Brown Algae Extracts Increase the Tolerance of Tomato Plants to High Temperatures by Improving Morphological, Physiological, Metabolomic, and Transcriptional Parameters
Abstract
1. Introduction
2. Results
2.1. Plant Growth and Biomass
2.2. Stomatal Conductance
2.3. Photosynthetic Pigments
2.4. Enzymatic Activity
2.5. Total Proteins
2.6. Non-Enzymatic Antioxidants and Antioxidant Capacity
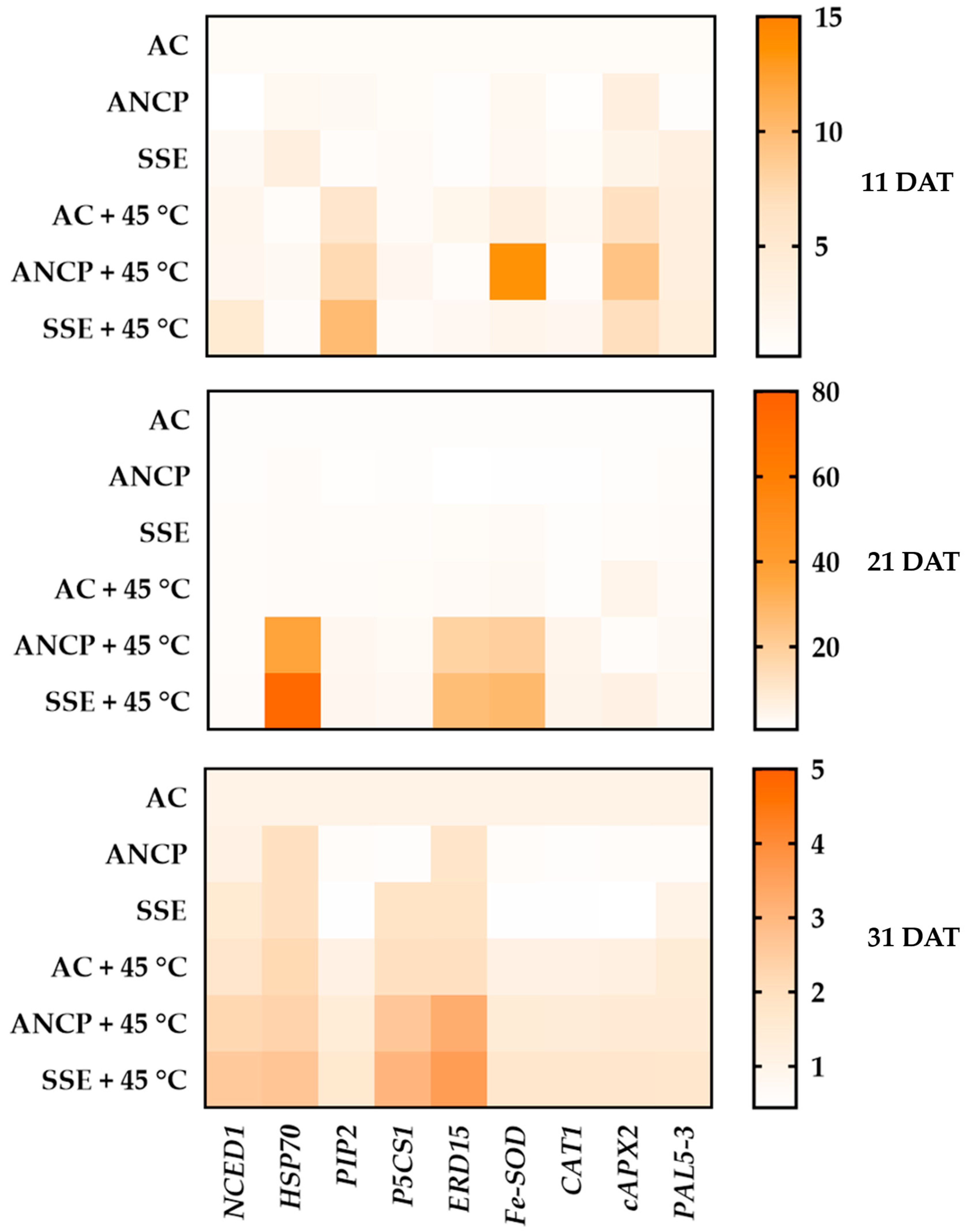
2.7. Expression of Defense Genes
3. Discussion
4. Materials and Methods
4.1. Plant Matter and Experimental Conditions
4.2. Treatments
4.3. Sampling and Evaluations
4.4. Biomolecule Analysis
4.5. Real-Time Reverse Transcription PCR
4.6. Experimental Design and Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| HSP | Heat shock proteins |
| DAT | Days after transplant |
| SSE | Sargassum spp. seaweed extract |
| AC | Control |
| SD | Standard deviation |
| ANCP | Ascophyllum nodosum commercial product |
| FW | Fresh weight |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| APX | Ascorbate peroxidase |
| DW | Dry weight |
| GAE | Gallic acid equivalent |
| AAE | Ascorbic acid equivalent |
| ACT | Actin |
| NCED1 | Nine-cis-expoxycarotenoid dioxygenase 1 |
| HSP70 | Heat shock protein 70 |
| PIP2 | Plasma membrane intrinsic protein 2 |
| P5CS1 | Delta1-pyrroline-5-carboxylate synthase 1 |
| ERD15 | Protein of early response to dehydration 15 |
| Fe-SOD | Iron superoxide dismutase |
| CAT1 | Catalase 1 |
| cAPX2 | Cytosolic ascorbate peroxidase 2 |
| PAL5-3 | Phenylalanine ammonia lyase 5-3 |
| ABA | Abscisic acid |
| RUBISCO | Ribulose-1,5-bisphosphate carboxylase/oxygenase |
| POD | Peroxidase |
| GPX | Glutathione peroxidase |
| PAL | Phenylalanine ammonia lyase |
| H2O2 | Hydrogen peroxide |
| TP | Total proteins |
| HPLC | High-performance liquid chromatography |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| PCR | Polymerase chain reaction |
| RNA | Ribonucleic acid |
| cDNA | Complementary deoxyribonucleic acid |
References
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Shi, Y.; Yang, S. Molecular Regulation of Plant Responses to Environmental Temperatures. Mol. Plant 2020, 13, 544–564. [Google Scholar] [CrossRef]
- Hernández-Hernández, H.; Pérez-Labrada, F.; Garcia Enciso, E.L.; Leija-Martínez, P.; López-Pérez, M.C.; Medrano-Macías, J.; González-Morales, S.; Juárez Maldonado, A.; García Dávila, L.R.; Benavidez Mendoza, A. Tolerance-Induction Techniques and Agronomical Practices to Mitigate Stress in Extensive Crops and Vegetables. In Plant, Abiotic Stress and Responses to Climate Change; Andjelkovic, V., Ed.; InTech: Rijeka, Croatia, 2018; pp. 145–181. [Google Scholar]
