Potential for Drought Stress Alleviation in Lettuce (Lactuca sativa L.) with Humic Substance-Based Biostimulant Applications
Abstract
1. Introduction
2. Results
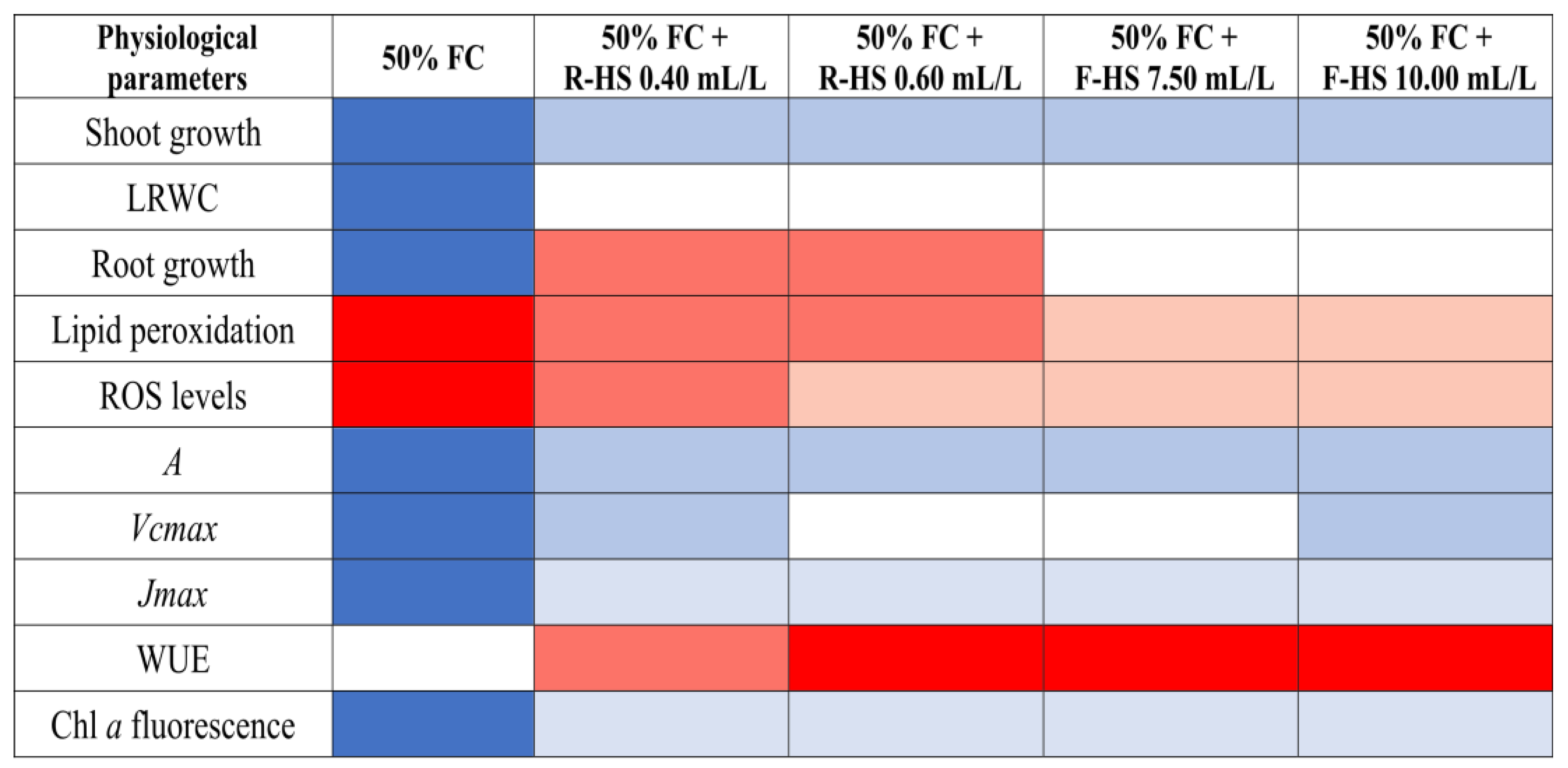
2.1. Shoot and Root Growth, LRWC, and DTI
2.2. Nutrient Accumulation
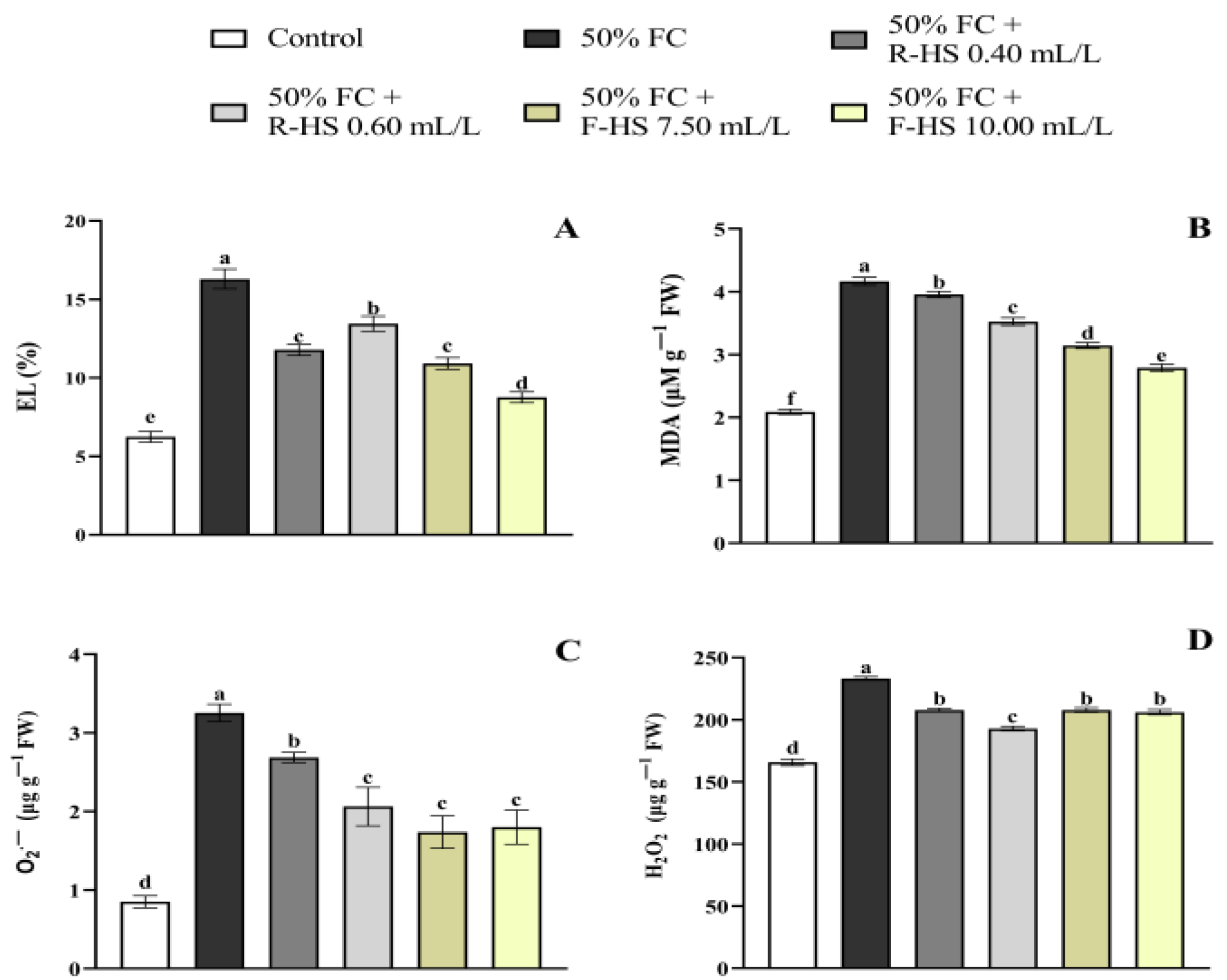
2.3. Stress Indicators
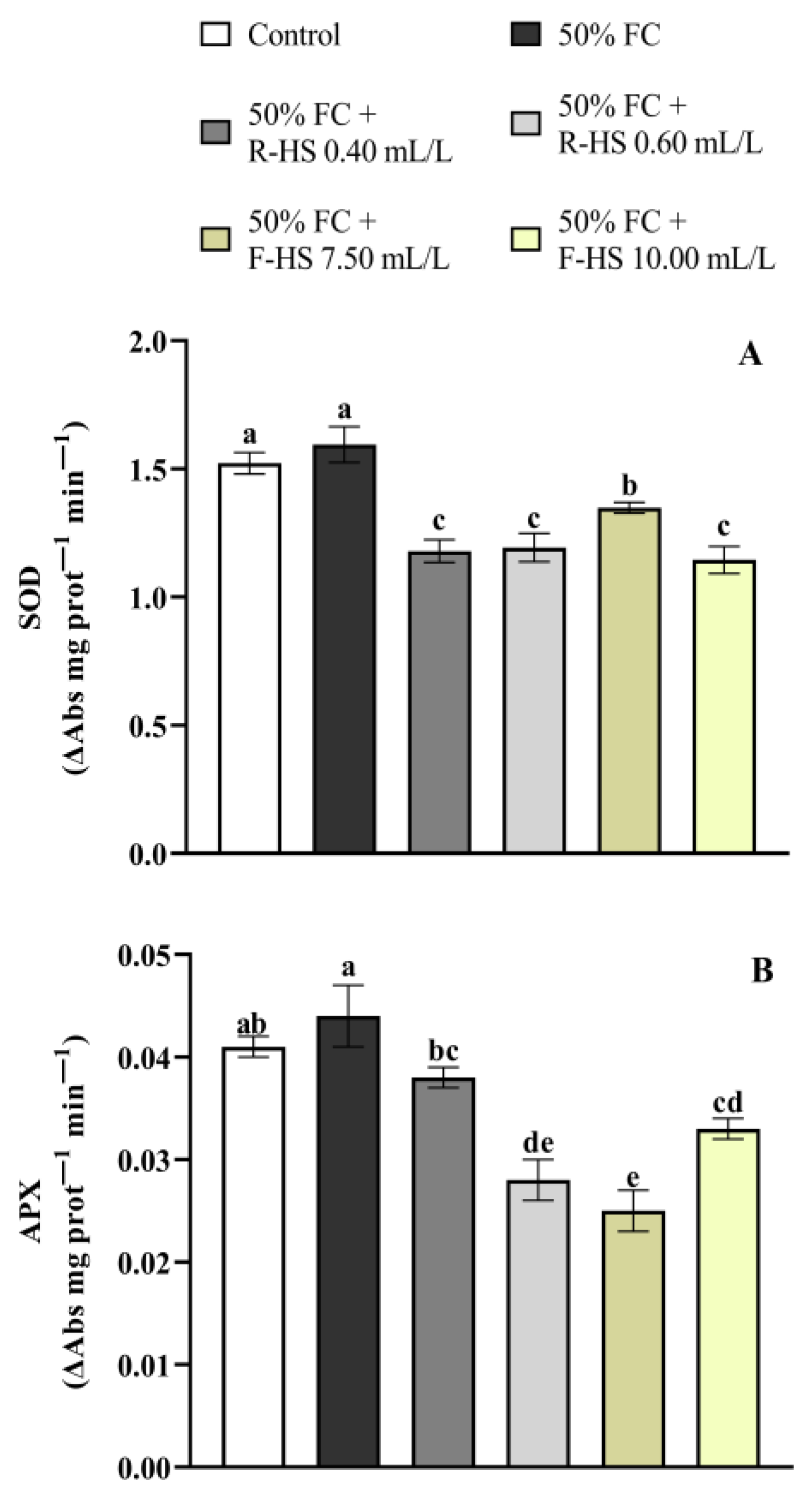
2.4. Non-Enzymatic and Enzymatic Antioxidants
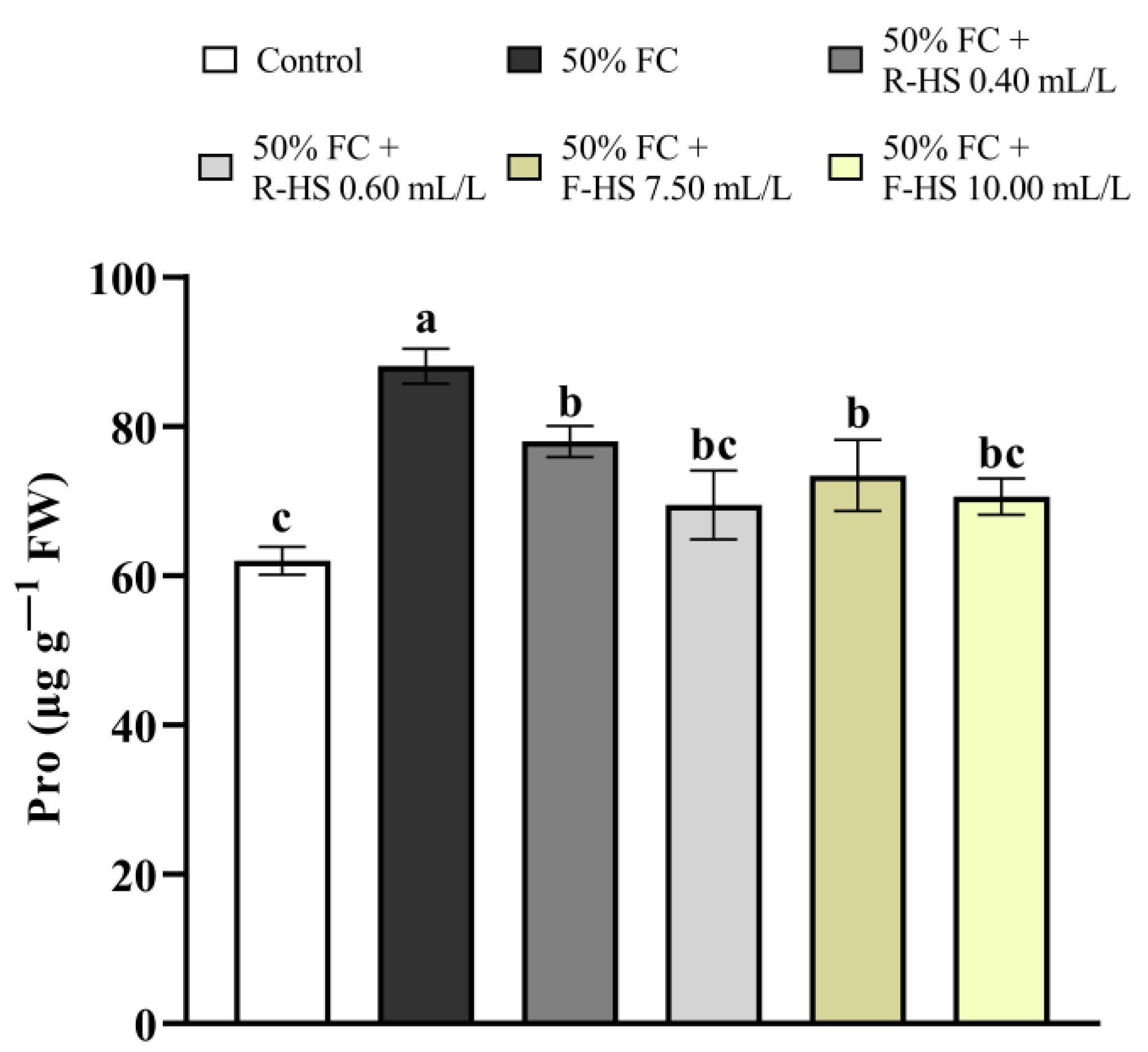
2.5. Pro Concentration
2.6. Leaf Gas Exchange Parameters
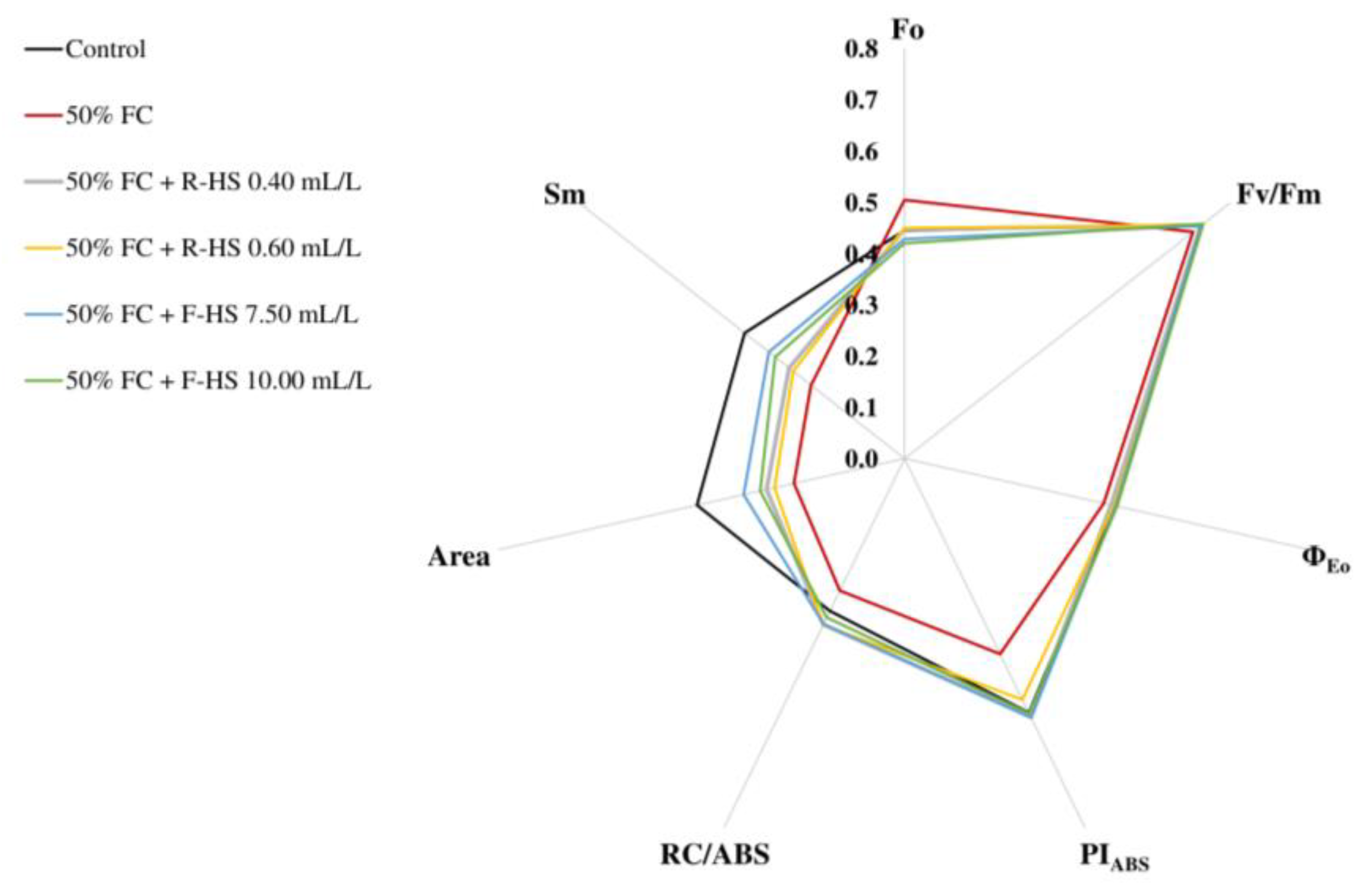
2.7. Chl a Fluorescence
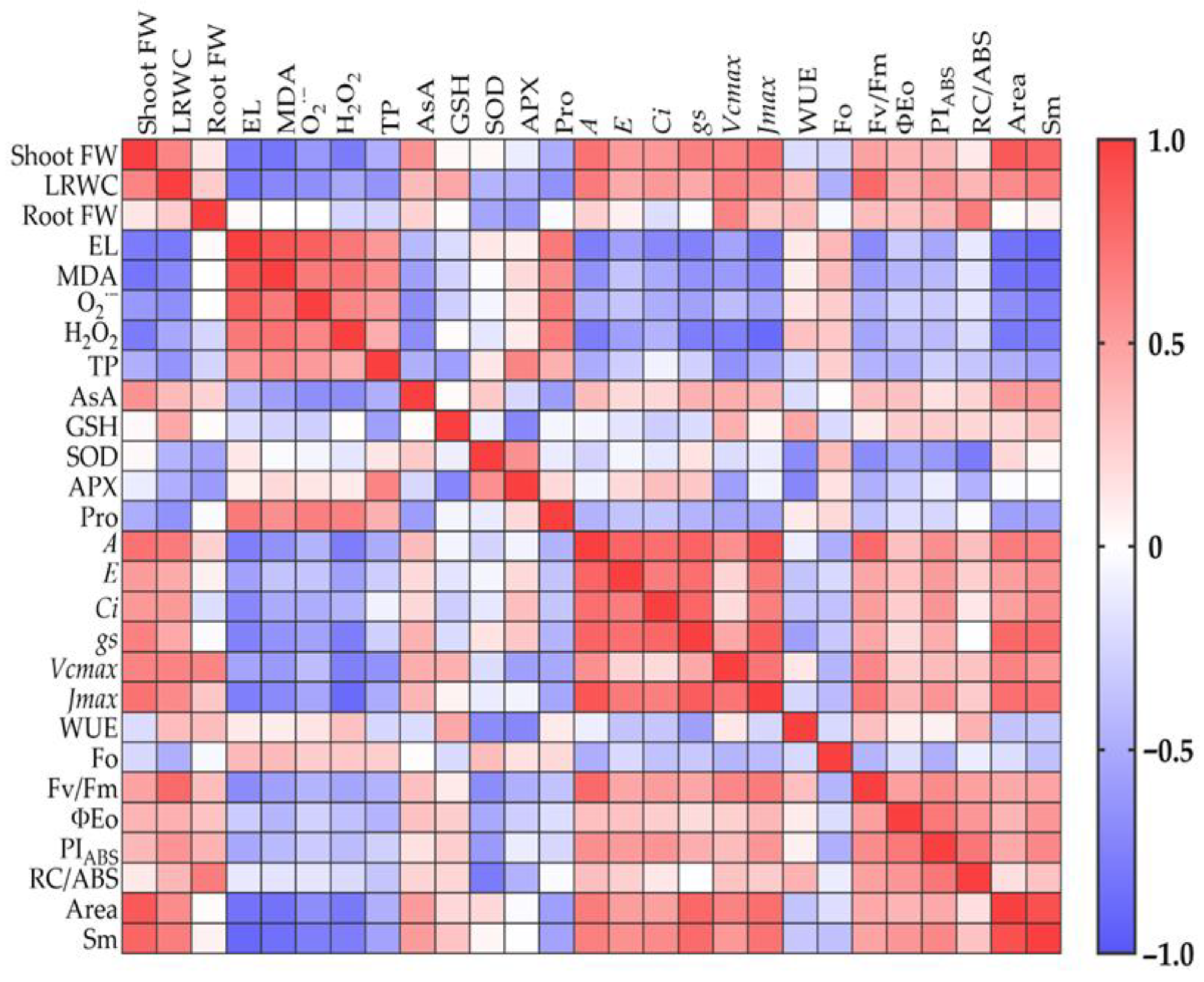
2.8. Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growing Conditions
4.2. Description of Treatments and Experimental Design
4.3. Plant Harvesting and Analysis of Growth Parameters
4.4. Determination of Nutrient Concentration
4.5. Determination of Oxidative Stress Indicators
4.6. Determination of Total Phenol, Flavonoids, AsA, and GSH
4.7. Determination of the SOD and APX Activities
4.8. Quantification of the Pro Concentration
4.9. Photosynthetic Gas Exchange Parameters
4.10. Analysis of Chlorophyll a Fluorescence
4.11. Statistical Procedures
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| A | Net photosynthetic rate |
| APX | Ascorbate peroxidase |
| AsA | Ascorbate |
| Ci | Intercellular CO2 |
| DHA | Dehydroascorbate |
| DTI | Drought tolerance index |
| E | Transpiration rate |
| EL | Electrolyte leakage |
| ΦEo | Maximum quantum yield of electron transport |
| Fo | Initial fluorescence |
| Fv/Fm | Maximum quantum yield of primary photochemistry |
| gs | Stomatal conductance |
| GSH | Glutathione |
| GSSG | Oxidized glutathione |
| Jmax | Maximum rate of electron transport |
| LRWC | Leaf relative water content |
| MDA | Malondialdehyde |
| PIABS | Performance index |
| Pro | Proline |
| RC/ABS | Proportion of active reaction centers |
| Sm | Energy needed to reduce reaction centers |
| SOD | Superoxide dismutase |
| Vcmax | Rubisco maximum carboxylation rate |
| WUE | Water use efficiency |
References
- Peláez-Vico, M.Á.; Sinha, R.; Preethi Induri, S.; Lyu, Z.; Darahas Venigalla, S.; Vasireddy, D.; Singh, P.; Sridhar Immadi, M.; Pascual, L.S.; Shostak, B.; et al. The Impact of Multifactorial Stress Combination on Reproductive Tissues and Grain Yield of a Crop Plant. Plant J. 2023, 117, 1728–1745. [Google Scholar] [CrossRef]
- Rivero, R.M.; Mittler, R.; Blumwald, E.; Zandalinas, S.I. Developing Climate-Resilient Crops: Improving Plant Tolerance to Stress Combination. Plant J. 2022, 109, 373–389. [Google Scholar] [CrossRef]
- Kandhol, N.; Jain, M.; Tripathi, D.K. Nanoparticles as Potential Hallmarks of Drought Stress Tolerance in Plants. Physiol. Plant. 2022, 174, e13665. [Google Scholar] [CrossRef]
- Kurunc, A. Effects of Water and Salinity Stresses on Growth, Yield, and Water Use of Iceberg Lettuce. J. Sci. Food Agric. 2021, 101, 5688–5696. [Google Scholar] [CrossRef]
- Ilyas, M.; Nisar, M.; Khan, N.; Hazrat, A.; Hamid Khan, A.; Hayat, K.; Fahad, S.; Khan, A.; Ullah, A. Drought Tolerance Strategies in Plants: A Mechanistic Approach. J. Plant Growth Regul. 2021, 40, 926–944. [Google Scholar] [CrossRef]
- Dinar, A.; Tieu, A.; Huynh, H. Water Scarcity Impacts on Global Food Production. Glob. Food Secur. 2019, 23, 212–226. [Google Scholar] [CrossRef]
