ToF Mass Spectrometry. J. Agric. Food Chem. 2018, 66, 11355–11361. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Serio, G.; Gaglio, R.; Busetta, G.; La Rosa, L.; Lauria, A.; Settanni, L.; Gentile, C. Phytochemical Profile and Antioxidant, Antiproliferative, and Antimicrobial Properties of Rubus Idaeus Seed Powder. Foods 2022, 11, 2605. [Google Scholar] [CrossRef] [PubMed]
- Geng, P.; Harnly, J.M.; Sun, J.; Polashock, J.; Vorsa, N.; Chen, P. Variability and Determinants of Secondary Metabolite Profiles in Cranberry (Vaccinium Macrocarpon) from Wisconsin and New Jersey. J. Agric. Food Res. 2024, 15, 100983. [Google Scholar] [CrossRef]
- Tang, H.; Cao, Y.; Liu, L.; Zhang, Y.; Li, W.; Tu, P.; Li, J.; Song, Y. High-Level Structural Analysis of Proanthocyanidins Using Full Collision Energy Ramp-MS2 Spectrum. J. Pharm. Biomed. Anal. 2022, 211, 114634. [Google Scholar] [CrossRef]
- Gevrenova, R.; Zheleva-Dimitrova, D.; Balabanova, V.; Voynikov, Y.; Sinan, K.I.; Mahomoodally, M.F.; Zengin, G. Integrated Phytochemistry, Bio-Functional Potential and Multivariate Analysis of Tanacetum Macrophyllum (Waldst. & Kit.) Sch.Bip. and Telekia Speciosa (Schreb.) Baumg. (Asteraceae). Ind. Crops Prod. 2020, 155, 112817. [Google Scholar] [CrossRef]
- Gevrenova, R.; Zengin, G.; Sinan, K.I.; Zheleva-Dimitrova, D.; Balabanova, V.; Kolmayer, M.; Voynikov, Y.; Joubert, O. An In-Depth Study of Metabolite Profile and Biological Potential of Tanacetum balsamita L. (Costmary). Plants 2022, 12, 22. [Google Scholar] [CrossRef]
- Ak, G.; Gevrenova, R.; Sinan, K.I.; Zengin, G.; Zheleva, D.; Mahomoodally, M.F.; Senkardes, I.; Brunetti, L.; Leone, S.; Di Simone, S.C.; et al. Tanacetum vulgare L. (Tansy) as an Effective Bioresource with Promising Pharmacological Effects from Natural Arsenal. Food Chem. Toxicol. 2021, 153, 112268. [Google Scholar] [CrossRef]
- Pancost, R.D.; Baas, M.; Van Geel, B.; Sinninghe Damsté, J.S. Biomarkers as Proxies for Plant Inputs to Peats: An Example from a Sub-Boreal Ombrotrophic Bog. Org. Geochem. 2002, 33, 675–690. [Google Scholar] [CrossRef]
- Szakiel, A.; Niżyński, B.; Pączkowski, C. Triterpenoid Profile of Flower and Leaf Cuticular Waxes of Heather Calluna Vulgaris. Nat. Prod. Res. 2013, 27, 1404–1407. [Google Scholar] [CrossRef] [PubMed]
- Edorh Tossa, P.; Belorgey, M.; Dashbaldan, S.; Pączkowski, C.; Szakiel, A. Flowers and Inflorescences of Selected Medicinal Plants as a Source of Triterpenoids and Phytosterols. Plants 2023, 12, 1838. [Google Scholar] [CrossRef]
- Jabal, K.; Walsh, J. A Study on the Triterpenoid Constituents in Erica Erigena. Planta Med. 2023, 89, 1326. [Google Scholar] [CrossRef]
- Gadouche, L.; Alsoufi, A.S.M.; Pacholska, D.; Skotarek, A.; Pączkowski, C.; Szakiel, A. Triterpenoid and Steroid Content of Lipophilic Extracts of Selected Medicinal Plants of the Mediterranean Region. Molecules 2023, 28, 697. [Google Scholar] [CrossRef]
- Lemma, B.; Bromm, T.; Zech, W.; Zech, M.; Nemomissa, S.; Glaser, B. Terpenoid Profiling of Keystone Plant Species of the Bale Mountains, Ethiopia: Implications for Chemotaxonomy and Paleovegetation Studies. Biochem. Syst. Ecol. 2024, 116, 104865. [Google Scholar] [CrossRef]
- Benmanseur, A.; Tacherfiout, M.; Benguerba, Y.; Hab, F.Z.; Tachour, R.A.; Khettal, B.; Derguine, R.; Bachir-Bey, M.; Rezgui, A.; Sobhi, W. Optimization of Ultrasound Phenolic Extraction from Erica Multiflora Leaves Using Response Surface Methodology and Artificial Neural Networks. J. Appl. Res. Med. Aromat. Plants 2025, 45, 100627. [Google Scholar] [CrossRef]
