Study on Rice Submergence Germination Through the Combination of RNA-Seq and Genome Resequencing Strategies
Abstract
1. Introduction
2. Results
2.1. Screening of Rice Cultivars with Contrasting Submergence Germination Ability
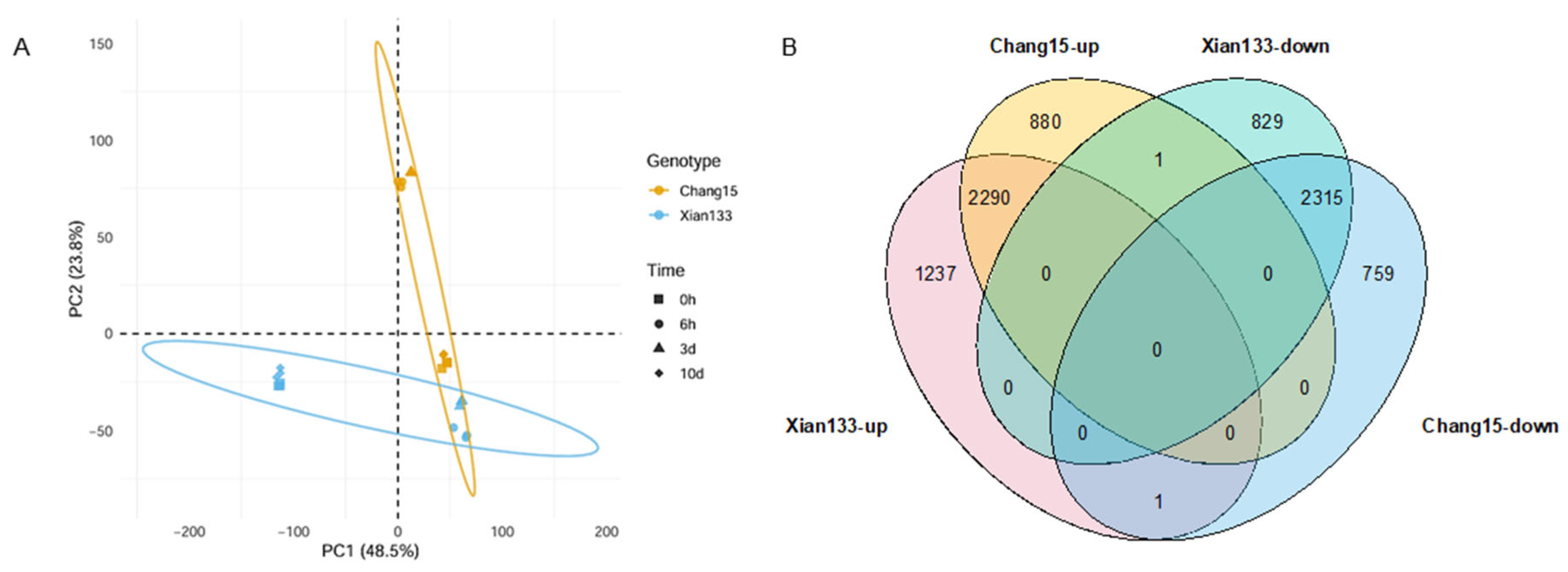
2.2. RNA-Seq Analyses on Xian133 and Chang15 During Submergence Germination
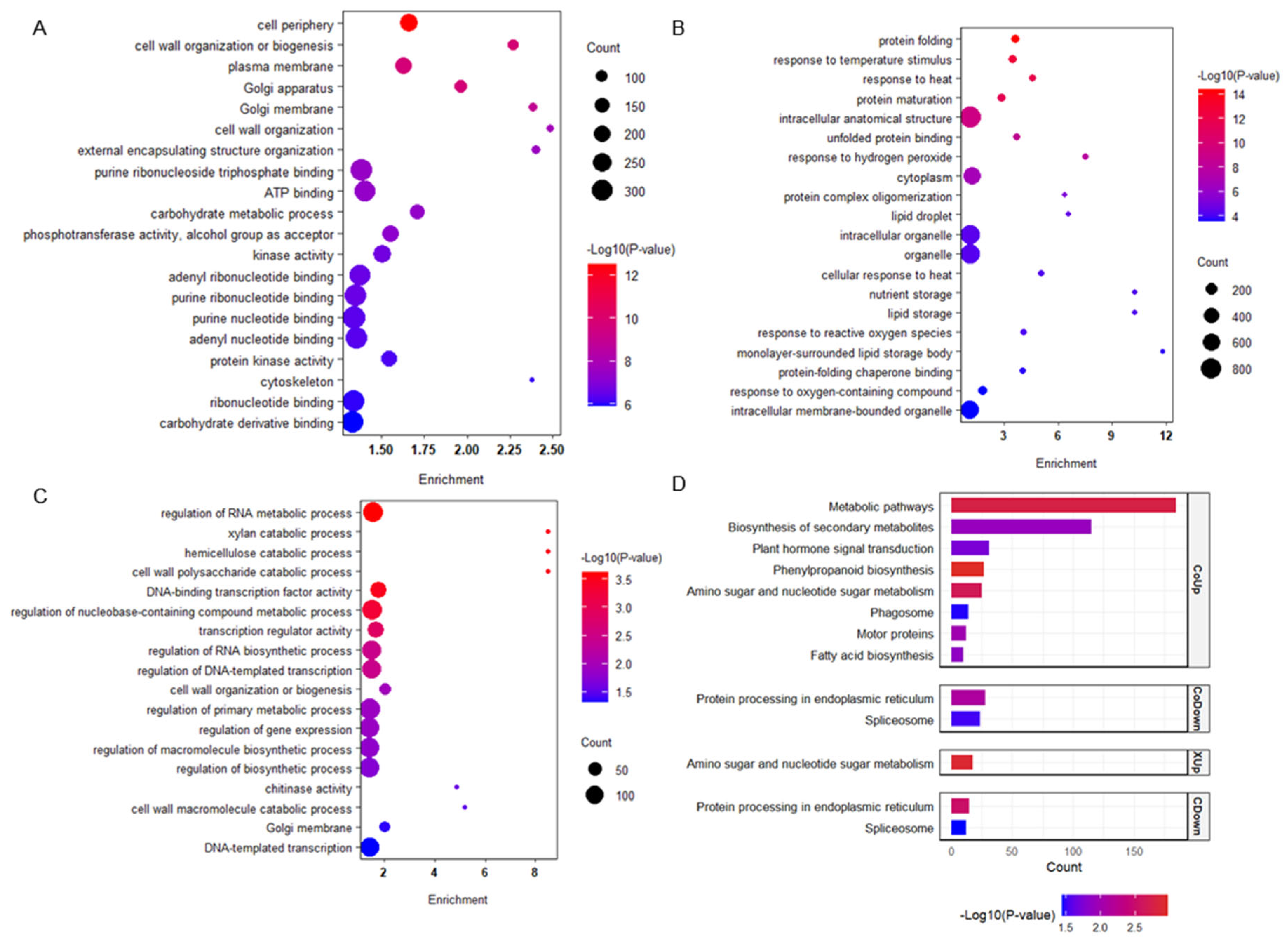
2.3. Functional Enrichment Reveals Divergent Transcriptional Strategies Underlying Coleoptile Elongation in Response to Submergence
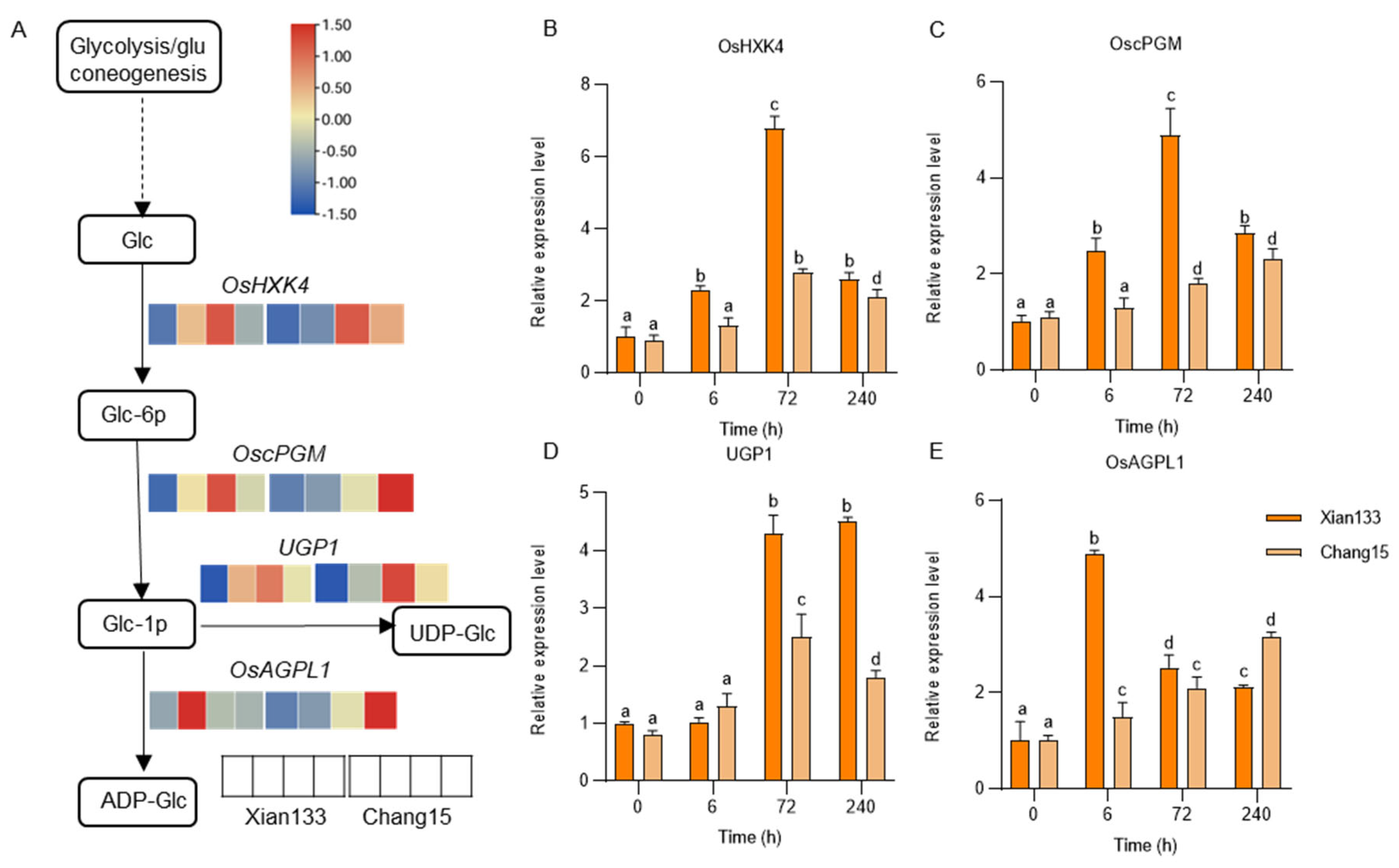
2.4. Amino Sugar Metabolism Pathway Key Gene Expression Patterns Validated Against Transcriptomic Data
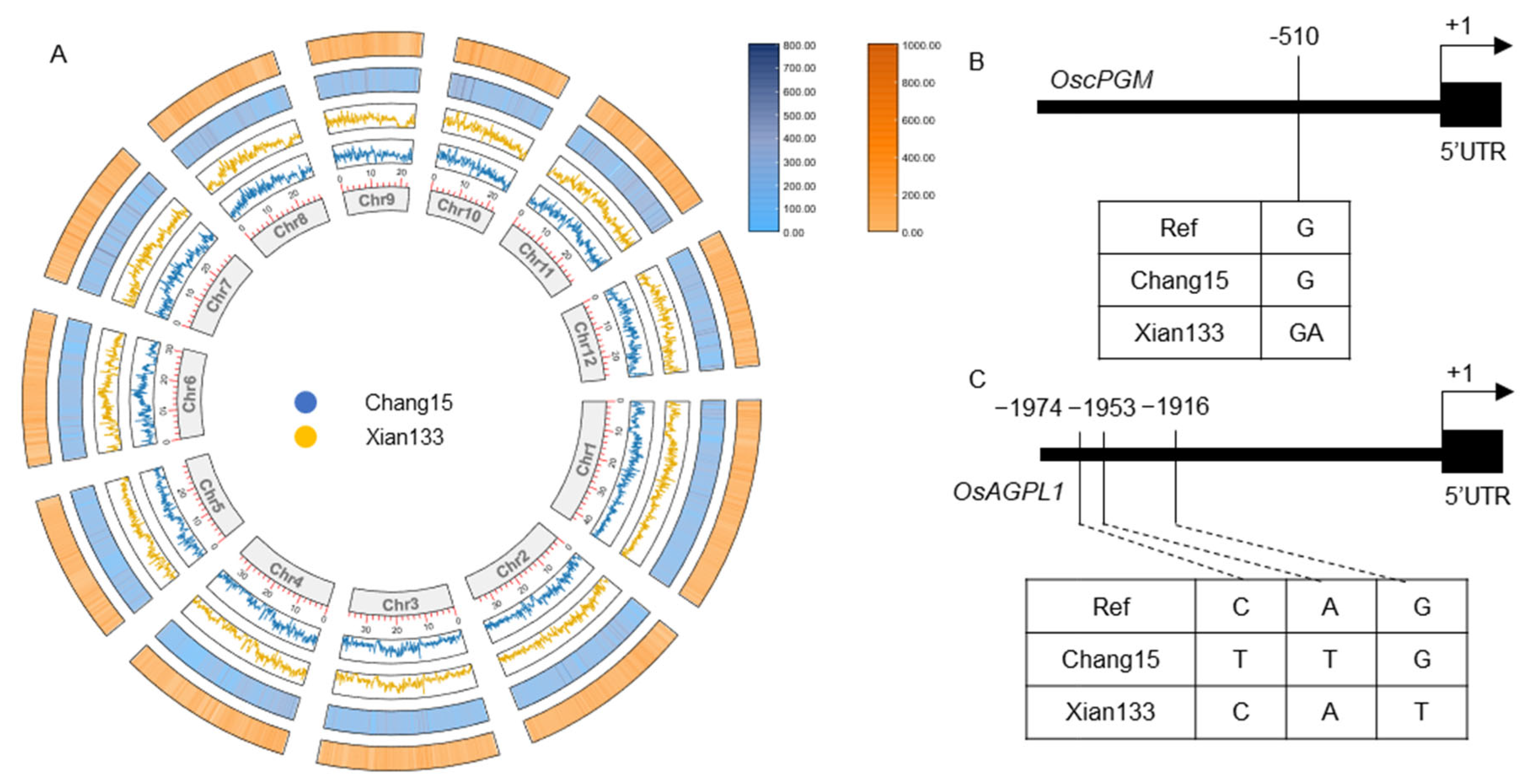
2.5. Genomic Landscape of Variation Between Xian133 and Chang15
3. Discussion
4. Materials and Methods
4.1. Rice Cultivars and Germination Assay
4.2. Transcriptomic Analysis of Xian133 and Chang15
4.3. RNA Extraction and qRT-PCR
4.4. Resequencing and Variant Calling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ismail, A.M. Flooding and Submergence Tolerance. In Genomics and Breeding for Climate-Resilient Crops; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 269–290. ISBN 978-3-642-37047-2. [Google Scholar]
- Voesenek, L.A.C.J.; Bailey-Serres, J. Flood Adaptive Traits and Processes: An Overview. New Phytol. 2015, 206, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.B. Physiological and Molecular Basis of Susceptibility and Tolerance of Rice Plants to Complete Submergence. Ann. Bot. 2003, 91, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Miro, B.; Ismail, A.M. Tolerance of Anaerobic Conditions Caused by Flooding during Germination and Early Growth in Rice (Oryza Sativa L.). Front. Plant Sci. 2013, 4, 269. [Google Scholar] [CrossRef] [PubMed]