- Del Buono, D. Can Biostimulants Be Used to Mitigate the Effect of Anthropogenic Climate Change on Agriculture? It Is Time to Respond. Sci. Total Environ. 2020, 751, 141763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic Stress Responses in Plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, T.; Liu, C.; Mao, K.; Gong, X.; Li, C.; Ma, F. Fruit Crops Combating Drought: Physiological Responses and Regulatory Pathways. Plant Physiol. 2023, 192, 1768–1784. [Google Scholar] [CrossRef]
- Mansoor, S.; Wani, O.A.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive Oxygen Species in Plants: From Source to Sink. Antioxidants 2022, 11, 225. [Google Scholar] [CrossRef] [PubMed]
- Lv, A.; Su, L.; Fan, N.; Wen, W.; Wang, Z.; Zhou, P.; An, Y. Chloroplast-Targeted Late Embryogenesis Abundant 1 Increases Alfalfa Tolerance to Drought and Aluminum. Plant Physiol. 2023, 193, 2750–2767. [Google Scholar] [CrossRef]
- Yang, Z.; Qin, F. The Battle of Crops against Drought: Genetic Dissection and Improvement. J. Integr. Plant Biol. 2023, 65, 496–525. [Google Scholar] [CrossRef] [PubMed]
- Vadez, V.; Pilloni, R.; Grondin, A.; Hajjarpoor, A.; Belhouchette, H.; Brouziyne, Y.; Chehbouni, G.; Kharrou, M.H.; Zitouna-Chebbi, R.; Mekki, I.; et al. Water Use Efficiency across Scales: From Genes to Landscapes. J. Exp. Bot. 2023, 74, 4770–4788. [Google Scholar] [CrossRef] [PubMed]
- Asayesh, Z.M.; Arzani, K.; Mokhtassi-Bidgoli, A.; Abdollahi, H. Enzymatic and Non-Enzymatic Response of Grafted and Ungrafted Young European Pear (Pyrus communis L.) Trees to Drought Stress. Sci. Hortic. 2022, 310, 111745. [Google Scholar] [CrossRef]
- Wahab, A.; Abdi, G.; Saleem, M.H.; Ali, B.; Ullah, S.; Shah, W.; Mumtaz, S.; Yasin, G.; Muresan, C.C.; Marc, R.A. Plants’ Physio-Biochemical and Phyto-Hormonal Responses to Alleviate the Adverse Effects of Drought Stress: A Comprehensive Review. Plants 2022, 11, 1620. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Santiago, L.; Navarro-León, E.; Javier López-Moreno, F.; Arjó, G.; María González, L.; Ruiz, J.M.; Blasco, B. The Application of the Silicon-Based Biostimulant Codasil® Offset Water Deficit of Lettuce Plants. Sci. Hortic. 2021, 285, 110177. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, L.; Hao, R.; Bai, X.; Wang, Y.; Yu, X. Drought-Tolerant Plant Growth-Promoting Rhizobacteria Isolated from Jujube (Ziziphus jujuba) and Their Potential to Enhance Drought Tolerance. Plant Soil 2020, 452, 423–440. [Google Scholar] [CrossRef]
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Feoktistova, A.; Timergalin, M.; Chetverikov, S.; Nazarov, A.; Kudoyarova, G. Effects on Pseudomonas Plecoglossicida 2,4-D and Humic Substances on the Growth, Pigment Indices and Concentration of Hormones in Wheat Seedlings Grown Under Water Deficit. Microorganisms 2023, 11, 549. [Google Scholar] [CrossRef]
- Ore, O.T.; Adeola, A.O.; Fapohunda, O.; Adedipe, D.T.; Bayode, A.A.; Adebiyi, F.M. Humic Substances Derived from Unconventional Resources: Extraction, Properties, Environmental Impacts, and Prospects. Environ. Sci. Pollut. Res. 2023, 30, 59106–59127. [Google Scholar] [CrossRef]
- Tiwari, J.; Ramanathan, A.L.; Bauddh, K.; Korstad, J. Humic Substances: Structure, Function and Benefits for Agroecosystems—A Review. Pedosphere 2023, 33, 237–249. [Google Scholar] [CrossRef]
- Al-Akbari, R.; Manasrah, A.D.; Nassar, N.N. Production of Humic and Fulvic Acid Analogs through the Ultrasonication of Low-Rank Lignite Coals. React. Chem. Eng. 2023, 9, 566–582. [Google Scholar] [CrossRef]
