Optimizing Resource Management with Organic Fertilizer and Microbial Inoculants to Enhance Soil Quality, Microbial Diversity, and Crop Productivity in Newly Cultivated Land
Abstract
1. Introduction
2. Results
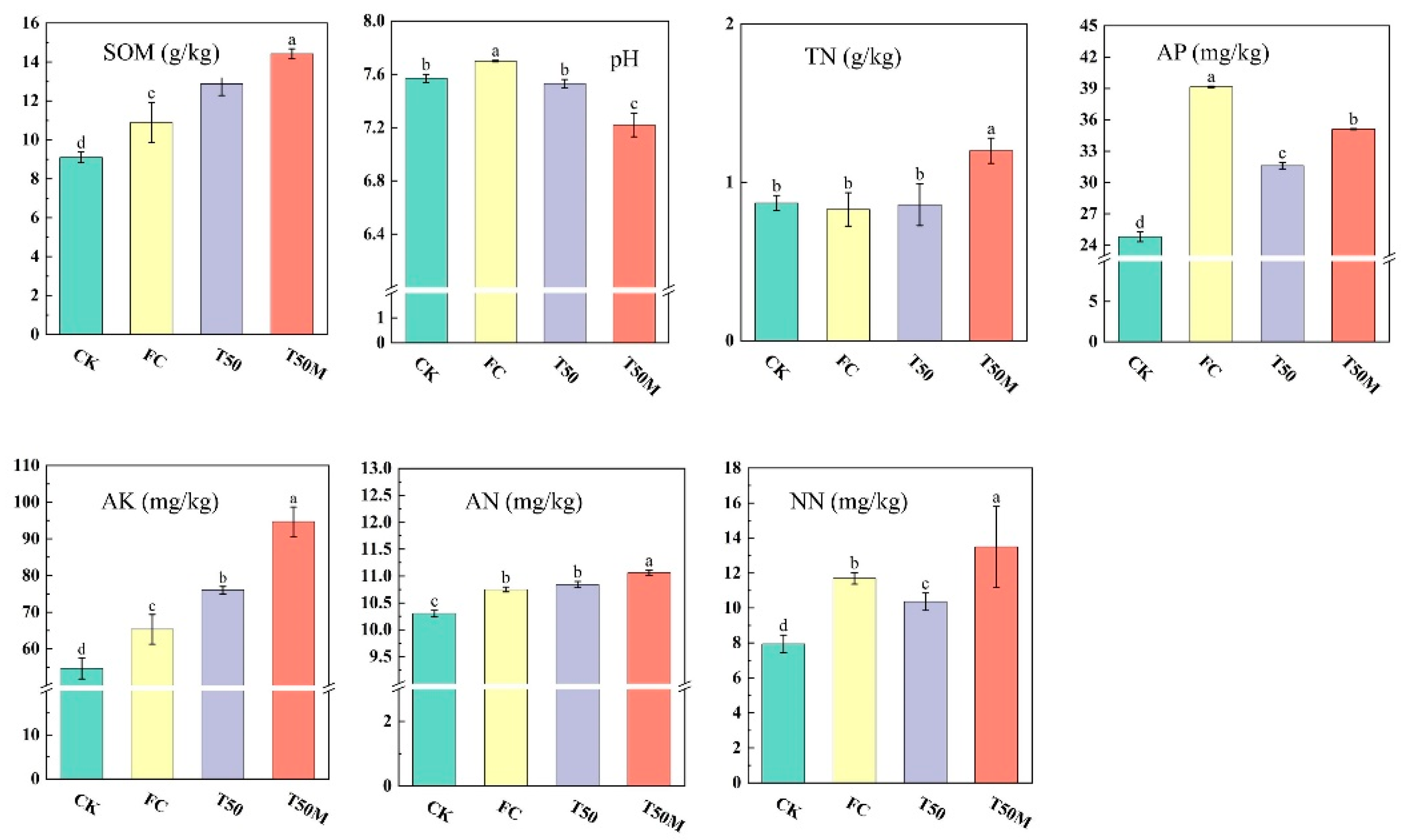
2.1. Soil Properties
2.2. Soil Bacterial Community Structure and Diversity
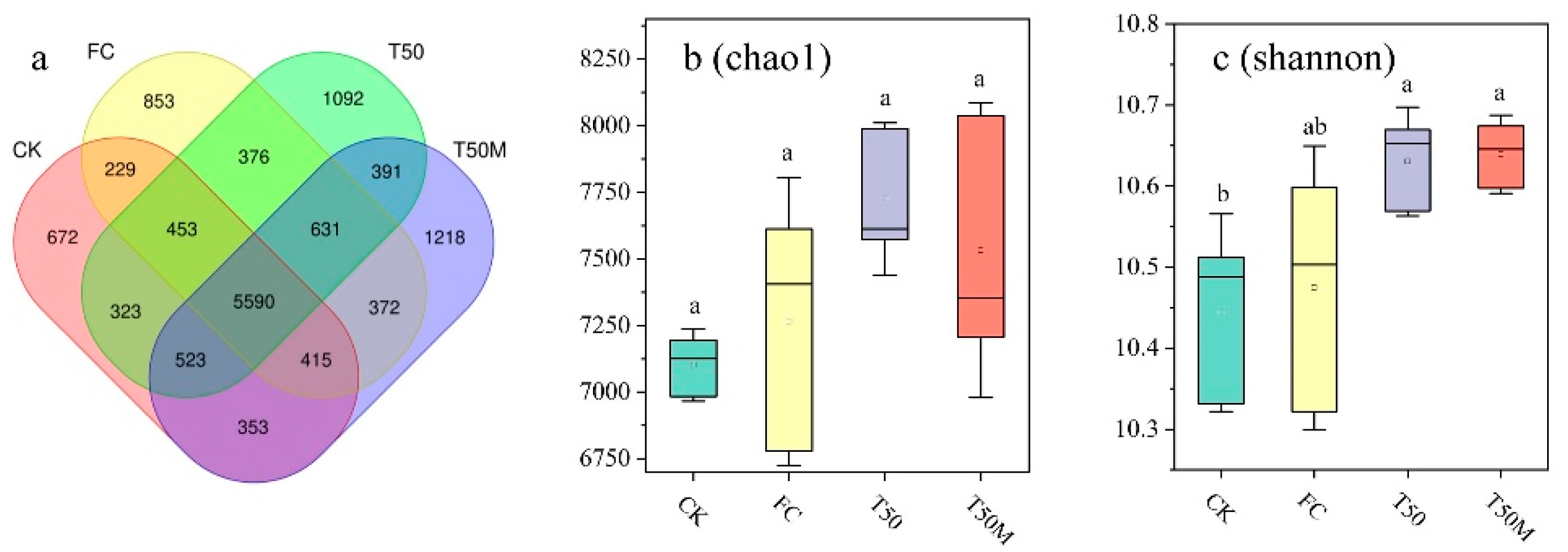
2.2.1. Analysis of Soil Bacterial Community α-Diversity Index, and OTU Composition
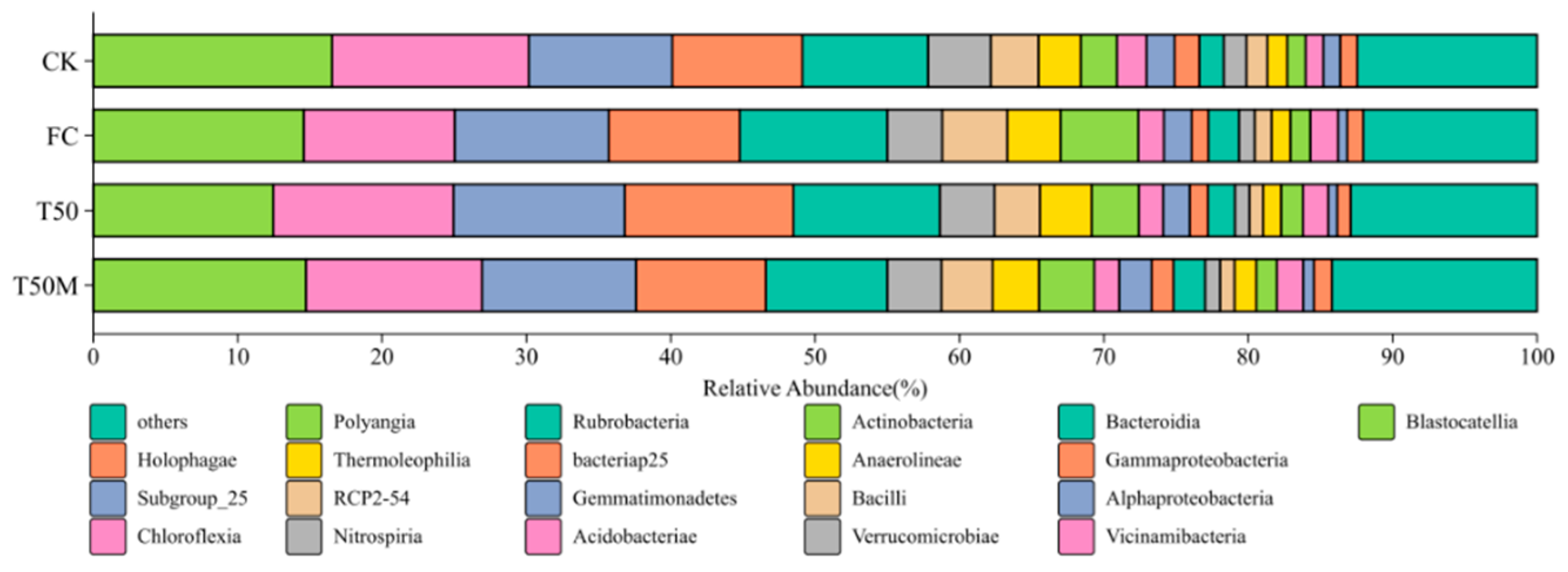
2.2.2. Structural Composition of Soil Bacterial Communities
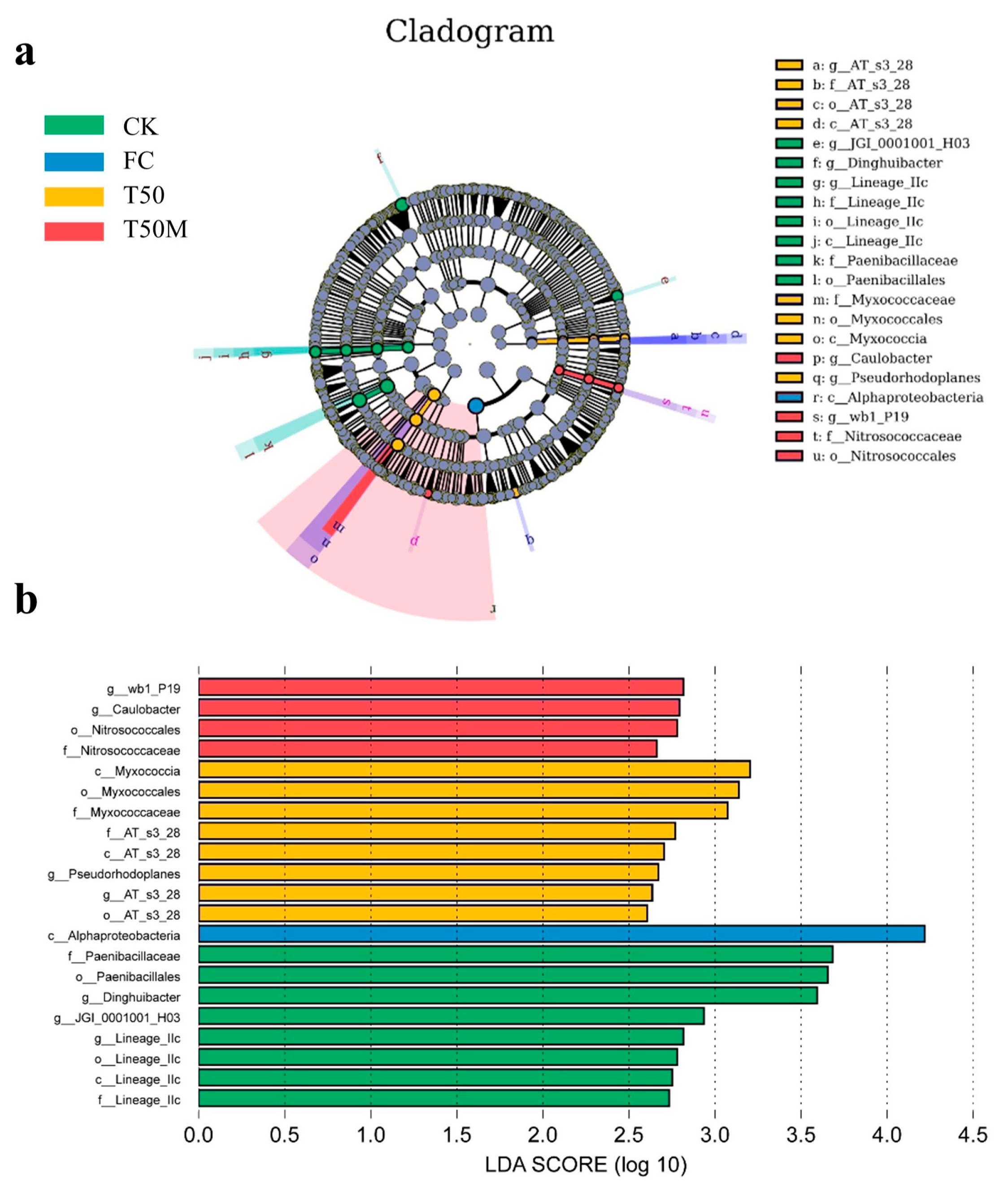
2.2.3. Analysis of Bacterial Community Structure Differences and Correlation Analysis
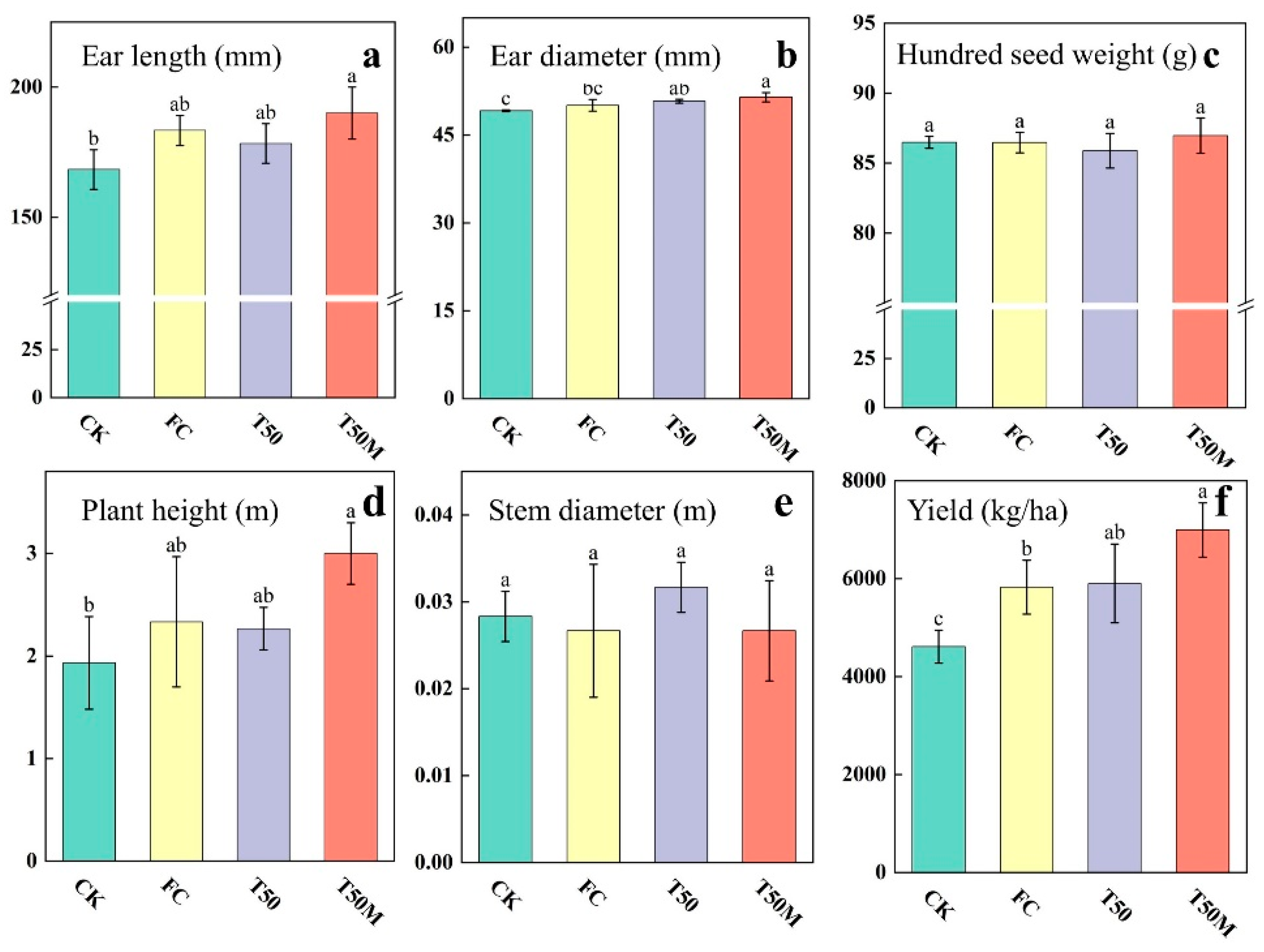
2.3. Maize Morphology and Yield
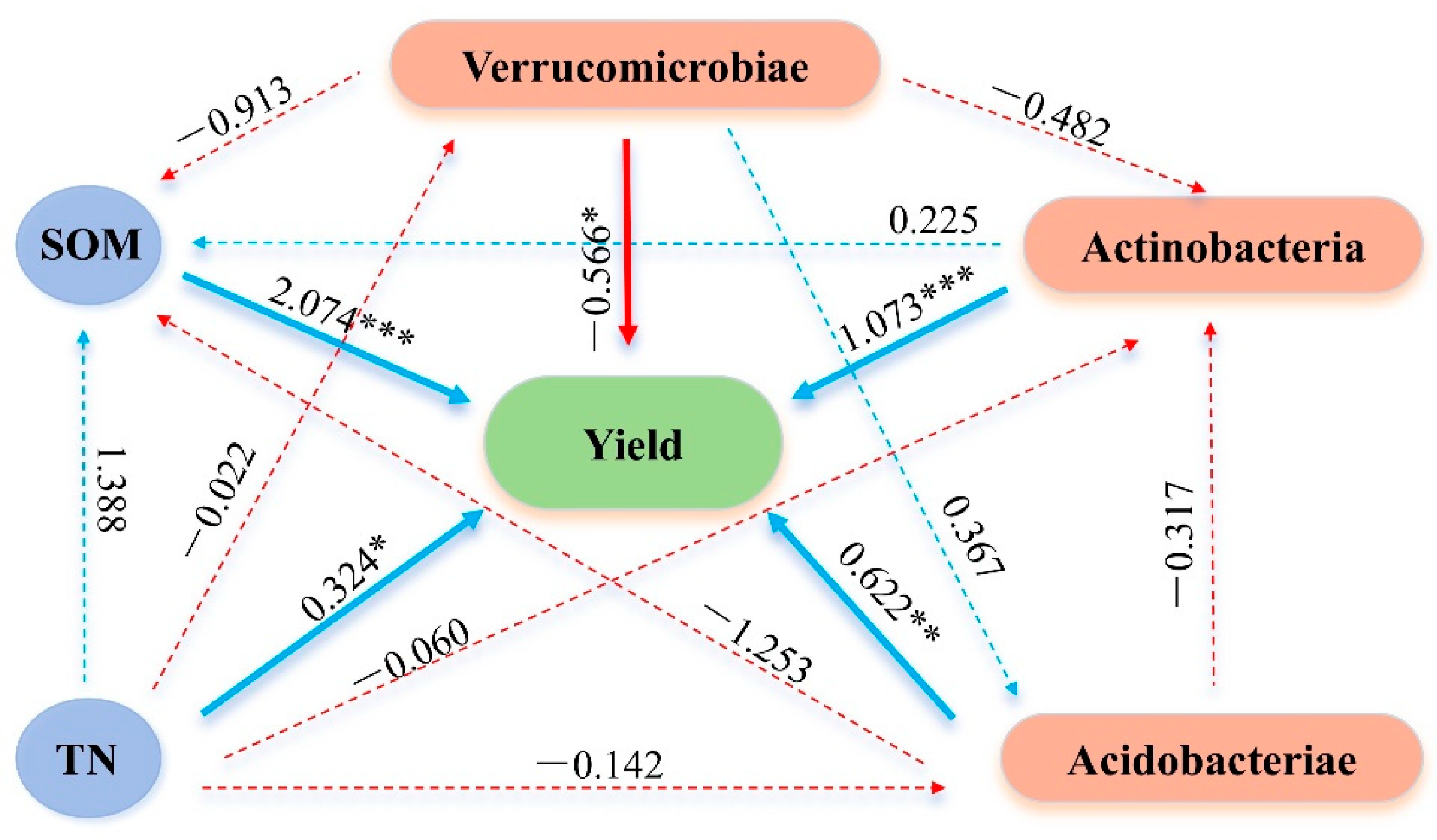
2.4. Direct and Indirect Effects of Soil Properties and Bacterial Communities on Yield (Path Analysis)
3. Discussion
3.1. Responses of Different Treatments on the Soil Properties of Newly Cultivated Land
3.2. Effects of Different Treatments on Soil Bacterial Community in Newly Cultivated Land
3.3. Relationship Between Soil Properties, Bacterial Community Structure, and Crop Yield
4. Materials and Methods
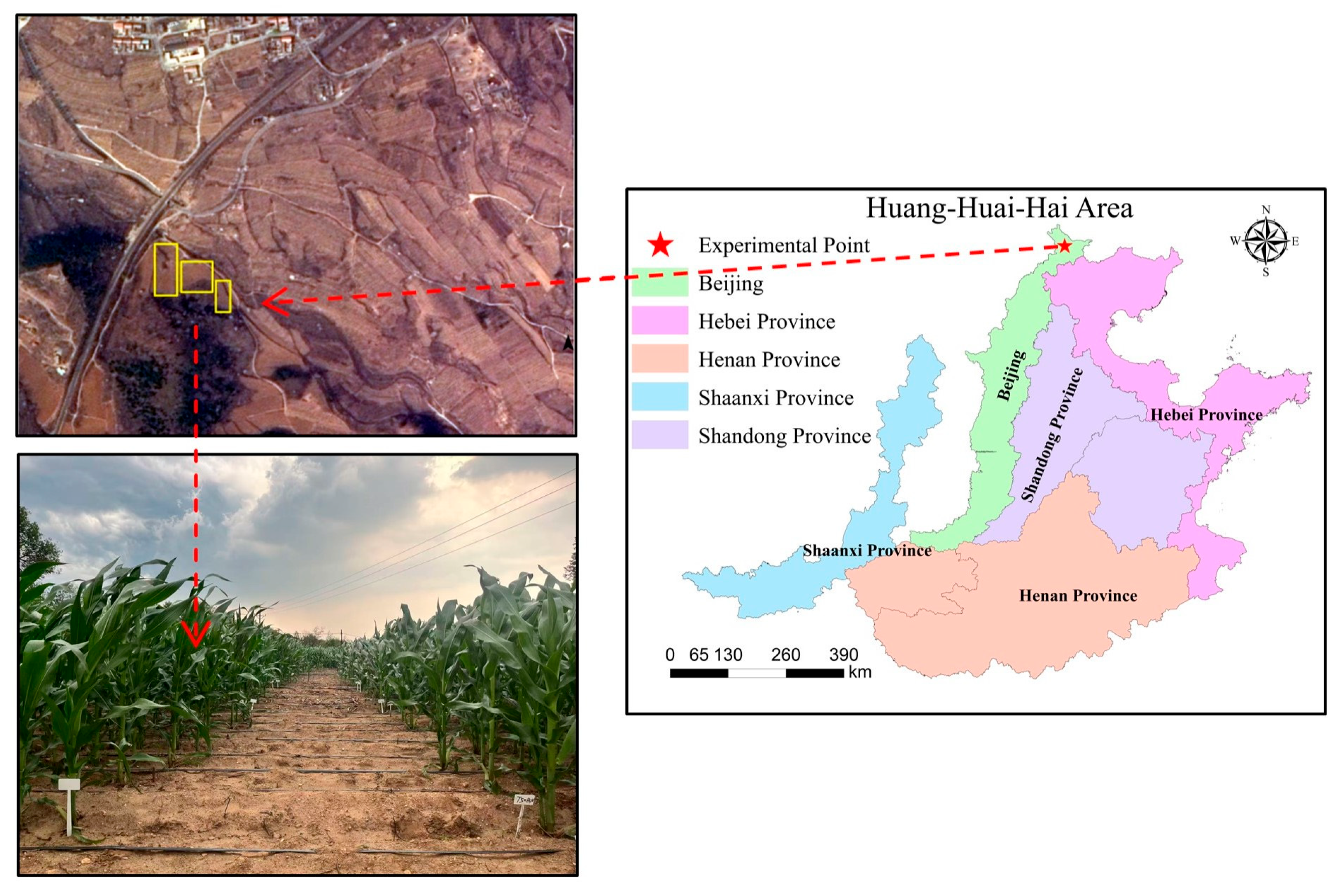
4.1. Study Site
4.2. Experimental Design and Materials
4.3. Sample Collection and Processing
4.4. Microbial Measurement and Analysis Methods
4.5. Statistical Analysis and Data Processing
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FC | 100% Chemical Fertilizer |
| T50 | 50% Organic Fertilizer + 50% Chemical Fertilizer |
| T50M | 50% Organic Fertilizer +50% Chemical Fertilizer + Microbial Inoculants |
| CK | no fertilizer |
| SOM | Soil Organic Matter |
| TN | Total Nitrogen |
| AN | Ammonium Nitrogen |
| AP | Available Phosphorus |
| NN | Nitrate Nitrogen |
| AK | Available Potassium |
References
- Wang, X.; Li, X.B. China’s agricultural land use change and its underlying drivers: A literature review. J. Geogr. Sci. 2021, 31, 1222–1242. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.; Liu, Y. Cultivated land protection and rational use in China. Land Use Policy 2021, 106, 105454. [Google Scholar] [CrossRef]
- Xu, X.L.; Wang, L.; Cai, H.Y.; Wang, L.Y.; Liu, L.; Wang, H.Z. The influences of spatiotemporal change of cultivated land on food crop production potential in China. Food Secur. 2017, 9, 485–495. [Google Scholar] [CrossRef]
- Wang, Y.F. Effects of urbanization on spatial-temporal changes of cultivated land in Bohai Rim region. Environ. Dev. Sustain. 2023, 25, 8469–8486. [Google Scholar] [CrossRef]
