Assessing the Insecticidal Performance of Commiphora myrrha Essential Oil Against Prostephanus truncatus and Sitophilus zeamais Using a Metabolomic Approach
Abstract
1. Introduction
2. Results
2.1. Chemical Analysis of the Essential Oil (EO)
2.2. Main Effects and Interactions
2.3. Efficacy of C. myrra EO Against S. zeamais Adults
2.4. Efficacy of C. myrra EO Against P. truncatus Adults
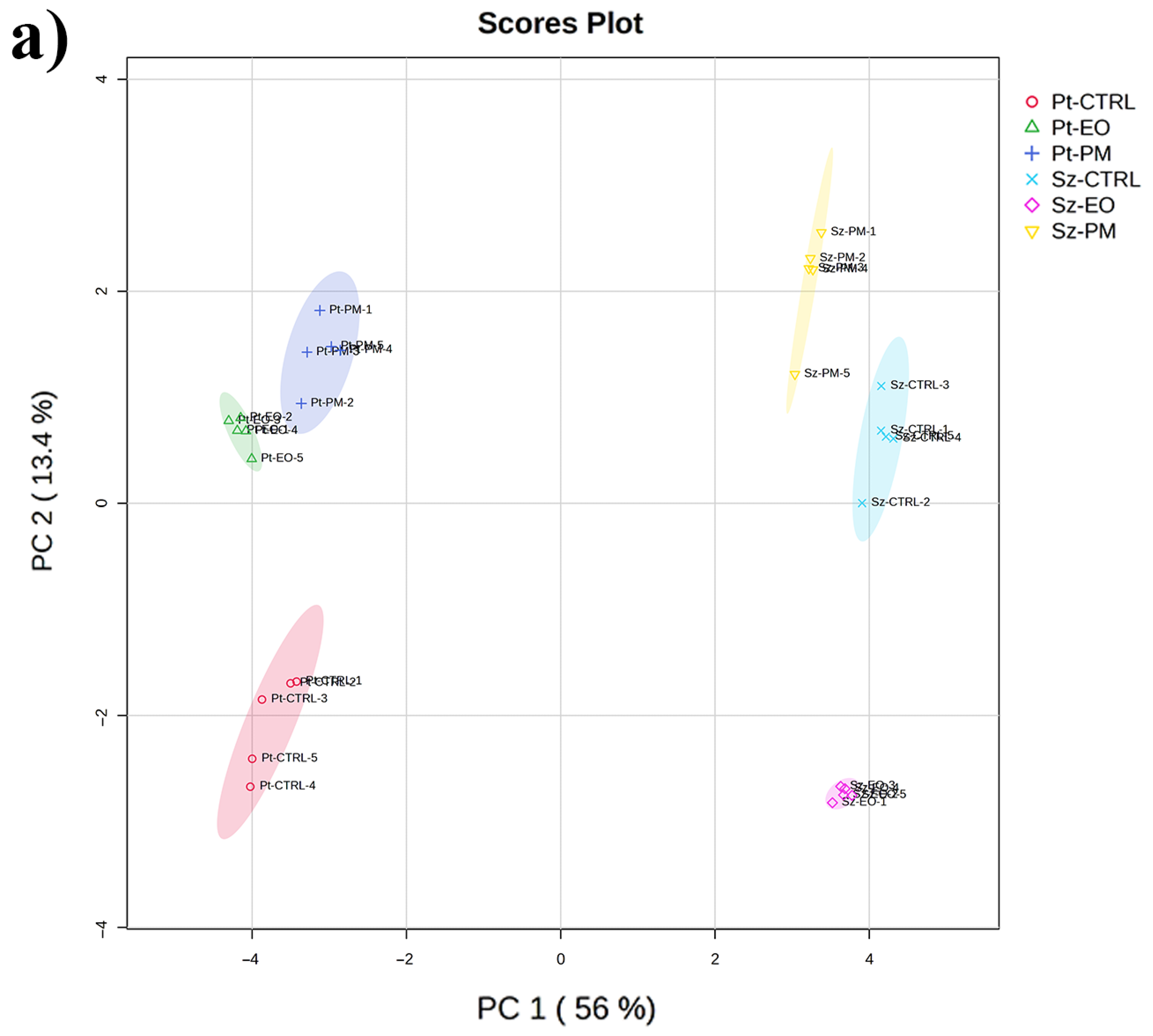
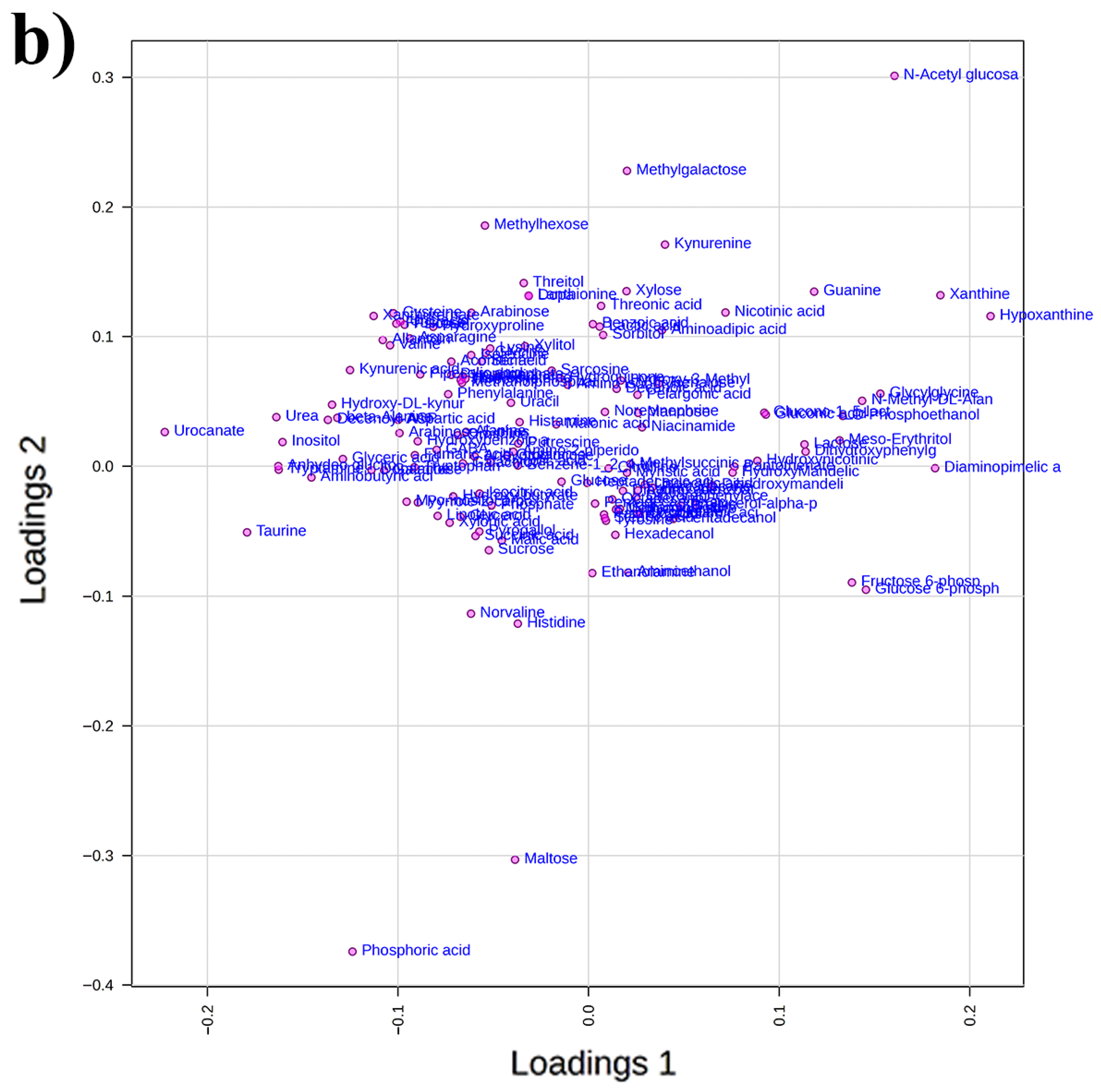
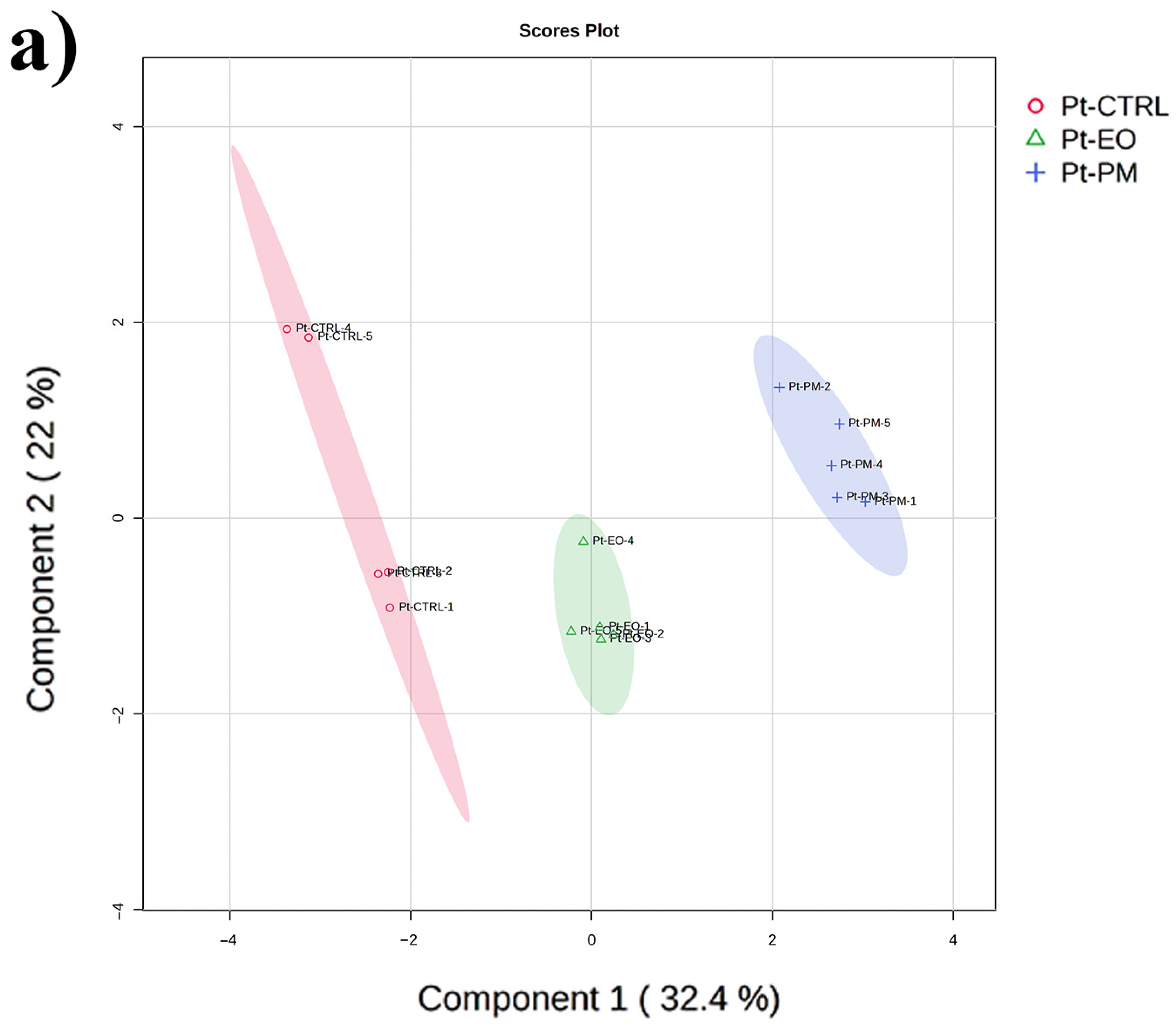
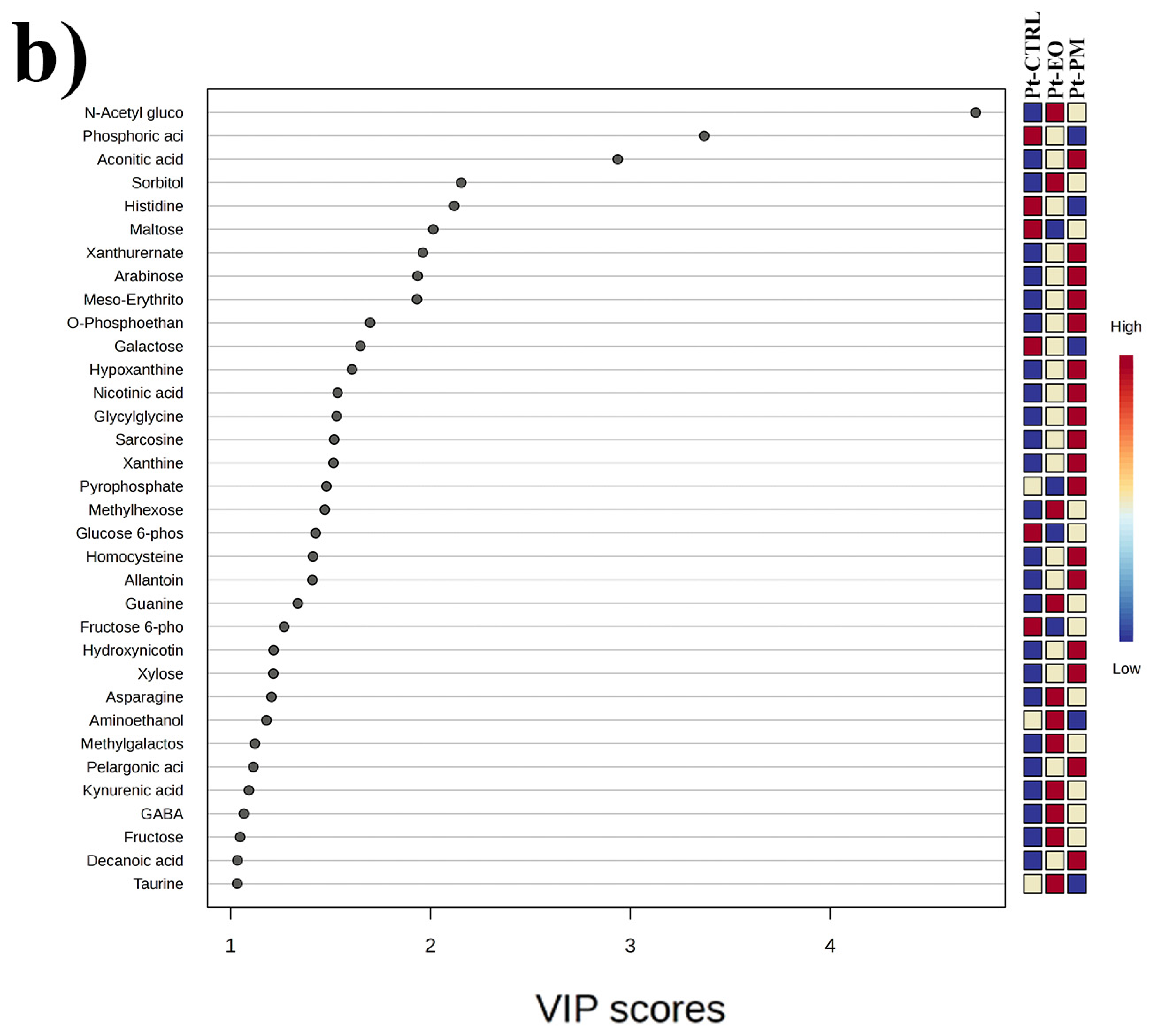
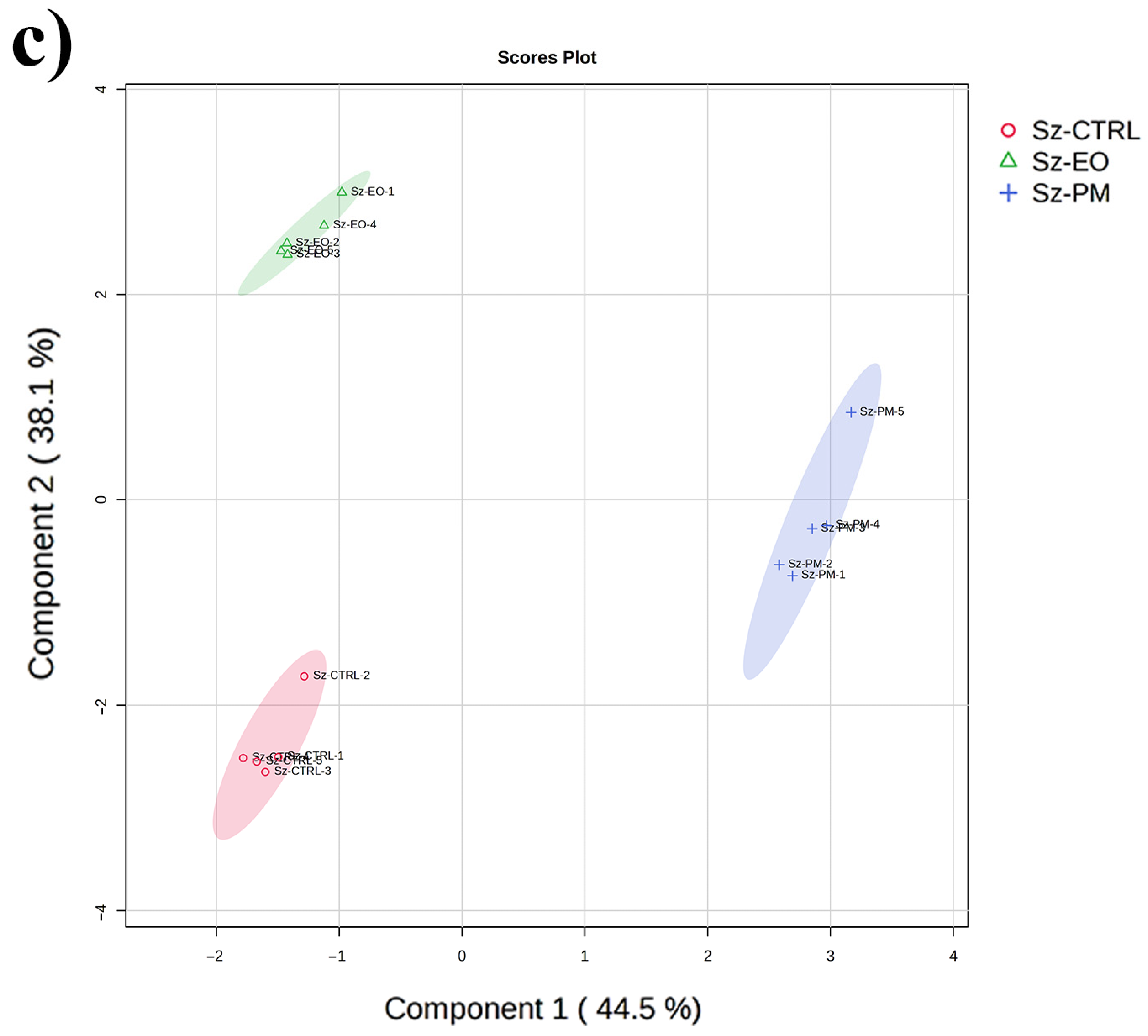
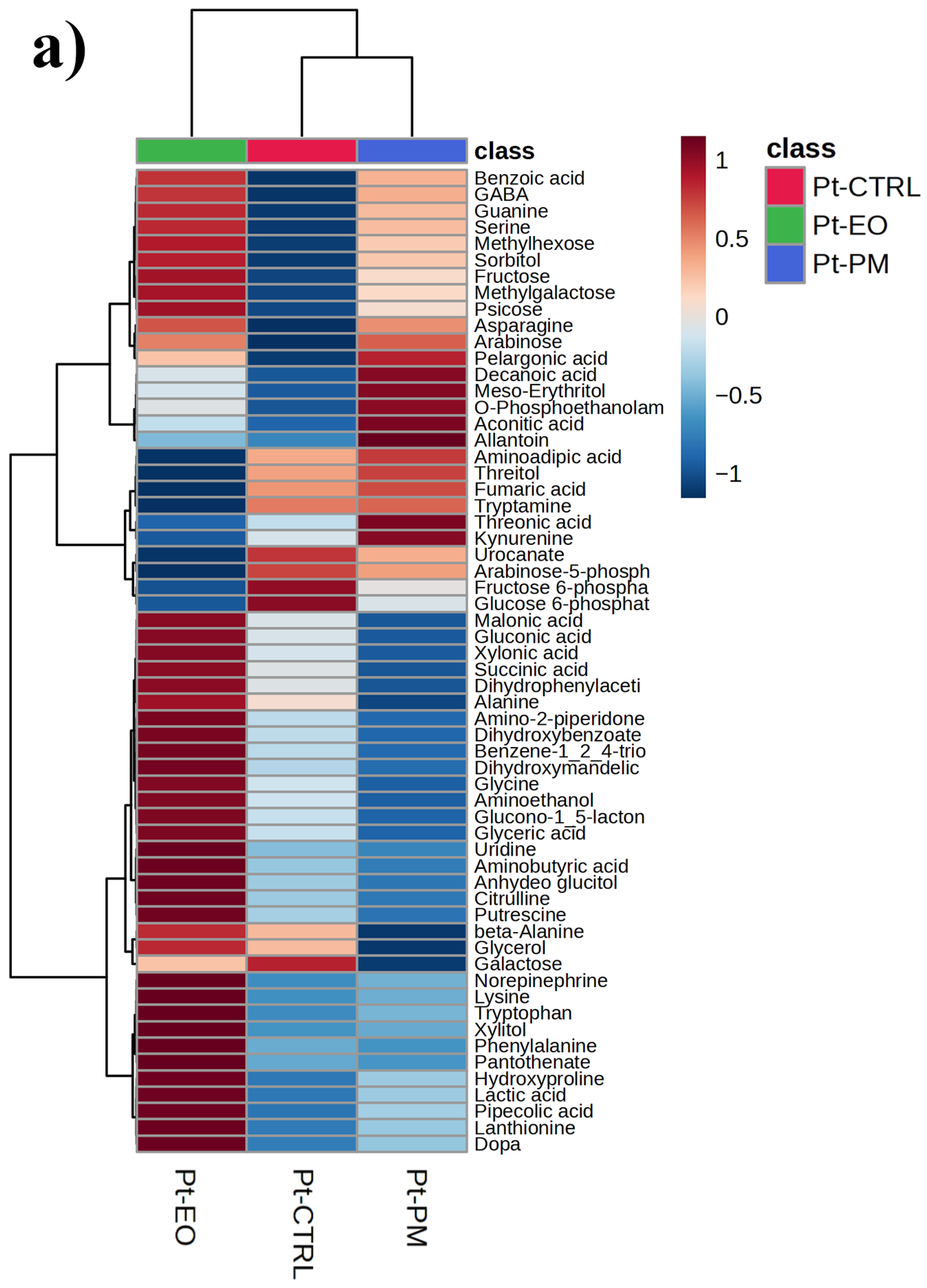
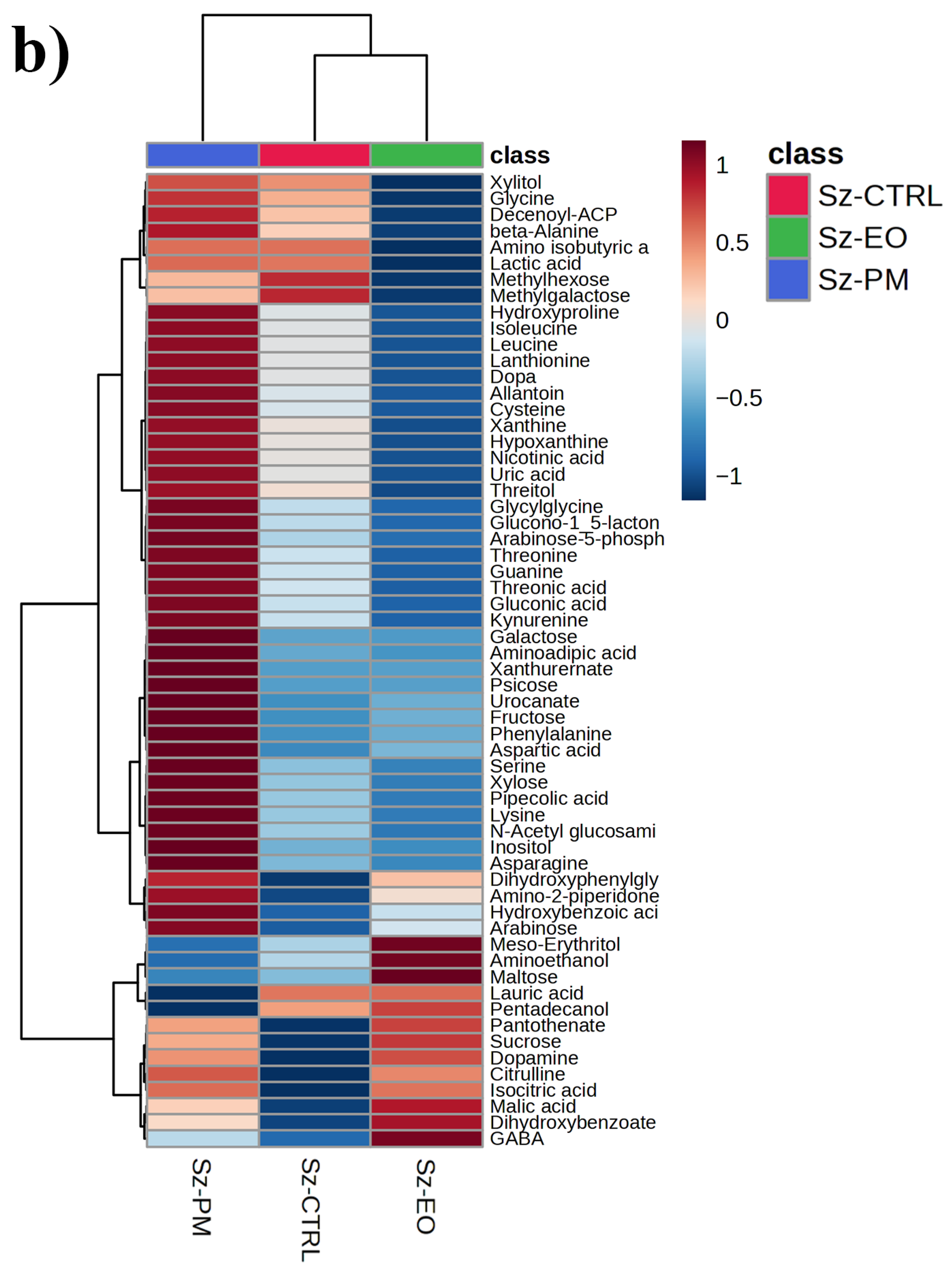
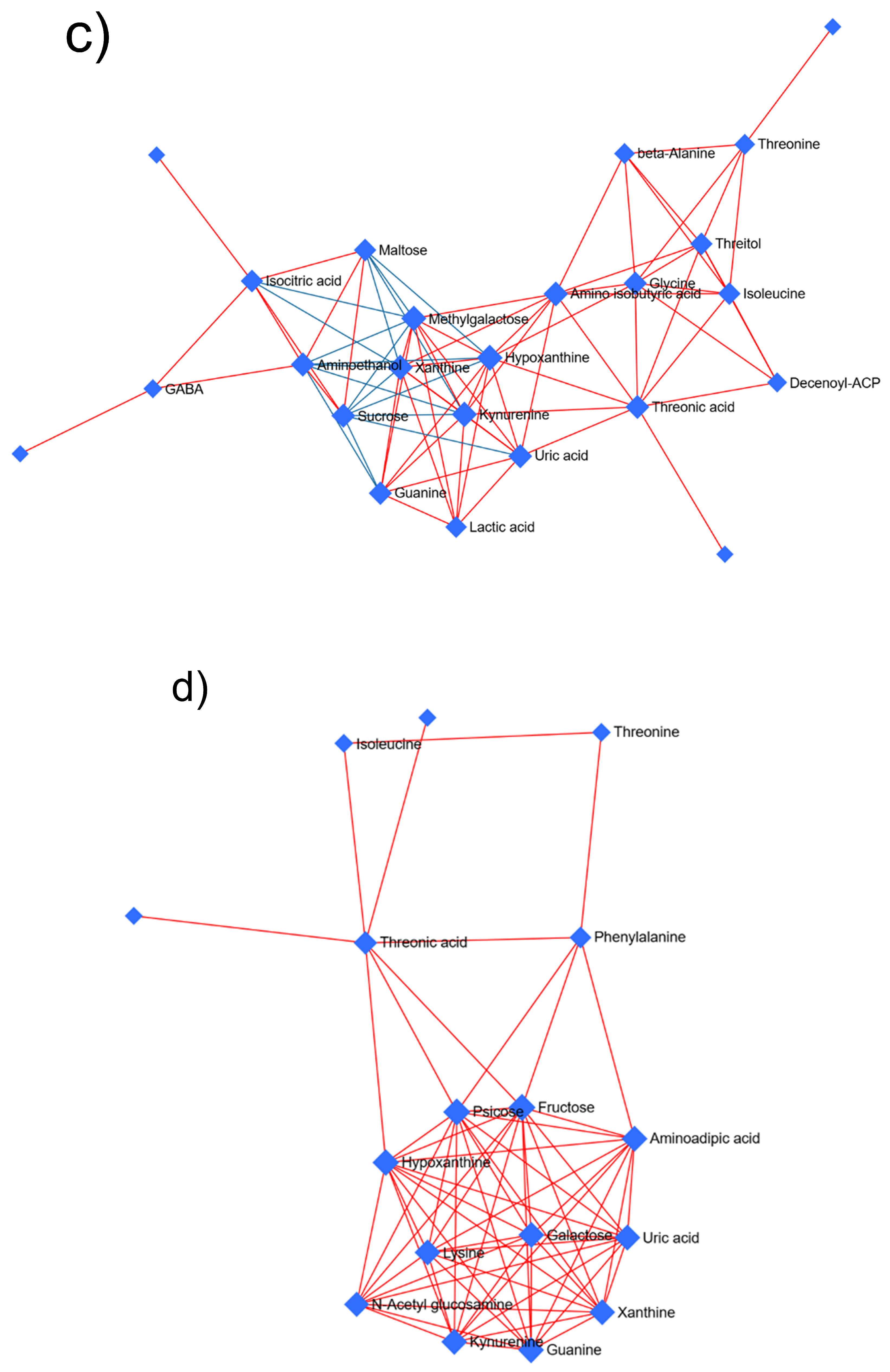
2.5. GC-MS-Driven Untargeted Metabolomic Analysis
3. Discussion
4. Materials and Methods
4.1. Chemical Analysis of C. myrrha Essential Oil (EO)
4.2. Insects Rearing and Grain Substrate
4.3. Mortality Bioassays
4.4. Metabolomic Analysis Bioassays
4.5. Metabolite Extraction and GC–MS Untargeted Metabolomic Analysis
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Langenheim, J.H. Plant Resins: Chemistry, Evolution, Ecology, and Ethnobotany; Timber Press: Portland, OR, USA, 2003. [Google Scholar]
- Mutiga, I.M. Ecological restoration of pastoral landscapes in the drylands of East Africa. J. Dryland Agric. 2021, 7, 34–41. [Google Scholar] [CrossRef]
- Başer, K.H.C.; Demirci, B.E.T.Ü.L.; Dekebo, A.; Dagne, E. Essential oils of some Boswellia spp., myrrh and opopanax. Flavour Fragr. J. 2003, 18, 153–156. [Google Scholar] [CrossRef]
- Alsherif, E.A. Ecological studies of Commiphora genus (myrrha) in Makkah region, Saudi Arabia. Heliyon 2019, 5, e01615. [Google Scholar] [PubMed]
- Hanuš, L.O.; Řezanka, T.; Dembitsky, V.M.; Moussaieff, A. Myrrh—Commiphora chemistry. Biomed. Papers 2005, 149, 3–28. [Google Scholar] [CrossRef] [PubMed]
- Shalabi, L.F.; Otaif, F.S. Commiphora Jacq (Burseraceae) in Saudi Arabia, botanical, phytochemical and ethnobotanical notes. Ecologies 2022, 3, 38–57. [Google Scholar] [CrossRef]
- Hassan, B.; Glover, E.K.; Luukkanen, O.; Chikamai, B.; Jamnadass, R.; Iiyam, M.; Kanninen, M. The role of Boswellia and Commiphora species in rural livelihood security and climate change adaptation in the Horn of Africa: Case study of northeastern Kenya. Int. J. Soc. For. 2011, 4, 86–112. [Google Scholar]
- Latha, S.; Selvamani, P.; Prabha, T. Pharmacological uses of the plants belonging to the genus Commiphora. Cardiovasc. Hematol. Agents Med. Chem. 2021, 19, 101–117. [Google Scholar] [CrossRef]
- Cao, B.; Wei, X.C.; Xu, X.R.; Zhang, H.Z.; Luo, C.H.; Feng, B.; Xu, R.C.; Zhao, S.Y.; Du, X.J.; Han, L.; et al. Seeing the unseen of the combination of two natural resins, frankincense and myrrh: Changes in chemical constituents and pharmacological activities. Molecules 2019, 24, 3076. [Google Scholar] [CrossRef]