- Xu, Y.; Li, T.; Xu, M.; Tan, L.; Shen, S. Assessing Climate Change Effects on Winter Wheat Production in the 3H Plain: Insights from Bias-Corrected CMIP6 Projections. Agriculture 2024, 14, 469. [Google Scholar] [CrossRef]
- Rubio-Casal, A.E.; Ibrahim, M.F.M. Editorial: Physiological traits and stress detection in crops during global climate change: Availability and sustainable use of water resources. Front. Plant Sci. 2024, 15, 1371044. [Google Scholar] [CrossRef]
- Suliman, M.S.E.; Elradi, S.B.M.; Zhou, G.; Meng, T.; Zhu, G.; Xu, Y.; Nimir, N.E.A.; Elsiddig, A.M.I.; Awdelseid, A.H.M.; Ali, A.Y.A.; et al. Exogenous glutathione protected wheat seedling from high temperature and water deficit damages. Sci. Rep. 2024, 14, 5304. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, Z.; Wang, L.; Jin, B. Plant Responses to Heat Stress: Physiology, Transcription, Noncoding RNAs, and Epigenetics. Int. J. Mol. Sci. 2021, 22, 117. [Google Scholar] [CrossRef]
- Vital, R.G.; Müller, C.; Freire, F.B.S.; Silva, F.B.; Batista, P.F.; Fuentes, D.; Rodrigues, A.A.; Moura, L.M.F.; Daloso, D.M.; Silva, A.A.; et al. Metabolic, physiological and anatomical responses of soybean plants under water deficit and high temperature condition. Sci. Rep. 2022, 12, 16467. [Google Scholar] [CrossRef]
- Mishra, S.; Spaccarotella, K.; Gido, J.; Samanta, I.; Chowdhary, G. Effects of Heat Stress on Plant-Nutrient Relations: An Update on Nutrient Uptake, Transport, and Assimilation. Int. J. Mol. Sci. 2023, 24, 15670. [Google Scholar] [CrossRef]
- ul Haq, S.; Khan, A.; Ali, M.; Khattak, A.M.; Gai, W.-X.; Zhang, H.-X.; Wei, A.-M.; Gong, Z.-H. Heat shock proteins: Dynamic biomolecules to counter plant biotic and abiotic stresses. Int. J. Mol. Sci. 2019, 20, 5321. [Google Scholar] [CrossRef]
- Carmody, N.; Goñi, O.; Łangowski, Ł.; O’Connell, S. Ascophyllum nodosum Extract Biostimulant Processing and Its Impact on Enhancing Heat Stress Tolerance During Tomato Fruit Set. Front. Plant Sci. 2020, 11, 807. [Google Scholar] [CrossRef]
- Toro, M.-T.; Fustos-Toribio, R.; Ortiz, J.; Becerra, J.; Zapata, N.; López-Belchí, M.D. Antioxidant Responses and Phytochemical Accumulation in Raphanus Species Sprouts through Elicitors and Predictive Models under High Temperature Stress. Antioxidants 2024, 13, 333. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Sariñana-Aldaco, O.; Benavides-Mendoza, A.; Juárez-Maldonado, A.; Robledo-Olivo, A.; Rodríguez-Jasso, R.M.; Preciado-Rangel, P.; Gonzalez-Morales, S. Efecto de extractos de Sargassum spp. en el crecimiento y antioxidantes de plántulas de tomate. Ecosist. Recurs. Agropec. 2021, 8, e2814. [Google Scholar] [CrossRef]