- Ouhaddou, R.; Ech-Chatir, L.; Anli, M.; Ben-Laouane, R.; Boutasknit, A.; Meddich, A. Secondary Metabolites, Osmolytes and Antioxidant Activity as the Main Attributes Enhanced by Biostimulants for Growth and Resilience of Lettuce to Drought Stress. Gesunde Pflanz. 2023, 75, 1737–1753. [Google Scholar] [CrossRef]
- Ibrahim, E.A.; Ebrahim, N.E.S.; Mohamed, G.Z. Effect of Water Stress and Foliar Application of Chitosan and Glycine Betaine on Lettuce. Sci. Rep. 2023, 13, 17274. [Google Scholar] [CrossRef]
- Atero-Calvo, S.; Magro, F.; Masetti, G.; Navarro-León, E.; Rios, J.J.; Blasco, B.; Ruiz, J.M. Comparative Effects of Root and Foliar Leonardite-Suspension Concentrate Application on Plant Growth and Photosynthetic Efficiency of Lettuce Plants (Lactuca sativa L.). J. Plant Growth Regul. 2024, 43, 4667–4679. [Google Scholar] [CrossRef]
- Atero-Calvo, S.; Magro, F.; Masetti, G.; Navarro-León, E.; Rios, J.J.; Ruiz, J.M. Assaying the Use of a Leonardite-Suspension Concentrate-Based Product as a Potential Biostimulant to Enhance Growth, NPK Use Efficiency, and Antioxidant Capacity in Lactuca sativa L. Agronomy 2023, 14, 64. [Google Scholar] [CrossRef]
- Cheraghi, M.; Mousavi, S.M.; Zarebanadkouki, M. Functions of Rhizosheath on Facilitating the Uptake of Water and Nutrients under Drought Stress: A Review. Plant Soil 2023, 491, 239–263. [Google Scholar] [CrossRef]
- Maseko, I.; Mabhaudhi, T.; Ncube, B.; Tesfay, S.; Araya, H.T.; Fessehazion, M.K.; Chimonyo, V.G.P.; Ndhlala, A.R.; Du Plooy, C.P. Postharvest Drying Maintains Phenolic, Flavonoid and Gallotannin Content of Some Cultivated African Leafy Vegetables. Sci. Hortic. 2019, 255, 70–76. [Google Scholar] [CrossRef]
- Malejane, D.N.; Tinyani, P.; Soundy, P.; Sultanbawa, Y.; Sivakumar, D. Deficit Irrigation Improves Phenolic Content and Antioxidant Activity in Leafy Lettuce Varieties. Food Sci. Nutr. 2018, 6, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Olaetxea, M.; Mora, V.; Bacaicoa, E.; Baigorri, R.; Garnica, M.; Fuentes, M.; Zamarreño, A.M.; Spíchal, L.; García-Mina, J.M. Root ABA and H+-ATPase Are Key Players in the Root and Shoot Growth-Promoting Action of Humic Acids. Plant Direct 2019, 3, e00175. [Google Scholar] [CrossRef] [PubMed]
- Moloi, S.J.; Ngara, R. The Roles of Plant Proteases and Protease Inhibitors in Drought Response: A Review. Front. Plant Sci. 2023, 14, 1165845. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; Ruiz, J.M.; Ferreres, F.; Moreno, D.A. Phenolic Metabolism in Grafted versus Nongrafted Cherry Tomatoes under the Influence of Water Stress. J. Agric. Food Chem. 2011, 59, 8839–8846. [Google Scholar] [CrossRef] [PubMed]
- Kiran, S.U.; Kıran, S.; Furtana, G.B.; Talhouni, M.; Ellialtıoğlu, Ş.Ş. Drought Stress Mitigation with Humic Acid in Two Cucumis melo L. Genotypes Differ in Their Drought Tolerance. Bragantia 2019, 78, 490–497. [Google Scholar] [CrossRef]
- Shen, J.; Guo, M.; Wang, Y.; Yuan, X.; Dong, S.; Song, X.-E.; Guoid, P. An Investigation into the Beneficial Effects and Molecular Mechanisms of Humic Acid on Foxtail Millet under Drought Conditions. PLoS ONE 2020, 15, e0234029. [Google Scholar] [CrossRef]
- Sun, J.; Qiu, C.; Ding, Y.; Wang, Y.; Sun, L.; Fan, K.; Gai, Z.; Dong, G.; Wang, J.; Li, X.; et al. Fulvic Acid Ameliorates Drought Stress-Induced Damage in Tea Plants by Regulating the Ascorbate Metabolism and Flavonoids Biosynthesis. BMC Genom. 2020, 21, 411. [Google Scholar] [CrossRef]
