Conserved and Divergent Phytochemical Profiles in Native and Micropropagated Micromeria croatica (Pers.) Schott: An LC-HRMS Study Across Solvent Extracts
Abstract
1. Introduction
2. Results
2.1. Liquid Chromatography Coupled to High Resolution Mass Spectrometry
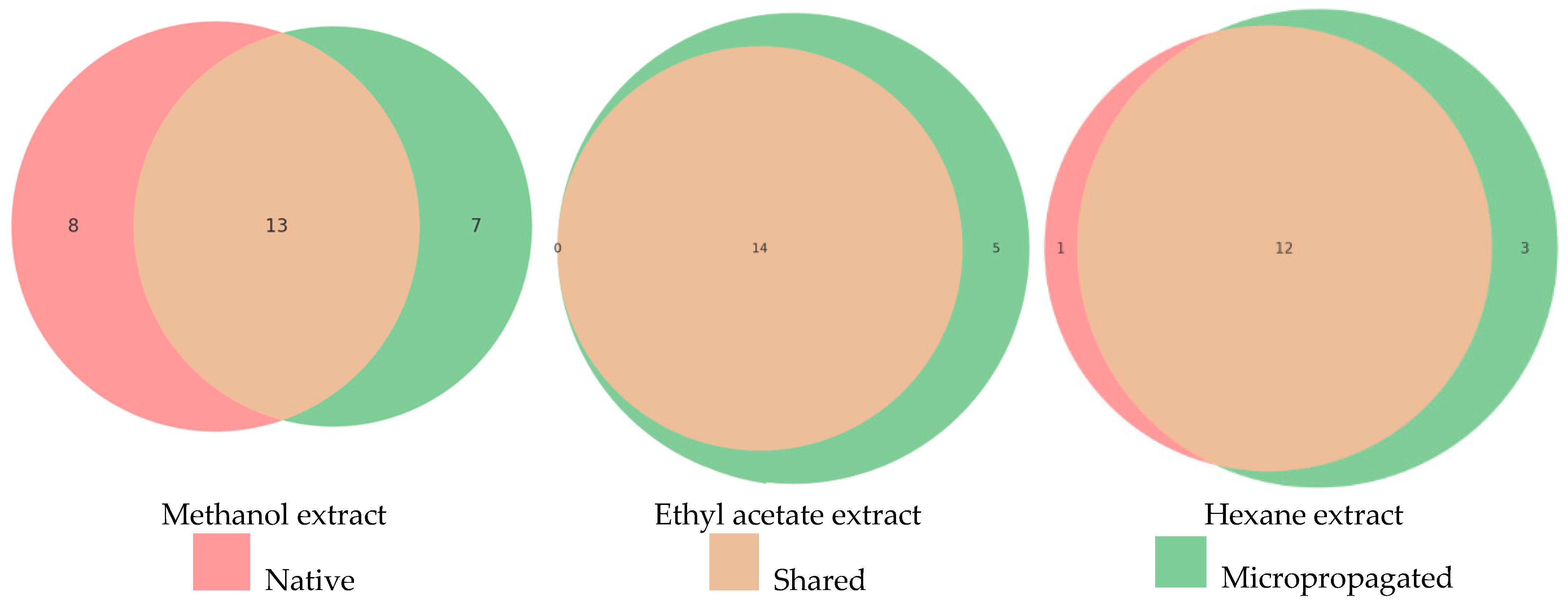
2.2. Statistical Comparison of Native and Micropropagated M. croatica Extracts
3. Discussion
3.1. Biological Significance of Shared Metabolites of Native and Micropropagated M. croatica
3.2. Comparison with Related Lamiaceae Species
4. Materials and Methods
4.1. Native Plant Material
4.2. Establishment and Maintenance of In Vitro Cultures
4.3. Extracts Preparation Protocol
4.4. Liquid Chromatography—High Resolution Mass Spectrometry (LC-HRMS)
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| LC-HRMS | Liquid Chromatography- High Resolution Mass Spectrometry |
| TIC | Total Ion Chromatogram |
| HMN | Herbarium collection of the Faculty of Science and Mathematics, University of Niš |
| HPLC | High Pressure Liquid Chromatography |
| ESI-LTQ | Electrospray Ionization- Linear Ion Trap |
| C18 | Octadecyl silica |
| ESI | Electrospray Ionization |
| FWHM | Full Width of Half Maximum intensity |
| BHT | Butylated Hydroxytoluene |
References
- Drew, B.T.; Sytsma, K.J. Phylogenetics, biogeography, and staminal evolution in the tribe Mentheae (Lamiaceae). Am. J. Bot. 2012, 99, 933–953. [Google Scholar] [CrossRef]
- Slavkovska, V.; Couladis, M.; Bojovic, S.; Tzakou, O.; Pavlovic, M.; Lakusic, B.; Jancic, R. Essential oil and its systematic significance in species of Micromeria Bentham from Serbia & Montenegro. Plant Syst. Evol. 2005, 255, 1–15. [Google Scholar] [CrossRef]
- Stanic, G.; Kalodjera, Z.; Petricic, J.; Todoric, A.; Blazevic, N. Essential oil content and composition of Micromeria croatica (Pers.) Schott and Micromeria thymifolia (Scop.) Fritsch. Acta Pharm. Jugosl. 1988, 38, 251–254. [Google Scholar]
- Das, S.; Prakash, B. Effect of Environmental Factors on Essential Oil Biosynthesis, Chemical Stability, and Yields. In Plant Essential Oils; Prakash, B., Dubey, N.K., de São José, J.F., Eds.; Springer Nature: Singapore, 2024; pp. 225–247. [Google Scholar]
- Zheljazkov, V.D.; Astatkie, T.; Jeliazkova, E. Year-round Variations in Essential Oil Content and Composition of Male and Female Juniper. HortScience 2013, 48, 883–886. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Wolfender, J.-L.; Rudaz, S.; Choi, Y.H.; Kim, H.K. Plant metabolomics: From holistic data to biochemical insight. Curr. Opin. Biotechnol. 2015, 31, 51–59. [Google Scholar]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arráez-Román, D.; Segura-Carretero, A. Metabolic profiling of Micromeria fruticosa extracts. Food Chem. 2019, 279, 128–143. [Google Scholar] [CrossRef]
- Ramachandra Rao, S.; Ravishankar, G.A. Plant cell cultures: Chemical factories of secondary metabolites. Biotechnol. Adv. 2002, 20, 101–153. [Google Scholar] [CrossRef]
- Marcelino, S.; Hamdane, S.; Gaspar, P.D.; Paço, A. Sustainable Agricultural Practices for the Production of Medicinal and Aromatic Plants: Evidence and Recommendations. Sustainability 2023, 15, 14095. [Google Scholar] [CrossRef]
- Šedivá, J.; Velebil, J.; Zahradník, D. Micropropagation as a Tool for the Conservation of Autochthonous Sorbus Species of Czechia. Plants 2023, 12, 488. [Google Scholar] [CrossRef] [PubMed]
- Bapat, V.A.; Kavi Kishor, P.B.; Jalaja, N.; Jain, S.M.; Penna, S. Plant Cell Cultures: Biofactories for the Production of Bioactive Compounds. Agronomy 2023, 13, 858. [Google Scholar] [CrossRef]