- Zengin, G.; Cvetanović, A.; Gašić, U.; Stupar, A.; Bulut, G.; Senkardes, I.; Dogan, A.; Seebaluck-Sandoram, R.; Rengasamy, K.R.R.; Sinan, K.I.; et al. Chemical Composition and Bio-Functional Perspectives of Erica Arborea L. Extracts Obtained by Different Extraction Techniques: Innovative Insights. Ind. Crops Prod. 2019, 142, 111843. [Google Scholar] [CrossRef]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The Versatility of Antioxidant Assays in Food Science and Safety—Chemistry, Applications, Strengths, and Limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef]
- Świderski, G.; Gołębiewska, E.; Kalinowska, M.; Świsłocka, R.; Kowalczyk, N.; Jabłońska-Trypuć, A.; Lewandowski, W. Comparison of Physicochemical, Antioxidant, and Cytotoxic Properties of Caffeic Acid Conjugates. Materials 2024, 17, 2575. [Google Scholar] [CrossRef]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Chlorogenic Acid: A Systematic Review on the Biological Functions, Mechanistic Actions, and Therapeutic Potentials. Nutrients 2024, 16, 924. [Google Scholar] [CrossRef]
- Yang, H.; Tuo, X.; Wang, L.; Tundis, R.; Portillo, M.P.; Simal-Gandara, J.; Yu, Y.; Zou, L.; Xiao, J.; Deng, J. Bioactive Proanthocyanidins from Dietary Sources: The Relationship between Bioactivity and Polymerization Degree. Trends Food Sci. Technol. 2021, 111, 114–127. [Google Scholar] [CrossRef]
- Luo, X.; Chen, M.; Duan, Y.; Duan, W.; Zhang, H.; He, Y.; Yin, C.; Sun, G.; Sun, X. Chemoprotective Action of Lotus Seedpod Proanthocyanidins on Oxidative Stress in Mice Induced by Extremely Low-Frequency Electromagnetic Field Exposure. Biomed. Pharmacother. 2016, 82, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Sun, Y.; Tang, Y.; Yu, Y.; Wang, J.; Zheng, F.; Li, Y.; Sun, Y. Catechins: Protective Mechanism of Antioxidant Stress in Atherosclerosis. Front. Pharmacol. 2023, 14, 1144878. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-Z.; Deng, G.; Liang, Q.; Chen, D.-F.; Guo, R.; Lai, R.-C. Antioxidant Activity of Quercetin and Its Glucosides from Propolis: A Theoretical Study. Sci. Rep. 2017, 7, 7543. [Google Scholar] [CrossRef]
- Sunil, C.; Irudayaraj, S.S.; Duraipandiyan, V.; Al-Dhabi, N.A.; Agastian, P.; Ignacimuthu, S. Antioxidant and Free Radical Scavenging Effects of β-Amyrin Isolated from S. Cochinchinensis Moore. Leaves. Ind. Crops Prod. 2014, 61, 510–516. [Google Scholar] [CrossRef]
- Karen Cardoso, B.; Line Marko De Oliveira, H.; Zonta Melo, U.; Mariano Fernandez, C.M.; Franco De Araújo Almeida Campo, C.; Gonçalves, J.E.; Laverde Jr, A.; Barion Romagnolo, M.; Andrea Linde, G.; Cristiani Gazim, Z. Antioxidant Activity of α and β -Amyrin Isolated from Myrcianthes Pungens Leaves. Nat. Prod. Res. 2020, 34, 1777–1781. [Google Scholar] [CrossRef]
- Allouche, Y.; Beltrán, G.; Gaforio, J.J.; Uceda, M.; Mesa, M.D. Antioxidant and Antiatherogenic Activities of Pentacyclic Triterpenic Diols and Acids. Food Chem. Toxicol. 2010, 48, 2885–2890. [Google Scholar] [CrossRef]
- Pereira, D.M.; Andrade, C.; Valentão, P.; Andrade, P.B. Natural Products as Enzyme Inhibitors. In Natural Products Targeting Clinically Relevant Enzymes; Andrade, P.B., Valentão, P., Pereira, D.M., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 1–18. ISBN 978-3-527-34205-1. [Google Scholar]
- Kaur, N.; Kumar, V.; Nayak, S.K.; Wadhwa, P.; Kaur, P.; Sahu, S.K. Alpha-amylase as Molecular Target for Treatment of Diabetes Mellitus: A Comprehensive Review. Chem. Biol. Drug Des. 2021, 98, 539–560. [Google Scholar] [CrossRef]
- Patel, P.; Shah, D.; Bambharoliya, T.; Patel, V.; Patel, M.; Patel, D.; Bhavsar, V.; Padhiyar, S.; Patel, B.; Mahavar, A.; et al. A Review on the Development of Novel Heterocycles as α-GlucosidaseInhibitors for the Treatment of Type-2 Diabetes Mellitus. Med. Chem. 2024, 20, 503–536. [Google Scholar] [CrossRef]