- Mahmoud, S.H.; Salama, D.M.; El-Tanahy, A.M.M.; Abd El-Samad, E.H. Utilization of seaweed (Sargassum vulgare) extract to enhance growth, yield and nutritional quality of red radish plants. Ann. Agric. Sci. 2019, 64, 167–175. [Google Scholar] [CrossRef]
- Carillo, P.; Ciarmiello, L.F.; Woodrow, P.; Corrado, G.; Chiaiese, P.; Rouphael, Y. Enhancing Sustainability by Improving Plant Salt Tolerance through Macro- and Micro-Algal Biostimulants. Biology 2020, 9, 253. [Google Scholar] [CrossRef]
- Smith, J.; Pilsbury, A.; Kumar, V.; Karamerou, E.E.; Chuck, C.J.; Herrera-Rodriguez, L.; Suarez, J.V.; Allen, M.J. Accessing the Efficacy of Sargassum-Based Aqueous Phase Products Derived from Hydrothermal Carbonisation and Hydrothermal Liquefaction on Plant Growth. Phycology 2024, 4, 53–64. [Google Scholar] [CrossRef]
- Hernández-Herrera, R.M.; González-González, M.F.; Velasco-Ramírez, A.P.; Velasco-Ramírez, S.F.; Santacruz-Ruvalcaba, F.; Zamora-Natera, J.F. Seaweed Extract Components Are Correlated with the Seeds Germination and Growth of Tomato Seedlings. Seeds 2023, 2, 436–448. [Google Scholar] [CrossRef]
- Chávez, V.; Uribe-Martínez, A.; Cuevas, E.; Rodríguez-Martínez, R.E.; van Tussenbroek, B.I.; Francisco, V.; Estévez, M.; Celis, L.B.; Monroy-Velázquez, V.; Leal-Bautista, R.; et al. Massive Influx of Pelagic Sargassum spp. on the Coasts of the Mexican Caribbean 2014–2020: Challenges and Opportunities. Water 2020, 12, 2908. [Google Scholar] [CrossRef]
- Liranzo-Gómez, R.E.; Torres-Valle, A.; Jauregui-Haza, U.J. Risk Perception Assessment of Sargassum Blooms in Dominican Republic. Sustainability 2024, 16, 2186. [Google Scholar] [CrossRef]
- Kumari, S.; Sehrawat, K.D.; Phogat, D.; Sehrawat, A.R.; Chaudhary, R.; Sushkova, S.N.; Voloshina, M.S.; Rajput, V.D.; Shmaraeva, A.N.; Marc, R.A.; et al. Ascophyllum nodosum (L.) Le Jolis, a Pivotal Biostimulant toward Sustainable Agriculture: A Comprehensive Review. Agriculture 2023, 13, 1179. [Google Scholar] [CrossRef]
- Melo, P.; Abreu, C.; Bahcevandziev, K.; Araujo, G.; Pereira, L. Biostimulant effect of marine macroalgae bioextract on pepper grown in greenhouse. Appl. Sci. 2020, 10, 4052. [Google Scholar] [CrossRef]
- Repke, R.A.; Ribeiro Silva, D.M.; Camilo dos Santos, J.C.; de Almeida Silva, M. Increased soybean tolerance to high-temperature through biostimulant based on Ascophyllum nodosum (L.) seaweed extract. J. Appl. Psychol. 2022, 34, 3205–3218. [Google Scholar] [CrossRef]
- Shedeed, Z.A.; Gheda, S.; Elsanadily, S.; Alharbi, K.; Osman, M.E.H. Spirulina platensis Biofertilization for Enhancing Growth, Photosynthetic Capacity and Yield of Lupinus luteus. Agriculture 2022, 12, 781. [Google Scholar] [CrossRef]