- Abdul-Ghani, R.A.; Loutfy, N.; Hassan, A. Myrrh and trematodoses in Egypt: An overview of safety, efficacy and effectiveness profiles. Parasitol. Int. 2009, 58, 210–214. [Google Scholar] [CrossRef]
- Nohr, L.A.; Rasmussen, L.B.; Straand, J. Resin from the mukul myrrh tree, guggul, can it be used for treating hypercholesterolemia? A randomized, controlled study. Complement. Ther. Med. 2009, 17, 16–22. [Google Scholar] [CrossRef]
- Haffor, A.S.A. Effect of myrrh (Commiphora molmol) on leukocyte levels before and during healing from gastric ulcer or skin injury. J. Immunotoxicol. 2010, 7, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Wang, T.; Duan, J.A.; Zhou, W.; Hua, Y.Q.; Tang, Y.P.; Yu, L.; Qian, D.W. Anti-inflammatory and analgesic activity of different extracts of Commiphora myrrha. J. Ethnopharmacol. 2011, 134, 251–258. [Google Scholar] [CrossRef] [PubMed]
- De Rapper, S.; Van Vuuren, S.F.; Kamatou, G.P.P.; Viljoen, A.M.; Dagne, E. The additive and synergistic antimicrobial effects of select frankincense and myrrh oils—A combination from the pharaonic pharmacopoeia. Lett. Appl. Microbiol. 2012, 54, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.K.; Tripathi, C.D. Commiphora mukul attenuates peripheral neuropathic pain induced by chronic constriction injury of sciatic nerve in rats. Nutr. Neurosci. 2015, 18, 97–102. [Google Scholar]
- Rahmani, A.H.; Anwar, S.; Raut, R.; Almatroudi, A.; Babiker, A.Y.; Khan, A.A.; Alsahli, M.; Almatroodi, S.A. Therapeutic potential of myrrh, a natural resin, in health management through modulation of oxidative stress, inflammation, and advanced glycation end products formation using in vitro and in silico analysis. Appl. Sci. 2022, 12, 9175. [Google Scholar] [CrossRef]
- Goda, A.A.; Aya, E.G.; Amin, M.H.; Youssef, M.; Shi, J.; Xu, J.; Liu, X.; Zhou, Y.; Xiao, L.; Ramzy, S. Evaluation of the antimicrobial and anticancer properties of myrrh resin extract and its application in cacao beverages. Discov. Food 2024, 4, 121. [Google Scholar] [CrossRef]
- Hamed, A.M.; Awad, A.A.; Abdel-Mobdy, A.E.; Alzahrani, A.; Salamatullah, A.M. Buffalo yogurt fortified with eucalyptus (Eucalyptus camaldulensis) and myrrh (Commiphora myrrha) essential oils: New insights into the functional properties and extended shelf life. Molecules 2021, 26, 6853. [Google Scholar] [CrossRef]
- Dekebo, A.; Juniedi, S.; Hu, X.; Jung, C. Ethnobotany, chemistry, and biological activities of some Commiphora species resins. In Gums, Resins and Latexes of Plant Origin: Chemistry, Biological Activities and Uses; Murthy, H.N., Ed.; Springer: Cham, Switzerland, 2021; pp. 581–607. [Google Scholar]