- Zuliani, G.; Zuin, M.; Romagnoli, T.; Polastri, M.; Cervellati, C.; Brombo, G. Acetyl-Cholinesterase-Inhibitors Reconsidered. A Narrative Review of Post-Marketing Studies on Alzheimer’s Disease. Aging Clin. Exp. Res. 2024, 36, 23. [Google Scholar] [CrossRef] [PubMed]
- Grenier, A.; Legault, J.; Pichette, A.; Jean, L.; Bélanger, A.; Pouliot, R. Antioxidant, Anti-Inflammatory, and Anti-Aging Potential of a Kalmia Angustifolia Extract and Identification of Some Major Compounds. Antioxidants 2021, 10, 1373. [Google Scholar] [CrossRef] [PubMed]
- Martău, G.A.; Bernadette-Emőke, T.; Odocheanu, R.; Soporan, D.A.; Bochiș, M.; Simon, E.; Vodnar, D.C. Vaccinium Species (Ericaceae): Phytochemistry and Biological Properties of Medicinal Plants. Molecules 2023, 28, 1533. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yang, W.; Sun, W.; Chen, S.; Liu, D.; Kong, X.; Tian, J.; Ye, X. Inhibition of Porcine Pancreatic α-Amylase Activity by Chlorogenic Acid. J. Funct. Foods 2020, 64, 103587. [Google Scholar] [CrossRef]
- Tshiyoyo, K.S.; Bester, M.J.; Serem, J.C.; Apostolides, Z. In-Silico Reverse Docking and in-Vitro Studies Identified Curcumin, 18α-Glycyrrhetinic Acid, Rosmarinic Acid, and Quercetin as Inhibitors of α-Glucosidase and Pancreatic α-Amylase and Lipid Accumulation in HepG2 Cells, Important Type 2 Diabetes Targets. J. Mol. Struct. 2022, 1266, 133492. [Google Scholar] [CrossRef]
- Fu, C.; Yang, X.; Lai, S.; Liu, C.; Huang, S.; Yang, H. Structure, Antioxidant and α-Amylase Inhibitory Activities of Longan Pericarp Proanthocyanidins. J. Funct. Foods 2015, 14, 23–32. [Google Scholar] [CrossRef]
- Reshma, A.; Subramanian, A.; Kumarasamy, V.; Tamilanban, T.; Sekar, M.; Gan, S.H.; Subramaniyan, V.; Wong, L.S.; Rani, N.N.I.M.; Wu, Y.S. Neurocognitive Effects of Proanthocyanidin in Alzheimer’s Disease: A Systematic Review of Preclinical Evidence. Braz. J. Med. Biol. Res. 2024, 57, e13587. [Google Scholar] [CrossRef]
- Baldión, P.A.; Díaz, C.A.; Betancourt, D.E. Myricetin Modulates Matrix Metalloproteinases Expression Induced by TEGDMA in Human Odontoblast–Like Cells. J. Biomed. Mater. Res. 2025, 113, e37872. [Google Scholar] [CrossRef]
- Li, X.; Sui, Y.; Li, S.; Xie, B.; Sun, Z. A-Type Proanthocyanidins from Litchi Pericarp Ameliorate Hyperglycaemia by Regulating Hepatic and Muscle Glucose Metabolism in Streptozotocin (STZ)-Induced Diabetic Mice Fed with High Fat Diet. J. Funct. Foods 2016, 27, 711–722. [Google Scholar] [CrossRef]
- Sugiyama, H.; Akazome, Y.; Shoji, T.; Yamaguchi, A.; Yasue, M.; Kanda, T.; Ohtake, Y. Oligomeric Proanthocyanidins in Apple Polyphenol Are Main Active Components for Inhibition of Pancreatic Lipase and Triglyceride Absorption. J. Agric. Food Chem. 2007, 55, 4604–4609. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, J.; Yan, Y.; Liu, D.; Wang, C.; Wang, H. Inhibition of Glycosidase by Ursolic Acid: In Vitro, in Vivo and in Silico Study. J. Sci. Food Agric. 2020, 100, 986–994. [Google Scholar] [CrossRef]
- Loesche, A.; Köwitsch, A.; Lucas, S.D.; Al-Halabi, Z.; Sippl, W.; Al-Harrasi, A.; Csuk, R. Ursolic and Oleanolic Acid Derivatives with Cholinesterase Inhibiting Potential. Bioorg. Chem. 2019, 85, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, J.; Tao, Y.; Zeng, G.; Yang, X.; Lai, Q. Discovery of Active Ingredients Oleanolic Acid, Ursolic Acid, and Arbutin for in Vitro Anti-Melanoma: Network Pharmacology and Experimental Validation. Nat. Prod. Commun. 2025, 20, 1934578X241310309. [Google Scholar] [CrossRef]
- The World Flora Online. Available online: https://www.worldfloraonline.org/ (accessed on 26 May 2025).