- Engel, D.C.H.; Feltrim, D.; Rodrigues, M.; Baptistella, J.L.C.; Mazzafera, P. Algae Extract Increases Seed Production of Soybean Plants and Alters Nitrogen Metabolism. Agriculture 2023, 13, 1296. [Google Scholar] [CrossRef]
- Song, X.; Bai, P.; Ding, J.; Li, J. Effect of vapor pressure deficit on growth and water status in muskmelon and cucumber. Plant Sci. 2021, 303, 110755. [Google Scholar] [CrossRef] [PubMed]
- Bisbis, M.B.; Gruda, N.S.; Blanke, M.M. Securing Horticulture in a Changing Climate-A Mini Review. Horticulturae 2019, 5, 56. [Google Scholar] [CrossRef]
- Giordano, M.; Petropoulos, S.A.; Rouphael, Y. Response and defence mechanisms of vegetable crops against drought, heat and salinity stress. Agriculture 2021, 11, 463. [Google Scholar] [CrossRef]
- Chaves-Barrantes, N.F.; Gutiérrez-Soto, M.V. Respuestas al estrés por calor en los cultivos. I. Aspectos moleculares, bioquímicos y fisiológicos. Agron. Mesoam. 2017, 28, 237–253. [Google Scholar] [CrossRef]
- Orzechowska, A.; Trtílek, M.; Tokarz, K.M.; Szymańska, R.; Niewiadomska, E.; Rozpądek, P.; Wątor, K. Thermal Analysis of Stomatal Response under Salinity and High Light. Int. J. Mol. Sci. 2021, 22, 4663. [Google Scholar] [CrossRef]
- Goyal, V.; Kumari, A.; Avtar, R.; Baliyan, V.; Mehrotra, S. Orthosilicic acid and Seaweed Extract Alleviate the Deteriorative Effects of High Temperature Stress in Brassica juncea (L.) Czern & Coss. Silicon 2023, 15, 4909–4919. [Google Scholar] [CrossRef]
- Zermeño Gonzalez, A.; López Rodríguez, B.R.; Melendres Alvarez, A.I.; Ramírez Rodríguez, H.; Cárdenas Palomo, J.O.; Munguía López, J.P. Extracto de alga marina y su relación con fotosíntesis y rendimiento de una plantación de vid. Rev. Mexicana Cienc. Agric. 2015, 6, 2437–2446. [Google Scholar] [CrossRef][Green Version]
- Masood, A.; Per, T.S.; Asgher, M.; Fatma, M.; Khan, M.I.R.; Rasheed, F.; Hussain, S.J.; Khan, N.A. Glycine Betaine: Role in Shifting Plants Toward Adaptation Under Extreme Environments. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Iqbal, N., Nazar, R., Khan, N.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 69–82. ISBN 978-81-322-2616-1. [Google Scholar][Green Version]
- Huang, S.; Zuo, T.; Ni, W. Important roles of glycinebetaine in stabilizing the structure and function of the photosystem II complex under abiotic stresses. Planta 2020, 251, 36. [Google Scholar] [CrossRef]
- Doğru, A. Effects of heat stress on photosystem II activity and antioxidant enzymes in two maize cultivars. Planta 2021, 253, 85. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fang, Z.; Zhao, D.; Tao, J. Effects of high-temperature stress on photosynthetic characteristics and antioxidant enzyme system of Paeonia ostii. Phyton-Int. J. Exp. Bot. 2022, 91, 599–615. [Google Scholar] [CrossRef]