- Krol, A.; Kokotkiewicz, A.; Gorniak, M.; Naczk, A.M.; Zabiegala, B.; Gebalski, J.; Graczyk, F.; Zaluski, D.; Bucinski, A.; Luczkiewicz, M. Evaluation of the yield, chemical composition and biological properties of essential oil from bioreactor-grown cultures of Salvia apiana microshoots. Sci. Rep. 2023, 13, 7141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morone-Fortunato, I.; Avato, P. Plant development and synthesis of essential oils in micropropagated and mycorrhiza inoculated plants of Origanum vulgare L. ssp. hirtum (Link) Ietswaart. Plant Cell Tissue Organ Cult. 2008, 93, 139–149. [Google Scholar] [CrossRef]
- Asensio, E.; de Medinacelli Juan-Méndez, R.; Juan-Vicedo, J. In Vitro Propagation and Phytochemistry of Thymol-Producing Plants from a Horticultural Form of Thymus × josephi-angeli Mansanet & Aguil. (Lamiaceae). Horticulturae 2022, 8, 1188. [Google Scholar] [CrossRef]
- Tasheva, K.; Georgieva, A.; Denev, P.; Dimitrova, L.; Dimitrova, M.; Misheva, S.; Petkova-Kirova, P.; Lazarova, M.; Petrova, M. Antioxidant and Antitumor Potential of Micropropagated Balkan Endemic Sideritis scardica Griseb. Plants 2023, 12, 3924. [Google Scholar] [CrossRef] [PubMed]
- Matkowski, A. Plant in vitro culture for the production of antioxidants- a review. Biotechnol. Adv. 2008, 26, 548–560. [Google Scholar] [CrossRef]
- Panattoni, A.; Luvisi, A.; Triolo, E. Review. Elimination of viruses in plants: Twenty years of progress. Span. J. Agric. Res. 2013, 11, 173–188. [Google Scholar] [CrossRef]
- Vladimir-Knežević, S.; Blažeković, B.; Bival-Štefan, M.; Alegro, A.; Kőszegi, T.; Petrik, J. Antioxidant Activities and Polyphenolic Contents of Three Selected Micromeria Species from Croatia. Molecules 2011, 16, 1454–1470. [Google Scholar] [CrossRef]
- Mladenova, T.; Stoyanov, P.; Denev, P.; Dimitrova, S.; Katsarova, M.; Teneva, D.; Todorov, K.; Bivolarska, A. Phytochemical Composition, Antioxidant and Antimicrobial Activity of the Balkan Endemic Micromeria frivaldszkyana (Degen) Velen. (Lamiaceae). Plants 2021, 10, 710. [Google Scholar] [CrossRef]
- Sarikürkçu, C.; Ceylan, O.; Ćavar Zeljković, S. Micromeria myrtifolia: Essential oil composition and biological activity. Nat. Prod. Commun. 2019, 14, 1934578X1985168. [Google Scholar] [CrossRef]
- Lee, J.-E.; Jayakody, J.T.M.; Kim, J.-I.; Jeong, J.-W.; Choi, K.-M.; Kim, T.-S.; Seo, C.; Azimi, I.; Hyun, J.; Ryu, B. The influence of solvent choice on the extraction of bioactive compounds from Asteraceae: A comparative review. Foods 2024, 13, 3151. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qi, J.; Chang, Y.-X.; Zhu, D.; Yu, B. Identification and determination of the major constituents in Traditional Chinese Medicinal formula Danggui-Shaoyao-San by HPLC–DAD–ESI-MS/MS. J. Pharm. Biomed. Anal. 2009, 50, 127–137. [Google Scholar] [CrossRef]
- Honda, A.; Miyazaki, T.; Ikegami, T.; Iwamoto, J.; Yamashita, K.; Numazawa, M.; Matsuzaki, Y. Highly sensitive and specific analysis of sterol profiles in biological samples by HPLC–ESI–MS/MS. J. Steroid Biochem. Mol. Biol. 2010, 121, 556–564. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Li, N.; Chea, Y.-Y.; Zhang, Y.; Liang, S.-X.; Zhao, M.-B.; Jiang, Y.; Tu, P.-F. Characterization of seventy polymethoxylated flavonoids (PMFs) in the leaves of Murraya paniculata by on-line high-performance liquid chromatography coupled to photodiode array detection and electrospray tandem mass spectrometry. J. Pharm. Biomed. Anal. 2011, 56, 950–961. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Reed, R.L.; Morr, J.T. Characterization of phytoecdysteroid glycosides in meadowfoam (Limnanthes alba) seed meal by positive and negative ion LC-MS/MS. J. Agric. Food Chem. 2008, 56, 3945–3952. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Harnly, J.; Upton, R. Comparison of the phenolic component profiles of skullcap (Scutellaria lateriflora) and germander (Teucrium canadense and T. chamaedrys), a potentially hepatotoxic adulterant. Phytochem. Anal. 2009, 20, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Kachlicki, P.; Stobiecki, M. Structural characterization of flavonoid glycoconjugates and their derivatives with mass spectrometric techniques. Molecules 2016, 21, 1494. [Google Scholar] [CrossRef]