- Zengin, G.; Aktumsek, A. Investigation of Antioxidant Potentials of Solvent Extracts From Different Anatomical Parts of Asphodeline Anatolica E. Tuzlaci: An Endemic Plant To Turkey. Afr. J. Trad. Compl. Alt. Med. 2014, 11, 481. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Nithiyanantham, S.; Locatelli, M.; Ceylan, R.; Uysal, S.; Aktumsek, A.; Selvi, P.K.; Maskovic, P. Screening of in Vitro Antioxidant and Enzyme Inhibitory Activities of Different Extracts from Two Uninvestigated Wild Plants: Centranthus longiflorus subsp. longiflorus and Cerinthe minor subsp. auriculata. Eur. J. Integr. Med. 2016, 8, 286–292. [Google Scholar] [CrossRef]
- Kurt-Celep, I.; Nilofar; Cetiz, M.V.; Zheleva-Dimitrova, D.; Gevrenova, R.; Celep, E.; Sinan, K.I.; Yildiztugay, E.; Ferrante, C.; Zengin, G. From Small-scale Studies to an Encompassing View: Inhibiting Inflammation and Clinically Relevant Enzymes with Various Extracts of Primula vulgaris Using in Vitro and in Silico Techniques. Food Front. 2025, 6, 329–359. [Google Scholar] [CrossRef]
- Lescano, L.; Cziáky, Z.; Kurt-Celep, İ.; Zengin, G.; Fernandes, E.; Trentin, R.; Pereira, C.G.; Custódio, L.; Rodrigues, M.J. Antioxidant Activity, Enzyme Inhibition, Photoprotection, Cytotoxicity, and Phytochemical Profiling of Sea Lavender (Limonium algarvense Erben) Seed Extracts for Dermo-Cosmetic Use. Beni-Suef Univ. J. Basic Appl. Sci. 2024, 13, 112. [Google Scholar] [CrossRef]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
- Roh, C.; Jung, U. Screening of crude plant extracts with anti-obesity activity. Int. J. Mol. Sci. 2012, 13, 1710–1719. [Google Scholar] [CrossRef]
- Barak, T.H.; Kurt Celep, İ.; Şentürk, T.B.; Bardakcı, H.; Celep, E. In Vitro Anti-Aging Potential Evaluation of Maclura pomifera (Rafin.) Schneider 80% Methanol Extract with Quantitative HPTLC Analysis. Turk. J. Pharm. Sci. 2022, 19, 400–407. [Google Scholar] [CrossRef] [PubMed]
| № | Identified/Tentatively Annotated Compound | Molecular Formula | Exact Mass [M-H]− | tR (min) | Distribution |
|---|---|---|---|---|---|
| Acylquinic acids | |||||
| 1. | neochlorogenic acid a | C16H18O9 | 353.0878 | 2.36 | ES1, ES2 |
| 2. | chlorogenic acid a | C16H18O9 | 353.0878 | 3.18 | ES1, ES2 |
| 3. | 4-caffeoylquinic acid | C16H18O9 | 353.0878 | 3.35 | ES1, ES2 |
| 4. | 3-feruloylquinic acid | C17H20O9 | 367.1035 | 3.44 | ES1, ES2 |
| 5. | 4-p-coumaroylquinic acid | C16H18O8 | 337.0929 | 3.79 | ES1, ES2 |
| 6. | 5-caffeoylquinic acid isomer | C16H18O9 | 353.0878 | 3.89 | ES1, ES2 |
| 7. | 5-p-coumaroylquinic acid | C16H18O8 | 337.0929 | 3.95 | ES1, ES2 |
| 8. | 4-p-coumaroylquinic acid isomer | C16H18O8 | 337.0929 | 4.03 | ES1, ES2 |
| 9. | 5-feruloylquinic acid | C17H20O9 | 367.1035 | 4.39 | ES1, ES2 |
| 10. | 5-p-coumaroylquinic acid isomer | C16H18O8 | 337.0929 | 4.61 | ES1, ES2 |
| 11. | 3, 5-dicaffeoylquinic acid | C25H24O12 | 515.1195 | 5.85 | ES1, ES2 |
| 12. | rosmarinic acid a | C18H16O8 | 359.0772 | 6.33 | ES1, ES2 |
| Proanthocyanidin oligomers (PACs) | |||||