- Mahender, A.; Anandan, A.; Pradhan, S.K. Early Seedling Vigour, an Imperative Trait for Direct-Seeded Rice: An Overview on Physio-Morphological Parameters and Molecular Markers. Planta 2015, 241, 1027–1050. [Google Scholar] [CrossRef]
- Nishiuchi, S.; Yamauchi, T.; Takahashi, H.; Kotula, L.; Nakazono, M. Mechanisms for Coping with Submergence and Waterlogging in Rice. Rice 2012, 5, 2. [Google Scholar] [CrossRef]
- Lee, K.; Chen, P.W.; Yu, S. Metabolic Adaptation to Sugar/O2 Deficiency for Anaerobic Germination and Seedling Growth in Rice. Plant Cell Environ. 2014, 37, 2234–2244. [Google Scholar] [CrossRef]
- Kretzschmar, T.; Pelayo, M.A.F.; Trijatmiko, K.R.; Gabunada, L.F.M.; Alam, R.; Jimenez, R.; Mendioro, M.S.; Slamet-Loedin, I.H.; Sreenivasulu, N.; Bailey-Serres, J.; et al. A Trehalose-6-Phosphate Phosphatase Enhances Anaerobic Germination Tolerance in Rice. Nat. Plants 2015, 1, 15124. [Google Scholar] [CrossRef]
- Jun, S.-H.; Han, M.-J.; Lee, S.; Seo, Y.S.; Kim, W.T.; An, G. OsEIN2 Is a Positive Component in Ethylene Signaling in Rice. Plant Cell Physiol. 2004, 45, 281–289. [Google Scholar] [CrossRef]
- Kuroha, T.; Nagai, K.; Gamuyao, R.; Wang, D.R.; Furuta, T.; Nakamori, M.; Kitaoka, T.; Adachi, K.; Minami, A.; Mori, Y.; et al. Ethylene-Gibberellin Signaling Underlies Adaptation of Rice to Periodic Flooding. Science 2018, 361, 181–186. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Fukao, T.; Ronald, P.; Ismail, A.; Heuer, S.; Mackill, D. Submergence Tolerant Rice: SUB1’s Journey from Landrace to Modern Cultivar. Rice 2010, 3, 138–147. [Google Scholar] [CrossRef]