- Tahir, N.I.; Shaari, K.; Abas, F.; Parveez, G.K.; Ishak, Z.; Ramli, U.S. Characterization of apigenin and luteolin derivatives from oil palm (Elaeis guineensis Jacq.) leaf using LC−ESI-MS/MS. J. Agric. Food Chem. 2012, 60, 11201–11210. [Google Scholar] [CrossRef]
- Medana, C.; Massolino, C.; Pazzi, M.; Baiocchi, C. Determination of salvinorins and divinatorins in Salvia divinorum leaves by liquid chromatography/multistage mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 131–136. [Google Scholar] [CrossRef]
- Fu, L.; Han, B.; Zhou, Y.; Ren, J.; Cao, W.; Patel, G.; Kai, G.; Zhang, J. The anticancer properties of tanshinones and the pharmacological effects of their active ingredients. Front. Pharmacol. 2020, 11, 193. [Google Scholar] [CrossRef]
- Al-Yousef, H.M.; Fantoukh, O.I.; El-Sayed, M.A.; Amina, M.; Adel, R.; Hassan, W.H.B.; Abdelaziz, S. Metabolic profiling and biological activities of the aerial parts of Micromeria imbricata Forssk. growing in Saudi Arabia. Saudi J. Biol. Sci. 2021, 28, 5609–5616. [Google Scholar] [CrossRef]
- Cai, Y.-Z.; Xing, J.; Sun, M.; Zhan, Z.-Q.; Corke, H. Phenolic antioxidants (hydrolyzable tannins, flavonols, and anthocyanins) identified by LC-ESI-MS and MALDI-QIT-TOF MS from Rosa chinensis flowers. J. Agric. Food Chem. 2005, 53, 9940–9948. [Google Scholar] [CrossRef]
- Li, R.; Zhou, Y.; Wu, Z.; Ding, L. ESI-QqTOF-MS/MS and APCI-IT-MS/MS analysis of steroid saponins from the rhizomes of Dioscorea panthaica. J. Mass Spectrom. 2006, 41, 1–22. [Google Scholar] [CrossRef]
- Kefi, S.; Essid, R.; Papetti, A.; Abid, G.; Bouslama, L.; Aouani, E.; Tabbene, O.; Limam, F. Antioxidant, Antibacterial, and Antileishmanial Potential of Micromeria nervosa Extracts and Molecular Mechanism of Action of the Bioactive Compound. J. Appl. Microbiol. 2023, 134, lxad007. [Google Scholar] [CrossRef]
- Lin, L.-Z.; He, X.-G.; Lindenmaier, M.; Nolan, G.; Yang, J.; Cleary, M.; Qiu, S.-X.; Cordell, G.A. Liquid chromatography–electrospray ionization mass spectrometry study of the flavonoids of the roots of Astragalus mongholicus and A. membranaceus. J. Chromatogr. A 2000, 876, 87–95. [Google Scholar] [CrossRef]
- Bolboacă, S.D.; Jäntschi, L.; Sestraş, A.F.; Sestraş, R.E.; Pamfil, D.C. Pearson-Fisher Chi-Square Statistic Revisited. Information 2011, 2, 528–545. [Google Scholar] [CrossRef]
- Tošić, S.; Stojičić, D.; Slavkovska, V.; Mihailov-Krstev, T.; Zlatković, B.; Budimir, S.; Uzelac, B. Phytochemical composition and biological activities of native and in vitro-propagated Micromeria croatica (Pers.) Schott (Lamiaceae). Planta 2019, 249, 1365–1377. [Google Scholar] [CrossRef] [PubMed]
- Šamec, D.; Gruz, J.; Kremer, D.; Kosalec, I.; Salopek-Sondi, B.; Piljac-Žegarac, J. Flavonoid profiles and antioxidant capacity of wild Micromeria croatica (Pers.) Schott populations. Nat. Prod. Res. 2015, 29, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Tošić, S.; Stojičić, D.; Stankov-Jovanović, V.; Mitić, V.; Mihajilov-Krstev, T.; Zlatković, B. Chemical composition, antioxidant and antimicrobial activities of micropropagated and native Micromeria pulegium (Lamiaceae) extracts. Oxid. Commun. 2015, 38, 55–66. [Google Scholar]
- Gruz, J.; Piljac-Žegarac, J.; Šamec, D.; Salopek-Sondi, B.; Vladimir-Knežević, S. Flavonoid glycoside variation in wild and micropropagated Micromeria croatica—An ethyl acetate extract study. Molecules 2024, 29, 2345. [Google Scholar]
- Aydoğan, F.; Ali, Z.; Zulfiqar, F.; Karaalp, C.; Khan, I.A.; Bedir, E. Neo-clerodanes from Teucrium divaricatum subsp. divaricatum and their biological activity assessment. Phytochem. Lett. 2023, 54, 45–49. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Liu, Y.; Zhang, Y.; Wang, Y.; Peng, Y.; Ding, Y. Diosmetin-7-O-β-D-glucopyranoside suppresses endothelial-mesenchymal transformation through endoplasmic reticulum stress in cardiac fibrosis. Clin. Exp. Pharmacol. Physiol. 2023, 50, 789–805. [Google Scholar] [CrossRef] [PubMed]