| 13. | proanthocyanidin tetramer A, B-type | C60H48O24 | 1151.2463 | 2.72 | ES1, ES2 |
| 14. | proanthocyanidin dimer B-type | C30H26O12 | 577.1351 | 2.77 | ES1, ES2 |
| 15. | proanthocyanidin dimer B-type | C30H26O12 | 577.1351 | 2.94 | ES1, ES2 |
| 16. | proanthocyanidin pentamer A, B-type | C75H60O30 | 719.1509 [M-2H]2− | 3.27 | ES1 |
| 17. | proanthocyanidin trimer B-type | C45H38O18 | 865.1985 | 3.25 | ES1 |
| 18. | proanthocyanidin dimer B-type | C30H26O12 | 577.1351 | 3.45 | ES1, ES2 |
| 19. | proanthocyanidin pentamer A, B-type | C75H60O30 | 719.1509 [M-2H]2− | 3.47 | ES1, ES2 |
| 20. | proanthocyanidin tetramer A, B-type | C60H48O24 | 1151.2463 | 3.53 | ES1, ES2 |
| 21. | proanthocyanidin trimer B-type | C45H38O18 | 865.1985 | 3.57 | ES1, ES2 |
| 22. | proanthocyanidin dimer B-type | C30H26O12 | 577.1351 | 3.58 | ES1, ES2 |
| 23. | proanthocyanidin tetramer B-type | C60H50O24 | 576.1273 [M-2H]2− | 3.79 | ES1, ES2 |
| 24. | proanthocyanidin pentamer A, B-type | C75H60O30 | 719.1512 [M-2H]2− | 3.84 | ES1, ES2 |
| 25. | proanthocyanidin trimer A, B-type | C45H36O18 | 863.1829 | 3.88 | ES1, ES2 |
| 26. | proanthocyanidin trimer A, B-type | C45H36O18 | 863.1829 | 4.11 | ES1, ES2 |
| 27. | proanthocyanidin trimer B-type | C45H38O18 | 865.1985 | 4.18 | ES1, ES2 |
| 28. | proanthocyanidin hexamer A, B-type | C90H72O36 | 863.1829 [M-2H]2− | 4.27 | ES1 |
| 29. | proanthocyanidin tetramer A, B-type | C60H48O24 | 1151.2462 | 4.32 | ES1 |
| 30. | proanthocyanidin dimer B-type | C30H26O12 | 577.1351 | 4.38 | ES1, ES2 |
| 31. | proanthocyanidin tetramer B-type | C60H50O24 | 1153.2619 | 4.38 | ES1, ES2 |
| 32. | proanthocyanidin pentamer B-type | C75H62O30 | 720.1590 [M-2H]2− | 4.46 | ES1, ES2 |
| 33. | proanthocyanidin trimer B-type | C45H38O18 | 865.1985 | 4.47 | ES1, ES2 |
| 34. | proanthocyanidin pentamer B-type | C75H62O30 | 720.1590 [M-2H]2− | 4.59 | ES1, ES2 |
| 35. | proanthocyanidin tetramer B-type | C60H50O24 | 576.1273 [M-2H]2− | 4.60 | ES1, ES2 |
| 36. | proanthocyanidin pentamer A, B-type | C75H60O30 | 719.1512 [M-2H]2− | 4.68 | ES1, ES2 |
| 37. | proanthocyanidin dimer A-type | C30H24O12 | 575.1195 | 4.74 | ES1, ES2 |
| 38. | proanthocyanidin tetramer A, B-type | C60H48O24 | 575.1195 [M-2H]2− | 4.83 | ES1, ES2 |
| 39. | proanthocyanidin heptamer A, B-type | C105H86O42 | 1008.7241 [M-2H]2− | 4.90 | ES1, ES2 |
| 40. | proanthocyanidin pentamer A, B-type | C75H60O30 | 719.1512 [M-2H]2− | 4.97 | ES1, ES2 |
| 41. | proanthocyanidin tetramer A, B-type | C60H48O24 | 575.1195 [M-2H]2− | 4.97 | ES1, ES2 |
| 42. | proanthocyanidin pentamer A, B-type | C75H60O30 | 719.1512 [M-2H]2− | 5.05 | ES1, ES2 |
| 43. | proanthocyanidin trimer B-type | C45H38O18 | 865.1985 | 5.17 | ES1, ES2 |
| 44. | proanthocyanidin dimer B-type | C30H26O12 | 577.1351 | 5.20 | ES1, ES2 |
| 45. | proanthocyanidin pentamer A-type | C75H58O30 | 718.1434 [M-2H]2− | 5.23 | ES1, ES2 |
| 46. | proanthocyanidin tetramer A, B-type | C60H48O24 | 575.1195 [M-2H]2− | 5.36 | ES1, ES2 |