- Ismail, A.M.; Ella, E.S.; Vergara, G.V.; Mackill, D.J. Mechanisms Associated with Tolerance to Flooding during Germination and Early Seedling Growth in Rice (Oryza sativa). Ann. Bot. 2009, 103, 197–209. [Google Scholar] [CrossRef]
- Pucciariello, C. Molecular Mechanisms Supporting Rice Germination and Coleoptile Elongation under Low Oxygen. Plants 2020, 9, 1037. [Google Scholar] [CrossRef]
- He, Y.; Sun, S.; Zhao, J.; Huang, Z.; Peng, L.; Huang, C.; Tang, Z.; Huang, Q.; Wang, Z. UDP-Glucosyltransferase OsUGT75A Promotes Submergence Tolerance during Rice Seed Germination. Nat. Commun. 2023, 14, 2296. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, G.; Song, S.; Jin, Y.; Wang, X.; Yang, S.; Shen, X.; Gan, Y.; Wang, Y.; Li, R.; et al. A Peroxisomal Cinnamate:CoA Ligase-Dependent Phytohormone Metabolic Cascade in Submerged Rice Germination. Dev. Cell 2024, 59, 1363–1378.e4. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, G.; Cui, Z.; Kong, X.; Yu, X.; Gui, R.; Han, Y.; Li, Z.; Lang, H.; Hua, Y.; et al. Regain Flood Adaptation in Rice through a 14-3-3 Protein OsGF14h. Nat. Commun. 2022, 13, 5664. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Wang, F.; Shi, L.; Chen, M.; Cao, Y.; Zhu, F.; Wu, Y.; Xie, L.; Liu, T.; Su, Z.; et al. Natural Variation in the Promoter of Rice Calcineurin B-like Protein10 (OsCBL10) Affects Flooding Tolerance during Seed Germination among Rice Subspecies. Plant J. 2018, 94, 612–625. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yang, L.; Shen, L.; Zhan, C.; Dai, L.; Huang, L.; Zhang, Q.; Fang, Y.; Ren, D.; Zhu, L.; et al. Genome-wide Association Study Uncovers a Novel Gene Responsible for Rice Seedling Submergence Tolerance. Plant Biotechnol. J. 2025, 23, 4092–4108. [Google Scholar] [CrossRef]