- Gunia-Krzyżak, A.; Słoczyńska, K.; Popiół, J.; Koczurkiewicz, P.; Marona, H.; Pękala, E. Cinnamic acid derivatives in cosmetics—Current use and future prospects. Int. J. Cosmet. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Matejczyk, M.; Ofman, P.; Juszczuk-Kubiak, E.; Świsłocka, R.; Shing, W.L.; Kesari, K.K.; Prakash, B.; Lewandowski, W. Biological effects of vanillic acid, iso-vanillic acid, and orto-vanillic acid as environmental pollutants. Ecotoxicol. Environ. Saf. 2024, 277, 116383. [Google Scholar] [CrossRef]
- Magiera, A.; Kołodziejczyk-Czepas, J.; Olszewska, M.A. Antioxidant and anti-inflammatory effects of vanillic acid in human plasma, human neutrophils, and non-cellular models in vitro. Molecules 2025, 30, 467. [Google Scholar] [CrossRef]
- Sathya, S.; Devi, K.P. Chapter 15—The use of polyphenols for the treatment of Alzheimer’s disease. In Role of the Mediterranean Diet in the Brain and Neurodegenerative Diseases; Academic Press: London, UK, 2018; pp. 239–252. [Google Scholar]
- Kong, J.; Han, L.; Su, H.; Hu, Y.; Huang, X.; Lou, Y. Riligustilide attenuated renal injury by the blockade of renin. Cell. Physiol. Biochem. 2018, 50, 654–667. [Google Scholar] [CrossRef]
- Bi, X.; Wang, P.; Ma, Q.; Han, L.; Wang, X.; Mu, Y.; Guan, P.; Qu, X.; Wang, Z.; Huang, X. Anti-inflammatory activities and liver protection of Alisol F and 25-anhydroalisol F through the inhibition of MAPK, STAT3, and NF-κB activation in vitro and in vivo. Molecules 2017, 22, 951. [Google Scholar] [CrossRef]
- Hua, F.; Li, J.Y.; Zhang, M.; Zhou, P.; Wang, L.; Ling, T.J.; Bao, G.H. Kaempferol-3-O-rutinoside Exerts Cardioprotective Effects through NF-κB/NLRP3/Caspase-1 Pathway in Ventricular Remodeling after Acute Myocardial Infarction. J. Food Biochem. 2022, 46, e14305. [Google Scholar] [CrossRef]
- Alrumaihi, F.; Almatroodi, S.A.; Alharbi, H.O.A.; Alwanian, W.M.; Alharbi, F.A.; Almatroudi, A.; Rahmani, A.H. Pharmacological Potential of Kaempferol, a Flavonoid in the Management of Pathogenesis via Modulation of Inflammation and Other Biological Activities. Molecules 2024, 29, 2007. [Google Scholar] [CrossRef]
- Sun, R.; Liu, J.; Yu, M.; Xia, M.; Zhang, Y.; Sun, X.; Xu, Y.; Cui, X. Paeoniflorin ameliorates BiPN by reducing IL6 levels and regulating PARKIN-mediated mitochondrial autophagy. Drug Des. Devel. Ther. 2022, 16, 2241–2259. [Google Scholar] [CrossRef]
- Garg, M.; Chaudhary, S.K.; Goyal, A.; Sarup, P.; Kumari, S.; Garg, N.; Vaid, L.; Shiveena, B. Comprehensive review on therapeutic and phytochemical exploration of diosmetin: A promising moiety. Phytomed. Plus 2022, 2, 100179. [Google Scholar] [CrossRef]
- Elloumi, W.; Mahmoudi, A.; Ortiz, S.; Boutefnouchet, S.; Chamkha, M.; Sayadi, S. Wound healing potential of quercetin-3-O-rhamnoside and myricetin-3-O-rhamnoside isolated from Pistacia lentiscus distilled leaves in rat models. Biomed. Pharmacother. 2022, 146, 112574. [Google Scholar] [CrossRef]
- Carrillo-Martinez, E.J.; Flores-Hernández, F.Y.; Salazar-Montes, A.M.; Nario-Chaidez, H.F.; Hernández-Ortega, L.D. Quercetin, a flavonoid with great pharmacological capacity. Molecules 2024, 29, 1000. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef] [PubMed]
- Valdés, L.J., 3rd; Chang, H.M.; Visger, D.C.; Koreeda, M. Salvinorin C, a new neoclerodane diterpene from a bioactive fraction of the hallucinogenic Mexican mint Salvia divinorum. Org. Lett. 2001, 3, 3935–3937. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, Y.; Liu, X.; Gao, X. Pharmacological properties of tanshinones, the natural products from Salvia miltiorrhiza. Adv. Pharmacol. 2020, 87, 43–70. [Google Scholar] [CrossRef] [PubMed]