- Wang, M.C.; Lin, N.N.; Huang, X.J.; Tang, Y.F. Mitigating farmland use carbon emissions: The dynamic role of farmland use transition. J. Clean. Prod. 2024, 450, 141866. [Google Scholar] [CrossRef]
- Zhao, Q. Deepening the Reform of Arable Land Occupancy and Compensation Balance to Guard the Red Line of Arable Land Protection—Interpretation of the Circular of the Ministry of Natural Resources and the Ministry of Agriculture and Rural Development on the Reform and Improvement of Arable Land Occupancy and Compensation Balance Management. Resour. Guide 2024, 19, 22–23. [Google Scholar]
- Li, C.J.; Cong, H.; Li, Q.; Li, Y.; Zhang, G.; Chen, W. Effects of different soil conditioner on soil quality and maize yield on new cultivated land. Soil Fertil. Sci. China 2023, 7, 15–28. (In Chinese) [Google Scholar]
- Otremba, K.; Kozlowski, M.; Tatusko-Krygier, N.; Korytowski, M.; Pajak, M.; Pietrzykowski, M.; Diatta, J.; Nili, M.S.; Zieba, A. Long-term agricultural reclamation on the chemical properties of Technosols at lignite postmining site—Efficiency of winter wheat and winter rape. Plant Soil 2024, 511, 223–244. [Google Scholar] [CrossRef]
- Yan, T.; Yang, H.E.; Liu, F.H.; Sun, Y.Y. Effects of Fertilization Measures on Nutrient Change and Crop Yield of Newly-cultivated Land. Chin. Agric. Sci. Bull. 2022, 38, 74–78. [Google Scholar]
- Igalavithana, A.D.; Ok, Y.S.; Usman, A.R.A. The Effects of Biochar Amendment on Soil Fertility. Agric. Environ. Appl. Biochar Adv. Barriers 2016, 63, 123–144. [Google Scholar]
- Zhang, S.C.; Zhao, R.; Wu, K.N.; Huang, Q.; Kang, L. Effects of the Rapid Construction of a High-Quality Plough Layer Based on Woody Peat in a Newly Reclaimed Cultivated Land Area. Agriculture 2022, 12, 31. [Google Scholar] [CrossRef]
- Wang, D.; Liu, S.; Guo, M.; Cheng, Y.; Shi, L.; Li, J.; Yu, Y.; Wu, S.; Dong, Q.; Ge, J.; et al. Optimizing Nitrogen Fertilization and Irrigation Practices for Enhanced Winter Wheat Productivity in the North China Plain: A Meta-Analysis. Plants 2025, 14, 1686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Batchelor, W.D.; Hu, K.; Han, H.; Li, J. Modeling Nitrogen Fate and Water and Nitrogen Use Efficiencies under Different Greenhouse Vegetable Production Systems Using the WHCNS-Veg Model. Plants 2024, 13, 1384. [Google Scholar] [CrossRef]