- Chen, Q.; Qu, Z.; Ma, G.; Wang, W.; Dai, J.; Zhang, M.; Wei, Z.; Liu, Z. Humic Acid Modulates Growth, Photosynthesis, Hormone and Osmolytes System of Maize under Drought Conditions. Agric. Water Manag. 2022, 263, 411. [Google Scholar] [CrossRef]
- Rehman, A.; Weng, J.; Li, P.; Hussain Shah, I.; Ur Rahman, S.; Khalid, M.; Manzoor, M.A.; Chang, L.; Niu, Q. Green Synthesized Zinc Oxide Nanoparticles Confer Drought Tolerance in Melon (Cucumis melo L.). Environ. Exp. Bot. 2023, 263, 107444. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and Fulvic Acids as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Kaya, C.; Şenbayram, M.; Akram, A.; Ashraf, M.; Nasser Alyemeni, M.; Ahmad, P. Sulfur-Enriched Leonardite and Humic Acid Soil Amendments Enhance Tolerance to Drought and Phosphorus Deficiency Stress in Maize (Zea mays L.). Sci. Rep. 2020, 10, 6432. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Z.; Xiemuxiding, A.; Zhang, X.; Duan, L.; Li, R. Fulvic Acid, Brassinolide, and Uniconazole Mediated Regulation of Morphological and Physiological Traits in Maize Seedlings Under Water Stress. J. Plant Growth Regul. 2023, 42, 1762–1774. [Google Scholar] [CrossRef]
- Navarro-León, E.; Grazioso, A.; Atero-Calvo, S.; Rios, J.J.; Esposito, S.; Blasco, B. Evaluation of the Alkalinity Stress Tolerance of Three Brassica rapa CAX1 TILLING Mutants. Plant Physiol. Biochem. 2023, 198, 107712. [Google Scholar] [CrossRef]
- Liu, C.; Mao, B.; Yuan, D.; Chu, C.; Duan, M. Salt Tolerance in Rice: Physiological Responses and Molecular Mechanisms. Crop J. 2022, 10, 13–25. [Google Scholar] [CrossRef]
- Hosseinifard, M.; Stefaniak, S.; Javid, M.G.; Soltani, E.; Wojtyla, Ł.; Garnczarska, M. Contribution of Exogenous Proline to Abiotic Stresses Tolerance in Plants: A Review. Int. J. Mol. Sci. 2022, 23, 5186. [Google Scholar] [CrossRef]
- Verslues, P.E.; Bailey-Serres, J.; Brodersen, C.; Buckley, T.N.; Conti, L.; Christmann, A.; Dinneny, J.D.S.R.; Grill, E.; Hayes, S.; Heckman, R.W.; et al. Burning Questions for a Warming and Changing World: 15 Unknowns in Plant Abiotic Stress. Plant Cell 2023, 35, 67–108. [Google Scholar] [CrossRef]
- Xiao, M.; Li, Z.; Zhu, L.; Wang, J.; Zhang, B.; Zheng, F.; Zhao, B.; Zhang, H.; Wang, Y.; Zhang, Z. The Multiple Roles of Ascorbate in the Abiotic Stress Response of Plants: Antioxidant, Cofactor, and Regulator. Front. Plant Sci. 2021, 12, 598173. [Google Scholar] [CrossRef] [PubMed]
- Dorion, S.; Ouellet, J.C.; Rivoal, J. Glutathione Metabolism in Plants under Stress: Beyond Reactive Oxygen Species Detoxification. Metabolites 2021, 11, 641. [Google Scholar] [CrossRef] [PubMed]
- Saidimoradi, D.; Ghaderi, N.; Javadi, T. Salinity Stress Mitigation by Humic Acid Application in Strawberry (Fragaria x ananassa Duch.). Sci. Hortic. 2019, 256, 108594. [Google Scholar] [CrossRef]
- Shukry, W.M.; Abu-Ria, M.E.; Abo-Hamed, S.A.; Anis, G.B.; Ibraheem, F. The Efficiency of Humic Acid for Improving Salinity Tolerance in Salt Sensitive Rice (Oryza sativa): Growth Responses and Physiological Mechanisms. Gesunde Pflanz. 2023, 75, 2639–2653. [Google Scholar] [CrossRef]