- Kong, W.; Li, S.; Zhang, C.; Qiang, Y.; Li, Y. Combination of Quantitative Trait Locus (QTL) Mapping and Transcriptome Analysis Reveals Submerged Germination QTLs and Candidate Genes Controlling Coleoptile Length in Rice. Food Energy Secur. 2022, 11, e354. [Google Scholar] [CrossRef]
- Ghosal, S.; Casal, C.; Quilloy, F.A.; Septiningsih, E.M.; Mendioro, M.S.; Dixit, S. Deciphering Genetics Underlying Stable Anaerobic Germination in Rice: Phenotyping, QTL Identification, and Interaction Analysis. Rice 2019, 12, 50. [Google Scholar] [CrossRef]
- Hsu, S.-K.; Tung, C.-W. Genetic Mapping of Anaerobic Germination-Associated QTLs Controlling Coleoptile Elongation in Rice. Rice 2015, 8, 38. [Google Scholar] [CrossRef]
- Rohilla, M.; Singh, N.; Mazumder, A.; Sen, P.; Roy, P.; Chowdhury, D.; Singh, N.K.; Mondal, T.K. Genome-Wide Association Studies Using 50 K Rice Genic SNP Chip Unveil Genetic Architecture for Anaerobic Germination of Deep-Water Rice Population of Assam, India. Mol. Genet. Genom. 2020, 295, 1211–1226. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, J.; Fu, H.; Yang, T.; Dong, J.; Yang, W.; Chen, L.; Zhou, L.; Wang, J.; Liu, B.; et al. Genome-Wide Identification, Expression and Functional Analysis Reveal the Involvement of FCS-like Zinc Finger Gene Family in Submergence Response in Rice. Rice 2021, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; He, S.-J.; Duan, K.-X.; Yin, C.-C.; Chen, H.; Yang, C.; Xiong, Q.; Song, Q.-X.; Lu, X.; Chen, H.