- Xiao, G.M.; Zhao, Z.C.; Liang, L.; Meng, F.Q.; Wu, W.L.; Guo, Y.B. Improving nitrogen and water use efficiency in a wheat-maize rotation system in the North China Plain using optimized farming practices. Agric. Water Manag. 2019, 212, 172–180. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, J.; Manuel, D.-B.; Op de Beeck, M.; Shahbaz, M.; Chen, Y.; Deng, X.; Xu, Z.; Li, J.; Liu, Z. Rotation cropping and organic fertilizer jointly promote soil health and crop production. J. Environ. Manag. 2022, 315, 115190. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhou, Z.Y.; Sun, S.; Zhang, Q.; Sun, S.Q.; Hang, X.N.; Ravanbakhsh, M.; Wei, Z.; Li, R.; Wang, S.M.; et al. Investigating protistan predators and bacteria within soil microbiomes in agricultural ecosystems under organic and chemical fertilizer applications. Biol. Fertil. Soils 2024, 60, 1009–1024. [Google Scholar] [CrossRef]
- Ning, C.C.; Gao, P.D.; Wang, B.Q.; Lin, W.P.; Jiang, N.H.; Cai, K.Z. Impacts of chemical fertilizer reduction and organic amendments supplementation on soil nutrient, enzyme activity and heavy metal content. J. Integr. Agric. 2017, 16, 1819–1831. [Google Scholar] [CrossRef]
- Xu, M.G.; Lu, C.A.; Zhang, W.J.; Li, L.; Duan, Y.H. Situation of the Quality of Arable Land in China and Improvement Strategy. Chin. J. Agric. Resour. Reg. Plan. 2016, 37, 8–14. (In Chinese) [Google Scholar]
- Tao, R.; Liang, Y.C.; Wakelin, S.A.; Chu, G.X. Supplementing chemical fertilizer with an organic component increases soil biological function and quality. Appl. Soil Ecol. 2015, 96, 42–51. [Google Scholar] [CrossRef]
- Ahmad, R.; Arshad, M.; Khalid, A.; Zahir, Z.A. Effectiveness of organic-/bio-fertilizer supplemented with chemical fertilizers for improving soil water retention, aggregate stability, growth and nutrient uptake of maize. J. Sustain. Agric. 2008, 31, 57–77. [Google Scholar] [CrossRef]
- Lehmann, J.; Bossio, D.A.; Kögel-Knabner, I.; Rillig, M.C. The concept and future prospects of soil health. Nat. Rev. Earth Environ. 2020, 1, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Hijbeek, R.; van Ittersum, M.K.; ten Berge, H.F.M.; Gort, G.; Spiegel, H.; Whitmore, A.P. Do organic inputs matter—A meta-analysis of additional yield effects for arable crops in Europe. Plant Soil 2017, 411, 293–303. [Google Scholar] [CrossRef]
- Tao, C.Y.; Li, R.; Xiong, W.; Shen, Z.Z.; Liu, S.S.; Wang, B.B.; Ruan, Y.Z.; Geisen, S.; Shen, Q.R.; Kowalchuk, G.A. Bio-organic fertilizers stimulate indigenous soil populations to enhance plant disease suppression. Microbiome 2020, 8, 137. [Google Scholar] [CrossRef]
- Amoah, A.A.; Miyagawa, S.; Kawakubo, N. Effect of Supplementing Inorganic Fertilizer with Organic Fertilizer on Growth and Yield of Rice-Cowpea Mixed Crop. Plant Prod. Sci. 2012, 15, 109–117. [Google Scholar] [CrossRef][Green Version]
- Luo, G.W.; Li, L.; Friman, V.P.; Guo, J.J.; Guo, S.W.; Shen, Q.R.; Ling, N. Organic amendments increase crop yields by improving microbe-mediated soil functioning of agroecosystems: A meta-analysis. Soil Biol. Biochem. 2018, 124, 105–115. [Google Scholar] [CrossRef]