- Baz, M.M.; Khater, H.F.; Baeshen, R.S.; Selim, A.; Shaheen, E.S.; El-Sayed, Y.A.; Salama, A.S.; Hegazy, M.M. Novel pesticidal efficacy of Araucaria heterophylla and Commiphora molmol extracts against camel and cattle blood-sucking ectoparasites. Plants 2022, 11, 1682. [Google Scholar] [CrossRef]
- Dinku, W.; Park, S.B.; Jeong, J.B.; Jung, C.; Dekebo, A. Chemical composition and anti-inflammatory activity of essential oils from resin of Commiphora species. Bull. Chem. Soc. Ethiop. 2022, 36, 399–415. [Google Scholar] [CrossRef]
- Shah, M.; Khan, F.; Ullah, S.; Khan, A.; Alsabahi, J.N.; Zainab, R.; Bilal, M.; Khan, S.; Rafiq, N.; Rehman, N.U.; et al. Chemical composition and biomedical effects of essential oil from Commiphora foliacea Sprague stem. J. Essent. Oil-Bear. Plants 2023, 26, 1319–1337. [Google Scholar] [CrossRef]
- Alanazi, N.A.H.; Alamri, A.A.; Mashlawi, A.M.; Almuzaini, N.; Mohamed, G.; Salama, S.A. Gas chromatography–mass spectrometry chemical profiling of Commiphora myrrha resin extracts and evaluation of larvicidal, antioxidant, and cytotoxic activities. Molecules 2024, 29, 1778. [Google Scholar] [CrossRef]
- Hosseinkhani, A.; Ghavidel, F.; Mohagheghzadeh, A.; Zarshenas, M.M. Analysis of six populations of Commiphora myrrha (Nees) Engl. oleo-gum resin. Trends Pharm. Sci. Technol. 2017, 3, 7–12. [Google Scholar]
- Batiha, G.E.S.; Wasef, L.; Teibo, J.O.; Shaheen, H.M.; Zakariya, A.M.; Akinfe, O.A.; Teibo, T.K.A.; Alkuraishy, H.M.; Al-Garbee, A.I.; Alexiou, A.; et al. Commiphora myrrh: A phytochemical and pharmacological update. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Al-Fuhaid, N. Repellency and fumigant toxicity of Aloe vera, Astragalus sarcocolla, Commiphora myrrha and Ferula assafoetida L gum resin powders and methanol extracts against Trogoderma granarium Everts. Int. J. Agric. Sci. 2017, 7, 597–604. [Google Scholar]
- Wahba, T.F.; Aly, H.M.; Hassan, N.A. The antifeedant properties of bio-oil from Cupressus sempervirens against rice weevil (Sitophilus oryzae) compared to that of myrrh and frankincense oils. Egypt. J. Agric. Res. 2023, 101, 331–341. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Boukouvala, M.C.; Gidari, D.L.S.; Filintas, C.S.; Skourti, A.; Maggi, F.; Ferrati, M.; Petrelli, R.; Spinozzi, E.; Teruzzi, C.; et al. Advances in stored-product pests control: Evaluation of the efficacy of myrrh essential oil on two Tenebrionidae species through a metabolomic approach. J. Stord Prod. Res. 2025, 114, 102772. [Google Scholar] [CrossRef]
- Spinozzi, E.; Ferrati, M.; Baldassarri, C.; Rossi, P.; Favia, G.; Cameli, G.; Benelli, G.; Canale, A.; De Fazi, L.; Pavela, R.; et al. Essential oil and furanosesquiterpenes from myrrh oleo-gum resin: A breakthrough in mosquito vector management. Nat. Prod. Bioprospect. 2025, 15, 12. [Google Scholar] [CrossRef]
- Hagstrum, D.W.; Phillips, T.W. Evolution of stored-product entomology: Protecting the world food supply. Annu. Rev. Entomol. 2017, 62, 379–397. [Google Scholar] [CrossRef]