- Li, Q.; Gao, Y.; Hamani, A.K.M.; Fu, Y.; Liu, J.; Wang, H.; Wang, X. Effects of Warming and Drought Stress on the Coupling of Photosynthesis and Transpiration in Winter Wheat (Triticum aestivum L.). Appl. Sci. 2023, 13, 2759. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response Mechanism of Plants to Drought Stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Shen, J.; Guo, M.J.; Wang, Y.G.; Yuan, X.Y.; Wen, Y.Y.; Song, X.E.; Dong, S.Q.; Guo, P.Y. Humic Acid Improves the Physiological and Photosynthetic Characteristics of Millet Seedlings under Drought Stress. Plant Signal. Behav. 2020, 15, 1774212. [Google Scholar] [CrossRef] [PubMed]
- Lahbouki, S.; Fernando, A.L.; Rodrigues, C.; Ben-Laouane, R.; Ait-El-Mokhtar, M.; Outzourhit, A.; Meddich, A. Effects of Humic Substances and Mycorrhizal Fungi on Drought-Stressed Cactus: Focus on Growth, Physiology, and Biochemistry. Plants 2023, 12, 4156. [Google Scholar] [CrossRef]
- Velasco-Clares, D.; Navarro-León, E.; Atero-Calvo, S.; Ruiz, J.M.; Blasco, B. Is the Application of Bioactive Anti-Stress Substances with a Seaweed-Derived Biostimulant Effective under Adequate Growth Conditions? Physiol. Plant. 2024, 176, e14193. [Google Scholar] [CrossRef]
- Nxele, X.; Klein, A.; Ndimba, B.K. Drought and Salinity Stress Alters ROS Accumulation, Water Retention, and Osmolyte Content in Sorghum Plants. S. Afr. J. Bot. 2017, 108, 261–266. [Google Scholar] [CrossRef]
- Saathoff, A.J.; Welles, J. Gas Exchange Measurements in the Unsteady State. Plant Cell Environ. 2021, 44, 3509–3523. [Google Scholar] [CrossRef]
- Ye, Z.P.; Kang, H.J.; An, T.; Duan, H.L.; Wang, F.B.; Yang, X.L.; Zhou, S.X. Modeling Light Response of Electron Transport Rate and Its Allocation for Ribulose Biphosphate Carboxylation and Oxygenation. Front. Plant Sci. 2020, 11, 581851. [Google Scholar] [CrossRef] [PubMed]
- Shah Jahan, M.; Guo, S.; Sun, J.; Shu, S.; Wang, Y.; Abou El-Yazied, A.; Alabdallah, N.M.; Hikal, M.; Mohamed, M.H.M.; Ibrahim, M.F.M.; et al. Melatonin-Mediated Photosynthetic Performance of Tomato Seedlings under High-Temperature Stress. Plant Physiol. Biochem. 2021, 167, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Wang, Y.; Chi, Y.; Zhou, L.; Chen, J.; Zhou, W.; Song, J.; Zhao, N.; Ding, J. Drought Stress Strengthens the Link between Chlorophyll Fluorescence Parameters and Photosynthetic Traits. PeerJ 2020, 8, e10046. [Google Scholar] [CrossRef] [PubMed]
- Semida, W.M.; Abdelkhalik, A.; Rady, M.O.A.; Marey, R.A.; Abd El-Mageed, T.A. Exogenously Applied Proline Enhances Growth and Productivity of Drought Stressed Onion by Improving Photosynthetic Efficiency, Water Use Efficiency and up-Regulating Osmoprotectants. Sci. Hortic. 2020, 272, 109580. [Google Scholar] [CrossRef]
- Mininni, A.N.; Tuzio, A.C.; Brugnoli, E.; Dichio, B.; Sofo, A. Carbon Isotope Discrimination and Water Use Efficiency in Interspecific Prunus Hybrids Subjected to Drought Stress. Plant Physiol. Biochem. 2022, 175, 33–43. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; Semida, W.M.; Rady, M.M. Moringa Leaf Extract as Biostimulant Improves Water Use Efficiency, Physio-Biochemical Attributes of Squash Plants under Deficit Irrigation. Agric. Water Manag. 2017, 193, 46–54. [Google Scholar] [CrossRef]
- Navarro-Morillo, I.; Navarro-León, E.; Atero-Calvo, S.; Rios, J.J.; Ruiz, J.M.; Blasco, B. Biostimulant-Induced Mitigation of Cold and Drought Stresses in Zucchini Plants. Sci. Hortic. 2024, 331, 113114. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Liu, S. Elevated CO2 Alleviates Adverse Effects of Drought on Plant Water Relations and Photosynthesis: A Global Meta-Analysis. J. Ecol. 2022, 110, 2836–2849. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar] [CrossRef]