- Fan, S.; Tang, Y.; Yang, H.; Hu, Y.; Zeng, Y.; Wang, Y.; Zhao, Y.; Chen, X.; Wu, Y.; Wang, G. Effects of fertilization and planting modes on soil organic carbon and microbial community formation of tree seedlings. Plants 2024, 13, 2665. [Google Scholar] [CrossRef]
- Chen, Z.L.; Zhang, J.Q.; Lv, W.G.; Zhang, H.L.; Li, S.X.; Zhang, H.Y.; Shen, Y.; Geng, C.N.; Bai, N.L. The unexpected effect of the compound microbial agent NP-M2 on microbial community dynamics in a nonylphenol-contaminated soil: The self-stability of soil ecosystem. Peerj 2024, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Darbon, G.; Declerck, S.; Riot, G.; Doubell, M.; Dupuis, B. Inoculation and tracking of beneficial microbes reveal they can establish in field-grown potato roots and decrease blemish diseases. Biol. Fertil. Soils 2024, 60, 699–712. [Google Scholar] [CrossRef]
- Mengual, C.; Schoebitz, M.; Azcón, R.; Roldán, A. Microbial inoculants and organic amendment improves plant establishment and soil rehabilitation under semiarid conditions. J. Environ. Manag. 2014, 134, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.X.; Yan, C.R.; Xue, Y.H.; Xu, Z.Y.; Jin, T.; Liu, Q. Effects of Exogenous Microbial Agents on Soil Nutrient and Microbial Community Composition in Greenhouse-Derived Vegetable Straw Composts. Sustainability 2021, 13, 2925. [Google Scholar] [CrossRef]
- Ambrosini, A.; de Souza, R.; Passaglia, L.M.P. Ecological role of bacterial inoculants and their potential impact on soil microbial diversity. Plant Soil 2016, 400, 193–207. [Google Scholar] [CrossRef]
- Yang, Z.C.; Zhao, N.; Huang, F.; Lv, Y. Long-term effects of different organic and inorganic fertilizer treatments on soil organic carbon sequestration and crop yields on the North China Plain. Soil Tillage Res. 2015, 146, 47–52. [Google Scholar] [CrossRef]
- Alori, E.T.; Dare, M.O.; Babalola, O.O. Microbial Inoculants for Soil Quality and Plant Health. In Sustainable Agriculture Reviews; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Li, X.Q.; Su, Y.; Ahmed, T.; Ren, H.Y.; Javed, M.R.; Yao, Y.L.; An, Q.L.; Yan, J.L.; Li, B. Effects of Different Organic Fertilizers on Improving Soil from Newly Reclaimed Land to Crop Soil. Agriculture 2021, 11, 560. [Google Scholar] [CrossRef]
- Luo, J.P.; Banerjee, S.; Ma, Q.X.; Liao, G.C.; Hu, B.F.; Zhao, H.P.; Li, T.Q. Organic fertilization drives shifts in microbiome complexity and keystone taxa increase the resistance of microbial mediated functions to biodiversity loss. Biol. Fertil. Soils 2023, 59, 441–458. [Google Scholar] [CrossRef]