- Belluco, S.; Bertola, M.; Montarsi, F.; Di Martino, G.; Granato, A.; Stella, R.; Martinello, M.; Bordin, F.; Mutinelli, F. Insects and public health: An overview. Insects 2023, 14, 240. [Google Scholar] [CrossRef]
- Rosentrater, K.A. Storage of Cereal Grains and their Products; Woodhead Publishing: Sawston, UK, 2022. [Google Scholar]
- Muatinte, B.L.; Kavallieratos, N.G.; Boukouvala, M.C.; García-Lara, S.; López-Castillo, L.M.; Mvumi, B.M. The threat of the larger grain borer, Prostephanus truncatus (Coleoptera: Bostrichidae) and practical control options for the pest. In Invasive Species Reviews 2018–2024; Hemming, D., Ed.; CABI: Boston, MA, USA, 2025; pp. 173–207. [Google Scholar]
- Hacham, Y.; Hershenhorn, J.; Dor, E.; Amir, R. Primary metabolic profiling of Egyptian broomrape (Phelipanche aegyptiaca) compared to its host tomato roots. J. Plant. Physiol. 2016, 205, 11–19. [Google Scholar] [CrossRef]
- Cui, S.F.; Wang, L.; Qiu, J.P.; Geng, X.Q.; Liu, Z.C. Effects of hypoxia/hypercapnia on the metabolism of Callosobruchus chinensis (L.) larvae. J. Stored Prod. Res. 2019, 83, 322–330. [Google Scholar] [CrossRef]
- Ferri, I.; Dell’Anno, M.; Spano, M.; Canala, B.; Petrali, B.; Dametti, M.; Magnaghi, S.; Rossi, L. Characterisation of Tenebrio molitor reared on substrates supplemented with chestnut shell. Insects 2024, 15, 512. [Google Scholar] [CrossRef]
- Marongiu, B.; Piras, A.; Porcedda, S.; Scorciapino, A. Chemical composition of the essential oil and supercritical CO2 extract of Commiphora myrrha (Nees) Engl. and of Acorus calamus L. J. Agric. Food Chem. 2005, 53, 7939–7943. [Google Scholar] [CrossRef]
- Morteza-Semnani, K.; Saeedi, M. Constituents of the essential oil of Commiphora myrrha (Nees) Engl. var. molmol. J. Essent Oil Res. 2003, 15, 50–51. [Google Scholar] [CrossRef]
- Maggi, F.; Barboni, L.; Papa, F.; Caprioli, G.; Ricciutelli, M.; Sagratini, G.; Vittori, S. A forgotten vegetable (Smyrnium olusatrum L., Apiaceae) as a rich source of isofuranodiene. Food Chem. 2012, 135, 2852–2862. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Iannarelli, R.; Petrelli, R.; Cappellacci, L.; Cianfaglione, K.; Afshar, F.H.; Nicoletti, M.; Canale, A.; Maggi, F. Synergized mixtures of Apiaceae essential oils and related plant-borne compounds: Larvicidal effectiveness on the filariasis vector Culex quinquefasciatus Say. Ind. Crops Prod. 2017, 96, 186–195. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Boukouvala, M.C.; Ntalli, N.; Skourti, A.; Karagianni, E.S.; Nika, E.P.; Kontodimas, D.C.; Cappellacci, L.; Petrelli, R.; Cianfaglione, K.; et al. Effectiveness of eight essential oils against two key stored-product beetles, Prostephanus truncatus (Horn) and Trogoderma granarium Everts. Food Chem. Toxicol. 2020, 139, 111255. [Google Scholar] [CrossRef] [PubMed]