- Mohanty, S.; Grimm, B.; Tripathy, B.C. Light and dark modulation of chlorophyll biosynthetic genes in response to temperature. Planta 2006, 224, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; El-Sheekh, M.M.; Hamed Aly, S.; Al-Harbi, M.; Elkelish, A.; Nagah, A. Inductive role of the brown alga Sargassum polycystum on growth and biosynthesis of imperative metabolites and antioxidants of two crop plants. Front. Plant Sci. 2023, 14, 1136325. [Google Scholar] [CrossRef]
- González-Morales, S.; Solís-Gaona, S.; Valdés-Caballero, M.V.; Juárez-Maldonado, A.; Loredo-Treviño, A.; Benavides-Mendoza, A. Transcriptomics of Biostimulation of Plants Under Abiotic Stress. Front. Genet. 2021, 12, 583888. [Google Scholar] [CrossRef]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef]
- Dvořák, P.; Krasylenko, Y.; Zeiner, A.; Šamaj, J.; Takáč, T. Signaling Toward Reactive Oxygen Species-Scavenging Enzymes in Plants. Front. Plant Sci. 2021, 11, 618835. [Google Scholar] [CrossRef]
- Smirnoff, N.; Conklin, P.L.; Loewus, F.A. Biosynthesis of Ascorbic Acid in Plants: A Renaissance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 437–467. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, J.; Li, X.; Zhang, J.; Liu, Y.; Chen, Y.; Yu, Q.; Li, N. Genome-wide identification and expression analyses of phenylalanine ammonia-lyase gene family members from tomato (Solanum lycopersicum) reveal their role in root-knot nematode infection. Front. Plant Sci. 2023, 14, 1204990. [Google Scholar] [CrossRef]
- Sariñana-Aldaco, O.; Rivera-Solís, L.L.; Benavides-Mendoza, A.; Robledo-Olivo, A.; Rodríguez-Jasso, R.M.; González-Morales, S. Using Brown Algae in the Plant–Soil System: A Sustainable Approach to Improving the Yield and Quality of Agricultural Crops. Horticulturae 2025, 11, 94. [Google Scholar] [CrossRef]
- Ding, F.; Wang, X.; Li, Z.; Wang, M. Jasmonate Positively Regulates Cold Tolerance by Promoting ABA Biosynthesis in Tomato. Plants 2023, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Hu, R.; Song, A.; Guan, Z.; Chen, F.; Jiang, J. Genome-Wide Identification and Expression Analysis of HSP70 Gene Family in Chrysanthemum lavandulifolium under Heat Stress. Horticulturae 2023, 9, 238. [Google Scholar] [CrossRef]
- Yaghobi, M.; Heidari, P. Genome-Wide Analysis of Aquaporin Gene Family in Triticum turgidum and Its Expression Profile in Response to Salt Stress. Genes 2023, 14, 202. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Fendel, A.; Nguyen, T.H.; Adebabay, A.; Kullik, A.S.; Benndorf, J.; Leon, J.; Naz, A.A. Natural diversity uncovers P5CS1 regulation and its role in drought stress tolerance and yield sustainability in barley. Plant Cell Environ. 2022, 45, 3523–3536. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Tian, N.; She, F.; Cao, A.; Wu, W.; Zheng, S.; Yang, N. Characteristics analysis of Early Responsive to Dehydration genes in Arabidopsis thaliana (AtERD). Plant Signal. Behav. 2022, 18, 2105021. [Google Scholar] [CrossRef]