- Xiao, Z.W.; Zhixi, Z.; Zhao, Y.; Cheng, H.; Ma, Y.; Pu, C.; Fu, Y. The ecological restoration effect of organic fertilizer combined with soil conditioner and biological fertilizer on continuous cropping soil of maize seed production under chemical fertilizer reduction. Soil Fertil. Sci. China 2023, 10, 48–54. (In Chinese) [Google Scholar]
- Hermans, S.M.; Buckley, H.L.; Case, B.S.; Curran-Cournane, F.; Taylor, M.; Lear, G. Using soil bacterial communities to predict physico-chemical variables and soil quality. Microbiome 2020, 8, 13. [Google Scholar] [CrossRef]
- Wang, R.; Hou, T.; Sun, Q.; Ji, L.D.; Lei, J.Y.; Zhang, J.X. Organic Fertilizers and Soil Conditioner Recover Chemical Fertilizer-Induced Changes in Soil Bacterial Community Diversity in Wine Grape Rhizosphere Soil. Pol. J. Environ. Stud. 2021, 30, 1853–1863. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, T.; Yu, S.; Zhou, C.; Teng, A.; Lei, L.; Li, F. Optimizing the mulching pattern and nitrogen application rate to improve maize photosynthetic capacity, yield, and nitrogen fertilizer utilization efficiency. Plants 2024, 13, 1241. [Google Scholar] [CrossRef]
- Shi, Y.W.; Niu, X.X.; Chen, B.Z.; Pu, S.H.; Ma, H.H.; Li, P.; Feng, G.P.; Ma, X.W. Chemical fertilizer reduction combined with organic fertilizer affects the soil microbial community and diversity and yield of cotton. Front. Microbiol. 2023, 14, 1295722. [Google Scholar] [CrossRef]
- Chen, H.S.; Zhang, W.; Wang, K.L.; Hou, Y. Soil organic carbon and total nitrogen as affected by land use types in karst and non-karst areas of northwest Guangxi, China. J. Sci. Food Agric. 2012, 92, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Chodak, M.; Niklinska, M. The effect of different tree species on the chemical and microbial properties of reclaimed mine soils. Biol. Fertil. Soils 2010, 46, 555–566. [Google Scholar] [CrossRef]
- Tofanelli, M.B.D.; de Jesus, G.L.; Silva, R.S.A. Organic fertilization for the improvement of production and quality of ripe figs. Span. J. Agric. Res. 2022, 20, 5. [Google Scholar] [CrossRef]
- Yu, Q.G.; Hu, X.; Ma, J.W.; Ye, J.; Sun, W.C.; Wang, Q.; Lin, H. Effects of long-term organic material applications on soil carbon and nitrogen fractions in paddy fields. Soil Tillage Res. 2020, 196, 7. [Google Scholar] [CrossRef]
- Zhou, S.; Chang, T.; Zhang, Y.; Shaghaleh, H.; Zhang, J.; Yang, X.; Qin, H.; Talpur, M.M.A.; Hamoud, Y.A. Organic fertilizer compost alters the microbial composition and network structure in strongly acidic soil. Appl. Soil Ecol. 2024, 195, 105263. [Google Scholar] [CrossRef]