- Navarro-León, E.; Ruiz, J.M.; Graham, N.; Blasco, B. Physiological Profile of CAX1a TILLING Mutants of Brassica rapa Exposed to Different Calcium Doses. Plant Sci. 2018, 272, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Bano, H.; Athar, H.U.R.; Zafar, Z.U.; Kalaji, H.M.; Ashraf, M. Linking Changes in Chlorophyll a Fluorescence with Drought Stress Susceptibility in Mung Bean [Vigna radiata (L.) Wilczek]. Physiol. Plant 2021, 172, 1244–1254. [Google Scholar] [CrossRef]
- van Tol de Castro, T.A.; García, A.C.; Tavares, O.C.H.; Pereira, E.G.; de Souza, C.d.C.B.; Torchia, D.F.d.O.; de Pinho, C.F.; Castro, R.N. Humic Acids Affect Photosynthetic Quantum Efficiency in Rice under Water Deficit. Theor. Exp. Plant Physiol. 2022, 34, 463–483. [Google Scholar] [CrossRef]
- Lotfi, R.; Pessarakli, M.; Gharavi-Kouchebagh, P.; Khoshvaghti, H. Physiological Responses of Brassica napus to Fulvic Acid Under Water Stress: Chlorophyll a Fluorescence and Antioxidant Enzyme Activity. Crop J. 2015, 3, 434–439. [Google Scholar] [CrossRef]
- Barone, V.; Bertoldo, G.; Magro, F.; Broccanello, C.; Puglisi, I.; Baglieri, A.; Cagnin, M.; Concheri, G.; Squartini, A.; Pizzeghello, D.; et al. Molecular and Morphological Changes Induced by Leonardite-Based Biostimulant in Beta vulgaris L. Plants 2019, 8, 181. [Google Scholar] [CrossRef]
- Da Silva, T.I.; Gomes Dias, M.; Oliveira De Araújo, N.; Nayana, M.; Santos, S.; Ribeiro, S.; Batista, F.; Filho, S.; Dias, J.; Barbosa, G.; et al. Spermine Reduces the Harmful Effects of Drought Stress in Tropaeolum majus. Sci. Hortic. 2022, 304, 111390. [Google Scholar] [CrossRef]
- Navarro-León, E.; López-Moreno, F.J.; de la Torre-González, A.; Ruiz, J.M.; Esposito, S.; Blasco, B. Study of Salt-Stress Tolerance and Defensive Mechanisms in Brassica rapa CAX1a TILLING Mutants. Environ. Exp. Bot. 2020, 175, 104061. [Google Scholar] [CrossRef]
- Wolf, B. A Comprehensive System of Leaf Analyses and Its Use for Diagnosing Crop Nutrient Status. Commun. Soil Sci. Plant Anal. 1982, 13, 1035–1059. [Google Scholar] [CrossRef]
- Fu, J.; Huang, B. Involvement of Antioxidants and Lipid Peroxidation in the Adaptation of Two Cool-Season Grasses to Localized Drought Stress. Environ. Exp. Bot. 2001, 45, 105–114. [Google Scholar] [CrossRef]
- Junglee, S.; Urban, L.; Sallanon, H.; Lopez-Lauri, F. Optimized Assay for Hydrogen Perox-Ide Determination in Plant Tissue Using Potassium Iodide. Am. J. Analyt. Chem. 2014, 5, 730–736. [Google Scholar] [CrossRef]
- Kubiś, J. Exogenous Spermidine Differentially Alters Activities of Some Scavenging System Enzymes, H2O2 and Superoxide Radical Levels in Water-Stressed Cucumber Leaves. J. Plant Physiol. 2008, 165, 397–406. [Google Scholar] [CrossRef]
- Rivero, R.M.; Ruiz, J.M.; García, P.C.; López-Lefebre, L.R.; Sánchez, E.; Romero, L. Resistance to Cold and Heat Stress: Accumulation of Phenolic Compounds in Tomato and Watermelon Plants. Plant Sci. 2001, 160, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Law, M.Y.; Charles, S.A.; Halliwell, B. Glutathione and Ascorbic Acid in Spinach (Spinacia oleracea) Chloroplasts The Effect of Hydrogen Peroxide and of Paraquat. J. Biochem. 