-W.; et al. Identification of Rice Ethylene-Response Mutants and Characterization of MHZ7/OsEIN2 in Distinct Ethylene Response and Yield Trait Regulation. Mol. Plant 2013, 6, 1830–1848. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Wang, S.; Jia, Q.; Wu, P. OsEIL1, a Rice Homolog of the Arabidopsis EIN3 Regulates the Ethylene Response as a Positive Component. Plant Mol. Biol. 2006, 61, 141–152. [Google Scholar] [CrossRef]
- Sauter, M. Rice in Deep Water: “How to Take Heed against a Sea of Troubles”. Naturwissenschaften 2000, 87, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Djurhuus, D.L.E.; Song, Z.; Andersen, A.G.; Gargiulo, S.; Casolo, V.; Ismail, A.M.; Nchimbi-Msolla, S.; De La Cruz Jiménez, J.; Pedersen, O. The Relationship between Anaerobic Germination Capacity and Submergence Tolerance in Rice Seedlings. Rice 2025, 18, 45. [Google Scholar] [CrossRef]
- Vriezen, W.H. Regulation of Submergence-Induced Enhanced Shoot Elongation in Oryza sativa L. Ann. Bot. 2003, 91, 263–270. [Google Scholar] [CrossRef]
- Sasidharan, R.; Hartman, S.; Liu, Z.; Martopawiro, S.; Sajeev, N.; Van Veen, H.; Yeung, E.; Voesenek, L.A.C.J. Signal Dynamics and Interactions during Flooding Stress. Plant Physiol. 2018, 176, 1106–1117. [Google Scholar] [CrossRef]
- Mondal, S.; Khan, M.I.R.; Dixit, S.; Cruz, P.C.S.; Septiningsih, E.M.; Ismail, A.M. Growth, Productivity and Grain Quality of AG1 and AG2 QTLs Introgression Lines under Flooding in Direct-Seeded Rice System. Field Crops Res. 2020, 248, 107713. [Google Scholar] [CrossRef]
- Rahman, M.; Grover, A.; Peacock, W.J.; Dennis, E.S.; Ellis, M.H. Effects of Manipulation of Pyruvate Decarboxylase and Alcohol Dehydrogenase Levels on the Submergence Tolerance of Rice. Funct. Plant Biol. 2001, 28, 1231. [Google Scholar] [CrossRef]