- Steiner, A.A. A universal method for preparing nutrient solutions of a certain desired composition. Plant Soil 1961, 15, 134–154. [Google Scholar] [CrossRef]
- Ramya, S.S.; Nagaraj, S.; Vijayanand, N. Influence of Seaweed Liquid Extracts on Growth, Biochemical and Yield Characteristics of Cyamopsis tetragonolaba (L.) Taub. J. Phytol. 2011, 3, 37–41. [Google Scholar]
- Ramya, S.S.; Vijayanand, N.; Rathinavel, S. Foliar application of liquid biofertilizer of brown alga Stoechospermum marginatum on growth, biochemical and yield of Solanum melongena. Int. J. Recycl. Org. Waste Agric. 2015, 4, 167–173. [Google Scholar] [CrossRef]
- Kasim, W.A.; Hamada, E.A.M.; El-Din, N.G.S.; Eskander, S. Influence of seaweed extracts on the growth, some metabolic activities and yield of wheat grown under drought stress. Int. J. Agron. Agric. Res. 2015, 7, 173–189. [Google Scholar]
- Sariñana-Aldaco, O.; Benavides-Mendoza, A.; Robledo-Olivo, A.; González-Morales, S. The Biostimulant Effect of Hydroalcoholic Extracts of Sargassum spp. in Tomato Seedlings under Salt Stress. Plants 2022, 11, 3180. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Medrano Macías, J.; López Caltzontzit, M.G.; Rivas Martínez, E.N.; Narváez Ortiz, W.A.; Benavides Mendoza, A.; Martínez Lagunes, P. Enhancement to salt stress tolerance in strawberry plants by iodine products application. Agronomy 2021, 11, 602. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf Senescence: Correlated with Increased Levels of Membrane Permeability and Lipid Peroxidation, and Decreased Levels of Superoxide Dismutase and Catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Purification of ascorbate peroxidase in spinach chloroplasts; its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol. 1987, 28, 131–140. [Google Scholar] [CrossRef]
- Nour, V.; Trandafir, I.; Ionica, M.E. HPLC Organic Acid Analysis in Different Citrus Juices under Reversed Phase Conditions. Not. Bot. Horti Agrobot. Cluj-Na. 2010, 38, 44–48. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Cui, X.; Tao, X.; Xie, Y.; Fauquet, C.M.; Zhou, X. A DNAβ Associated with Tomato Yellow Leaf Curl China Virus Is Required for Symptom Induction. J. Virol. 2004, 78, 13966–13974. [Google Scholar] [CrossRef] [PubMed]
- Ziaf, K.; Munis, M.F.H.; Samin, G.; Zhang, X.; Li, J.; Zhang, J.; Ye, Z. Characterization of ERD15 gene from cultivated tomato (Solanum lycopersicum). Pak. J. Agric. Sci. 2016, 53, 27–33. [Google Scholar] [CrossRef]