- Boenzi, S.; Deodato, F.; Taurisano, R.; Goffredo, B.M.; Rizzo, C.; Dionisi-Vici, C. Evaluation of plasma cholestane-3β,5α,6β-triol and 7-ketocholesterol in inherited disorders related to cholesterol metabolism. J. Lipid Res. 2016, 57, 361–367. [Google Scholar] [CrossRef]
- Fontana, G.; Bruno, M.; Sottile, F.; Badalamenti, N. The chemistry and the anti-inflammatory activity of polymethoxyflavonoids from Citrus genus. Antioxidants 2023, 12, 23. [Google Scholar] [CrossRef]
- Lv, J.; Song, X.; Luo, Z.; Huang, D.; Xiao, L.; Zou, K. Luteolin: Exploring its therapeutic potential and molecular mechanisms in pulmonary diseases. Front. Pharmacol. 2025, 16, 1535555. [Google Scholar] [CrossRef]
- Fang, P.; Wang, Y.; Sun, F.; Lin, H.; Zhang, X. Effects of albiflorin on oxidative stress and inflammatory responses in rats with acute spinal cord injury. Immun. Inflamm. Dis. 2023, 11, e1015. [Google Scholar] [CrossRef]
- Ren, S.-C.; Qiao, Q.-Q.; Ding, X.-L. Antioxidative activity of five flavone glycosides from corn silk (Stigma maydis). Czech J. Food Sci. 2013, 31, 148–155. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Cheng, Y.; Gao, H.; Chen, X. A review of classification, biosynthesis, biological activities and potential applications of flavonoids. Molecules 2023, 28, 4982. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Andrade, J.C.; Ozer, M.S.; de Lima Silva, J.M.F.; Ceylan, O.; de Sousa, E.O.; Coutinho, H.D.M. LC-MS/MS profiles and interrelationships between the enzyme inhibition activity, total phenolic content and antioxidant potential of Micromeria nervosa extracts. Food Chem. 2020, 328, 126930. [Google Scholar] [CrossRef]
- Stavrakeva, K.; Metodieva, K.; Benina, M.; Bivolarska, A.; Dimov, I.; Choneva, M.; Kokova, V.; Alseekh, S.; Ivanova, V.; Vatov, E.; et al. Metabolic composition of methanolic extract of the Balkan endemic species Micromeria frivaldszkyana (Degen) Velen. and its anti-inflammatory effect on male Wistar rats. Int. J. Mol. Sci. 2024, 25, 5396. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, M.A.; Çakır, O.; Zengin, G.; İzol, E.; Behçet, L. The uprisal of a lost endemic edible species, Micromeria cymuligera: Comprehensive elucidation of its biological activities and phytochemical composition. Food Biosci. 2024, 61, 104690. [Google Scholar] [CrossRef]
- Li, W.; Pan, J.; Chen, X.; Guo, S.; Ouyang, X. The chemical composition, pharmacological activity, quality control, toxicity, and pharmacokinetics of the genus Clinopodium L. Molecules 2025, 30, 2425. [Google Scholar] [CrossRef]
- Tošić, S.M.; Stojičić, D.D.; Zlatković, B.K.; Mitić, V.D.; Ilić, M.D.; Marković, M.S.; Stankov Jovanović, V.P. Antioxidant activity of Micromeria croatica grown in plant tissue culture in vitro versus natural habitats. Chem. Naissensis 2015, 3, 121–130. [Google Scholar] [CrossRef]
- Stankov-Jovanović, V.P.; Ilić, M.D.; Mitić, V.D.; Mihajilov-Krstev, T.M.; Simonović, S.R.; Nikolić Mandić, S.D.; Tabet, J.C.; Cole, R.B. Secondary metabolites of Seseli rigidum: Chemical composition plus antioxidant, antimicrobial and cholinesterase inhibition activity. J. Pharm. Biomed. Anal. 2015, 111, 78–90. [Google Scholar] [CrossRef]



| RT | M. croatica Native | Abundance of Compounds (%) | RT | M. croatica Micropropagated | Abundance of Compounds (%) |
|---|---|---|---|---|---|
| 1.0 | Albiflorin | 0.52 | 0.98 | Albiflorin | 0.81 |
| 1.03 | Dihydroxycholesterol | 1.23 | 1.05 | Apigenin-7-O-glucuronide | 1.12 |
| 1.61 | Trans-cinnamic acid | 0.29 | 1.61 | Trans-cinnamic acid | 0.88 |
| 2.03 | Dihydroxymethoxyflavone-glucoside | 0.49 | 2.05 | Vanillic acid | 0.98 |
| 2.09 | Vanillic acid | 0.57 | 2.48 | 5β-cholestane-3α7α12α25-tetrol | 0.68 |
| 2.51 | Luteolin-7-O-rutinoside | 0.82 | 2.51 | Luteolin-7-O-rutinoside | 1.00 |
| 2.48 | 5β-Cholestane-3α7α12α25-tetrol | 0.77 | 3.38 | Apigenin | 0.74 |
| 3.67 | Cycloartenol trans-ferulate | 0.78 | 6.42 | Teucrin G | 0.38 |