- Harrison, B.R.; Hoffman, J.M.; Samuelson, A.; Raftery, D.; Promislow, D.E. Modular evolution of the Drosophila metabolome. Mol. Biol. Evol. 2022, 39, 307. [Google Scholar] [CrossRef]
- Koul, O.; Singh, R.; Kaur, B.; Kanda, D. Comparative study on the behavioral response and acute toxicity of some essential oil compounds and their binary mixtures to larvae of Helicoverpa armigera, Spodoptera litura and Chilo partellus. Ind. Crop Prod. 2013, 49, 428–436. [Google Scholar] [CrossRef]
- Brinzer, R.A. Drosophila, Metabolomics and Insecticide Action. Doctoral Dissertation, University of Glasgow, Glasgow, UK, 2015. [Google Scholar]
- Storey, K.B. Organic solutes in freezing tolerance. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 1997, 117, 319–5326. [Google Scholar]
- Lv, N.; Ma, K.; Li, R.; Liang, P.; Liang, P.; Gao, X. Sublethal and lethal effects of the imidacloprid on the metabolic characteristics based on high-throughput non-targeted metabolomics in Aphis gossypii Glover. Ecotoxicol. Environ. Saf. 2021, 212, 111969. [Google Scholar]
- Ren, H.; Pu, Q.; Yang, X.; Kashyap, S.; Liu, S. Regulatory mechanisms of nitrogen homeostasis in insect growth and development. Insect Sci. 2025, 0, 1–21. [Google Scholar] [CrossRef]
- Yousuf, P.Y.; Shabir, P.A.; Hakeem, K.R. miRNAomic approach to plant nitrogen starvation. Int. J. Genom. 2021, 2021, 8560323. [Google Scholar] [CrossRef]
- Ren, X.; Guo, R.; Akami, M.; Niu, C. Nitrogen acquisition strategies mediated by insect symbionts: A review of their mechanisms, methodologies, and case studies. Insects 2022, 13, 84. [Google Scholar] [CrossRef]
- Aqueel, M.A.; Raza, A.B.M.; Balal, R.M.; Shahid, M.A.; Mustafa, I.; Javaid, M.M.; Leather, S.R. Tritrophic interactions between parasitoids and cereal aphids are mediated by nitrogen fertilizer. Insect Sci. 2015, 22, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Isoe, J.; Petchampai, N.; Isoe, Y.E.; Co, K.; Mazzalupo, S.; Scaraffia, P.Y. Xanthine dehydrogenase-1 silencing in Aedes aegypti mosquitoes promotes a blood feeding–induced adulticidal activity. FASEB J. 2017, 31, 2276. [Google Scholar] [PubMed]
- Li, Z.; Zhao, M.; Li, L.; Yuan, Y.Y.; Chen, F.J.; Parajulee, M.N.; Ge, F. Azotobacter inoculation can enhance the resistance of Bt cotton to cotton bollworm, Helicoverpa armigera. Insect Sci. 2023, 30, 1648–1662. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.B.; Mackey, T.; Juba, A.N.; Amin, K.; Atyam, A.; McDole, M.; Yancy, J.; Thomas, T.C.; Buhlman, L.M. Mild traumatic brain injury in Drosophila melanogaster alters reactive oxygen and nitrogen species in a sex-dependent manner. Exp. Neurol. 2024, 372, 114621. [Google Scholar]