- Wato, T.; Negash, T.; Andualem, A.; Bitew, A. Significance of organic and inorganic fertilizers in maintaining soil fertility and increasing crop productivity in Ethiopia: A review. Environ. Res. Commun. 2024, 6, 102002. [Google Scholar] [CrossRef]
- Wang, Q.; Song, J.; Zhang, J.L.; Qin, X.J.; Kang, Y.H.; Huang, S.L.; Zhou, S.M.; Chen, T.S. Effects of microbial agent application on the bacterial community in ginger rhizosphere soil under different planting years. Front. Microbiol. 2023, 14, 13. [Google Scholar] [CrossRef]
- Lin, Y.B.; Ye, Y.; Yang, J.; Hu, Y.; Shi, H. The effect of land consolidation on soil microbial diversity. Acta Sci. Circumstantiae 2019, 39, 2644–2653. (In Chinese) [Google Scholar]
- Zhu, Y.H.; Li, Q.M.; Liu, X.L.; Li, N.; Song, F.L.; Chen, W.F. Characteristics of Soil Microbial Community in Newly Cultivated Land under Different Land Consolidation Types. Ecol. Environ. Sci. 2022, 31, 909–917. (In Chinese) [Google Scholar]
- Pan, H.; Chen, M.M.; Feng, H.J.; Wei, M.; Song, F.P.; Lou, Y.H.; Cui, X.M.; Wang, H.; Zhuge, Y.P. Organic and inorganic fertilizers respectively drive bacterial and fungal community compositions in a fluvo-aquic soil in northern China. Soil Tillage Res. 2020, 198, 9. [Google Scholar] [CrossRef]
- Zhuo, S.; Fang, Y.; Chen, Y.; Vancov, T.; Du, H.; Li, Y.; Yu, B.; Chang, S.X.; Cai, Y. Interactive effects of plant litter chemistry and organic/inorganic forms of nitrogen addition on Moso bamboo (Phyllostachys edulis) soil respiration. Biol. Fertil. Soils 2025, 65, 109–123. [Google Scholar] [CrossRef]
- Guan, X.; Cheng, Z.; Li, Y.; Wang, J.; Zhao, R.; Guo, Z.; Zhao, T.; Huang, L.; Qiu, C.; Shi, W.; et al. Mixed organic and inorganic amendments enhance soil microbial interactions and environmental stress resistance of Tibetan barley on plateau farmland. J. Environ. Manag. 2023, 330, 117137. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.L.; Zhang, J.G.; Shi, A.D.; Yao, S.H.; Zhang, B. Manure substitution of mineral fertilizers increased functional stability through changing structure and physiology of microbial communities. Eur. J. Soil Biol. 2016, 77, 34–43. [Google Scholar] [CrossRef]
- Nemergut, D.R.; Cleveland, C.C.; Wieder, W.R.; Washenberger, C.L.; Townsend, A.R. Plot-scale manipulations of organic matter inputs to soils correlate with shifts in microbial community composition in a lowland tropical rain forest. Soil Biol. Biochem. 2011, 42, 2153–2160. [Google Scholar] [CrossRef]