| 6.42 | Teucrin G | 1.37 | 7.50 | Kaempferol-O-rutinoside | 0.52 |
| 7.50 | Kaempferol-O-rutinoside | 0.62 | 14.41 | Gallic acid monohydrate | 0.70 |
| 9.91 | Salvinorin C | 1.05 | 17.27 | Diosmetin-7-O-glucoside | 0.95 |
| 10.93 | Eriocitrin | 0.63 | 19.80 | Diosmetin-6-C-glucoside | 1.13 |
| 14.38 | Gallic acid monohydrate | 1.14 | 19.90 | Kaempferol-O-rutinoside-3-O-rhamnoside | 0.45 |
| 17.30 | Diosmetin-7-O-glucoside | 0.60 | 27.78 | Kaempferol-3-O-rhamnside | 3.49 |
| 19.84 | Diosmetin-6-C-glucoside | 0.56 | 28.55 | Alisol F | 0.37 |
| 19.90 | Kaempferol-O-rutinoside-3-O-rhamnoside | 1.59 | 32.15 | Hexamethoxyflavone | 0.55 |
| 23.15 | Isomaltopaeoniflorin | 0.57 | 33.93 | Teuflin | 2.58 |
| 28.55 | Alisol F | 0.36 | 35.94 | Riligustilide | 0.49 |
| 35.92 | Ursolic Acid | 2.93 | 35.99 | Tanshinone I | 0.41 |
| 35.99 | Tanshinone I | 0.56 | 36.04 | Ursolic acid | 0.31 |
| 35.99 | Riligustilide | 0.14 |
| RT | M. croatica Native | Abundance of Compounds (%) | RT | M. croatica Micropropagated | Abundance of Compounds (%) |
|---|---|---|---|---|---|
| 1.02 | Albiflorin | 0.18 | 0.98 | Apigenin-7-O-glucuronide | 0.31 |
| 1.62 | Trans-cinnamic acid | 0.80 | 1.07 | Albiflorin | 0.47 |
| 2.52 | Luteolin-7-O-rutinoside | 1.43 | 2.55 | Luteolin-7-O-rutinoside | 1.01 |
| 6.51 | Teucrin G | 0.18 | 1.55 | Trans-cinnamic acid | 0.67 |
| 7.56 | Kaempferol-O-rutinoside | 0.49 | 3.37 | Apigenin | 1.52 |
| 9.91 | Salvinorin C | 0.95 | 6.54 | Teucrin G | 0.66 |
| 14.41 | Gallic acid monohydrate | 0.65 | 7.58 | Kaempferol-O-rutinoside | 0.54 |
| 17.36 | Diosmetin-7-O-glucoside | 0.98 | 9.98 | Salvinorin C | 0.47 |
| 19.88 | Diosmetin-6-C-glucoside | 0.54 | 14.42 | Gallic acid monohydrate | 1.54 |
| 19.88 | Kaempferol-O-rutinoside-3-O-rhamnoside | 0.40 | 17.35 | Diosmetin-7-O-glucoside | 0.81 |
| 28.56 | Alisol F | 1.28 | 19.84 | Diosmetin-6-C-glucoside | 1.34 |
| 35.91 | Ursolic acid | 0.73 | 19.93 | Kaempferol-O-rutinoside-3-O- rhamnoside | 0.33 |
| 35.98 | Tanshinone I | 0.47 | 23.09 | Isomaltopaeoniflorin | 0.14 |
| 35.98 | Riligustilide | 2.16 | 28.49 | Alisol F | 0.90 |
| 32.08 | Monohydroxyexamethoxyflavone | 3.32 | |||
| 32.45 | Dihydroxymethoxyflavanone | 0.74 | |||
| 35.97 | Riligustilide | 0.37 | |||
| 35.99 | Tanshinone I | 0.44 | |||
| 36.07 | Ursolic acid | 0.39 |
| RT | M. croatica Native | Abundance of Compounds (%) | RT | M. croatica Micropropagated | Abundance of Compounds (%) |
|---|---|---|---|---|---|
| 1.48 | Trans-cinnamic acid | 0.24 | 0.97 | Albiflorin | 0.49 |
| 2.54 | Luteolin-7-O-rutinoside | 0.28 | 1.52 | Trans-cinnamic acid | 1.12 |
| 3.53 | Apigenin | 0.48 | 2.57 | Luteolin-7-O-rutinoside | 0.67 |
| 6.46 | Teucrin G | 0.49 | 3.41 | Apigenin | 0.45 |
| 7.40 | Kaempferol-O-rutinoside | 0.43 | 6.50 | Teucrin G | 0.60 |
| 9.96 | Salvinorin C | 0.34 | 7.54 | Kaempferol-O-rutinoside | 0.79 |
| 14.48 | Gallic acid monohydrate | 0.36 | 9.84 | Salvinorin C | 0.54 |
| 17.40 | Diosmetin-7-O-glucoside | 1.61 | 14.44 | Gallic acid monohydrate | 0.56 |
| 19.96 | Diosmetin-6-C-glucoside | 0.57 | 17.35 | Diosmetin-7-O-glucoside | 0.53 |
| 23.10 | Isomaltopaeoniflorin | 0.53 | 19.85 | Diosmetin-6-C-glucoside | 0.72 |
| 32.02 | Monohydroxyhexamethoxyflavone | 3.44 | 19.85 | Kaempferol-O-rutinoside-3-O-rhamnoside | 0.55 |
| 35.97 | Tanshinone I | 1.19 | 23.17 | Isomaltopaeoniflorin | 1.33 |
| 35.97 | Riligustilide | 0.53 | 28.49 | Alisol F | 0.45 |
| 35.95 | Tanshinone I | 0.35 | |||
| 36.02 | Riligustilide | 0.52 |
| Number | Detected Compound | Mass to Charge Ratio (m/z), [M + H]+, [M + Na]+ # | Detected Fragmentions, m/z | Reference |
|---|---|---|---|---|
| 1. | Albiflorin (Mr 480.5) | 481.27 | 124.08, 214.09, 279.16, 329.14, 337.23, 359.22, 446.21, 453.12, 508.30, 595.30, 695.45, 787.41, 881.51, 940.82, 1146.48 | [23] |