1983, 210, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Foyer, C.H. Simultaneous Measurement of Foliar Glutathione, Gamma-Glutamylcysteine, and Amino Acids by High-Performance Liquid Chromatography: Comparison with Two Other Assay Methods for Glutathione. Anal. Biochem. 1998, 264, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Osborne, L.; Rengel, Z. Micronutrient Deficiency Changes Activities of Superoxide Dismutase and Ascorbate Peroxidase in Tobacco Plants. J. Plant Nutr. 1998, 21, 1427–1437. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P. Ultraviolet-9-and Ozone-Lnduced Biochemical Changes in Antioxidant Enzymes of Arabidopsis thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef]
- Irigoyen, J.J.; Emerich, D.W.; Sanchez-Diaz Irigoyen, M. Water Stress Induced Changes in Concentrations of Proline and Total Soluble Sugars in Nodulated Alfalfa (Medkago sativa). Physiol. Plant. 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Duursma, R.A. Plantecophys—An R Package for Analysing and Modelling Leaf Gas Exchange Data. PLoS ONE 2015, 10, e0143346. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; Von Caemmerer, S.; Berry, J.A. A Biochemical Model of Photosynthetic CO2 Assimilation in Leaves of C3 Species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [PubMed]
| Shoot FW | Shoot RGR | LRWC | Root FW | Root RGR | Root Length | Root Surface Area | DTI | |
|---|---|---|---|---|---|---|---|---|
| Control | 73.03 ± 2.44 a | 0.0988 ± 0.0011 a | 90.81 ± 0.49 ab | 6.03 ± 0.30 c | 0.0577 ± 0.0016 c | 26.82 ± 0.51 | 45.58 ± 1.50 a | |
| 50% FC | 39.27 ± 0.75 d | 0.0782 ± 0.0007 d | 85.60 ± 0.13 c | 4.91 ± 0.07 d | 0.0510 ± 0.0005 d | 25.51 ± 1.14 | 28.14 ± 3.71 c | 55.88 ± 1.00 c |
| 50% FC + R-HS 0.40 mL/L | 48.08 ± 1.26 c | 0.0850 ± 0.0009 c | 89.35 ± 0.59 b | 6.95 ± 0.16 ab | 0.0625 ± 0.0008 ab | 26.41 ± 1.70 | 36.27 ± 3.07 b | 69.61 ± 1.70 b |
| 50% FC + R-HS 0.60 mL/L | 50.26 ± 2.04 bc | 0.0863 ± 0.0013 bc | 89.25 ± 0.08 b | 7.68 ± 0.33 a | 0.0658 ± 0.0014 a | 27.52 ± 1.18 | 42.15 ± 2.24 ab | 73.29 ± 2.80 ab |
| 50% FC + F-HS 7.50 mL/L | 52.59 ± 0.55 b | 0.0880 ± 0.0003 b | 90.92 ± 0.82 ab | 6.64 ± 0.30 bc | 0.0609 ± 0.0015 bc | 28.19 ± 1.95 | 45.22 ± 1.46 a | 74.92 ± 0.91 a |
| 50% FC + F-HS 10.00 mL/L | 50.66 ± 0.95 bc | 0.0862 ± 0.0007 bc | 91.37 ± 0.94 a | 5.87 ± 0.47 c | 0.0565 ± 0.0027 c | 26.17 ± 0.70 | 38.20 ± 3.22 ab | 70.54 ± 1.68 ab |
| p-Value | *** | *** | *** | *** | *** | NS | *** | *** |
| LSD0.05 | 4.28 | 0.0026 | 1.86 | 0.88 | 0.005 | 3.80 | 7.81 | 5.04 |
| K | Ca | Mg | Fe | Mn | Zn | |
|---|---|---|---|---|---|---|
| Control | 62.25 ± 2.43 a | 9.25 ± 0.31 a | 4.92 ± 0.18 b | 0.221 ± 0.001 a | 0.178 ± 0.006 b | 56.14 ± 2.25 a |
| 50% FC | 56.25 ± 1.34 b | 8.30 ± 0.21 b | 6.08 ± 0.16 a | 0.230 ± 0.003 a | 0.179 ± 0.004 b | 50.31 ± 1.15 b |
| 50% FC + R-HS 0.40 mL/L | 54.81 ± 1.07 b | 7.95 ± 0.18 b | 5.75 ± 0.15 a | 0.143 ± 0.008 c | 0.190 ± 0.005 ab | 46.59 ± 0.73 bc |
| 50% FC + R-HS 0.60 mL/L | 52.59 ± 0.21 b | 8.39 ± 0.05 b | 6.03 ± 0.06 a | 0.199 ± 0.009 b | 0.192 ± 0.002 ab | 43.87 ± 0.91 c |
| 50% FC + F-HS 7.50 mL/L | 55.77 ± 1.96 b | 8.39 ± 0.31 b | 5.87 ± 0.26 a | 0.163 ± 0.008 c | 0.190 ± 0.008 ab | 48.07 ± 1.97 bc |
| 50% FC + F-HS 10.00 mL/L | 56.47 ± 1.72 b | 7.99 ± 0.25 b | 6.16 ± 0.21 a | 0.195 ± 0.007 b | 0.204 ± 0.006 a | 49.95 ± 1.16 b |
| p-Value | * | * | ** | *** | * | ** |
| LSD0.05 | 4.98 | 0.73 | 0.55 | 0.020 | 0.016 | 4.53 |
| Total Phenols | Flavonoids | Total AsA | AsA | DHA | Total GSH | GSH | GSSG | |