- Felici, C.; Vettori, L.; Giraldi, E.; Forino, L.M.C.; Toffanin, A.; Tagliasacchi, A.M.; Nuti, M. Single and co-inoculation of Bacillus subtilis and Azospirillum brasilense on Lycopersicon esculentum: Effects on plant growth and rhizosphere microbial community. Appl. Soil Ecol. 2008, 40, 260–270. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 8. [Google Scholar] [CrossRef]
- Jiang, M.; Dong, C.; Bian, W.; Zhang, W.; Wang, Y. Effects of different fertilization practices on maize yield, soil nutrients, soil moisture, and water use efficiency in northern China based on a meta-analysis. Sci. Rep. 2024, 14, 6480. [Google Scholar] [CrossRef]
- Shen, M.; Li, J.; Dong, Y.; Zhang, Z.; Zhao, Y.; Li, Q.; Dang, K.; Peng, J.; Liu, H. The Effects of Microbial Inoculants on Bacterial Communities of the Rhizosphere Soil of Maize. Agriculture 2021, 11, 389. [Google Scholar] [CrossRef]
- Wu, Z.; Xue, B.; Wang, S.; Xing, X.; Nuo, M.; Meng, X.; Wu, M.; Jiang, H.; Ma, H.; Yang, M.; et al. Rice under dry cultivation–maize intercropping improves soil environment and increases total yield by regulating belowground root growth. Plants 2024, 13, 2957. [Google Scholar] [CrossRef]
- Bao, Y.; Dolfing, J.; Guo, Z.; Chen, R.; Feng, Y. Important ecophysiological roles of non-dominant actinobacteria in plant residue decomposition, especially in less fertile soils. Microbiome 2021, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Araujo, R.; Gupta, V.V.; Reith, F.; Bissett, A.; Franco, C.M. Biogeography and emerging significance of Actinobacteria in Australia and Northern Antarctica soils. Soil Biol. Biochem. 2020, 146, 107805. [Google Scholar] [CrossRef]
- Gonçalves, O.S.; Fernandes, A.S.; Tupy, S.M.; Ferreira, T.G.; Almeida, L.N.; Creevey, C.J.; Santana, M.F. Insights into plant interactions and the biogeochemical role of the globally widespread Acidobacteriota phylum. Soil Biol. Biochem. 2024, 192, 109369. [Google Scholar] [CrossRef]
- Song, T.; Liu, J.; Han, S.; Li, Y.; Xu, T.; Xi, J.; Hou, L.; Lin, Y. Effect of conventional and biodegradable microplastics on the soil-soybean system: A perspective on rhizosphere microbial community and soil element cycling. Environ. Int. 2024, 190, 108781. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agricultural Chemical Analysis; China Agriculture Press: Beijing, China, 1999. (In Chinese) [Google Scholar]
- Lin, Y.; Ye, G.; Kuzyakov, Y.; Liu, D.; Fan, J.; Ding, W. Long-term manure application increases soil organic matter and aggregation, and alters microbial community structure and keystone taxa. Soil Biol. Biochem. 2019, 134, 187–196. [Google Scholar] [CrossRef]