| 2. | Dihydroxycholesterol (Mr 418.65) | 441.22 # | 148.82, 245.88, 329.14, 347.15, 508.23, 670.25, 757.32, 846.56, 857.36, 874.41, 874.41, 916.45, 1027.31, 1146.60 | [24] |
| 3. | Apigenin-7-O-glucuronide (Mr 446.36) | 447.36 | 187.12, 214.09, 225.19, 235.20, 318.28, 391.28, 595.33, 666.91, 809.18, 940.81, 1027.26, 1146.75 | [23] |
| 4. | Trans-cinnamic acid (Mr 148.16) | 171.19 # | 214.09, 277.202, 299.18, 322.26, 336.27, 435.17, 595.33, 666.90, 809.15, 940.81, 1027.28, 1146.52 | [25] |
| 5. | Dihydroxymethoxyflavoneglucoside (Mr 446.4) | 447.20 | 214.09, 271.09, 327.12, 382.20, 486.25, 597.16, 667.22, 753.30, 808.38, 853.35, 870.38, 877.36, 940.58, 1017.53, 1146.62 | [26] |
| 6. | Vanillic acid (Mr 168.14) | 169.14 | 214.09, 299.18, 336.27, 435.17, 528.95, 598.06, 729.49, 807.97, 940.38, 1027.55, 1146.91 | [25] |
| 7. | 5β-Cholestane-3α7α12α25-tetrol (Mr 711.37) | 712.25 | 203.03, 217.05, 287.09, 362.16, 382.45, 529.02, 597.58, 712.25, 954.62, 1047.48 | [24] |
| 8. | Luteolin-7-O-rutinoside (Mr 594.52) | 596.23 | 186.15, 205.09, 214.09, 272.14, 381.59, 444.26, 666.88, 808.87, 940.51, 1027.54, 1146.83 | [27,28] |
| 9. | Apigenin (Mr 270.24) | 271.10 | 1947.79, 203.03, 288.12, 330.17, 529.06, 558.21, 568.14, 666.88, 808.89, 940.55, 1017.52, 1146.77 | [29] |
| 10. | Cycloartenol trans-ferulate (Mr 602.9) | 604.32 | 182.98, 214.09, 234.13, 245.08, 345.13, 364.17, 406.22, 443.33, 529.16, 728.47, 809.14, 940.73, 1027.27, 1146.74 | [30] |
| 11. | Teucrin G (Mr 390.4) | 391.28 | 186.95, 214.09, 264.23, 316.15, 529.20, 595.30, 772.04, 940.80, 1027.27, 1146.69 | [25] |
| 12. | Diosmetin-7-O-glucoside (Mr 462.4) | 463.14 | 177.11, 187.04, 214.09, 245.08, 286.20, 362.16, 404.21, 529.16, 595.29, 706.29, 809.16, 940.74, 1017.43, 1146.85 | [25,28] |
| 13. | Gallic acid monohydrate (Mr 188.13) | 189.13 | 211.09, 225.19, 264.23, 318.28, 435.17, 529.03, 597.30, 771.19, 940.50, 1017.47, 1146.76 | [25,28] |
| 14. | Salvinorin C (Mr 475.29) | 476.31 | 179.06, 239.15, 300.20, 388.26, 432.28, 520.39, 564.36, 608.38, 666.89, 809.13, 940.78, 1027.28, 1146.46 | [27] |
| 15. | Diosmetin-6-C-glucoside (Mr 462.4) | 463.12 | 124.09, 214.09, 275.61, 327.12, 391.29, 488.27, 530.31, 616.16, 725.33, 808.98, 941.47, 958.49, 965.46, 1025.42, 1146.57 | [27,28] |
| 16. | Eriocitrin (Mr 596.5) | 597.58 | 203.3, 217.05, 287.09, 362.16, 382.45, 711.25, 808.17, 954.62. 1017.48, 1146.78 | [24] |
| 17. | Kaempferol-O-rutinoside-3-O-rhamnoside (Mr 740.70) | 763.93 # | 177.11, 187.04, 304.21, 362.16, 404.21, 528.99, 599.11, 711.25, 940.42, 1017.53, 1146.69 | [28,29] |
| 18. | Tanshinone I (Mr 276.29) | 277.20 | 171.14, 214.09, 299.19, 322.26, 335.28, 435.17, 595.33, 666.90, 809.15, 940.81, 1027.28, 1146.52 | [30,31] |
| 19. | Kaempferol-O-rutinoside (Mr 594.52) | 595.86 | 182.98, 234.14, 245.08, 329.14, 358.24, 488.3-27, 530.31, 770.38, 874.41, 940.59, 1146.74 | [32] |
| 20. | Kaempferol-3-O-rhamnoside (Mr 432.38) | 433.17 | 187.13, 214.09, 264.23, 391.29, 529.20, 595.28, 666.92, 809.16, 940.80, 1027.27, 1146.68 | [33] |
| 21. | Alisol F (Mr 488.7) | 489.16 | 187.04, 203.07, 245.08, 327.12, 362.16, 404.21, 589.21, 596.059, 706.29, 716.22, 829.31, 940.49, 1027.39, 1146.81 | [23] |
| 22. | Hexamethoxyflavone (Mr 402.39) | 403.21 | 177.11, 214.09, 294.13, 299.18, 362.16, 529.17, 595.32, 667.57, 691.53, 809.14, 940.73, 1076.49, 1146.61 | [25,28] |
| 23. | Monohydroxyhexamethoxyflavone (Mr 252.26) | 253.25 | 124.09, 214.09, 234.13, 299.18, 362.16, 529.31, 595.32, 667.57, 691.53, 809.14, 940.73, 1076.49, 1146.61 | [25] |
| 24. | Dihydroxymethoxyflavanone (Mr 284.27) | 285.18 | 182.99, 214.09, 234.14, 382.41, 429.38, 447.39, 528.96, 597.41, 666.77, 808.12, 940.39, 1075.07, 1146.83 | [25] |
| 25. | Isomaltopaeoniflorin (Mr 480.46) | 481.27 | 124.09, 214.09, 299.18, 299.18, 329.14, 446.22, 503.23, 516.26, 727.37, 809.07, 874.41, 916.45, 940.74, 1146.60 | [23] |