|---|---|---|---|---|---|---|---|---|
| Control | 1.71 ± 0.08 b | 1.55 ± 0.03 b | 93.31 ± 4.43 d | 54.29 ± 1.61 a | 39.03 ± 2.82 e | 79.01 ± 3.16 cd | 45.32 ± 2.90 c | 33.69 ± 0.31 c |
| 50% FC | 2.54 ± 0.08 a | 1.95 ± 0.10 a | 118.42 ± 2.41 b | 35.05 ± 1.00 c | 83.37 ± 1.90 c | 76.54 ± 7.33 d | 44.35 ± 5.19 c | 32.19 ± 2.14 c |
| 50% FC + R-HS 0.40 mL/L | 2.52 ± 0.07 a | 1.66 ± 0.08 b | 124.86 ± 1.59 b | 30.00 ± 0.97 d | 95.11 ± 1.69 a | 75.75 ± 3.31 d | 29.22 ± 2.13 d | 46.52 ± 1.36 b |
| 50% FC + R-HS 0.60 mL/L | 1.72 ± 0.07 b | 1.22 ± 0.03 c | 141.29 ± 4.73 a | 51.56 ± 2.82 a | 91.55 ± 2.00 ab | 91.13 ± 1.55 c | 38.18 ± 1.32 cd | 52.95 ± 2.63 a |
| 50% FC + F-HS 7.50 mL/L | 1.43 ± 0.05 c | 0.96 ± 0.04 d | 102.25 ± 1.11 c | 40.28 ± 0.48 b | 62.41 ± 0.88 d | 169.99 ± 5.08 a | 125.58 ± 4.43 a | 44.41 ± 0.66 b |
| 50% FC + F-HS 10.00 mL/L | 1.73 ± 0.09 b | 0.99 ± 0.05 d | 123.68 ± 1.86 b | 37.46 ± 0.58 bc | 86.23 ± 1.43 bc | 118.22 ± 3.43 b | 70.89 ± 2.30 b | 47.33 ± 1.45 b |
| p-Value | *** | *** | *** | *** | *** | *** | *** | *** |
| LSD0.05 | 0.21 | 0.18 | 8.62 | 4.20 | 5.36 | 12.62 | 9.62 | 4.71 |
| A | E | Ci | gs | Vcmax | Jmax | WUE | |
|---|---|---|---|---|---|---|---|
| Control | 9.35 ± 0.92 a | 3.58 ± 1.07 a | 359.55 ± 10.42 a | 0.148 ± 0.022 a | 44.21 ± 3.14 a | 101.88 ± 1.87 a | 2.29 ± 0.15 c |
| 50% FC | 4.64 ± 0.05 c | 1.59 ± 0.16 b | 275.48 ± 10.01 c | 0.039 ± 0.002 c | 24.13 ± 0.98 c | 43.05 ± 0.10 c | 2.61 ± 0.06 bc |
| 50% FC + R-HS 0.40 mL/L | 7.61 ± 0.26 b | 2.93 ± 0.41 ab | 325.11 ± 19.02 ab | 0.078 ± 0.010 b | 36.69 ± 1.87 b | 76.67 ± 4.32 b | 2.95 ± 0.24 ab |
| 50% FC + R-HS 0.60 mL/L | 7.63 ± 0.28 b | 2.25 ± 0.13 ab | 292.84 ± 12.94 bc | 0.063 ± 0.004 bc | 42.16 ± 1.41 a | 74.79 ± 3.30 b | 3.27 ± 0.04 a |
| 50% FC + F-HS 7.50 mL/L | 6.74 ± 0.47 b | 2.13 ± 0.10 ab | 272.65 ± 10.10 c | 0.055 ± 0.003 bc | 45.90 ± 0.09 a | 75.42 ± 1.62 b | 3.28 ± 0.19 a |
| 50% FC + F-HS 10.00 mL/L | 7.69 ± 0.65 b | 2.61 ± 0.26 ab | 345.13 ± 12.70 a | 0.077 ± 0.008 b | 33.39 ± 1.09 b | 73.07 ± 6.20 b | 3.27 ± 0.08 a |
| p-Value | *** | * | ** | *** | *** | *** | ** |
| LSD0.05 | 1.61 | 1.51 | 39.67 | 0.033 | 5.26 | 18.29 | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atero-Calvo, S.; Magro, F.; Masetti, G.; Navarro-León, E.; Blasco, B.; Ruiz, J.M. Potential for Drought Stress Alleviation in Lettuce (Lactuca sativa L.) with Humic Substance-Based Biostimulant Applications. Plants 2025, 14, 2386. https://doi.org/10.3390/plants14152386
Atero-Calvo S, Magro F, Masetti G, Navarro-León E, Blasco B, Ruiz JM. Potential for Drought Stress Alleviation in Lettuce (Lactuca sativa L.) with Humic Substance-Based Biostimulant Applications. Plants. 2025; 14(15):2386. https://doi.org/10.3390/plants14152386
Chicago/Turabian StyleAtero-Calvo, Santiago, Francesco Magro, Giacomo Masetti, Eloy Navarro-León, Begoña Blasco, and Juan Manuel Ruiz. 2025. "Potential for Drought Stress Alleviation in Lettuce (Lactuca sativa L.) with Humic Substance-Based Biostimulant Applications" Plants 14, no. 15: 2386. https://doi.org/10.3390/plants14152386
APA StyleAtero-Calvo, S., Magro, F., Masetti, G., Navarro-León, E., Blasco, B., & Ruiz, J. M. (2025). Potential for Drought Stress Alleviation in Lettuce (Lactuca sativa L.) with Humic Substance-Based Biostimulant Applications. Plants, 14(15), 2386. https://doi.org/10.3390/plants14152386