- Bar-Even, A.; Flamholz, A.; Noor, E.; Milo, R. Rethinking glycolysis: On the biochemical logic of metabolic pathways. Nat. Chem. Biol. 2012, 8, 509–517. [Google Scholar] [CrossRef]
- Ankrah, N.Y.; Wilkes, R.A.; Zhang, F.Q.; Zhu, D.; Kaweesi, T.; Aristilde, L.; Douglas, A.E. Syntrophic splitting of central carbon metabolism in host cells bearing functionally different symbiotic bacteria. ISME J. 2020, 14, 1982–1993. [Google Scholar] [CrossRef]
- Sadekuzzaman, M.D.; Gautam, N.; Kim, Y. A novel calcium-independent phospholipase A2 and its physiological roles in development and immunity of a lepidopteran insect, Spodoptera exigua. Dev. Comp. Immunol. 2017, 77, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Rajam, M.V. Insecticidal activity of inhibitors of polyamine biosynthesis on Spodoptera litura F. larvae. Indian J. Exp. Biol. 1991, 29, 881–882. [Google Scholar] [PubMed]
- Zawisza-Raszka, A.; Dolezych, B. Acetylcholinesterase, catalase and glutathione S-transferase activity in beet armyworm (Spodoptera exigua) exposed to nickel and/or diazinon. Acta Biol. Hung. 2008, 59, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Hu, S.; Huang, H.; Wei, Q.; Ren, M.; Huang, S.; Tian, X.; Su, J. Insecticides induce the co-expression of glutathione S-transferases through ROS/CncC pathway in Spodoptera exigua. Pestic. Biochem. Physiol. 2019, 155, 58–71. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef]
- Pavela, R. History, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects–a review. Plant Prot. Sci. 2016, 52, 229–241. [Google Scholar] [CrossRef]
- Chen, C.; Cai, N.; Chen, J.; Wan, C. Clove essential oil as an alternative approach to control postharvest blue mold caused by Penicillium italicum in citrus fruit. Biomolecules 2019, 9, 197. [Google Scholar] [CrossRef]
- Čolović, M.; Lazarević-Pašti, T.; Vasić, V. Toxic effects of chlorpyrifos and its metabolites on some physiologically important enzymes: ATPases, cholinesterases, peroxidases. In Biochemistry Research Trends. Chlorpyrifos. Toxicological Properties, Uses and Effects on Human Health and the Environment; Mayes, C., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2015; pp. 87–139. [Google Scholar]
- Hou, W.; Jiang, C.; Zhou, X.; Qian, K.; Wang, L.; Shen, Y.; Zhao, Y. Increased expression of P-glycoprotein is associated with chlorpyrifos resistance in the German cockroach (Blattodea: Blattellidae). J. Econ. Entomol. 2016, 109, 2500–2505. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, C.; Li, W.; Lan, R.; Chen, R.; Hu, J.; Yang, C.; Wang, P.; Tang, B.; Wang, S. Residues of chlorpyrifos in the environment induce resistance in Aedes albopictus by affecting its olfactory system and neurotoxicity. Sci. Total Environ. 2024, 930, 172425. [Google Scholar] [CrossRef]