- Mascia, T.; Santovito, E.; Gallitelli, D.; Cillo, F. Evaluation of reference genes for quantitative reverse-transcription polymerase chain reaction normalization in infected tomato plants. Mol. Plant Pathol. 2010, 11, 805–816. [Google Scholar] [CrossRef] [PubMed]
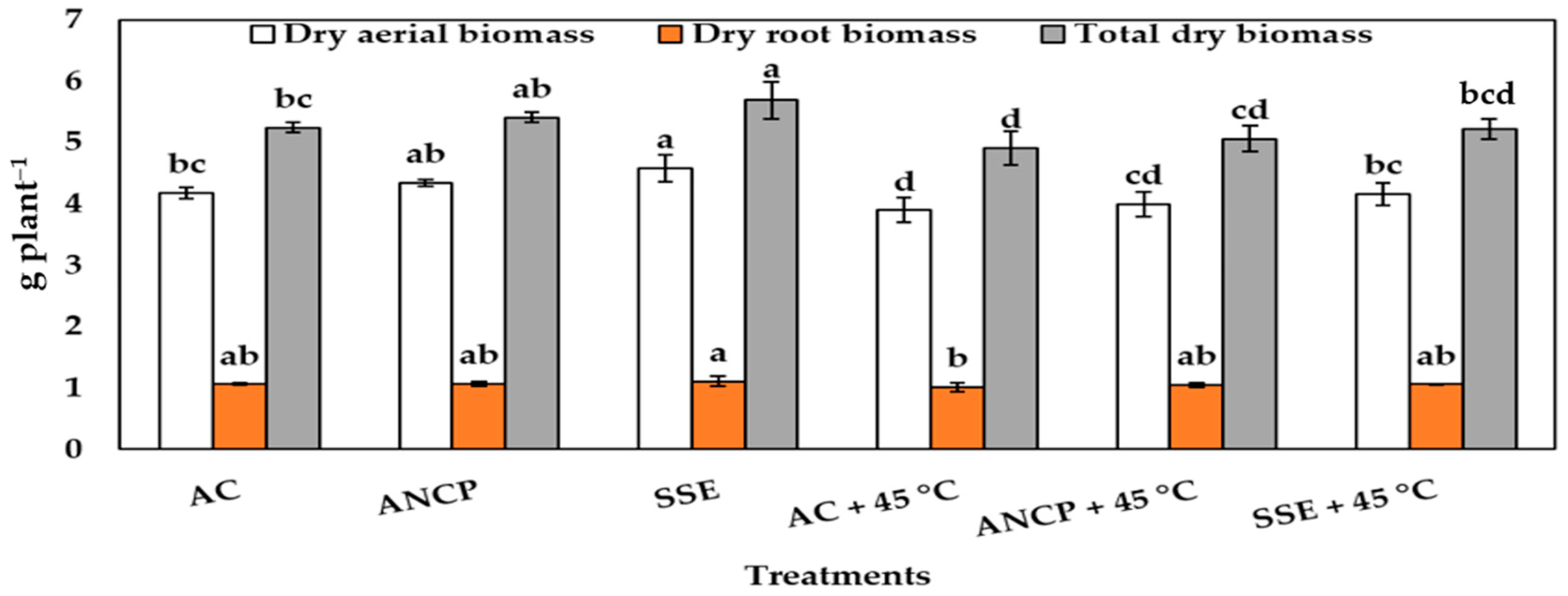

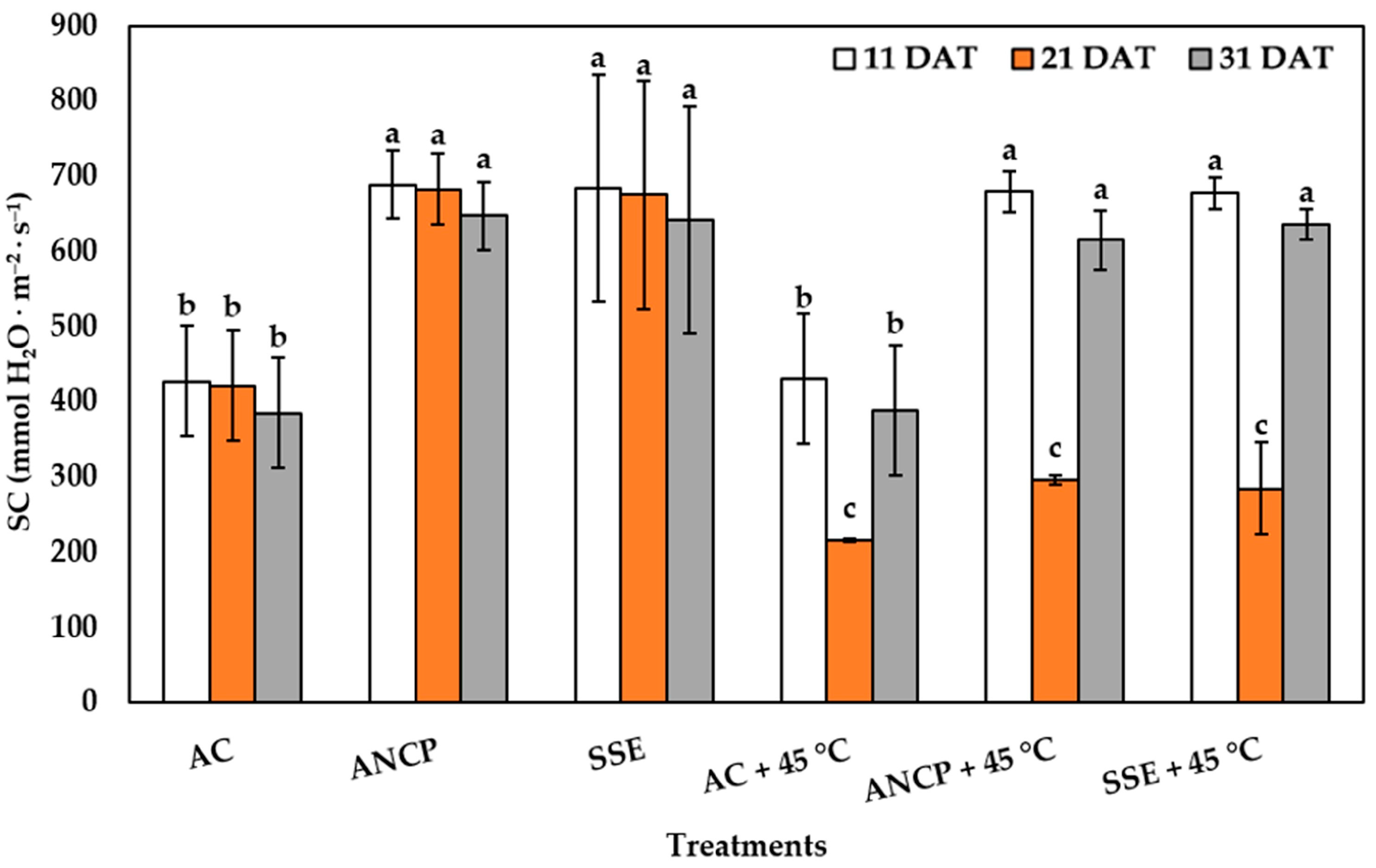
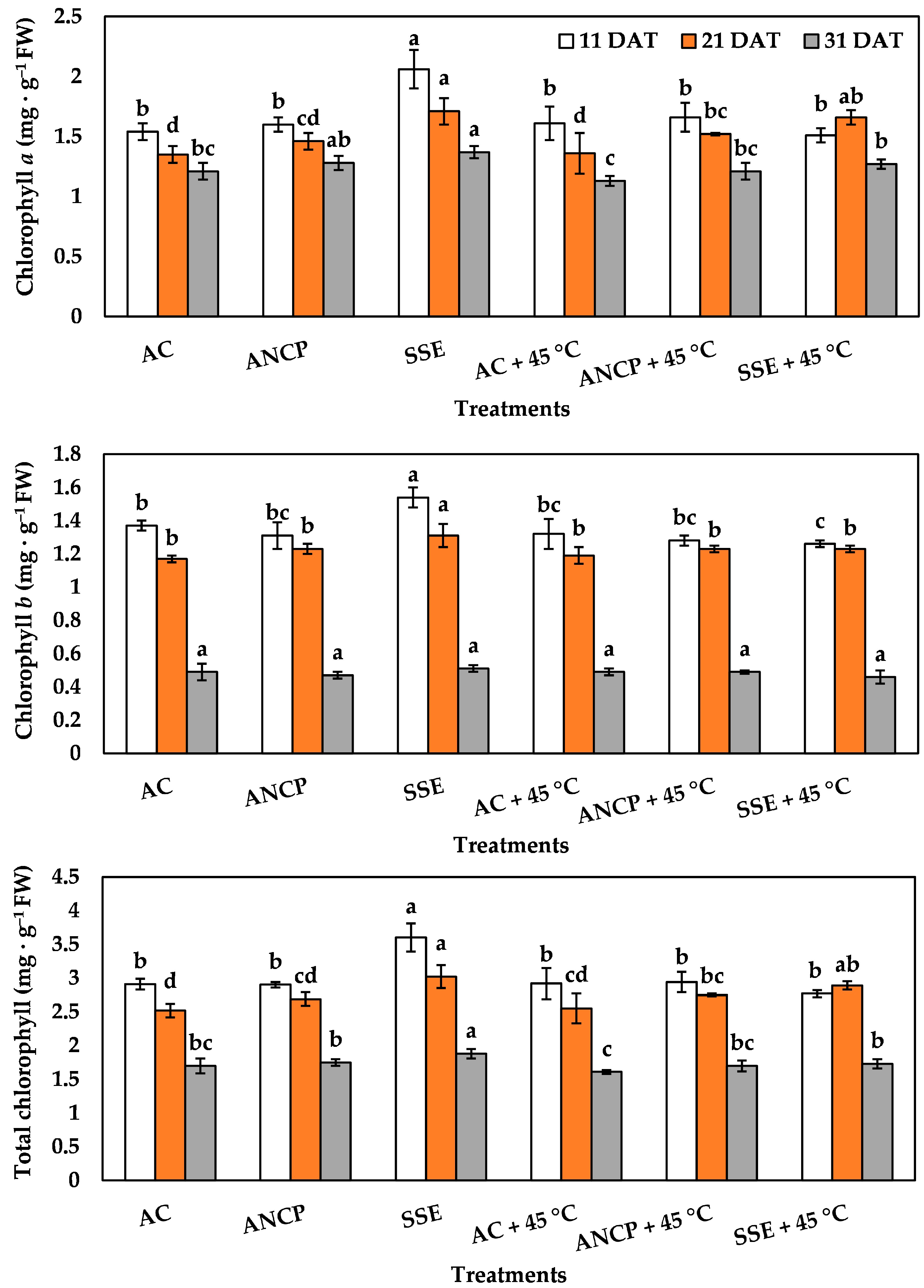
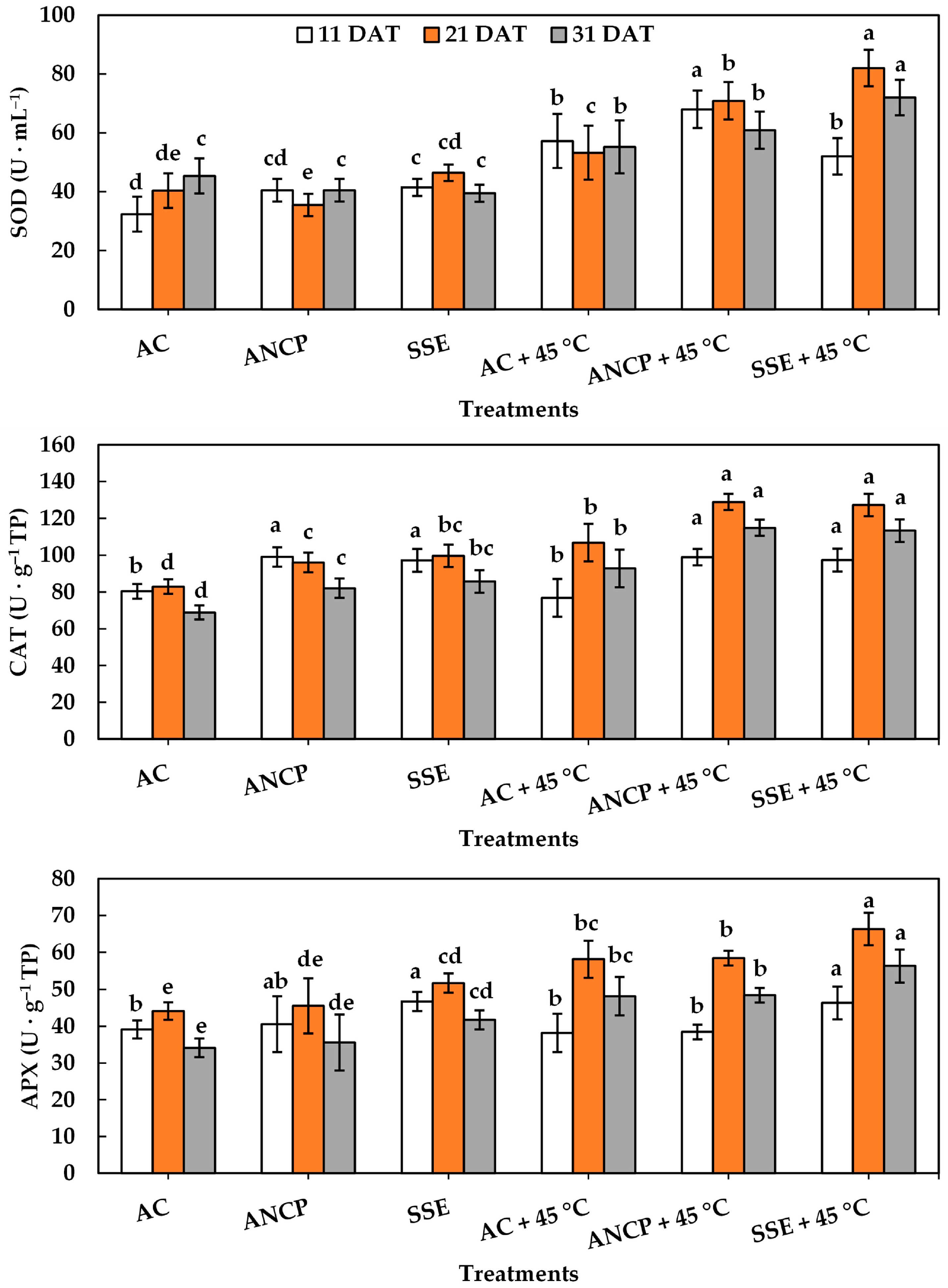


| Evaluation | Treatments | Plant Height (cm) | Stem Diameter (mm) | Leaf Number |
|---|---|---|---|---|
| AC | 12.08 ± 0.30 a | 3.33 ± 0.17 a | 6.75 ± 0.50 a | |
| ANCP | 12.58 ± 0.47 a | 3.40 ± 0.14 a | 6.75 ± 0.50 a | |
| 11 DAT | SSE | 12.65 ± 0.44 a | 3.48 ± 0.09 a | 6.75 ± 0.50 a |
| AC + 45 °C | 12.25 ± 0.47 a | 3.30 ± 0.14 a | 6.75 ± 0.50 a | |
| ANCP + 45 °C | 12.53 ± 0.45 a | 3.38 ± 0.09 a | 6.75 ± 0.50 a | |
| SSE + 45 °C | 12.55 ± 0.36 a | 3.43 ± 0.18 a | 7.00 ± 0.00 a | |
| AC | 19.78 ± 1.04 bc | 3.89 ± 0.18 abc | 10.50 ± 0.57 a | |
| ANCP | 20.40 ± 0.52 ab | 3.96 ± 0.15 ab | 10.75 ± 0.50 a | |
| 21 DAT | SSE | 20.75 ± 0.36 a | 4.04 ± 0.10 a | 10.50 ± 0.57 a |
| AC + 45 °C | 19.10 ± 0.43 c | 3.78 ± 0.09 c | 10.25 ± 0.50 a | |
| ANCP + 45 °C | 19.48 ± 0.28 c | 3.80 ± 0.08 bc | 10.25 ± 0.50 a | |
| SSE + 45 °C | 19.43 ± 0.72 c | 3.80 ± 0.08 bc | 10.25 ± 0.50 a | |
| AC | 35.78 ± 1.05 b | 5.04 ± 0.17 a | 14.50 ± 0.60 a | |
| ANCP | 36.40 ± 0.50 ab | 5.11 ± 0.15 a | 14.75 ± 0.50 a | |
| 31 DAT | SSE | 36.75 ± 0.40 a | 5.19 ± 0.09 a | 14.50 ± 0.60 a |
| AC + 45 °C | 34.10 ± 0.40 c | 4.78 ± 0.10 b | 14.25 ± 0.50 a | |
| ANCP + 45 °C | 34.48 ± 0.30 c | 4.83 ± 0.05 b | 14.25 ± 0.50 a | |
| SSE + 45 °C | 34.43 ± 0.70 c | 4.80 ± 0.09 b | 14.25 ± 0.50 a |
| Sampling | Treatments | Total Phenols (mg GAE g–1 DW) | Ascorbic Acid (mg 100 g–1 DW) | Antioxidant Capacity (mg AAE g–1 DW) |
|---|---|---|---|---|
| AC | 15.72 ± 1.42 a | 49.66 ± 1.45 c | 18.29 ± 1.50 c | |
| ANCP | 16.11 ± 0.64 a | 62.06 ± 4.20 b | 20.36 ± 0.80 b | |
| 11 DAT | SSE | 16.34 ± 1.15 a | 70.13 ± 2.60 a | 22.88 ± 0.74 a |
| AC + 45 °C | 15.77 ± 0.85 a | 50.26 ± 2.70 c | 18.13 ± 0.89 c | |
| ANCP + 45 °C | 15.69 ± 0.75 a | 61.54 ± 2.00 b | 20.68 ± 0.70 b | |
| SSE + 45 °C | 15.77 ± 0.73 a | 70.04 ± 3.00 a | 22.42 ± 1.28 a | |
| AC | 12.59 ± 1.40 c | 55.99 ± 4.28 e | 17.75 ± 1.48 d | |
| ANCP | 12.88 ± 0.65 c | 70.60 ± 3.14 d | 18.62 ± 0.67 d | |
| 21 DAT | SSE | 13.22 ± 1.20 c | 68.47 ± 5.49 d | 19.19 ± 0.52 cd |
| AC + 45 °C | 17.33 ± 0.90 b | 94.46 ± 5.50 c | 20.30 ± 0.90 c | |
| ANCP + 45 °C | 17.78 ± 0.80 b | 161.17 ± 7.97 a | 22.86 ± 0.71 b | |
| SSE + 45 °C | 20.48 ± 0.70 a | 132.86 ± 4.28 b | 25.68 ± 1.30 a | |