| 26. | Teuflin (Mr 328.4) | 329.14 | 124.09, 214.09, 279.16, 337.24, 359.22, 446.22, 453.32, 488.27, 508.23, 596.30, 695.45, 787.41, 881.51, 940.82, 1146.48 | [25,28] |
| 27. | Riligustilide (Mr 380.48) | 381.19 | 128.11, 203.03, 245.05, 320.11, 362.17, 488.27, 530.31, 622.20, 689.30, 738.35, 795.30, 951.45, 1027.32, 1146.78 | [23] |
| 28. | Ursolic acid (Mr 456.7) | 457.24 | 182.99, 248.15, 272.24, 338.34, 386.28, 565.43, 666.71, 807.91, 940.42, 1017.59, 1146.75 | [28,34,35,36] |
| Common Compound | Biological Activity | References |
|---|---|---|
| Teucrin G | Anti-inflammatory and antimicrobial effects. | [42] |
| Diosmetin-7-O-glucoside | Cardiovascular protection. | [43] |
| Trans-cinnamic acid | Antioxidant, antimicrobial, anti-aging activity. | [44] |
| Vanillic acid | Antioxidant, antimicrobial and anti-inflammatory activity. | [45,46] |
| Apigenin | Antioxidant, antimicrobial, anti-inflammatory, antitumor, neuroprotective activity. | [47] |
| Riligustilide | Antioxidant, anti-inflammatory, anticancer, neuroprotective, progestogenic activity and cardiovascular protection. | [48] |
| Alisol F | Anti-inflammatory activity. | [49] |
| Kaempferol-O-rutinoside | Antioxidant, anti-inflammatory, antidiabetic, anti-cancer, hepatoprotective, renoprotective, gastroprotective, neuroprotective, cardioprotective | [50,51] |
| Isomaltopaeoniflorin | Alleviating pain. | [52] |
| Diosmetin-6-C-glucoside | Antioxidant, anti-aging, anti-inflammatory, antidiabetic activity, potential in Alzheimer’s treatment, enhance spatial memory, contribute to memory-enhancing and has anxiolytic effects. | [53] |
| Kaempferol-O-rutinoside-3-O-rhamnoside | Effects on the swift healing of skin injuries. Antioxidant, anti-inflammatory, cytotoxic, neuroprotective effects, and cardiovascular protection. | [54,55] |
| Gallic acid monohydrate | Antioxidant, anti-inflammatory, anticancer and antiviral activity. | [56] |
| Salvinorin C | Psychoactive activity. | [57] |
| Tanshinone I | Anti-inflammatory, antioxidant, and anticancer effects. | [33,58] |
| 5β-Cholestane-3α7α12α25-tetrol | Applications in metabolic disorders | [59] |
| Monohydroxy hexamethoxy flavone | Anti-inflammatory activity | [60] |
| Luteolin-7-O-rutinoside | Antioxidant, antimicrobial, anti-inflammatory, antitumor, neuroprotective, anti-viral activity and cardiovascular protection. | [61] |
| Albiflorin | Anti-inflammatory, antioxidant, and neuroprotective effects. | [62] |
| Dihydroxymethoxy flavoneglucoside | Antioxidant, anti-inflammatory, and anticancer properties. | [63,64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tošić, S.M.; Ilić, M.; Svilar, L.; Nikolić, J.; Mitić, M.; Mitić, V.; Stankov Jovanović, V.P. Conserved and Divergent Phytochemical Profiles in Native and Micropropagated Micromeria croatica (Pers.) Schott: An LC-HRMS Study Across Solvent Extracts. Plants 2025, 14, 2971. https://doi.org/10.3390/plants14192971
Tošić SM, Ilić M, Svilar L, Nikolić J, Mitić M, Mitić V, Stankov Jovanović VP. Conserved and Divergent Phytochemical Profiles in Native and Micropropagated Micromeria croatica (Pers.) Schott: An LC-HRMS Study Across Solvent Extracts. Plants. 2025; 14(19):2971. https://doi.org/10.3390/plants14192971
Chicago/Turabian StyleTošić, Svetlana M., Marija Ilić, Ljubica Svilar, Jelena Nikolić, Milan Mitić, Violeta Mitić, and Vesna P. Stankov Jovanović. 2025. "Conserved and Divergent Phytochemical Profiles in Native and Micropropagated Micromeria croatica (Pers.) Schott: An LC-HRMS Study Across Solvent Extracts" Plants 14, no. 19: 2971. https://doi.org/10.3390/plants14192971
APA StyleTošić, S. M., Ilić, M., Svilar, L., Nikolić, J., Mitić, M., Mitić, V., & Stankov Jovanović, V. P. (2025). Conserved and Divergent Phytochemical Profiles in Native and Micropropagated Micromeria croatica (Pers.) Schott: An LC-HRMS Study Across Solvent Extracts. Plants, 14(19), 2971. https://doi.org/10.3390/plants14192971