| 47. | proanthocyanidin pentamer A, B-type | C75H60O30 | 1439.3096 | 5.36 | ES1, ES2 |
| 48. | Proanthocyanidin trimer A, B-type | C45H36O18 | 863.1829 | 5.58 | ES1, ES2 |
| 49. | proanthocyanidin dimer A-type | C30H24O12 | 575.1195 | 5.83 | ES1, ES2 |
| 50. | proanthocyanidin tetramer A, B-type | C60H46O24 | 1149.2306 | 6.22 | ES1, ES2 |
| 51. | proanthocyanidin pentamer A-type | C75H58O30 | 718.1434 [M-2H]2− | 6.36 | ES1, ES2 |
| Flavonoids | |||||
| 52. | gallocatechin | C15H14O7 | 305.0667 | 1.79 | ES1, ES2 |
| 53. | (+) catechin | C15H14O6 | 289.0718 | 3.12 | ES1, ES2 |
| 54. | naringenin 6, 8 diC-hexoside | C27H32O15 | 595.1678 | 3.64 | ES1, ES2 |
| 55. | eryodictiol O-hexoside 1 | C21H22O11 | 449.1089 | 3.73 | ES1, ES2 |
| 56. | gossypetin O-hexoside 1 | C21H20O13 | 479.0831 | 3.90 | ES1, ES2 |
| 57. | epicatechin | C15H14O6 | 289.0718 | 3.90 | ES1, ES2 |
| 58. | gossypetin O-rutinoside | C27H30O17 | 625.1410 | 4.01 | ES1, ES2 |
| 59. | eryodictiol O-hexoside 1 | C21H22O11 | 449.1089 | 4.02 | ES1, ES2 |
| 60. | gossypetin O-hexoside 2 | C21H20O13 | 479.0831 | 4.04 | ES1, ES2 |
| 61. | gossypetin O-pentoside 1 | C20H18O12 | 449.0725 | 4.18 | ES1, ES2 |
| 62. | gossypetin O-pentoside 2 | C20H18O12 | 449.0725 | 4.24 | ES1, ES2 |
| 63. | galangin methyl ether O-hexoside | C23H24O12 | 491.1195 | 4.36 | ES1, ES2 |
| 64. | quercetin O-dihexoside | C27H30O17 | 625.1410 | 4.40 | ES1, ES2 |
| 65. | saponarin a | C27H30O15 | 593.1512 | 4.40 | ES1, ES2 |
| 66. | gossypetin O-pentoside 3 | C20H18O12 | 449.0725 | 4.50 | ES1, ES2 |
| 67. | myricetin O-hexoside 1 | C21H20O13 | 479.0831 | 4.51 | ES1, ES2 |
| 68. | myricetin O-hexoside 2 | C21H20O13 | 479.0831 | 4.59 | ES1, ES2 |
| 69. | luteolin O-hexosyl-O-hexuronide | C27H28O17 | 623.1254 | 4.62 | ES1, ES2 |
| 70. | myricetin O-pentoside 1 | C20H18O12 | 449.0725 | 4.98 | ES1, ES2 |
| 71. | myricetin O-pentoside 2 | C20H18O12 | 449.0725 | 5.03 | ES1, ES2 |
| 72. | rutin a | C27H30O16 | 609.1464 | 5.08 | ES1, ES2 |
| 73. | quercetin O-pentosylhexoside | C26H28O16 | 595.1305 | 5.11 | ES1 |
| 74. | isoquercitrin a | C21H20O12 | 463.0886 | 5.18 | ES1, ES2 |
| 75. | quercetin O-hexuronide | C21H18O13 | 477.0675 | 5.22 | ES1, ES2 |
| 76. | luteolin 7-O-rutinside a | C27H30O15 | 593.1512 | 5.26 | ES1, ES2 |
| 77. | hyperoside a | C21H20O12 | 463.0887 | 5.29 | ES1, ES2 |
| 78. | luteolin O-hexuronide | C21H18O12 | 461.0725 | 5.39 | ES1, ES2 |
| 79. | luteolin 7-O-glucoside a | C21H20O11 | 447.0933 | 5.39 | ES1, ES2 |
| 80. | luteolin O-deoxyhexosyl-O-hexoside | C27H30O15 | 593.1512 | 5.44 | ES1, ES2 |
| 81. | quercetin 3-O-pentoside 1 | C20H18O11 | 433.0776 | 5.52 | ES1, ES2 |
| 82. | quercetin 3-O-pentoside 2 | C20H18O11 | 433.0776 | 5.63 | ES1, ES2 |
| 83. | kaempferol 3-O-rutinoside a | C27H30O15 | 593.1512 | 5.63 | ES1, ES2 |
| 84. | quercetin O-pentoside 3 | C20H18O11 | 433.0776 | 5.74 | ES1, ES2 |
| 85. | isorhamnetin 3-O-rutinoside a | C28H32O16 | 623.1618 | 5.80 | ES1, ES2 |