- Cui, S.; Wang, L.; Qiu, J.; Liu, Z.; Geng, X. Comparative metabolomics analysis of Callosobruchus chinensis larvae under hypoxia, hypoxia/hypercapnia and normoxia. Pest Manag. Sci. 2017, 73, 1267–1276. [Google Scholar] [CrossRef]
- Williamson, S.M.; Moffat, C.; Gomersall, M.A.; Saranzewa, N.; Connolly, C.N.; Wright, G.A. Exposure to acetylcholinesterase inhibitors alters the physiology and motor function of honeybees. Front. Physiol. 2013, 4, 13. [Google Scholar] [CrossRef]
- Brinzer, R.A.; Henderson, L.; Marchiondo, A.A.; Woods, D.J.; Davies, S.A.; Dow, J.A. Metabolomic profiling of permethrin-treated Drosophila melanogaster identifies a role for tryptophan catabolism in insecticide survival. Insect Biochem. Mol. Biol. 2015, 67, 74–86. [Google Scholar] [CrossRef]
- Cox, J.E.; Thummel, C.S.; Tennessen, J.M. Metabolomic studies in Drosophila. Genetics 2017, 206, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Vasamsetti, B.M.K.; Kim, J.; Chon, K.; Kim, B.S.; Yoon, C.Y.; Hwang, S.; Park, K.H. Molecular impact of sublethal spinetoram exposure on honeybee (Apis mellifera) larval and adult transcriptomes. Int. J. Mol. Sci. 2024, 25, 11923. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corp.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Mondello, L. Mass Spectra of Flavors and Fragrances of Natural and Synthetic Compounds; Wiley: New York, NY, USA, 2015. [Google Scholar]
- NIST23 (National Institute of Standards and Technology). NIST/EPA/NIH Mass Spectral Database; United States Department of Commerce: Gaithersburg, MD, USA, 2023. Available online: https://www.nist.gov/srd/nist-standard-reference-database-1a (accessed on 12 January 2025).
- Kar, S.; Nayak, R.N.; Sahoo, N.R.; Bakhara, C.K.; Panda, M.K.; Pal, U.S.; Bal, L.M. Rice weevil management through application of silica nano particle and physico-chemical and cooking characterization of the treated rice. J. Stored Prod. Res. 2021, 94, 101892. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Athanassiou, C.G.; Peteinatos, G.G.; Boukouvala, M.C.; Benelli, G. Insecticidal effect and impact of fitness of three diatomaceous earths on different maize hybrids for the eco-friendly control of the invasive stored-product pest Prostephanus truncatus (Horn). Environ. Sci. Pollut. Res. 2018, 25, 10407–10417. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Eleftheriadou, N.; Boukouvala, M.C.; Skourti, A.; Filintas, C.S.; Gidari, D.L.S.; Maggi, F.; Rossi, P.; Drenaggi, E.; Morshedloo, M.R.; et al. Exploring the efficacy of four Apiaceae essential oils against nine stored-product pests in wheat protection. Plants 2024, 13, 533. [Google Scholar] [CrossRef] [PubMed]
- Misra, B. Steps for Building an Open Source EI-MS Mass Spectral Library for GC-MS -Based Metabolomics. 2019. Available online: https://www.protocols.io/view/steps-for-building-an-open-source-ei-ms-mass-spect-eq2ly33rqgx9/v1 (accessed on 20 December 2022).