- Ma, M.; Cen, W.; Li, R.; Wang, S.; Luo, J. The Molecular Regulatory Pathways and Metabolic Adaptation in the Seed Germination and Early Seedling Growth of Rice in Response to Low O2 Stress. Plants 2020, 9, 1363. [Google Scholar] [CrossRef]
- Yang, J.; Sun, K.; Li, D.; Luo, L.; Liu, Y.; Huang, M.; Yang, G.; Liu, H.; Wang, H.; Chen, Z.; et al. Identification of Stable QTLs and Candidate Genes Involved in Anaerobic Germination Tolerance in Rice via High-Density Genetic Mapping and RNA-Seq. BMC Genom. 2019, 20, 355. [Google Scholar] [CrossRef] [PubMed]
- Rohilla, M.; Mazumder, A.; Chakraborty, K.; Chowdhury, D.; Prakash, N.; Mondal, T.K. The Private Life of Deepwater Rice: Unravelling Exclusive Features and Unexplored Mechanisms. Plant Stress 2025, 17, 100910. [Google Scholar] [CrossRef]
- Minami, A.; Yano, K.; Gamuyao, R.; Nagai, K.; Kuroha, T.; Ayano, M.; Nakamori, M.; Koike, M.; Kondo, Y.; Niimi, Y.; et al. Time-Course Transcriptomics Analysis Reveals Key Responses of Submerged Deepwater Rice to Flooding. Plant Physiol. 2018, 176, 3081–3102. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, M.; Chigira, K.; Nomura, T.; Adachi, S.; Asami, H.; Nakamura, T.; Motobayashi, T.; Ookawa, T. Identification of Genomic Regions for Deep-Water Resistance in Rice for Efficient Weed Control with Reduced Herbicide Use. Rice 2023, 16, 53. [Google Scholar] [CrossRef]