- Deng, L.; Zhao, M.; Bi, R.; Bello, A.; Egbeagu, U.; Zhang, J.; Li, S.; Chen, Y.; Han, Y.; Sun, Y.; et al. Insight into the influence of biochar on nitrification based on multi-level and multi-aspect analyses of ammonia-oxidizing microorganisms during cattle manure composting. Bioresour. Technol. 2021, 339, 125515. [Google Scholar] [CrossRef] [PubMed]
- Zi, R.; Zhao, L.; Fang, Q.; Qian, X.; Fang, F.; Fan, C. Path analysis of the effects of hydraulic conditions, soil properties and plant roots on the soil detachment capacity of karst hillslopes. Catena 2023, 228, 107177. [Google Scholar] [CrossRef]
- Xiao, M.; Jiang, S.; Li, J.; Li, W.; Fu, P.; Liu, G.; Chen, J. Synergistic effects of bio-organic fertilizer and different soil amendments on salt reduction, soil fertility, and yield enhancement in salt-affected coastal soils. Soil Tillage Res. 2025, 248, 106433. [Google Scholar] [CrossRef]

| Treatment | Base Fertilizer | Additional Fertilizer | ||||
|---|---|---|---|---|---|---|
| Organic Fertilizer | Chemical Fertilizer | Microbial Inoculant | Chemical Fertilizer | |||
| N (3.37%) | N (46.4%) | P2O5 (12%) | K2O (52%) | N (46.4%) | ||
| CK | 0 | 0 | 0 | 0 | 0 | 0 |
| FC | 0 | 175 | 1150 | 235 | 0 | 345 |
| T50 | 3560 | 0 | 0 | 235 | 0 | 260 |
| T50M | 3560 | 0 | 0 | 235 | 45 | 260 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Y.; Wu, X.; Li, S.; Li, Y.; Wang, L.; Hu, Y.; Liu, K.; Yang, Z.; Cai, L.; Xu, K.; et al. Optimizing Resource Management with Organic Fertilizer and Microbial Inoculants to Enhance Soil Quality, Microbial Diversity, and Crop Productivity in Newly Cultivated Land. Plants 2025, 14, 3032. https://doi.org/10.3390/plants14193032
Dai Y, Wu X, Li S, Li Y, Wang L, Hu Y, Liu K, Yang Z, Cai L, Xu K, et al. Optimizing Resource Management with Organic Fertilizer and Microbial Inoculants to Enhance Soil Quality, Microbial Diversity, and Crop Productivity in Newly Cultivated Land. Plants. 2025; 14(19):3032. https://doi.org/10.3390/plants14193032
Chicago/Turabian StyleDai, Yuling, Xiaoxiao Wu, Shuo Li, Yan Li, Lei Wang, Yu Hu, Kangmeng Liu, Zhenguo Yang, Lianfeng Cai, Kuifeng Xu, and et al. 2025. "Optimizing Resource Management with Organic Fertilizer and Microbial Inoculants to Enhance Soil Quality, Microbial Diversity, and Crop Productivity in Newly Cultivated Land" Plants 14, no. 19: 3032. https://doi.org/10.3390/plants14193032
APA StyleDai, Y., Wu, X., Li, S., Li, Y., Wang, L., Hu, Y., Liu, K., Yang, Z., Cai, L., Xu, K., Cui, M., Xu, X., Jia, Y., Wei, D., & Ding, J. (2025). Optimizing Resource Management with Organic Fertilizer and Microbial Inoculants to Enhance Soil Quality, Microbial Diversity, and Crop Productivity in Newly Cultivated Land. Plants, 14(19), 3032. https://doi.org/10.3390/plants14193032