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, W.M.T.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis: Chemical analysis working group (CAWG) metabolomics standards initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis; Pearson: Essex, UK, 2014. [Google Scholar]
- Scheff, D.S.; Arthur, F.H. Fecundity of Tribolium castaneum and Tribolium confusum adults after exposure to deltamethrin packaging. J. Pest Sci. 2018, 91, 717–725. [Google Scholar] [CrossRef]
- Sall, J.; Lehman, A.; Creighton, L. JMP Start Statistics. A Guide to Statistics and Data Analysis Using JMP and JMP IN Software; Duxbury Press: Belmont, CA, USA, 2001. [Google Scholar]
- SAS Institute Inc. Using JMP 16.2; SAS Institute Inc.: Cary, NC, USA, 2021. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research; Freeman & Company: New York, NY, USA, 1995. [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods; Iowa State University Press: Iowa, IA, USA, 1989. [Google Scholar]


| Between Exposures | Within Exposures | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intercept | Concentration | Formulation | Concentration x Formulation | Exposure | Exposure x Concentration | Exposure x Formulation | Exposure x Concentration x Formulation | ||
| df | 1 | 1 | 1 | 1 | 9 | 9 | 9 | 9 | |
| S. zeamais adults | F | 2957.5 | 27.2 | 1378.0 | 23.9 | 3616.5 | 131.1 | 2027.7 | 135.2 |
| p | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| P. truncatus adults | F | 3369.5 | 7.9 | 18.6 | 4.8 | 7263.7 | 7.6 | 39.3 | 8.0 |
| p | <0.01 | 0.01 | 0.01 | 0.04 | <0.01 | <0.01 | <0.01 | <0.01 | |
| C. myrrha EO | PM | C. myrrha EO | PM | |||||
|---|---|---|---|---|---|---|---|---|
| Dose | 500 ppm | 1000 ppm | ||||||
| Exposure | t | p | t | p | ||||
| 4 h | 0.0 ± 0.0 B | 0.0 ± 0.0 E | - | - | 0.0 ± 0.0 C | 0.0 ± 0.0 E | - | - |
| 8 h | 0.0 ± 0.0 B | 0.0 ± 0.0 E | - | - | 0.0 ± 0.0 C | 0.0 ± 0.0 E | - | - |
| 16 h | 0.0 ± 0.0 B | 4.4 ± 2.4 D | 2.0 | 0.08 | 0.0 ± 0.0 C | 5.6 ± 2.4 D* | 2.5 | 0.02 |
| 1 d | 0.0 ± 0.0 B | 13.3 ± 2.9 C* | 7.3 | <0.01 | 0.0 ± 0.0 C | 13.3 ± 3.3 C* | 5.1 | 0.01 |
| 2 d | 0.0 ± 0.0 B | 30.0 ± 4.4 B* | 27.2 | <0.01 | 0.0 ± 0.0 C | 31.1 ± 4.2 B* | 29.1 | <0.01 |
| 3 d | 0.0 ± 0.0 B | 46.7 ± 3.3 AB* | 61.3 | <0.01 | 0.0 ± 0.0 C | 48.9 ± 3.1 AB* | 67.4 | <0.01 |
| 4 d | 0.0 ± 0.0 B | 58.9 ± 3.1 AB* | 84.2 | <0.01 | 0.0 ± 0.0 C | 61.1 ± 3.1 AB* | 85.6 | <0.01 |
| 5 d | 0.0 ± 0.0 B | 77.8 ± 2.8 A* | 125.5 | <0.01 | 10.0 ± 1.7 B | 78.9 ± 2.6 A* | 7.6 | <0.01 |
| 6 d | 0.0 ± 0.0 B | 92.2 ± 2.2 A* | 187.9 | <0.01 | 18.9 ± 1.1 A | 91.1 ± 2.0 A* | 20.6 | <0.01 |
| 7 d | 10.0 ± 1.7 A | 100.0 ± 0.0 A* | 8.5 | <0.01 | 25.6 ± 2.4 A | 100.0 ± 0.0 A* | 16.0 | <0.01 |
| F | 60.0 | 107.8 | 203.9 | 82.7 | ||||
| p | <0.01 | <0.01 | <0.01 | <0.01 | ||||
| C. myrrha EO | PM | C. myrrha EO | PM | |||||
|---|---|---|---|---|---|---|---|---|
| Dose | 500 ppm | 1000 ppm | ||||||
| Exposure | t | p | t | p | ||||
| 4 h | 0.0 ± 0.0 E | 0.0 ± 0.0 E | - | - | 0.0 ± 0.0 G | 0.0 ± 0.0 E | - | - |
| 8 h | 0.0 ± 0.0 E | 2.2 ± 1.5 DE | 1.5 | 0.17 | 0.0 ± 0.0 G | 3.3 ± 1.7 DE | 2.0 | 0.06 |
| 16 h | 4.4 ± 1.8 D | 4.4 ± 1.8 D | 0.0 | 1.00 | 7.8 ± 1.5 F | 5.6 ± 1.8 D | −1.0 | 0.35 |
| 1 d | 10.0 ± 1.7 C | 16.7 ± 2.4 C | 1.0 | 0.34 | 14.4 ± 1.8 E | 16.7 ± 2.9 C | −0.2 | 0.83 |
| 2 d | 15.6 ± 2.9 BC | 33.3 ± 2.9 BC* | 2.8 | 0.02 | 25.6 ± 1.8 D | 34.4 ± 3.4 BC* | 2.3 | 0.03 |
| 3 d | 20.0 ± 1.7 ABC | 50.0 ± 1.7 AB* | 9.6 | <0.01 | 32.2 ± 1.5 CD | 51.1 ± 1.1 AB* | 10.0 | <0.01 |
| 4 d | 26.7 ± 2.4 AB | 61.1 ± 2.0 AB* | 9.3 | <0.01 | 42.2 ± 2.2 BCD | 62.2 ± 1.5 AB* | 6.7 | <0.01 |
| 5 d | 30.0 ± 1.7 AB | 77.8 ± 2.8 AB* | 14.2 | <0.01 | 55.6 ± 2.4 ABC | 78.9 ± 3.9 AB* | 5.5 | <0.01 |
| 6 d | 38.9 ± 3.1 A | 88.9 ± 3.1 A* | 8.6 | <0.01 | 70.0 ± 1.7 AB | 90.0 ± 2.9 AB* | 6.2 | <0.01 |
| 7 d | 48.9 ± 3.5 A | 97.8 ± 1.5 A* | 10.3 | <0.01 | 85.6 ± 2.4 A | 96.7 ± 1.7 A* | 3.8 | 0.01 |
| F | 53.0 | 72.3 | 182.7 | 61.4 | ||||
| p | <0.01 | <0.01 | <0.01 | <0.01 | ||||
| Treatment | Solutions |
|---|---|
| C. myrrha EO 500 ppm | 150 μL EO + 150 μL ethanol + 450 μL Tween 80 0.3% (v/v) |
| C. myrrha EO 1000 ppm | 250 μL EO + 250 μL ethanol + 250 μL Tween 80 0.3% (v/v) |
| Positive control | 5 ppm pirimiphos-methyl |
| Carrier control | 150 μL distilled water + 150 μL pure ethanol + 150 μL pure ethanol in water + 300 μL Tween 80 0.3% (v/v) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kavallieratos, N.G.; Boukouvala, M.C.; Filintas, C.S.; Gidari, D.L.S.; Skourti, A.; Kyrpislidi, V.P.C.; Maggi, F.; Petrelli, R.; Spinozzi, E.; Ferrati, M.; et al. Assessing the Insecticidal Performance of Commiphora myrrha Essential Oil Against Prostephanus truncatus and Sitophilus zeamais Using a Metabolomic Approach. Plants 2025, 14, 3031. https://doi.org/10.3390/plants14193031
Kavallieratos NG, Boukouvala MC, Filintas CS, Gidari DLS, Skourti A, Kyrpislidi VPC, Maggi F, Petrelli R, Spinozzi E, Ferrati M, et al. Assessing the Insecticidal Performance of Commiphora myrrha Essential Oil Against Prostephanus truncatus and Sitophilus zeamais Using a Metabolomic Approach. Plants. 2025; 14(19):3031. https://doi.org/10.3390/plants14193031
Chicago/Turabian StyleKavallieratos, Nickolas G., Maria C. Boukouvala, Constantin S. Filintas, Demeter Lorentha S. Gidari, Anna Skourti, Vasiliki Panagiota C. Kyrpislidi, Filippo Maggi, Riccardo Petrelli, Eleonora Spinozzi, Marta Ferrati, and et al. 2025. "Assessing the Insecticidal Performance of Commiphora myrrha Essential Oil Against Prostephanus truncatus and Sitophilus zeamais Using a Metabolomic Approach" Plants 14, no. 19: 3031. https://doi.org/10.3390/plants14193031
APA StyleKavallieratos, N. G., Boukouvala, M. C., Filintas, C. S., Gidari, D. L. S., Skourti, A., Kyrpislidi, V. P. C., Maggi, F., Petrelli, R., Spinozzi, E., Ferrati, M., Teruzzi, C., & Araniti, F. (2025). Assessing the Insecticidal Performance of Commiphora myrrha Essential Oil Against Prostephanus truncatus and Sitophilus zeamais Using a Metabolomic Approach. Plants, 14(19), 3031. https://doi.org/10.3390/plants14193031