- Chevilly, S.; Dolz-Edo, L.; Morcillo, L.; Vilagrosa, A.; López-Nicolás, J.M.; Yenush, L.; Mulet, J.M. Identification of Distinctive Physiological and Molecular Responses to Salt Stress among Tolerant and Sensitive Cultivars of Broccoli (Brassica oleracea Var. Ital.). BMC Plant Biol. 2021, 21, 488. [Google Scholar] [CrossRef]
- Liu, B.; Wang, X.; Cao, Y.; Arora, R.; Zhou, H.; Xia, Y. Factors Affecting Freezing Tolerance: A Comparative Transcriptomics Study between Field and Artificial Cold Acclimations in Overwintering Evergreens. Plant J. 2020, 103, 2279–2300. [Google Scholar] [CrossRef]
- Lee, D.-K.; Ahn, S.; Cho, H.Y.; Yun, H.Y.; Park, J.H.; Lim, J.; Lee, J.; Kwon, S.W. Metabolic Response Induced by Parasitic Plant-Fungus Interactions Hinder Amino Sugar and Nucleotide Sugar Metabolism in the Host. Sci. Rep. 2016, 6, 37434. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Yu, F.; Feng, L.; Zhu, M.; Yang, P. Study on Rice Submergence Germination Through the Combination of RNA-Seq and Genome Resequencing Strategies. Plants 2025, 14, 3033. https://doi.org/10.3390/plants14193033
Wang X, Yu F, Feng L, Zhu M, Yang P. Study on Rice Submergence Germination Through the Combination of RNA-Seq and Genome Resequencing Strategies. Plants. 2025; 14(19):3033. https://doi.org/10.3390/plants14193033
Chicago/Turabian StyleWang, Xin, Feng Yu, Linfeng Feng, Mingdong Zhu, and Pingfang Yang. 2025. "Study on Rice Submergence Germination Through the Combination of RNA-Seq and Genome Resequencing Strategies" Plants 14, no. 19: 3033. https://doi.org/10.3390/plants14193033
APA StyleWang, X., Yu, F., Feng, L., Zhu, M., & Yang, P. (2025). Study on Rice Submergence Germination Through the Combination of RNA-Seq and Genome Resequencing Strategies. Plants, 14(19), 3033. https://doi.org/10.3390/plants14193033