| AC | 11.55 ± 1.40 c | 99.13 ± 4.30 e | 16.66 ± 1.40 d | |
| ANCP | 1184 ± 0.60 c | 113.74 ± 3.15 d | 17.53 ± 0.70 d | |
| 31 DAT | SSE | 12.18 ± 1.10 c | 111.61 ± 5.50 d | 18.10 ± 0.50 cd |
| AC + 45 °C | 16.29 ± 0.80 b | 137.61 ± 5.50 c | 19.21 ± 0.89 c | |
| ANCP + 45 °C | 1673 ± 0.70 b | 204.31 ± 8.00 a | 21.77 ± 0.71 b | |
| SSE + 45 °C | 19.44 ± 0.72 a | 176.00 ± 4.30 b | 24.60 ± 1.28 a |
| Gene | Forward Primer 5′-3′ | Reverse Primer 5′-3′ |
|---|---|---|
| ACT | CCCAGGCACACAGGTGTTA | CAGGAGCAACTCGAAGCTC |
| NCED1 | CTTATTTGGCTATCGCTGAACC | CCTCCAACTTCAAACTCATTGC |
| HSP70 | TGCTGGAGGTGTTATGACCA | GACTCCTCTTGGTGCTGGAG |
| PIP2 | CTGCACCGTTGCTCGATTTT | GCGACAGTGACGTAGAGGAA |
| P5CS1 | CTGTTGTGGCTCGAGCTGAT | GACGACCAACACCTACAGCA |
| ERD15 | AGGCATCAAGTCATCACTCTCTGGT | GAGGTAAATGTGAGTAAGAACCAACG |
| Fe-SOD | CTGGGAATCTATGAAGCCCAACGGA | CAAATTGTGTTGCTGCAGCTGCCTT |
| CAT1 | TCGCGATGGTGCTATGAACA | CTCCCCTGCCTGTTTGAAGT |
| cAPX2 | GTGACCACTTGAGGGACGTGTTTGT | ACCAGAACGCTCCTTGTGGCATCTT |
| PAL5-3 | GGAGGAGAATTTGAAGAATGCTGTG | TCCCTTTCCACCACTTGTAGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sariñana-Aldaco, O.; Rodríguez-Jasso, R.M.; Benavides-Mendoza, A.; Robledo-Olivo, A.; Preciado-Rangel, P.; Juárez-Maldonado, A.; González-Morales, S. Brown Algae Extracts Increase the Tolerance of Tomato Plants to High Temperatures by Improving Morphological, Physiological, Metabolomic, and Transcriptional Parameters. Plants 2025, 14, 2996. https://doi.org/10.3390/plants14192996
Sariñana-Aldaco O, Rodríguez-Jasso RM, Benavides-Mendoza A, Robledo-Olivo A, Preciado-Rangel P, Juárez-Maldonado A, González-Morales S. Brown Algae Extracts Increase the Tolerance of Tomato Plants to High Temperatures by Improving Morphological, Physiological, Metabolomic, and Transcriptional Parameters. Plants. 2025; 14(19):2996. https://doi.org/10.3390/plants14192996
Chicago/Turabian StyleSariñana-Aldaco, Oscar, Rosa M. Rodríguez-Jasso, Adalberto Benavides-Mendoza, Armando Robledo-Olivo, Pablo Preciado-Rangel, Antonio Juárez-Maldonado, and Susana González-Morales. 2025. "Brown Algae Extracts Increase the Tolerance of Tomato Plants to High Temperatures by Improving Morphological, Physiological, Metabolomic, and Transcriptional Parameters" Plants 14, no. 19: 2996. https://doi.org/10.3390/plants14192996
APA StyleSariñana-Aldaco, O., Rodríguez-Jasso, R. M., Benavides-Mendoza, A., Robledo-Olivo, A., Preciado-Rangel, P., Juárez-Maldonado, A., & González-Morales, S. (2025). Brown Algae Extracts Increase the Tolerance of Tomato Plants to High Temperatures by Improving Morphological, Physiological, Metabolomic, and Transcriptional Parameters. Plants, 14(19), 2996. https://doi.org/10.3390/plants14192996