| 86. | quercitrin a | C21H20O11 | 447.0933 | 5.93 | ES1, ES2 |
| 87. | Isorhamnetin 3-O-glucoside a | C22H22O12 | 477.1044 | 6.02 | ES1, ES2 |
| 88. | luteolin O-hexoside | C21H20O11 | 447.0933 | 6.06 | ES1, ES2 |
| 89. | apigenin 7-O-glucoside a | C21H20O10 | 431.0983 | 6.09 | ES1, ES2 |
| 90. | apigenin O-hexuronide | C21H18O11 | 445.0776 | 6.13 | ES1, ES2 |
| 91. | gossypetin | C15H10O8 | 317.0303 | 6.19 | ES1, ES2 |
| 92. | chrysoeriol O-hexoside | C22H22O11 | 461.1089 | 6.31 | ES1, ES2 |
| 93. | quercetin O-caffeoylhexoside | C30H26O15 | 625.1199 | 6.44 | ES1, ES2 |
| 94. | kaempferol O-deoxyhexoside | C21H20O11 | 431.0983 | 6.61 | ES1, ES2 |
| 95. | isorhamnetin O-deoxyhexoside | C22H22O11 | 461.1089 | 6.75 | ES1, ES2 |
| 96. | luteolin O-acetylhexoside | C23H22O12 | 489.1038 | 6.87 | ES1, ES2 |
| 97. | quercetin O-pentoside 4 | C20H18O11 | 433.0776 | 7.04 | ES1, ES2 |
| 98. | luteolin a | C15H10O6 | 285.0405 | 7.58 | ES1, ES2 |
| 99. | quercetin a | C15H10O7 | 301.0354 | 7.62 | ES1, ES2 |
| 100. | apigenin a | C15H10O5 | 269.0457 | 8.63 | ES1, ES2 |
| 101. | kaempferol a | C15H9O7 | 285.0406 | 8.83 | ES1, ES2 |
| 102. | hispidulin (scutellarein-6-methyl ether) a | C16H12O6 | 299.0563 | 8.85 | ES1, ES2 |
| 103. | quercetin O-cinnamoylhexoside1 | C30H26O13 | 593.1301 | 8.86 | ES1, ES2 |
| 104. | chrysoeriol a | C16H12O6 | 299.0562 | 8.93 | ES1, ES2 |
| 105. | quercetin O-cinnamoylhexoside2 | C30H26O13 | 593.1301 | 9.13 | ES1, ES2 |
| Compound | Flowers (ES1) | Leaves (ES2) | Aerial Part (ES1) | Aerial Part (ES2) |
|---|---|---|---|---|
| steroids | ||||
| campesterol | 62.48 ± 4.42 | 32.31 ± 3.05 | 22.39 ± 2.11 | 17.28 ± 2.04 |
| sitosterol | 625.86 ± 58.04 | 652.60 ± 62.18 | 804.05 ± 76.60 | 728.58 ± 70.64 |
| sitostanol | 45.12 ± 5.36 | 50.04 ± 5.58 | 69.02 ± 6.80 | 62.18 ± 5.92 |
| tremulone | 71.88 ± 7.04 | 73.33. ± 6.95 | 97.45 ± 10.03 | 91.96 ± 9.28 |
| 24-methylenecycloartanol | 142.66 ± 12.86 | 127.20 ± 10.64 | 160.75 ± 15.50 | 148.01 ± 12.55 |
| sum | 948.00 | 935.48 | 1084.64 | 1048.02 |
| neutral triterpenoids | ||||
| α-amyrin | 2732.80 ± 252.64 | 3297.49 ± 310,50 | 3333.86 ± 296.12 | 3291.04 ± 315.68 |
| α-amyrenone | 1305.13 ± 108.90 | 1436.92 ± 140.24 | 1890.60 ± 166.48 | 1670.49 ±150.05 |
| α-amyrin acetate | 175.12 ± 15.48 | 151.44 ± 14.80 | 193.21 ± 20.50 | 216.66 ± 20.82 |
| β-amyrin | 1339.84 ± 128.16 | 1326.26 ± 122.38 | 1428.05 ± 140.10 | 1603.28 ± 155.86 |
| germanicol | 88.09 ± 7.50 | 75.16 ± 6.94 | 91.50 ± 9.01 | 84.41 ± 7.55 |
| taraxasterol | 94.71 ± 9.33 | 90.44 ± 8.86 | 98.32 ± 9.64 | 84.76 ±8.06 |
| erythrodiol | 18.45 ± 1.70 | 19.89 ± 2.05 | 23.12 ± 2.26 | 20.42 ± 2.18 |
| uvaol | 47.59 ± 4.11 | 50.21 ± 4.95 | 75.32 ± 7.18 | 62.41 ± 6.03 |
| oleanolic aldehyde | 91.01 ± 8.15 | 75.44 ± 7.08 | 98.32 ± 10.46 | 95.48 ± 9.60 |
| ursolic aldehyde | 487.05 ± 44.10 | 422.91 ± 38.15 | 468.39 ± 42.50 | 455.02 ± 41.98 |
| sum | 6379.79 | 6946.16 | 7700.69 | 7583.97 |
| triterpenoid acids | ||||
| olean-2,12-dien-28-oic acid | 115.84 ± 10.62 | 132.87 ± 12.85 | 106.73 ± 9.40 | 126.39 ± 10.55 |
| ursa-2,12-dien-28-oic acid | 471.10 ± 45.38 | 500.27 ± 48.05 | 579.38 ± 55.14 | 679.12 ± 63.08 |
| 3-oxo-oleanolic acid | 65.41 ± 6.55 | 86.98 ± 7.92 | 96.63 ± 9.01 | 105.89 ± 9.45 |
| 3-oxo-ursolic acid | 493.35 ± 45.70 | 693.93 ± 66.05 | 625.39 ± 58.91 | 703.78 ± 66.22 |
| oleanolic acid | 4783.35 ± 420.50 | 6138.27 ± 562.33 | 4717.10 ± 458.02 | 5508.94 ± 533.46 |
| ursolic acid | 19,428.66 ± 1859.12 | 32,214.84 ± 3006.28 | 20,911.64 ± 1894.92 | 21,546.75 ± 1909.15 |
| unidentified acid * | 3054.65 ± 282.01 | 5672.02 ± 508.14 | 4825.00 ± 456.86 | 5558.69 ± 527.50 |
| sum | 30,412.36 | 45,439.18 | 31,861.87 | 34,229.56 |
| Total | 37,740.15 | 53,320.82 | 40,647.20 | 42,861.55 |
| Total Bioactive Compounds | |
| Total phenolic content (mg GAE/g) | 83.85 ± 0.89 |
| Total flavonoid content (mg RE/g) | 78.91 ± 0.41 |
| Antioxidant Properties | |
| DPPH scavenging ability (mg TE/g) | 540.01 ± 9.68 |
| ABTS scavenging ability (mg TE/g) | 639.11 ± 8.51 |
| CUPRAC (mg TE/g) | 869.22 ± 25.02 |
| FRAP (mg TE/g) | 660.32 ± 17.15 |
| Metal chelating (mg EDTAE/g) | 15.57 ± 1.44 |
| Phosphomolybdenum (mmol TE/g) | 2.52 ± 0.03 |
| Enzyme Inhibitory Properties | |
| AChE inhibition (mg GALAE/g) | 2.56 0.06 |
| BChE inhibition (mg GALAE/g) | n.a. |
| Tyrosinase inhibition (mg KAE/g) | 71.90 ± 1.50 |
| Amylase inhibition (mmol ACAE/g) | 0.36 ± 0.002 |
| Glucosidase inhibition (mmol ACAE/g) | 1.35 ± 0.004 |
| Lipase inhibition (mg OE/g) | 53.26 ± 4.44 |
| Collagenase inhibition (IC50 μg/mL) | 17.18 ± 1.61 |
| Elastase inhibition (IC50 μg/mL) | 16.49 ± 1.60 |
| Hyaluronidase inhibition (IC50 μg/mL) | 22.83 ± 2.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gevrenova, R.; Szakiel, A.; Pączkowski, C.; Zengin, G.; Kurt-Celep, I.; Stefanova, A.; Zheleva-Dimitrova, D. Erica spiculifolia Salisb. (Balkan Heath): A Focus on Metabolic Profiling and Antioxidant and Enzyme Inhibitory Properties. Plants 2025, 14, 1648. https://doi.org/10.3390/plants14111648
Gevrenova R, Szakiel A, Pączkowski C, Zengin G, Kurt-Celep I, Stefanova A, Zheleva-Dimitrova D. Erica spiculifolia Salisb. (Balkan Heath): A Focus on Metabolic Profiling and Antioxidant and Enzyme Inhibitory Properties. Plants. 2025; 14(11):1648. https://doi.org/10.3390/plants14111648
Chicago/Turabian StyleGevrenova, Reneta, Anna Szakiel, Cezary Pączkowski, Gokhan Zengin, Inci Kurt-Celep, Alexandra Stefanova, and Dimitrina Zheleva-Dimitrova. 2025. "Erica spiculifolia Salisb. (Balkan Heath): A Focus on Metabolic Profiling and Antioxidant and Enzyme Inhibitory Properties" Plants 14, no. 11: 1648. https://doi.org/10.3390/plants14111648
APA StyleGevrenova, R., Szakiel, A., Pączkowski, C., Zengin, G., Kurt-Celep, I., Stefanova, A., & Zheleva-Dimitrova, D. (2025). Erica spiculifolia Salisb. (Balkan Heath): A Focus on Metabolic Profiling and Antioxidant and Enzyme Inhibitory Properties. Plants, 14(11), 1648. https://doi.org/10.3390/plants14111648










