Effects of Deep Shading on Agronomic Traits, Coloration, and Antioxidant Properties in Sweetpotato Leaves
Abstract
1. Introduction
2. Results
2.1. Agronomic Traits of Sweetpotato Aboveground Parts in Response to Deep Shading
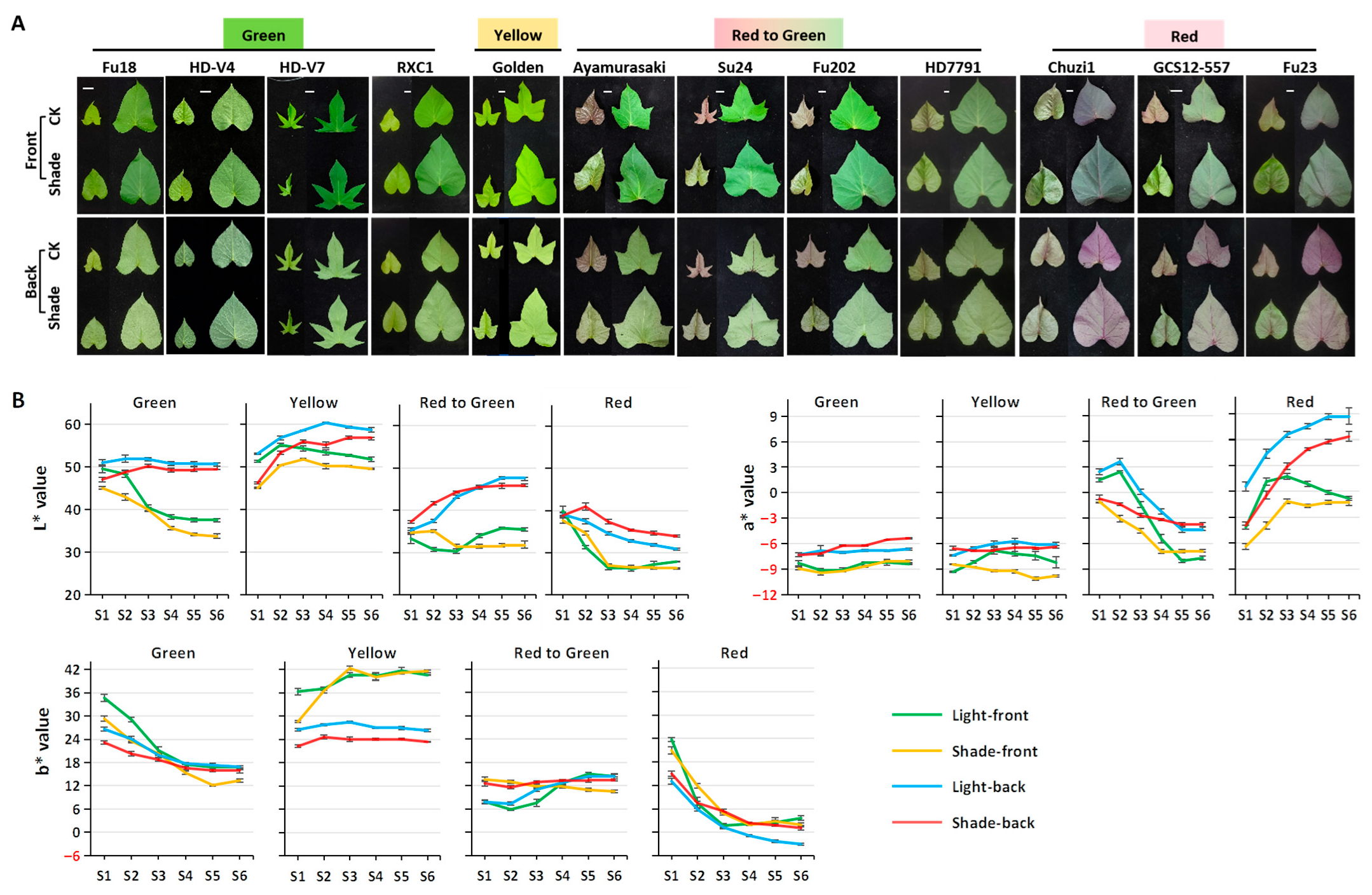
2.2. Morphological and Color Changes in Sweetpotato Leaves in Response to Deep Shading
2.2.1. Effects of Shading on Leaf Morphology and Color
2.2.2. Effects of Shading on Leaf Color Parameters
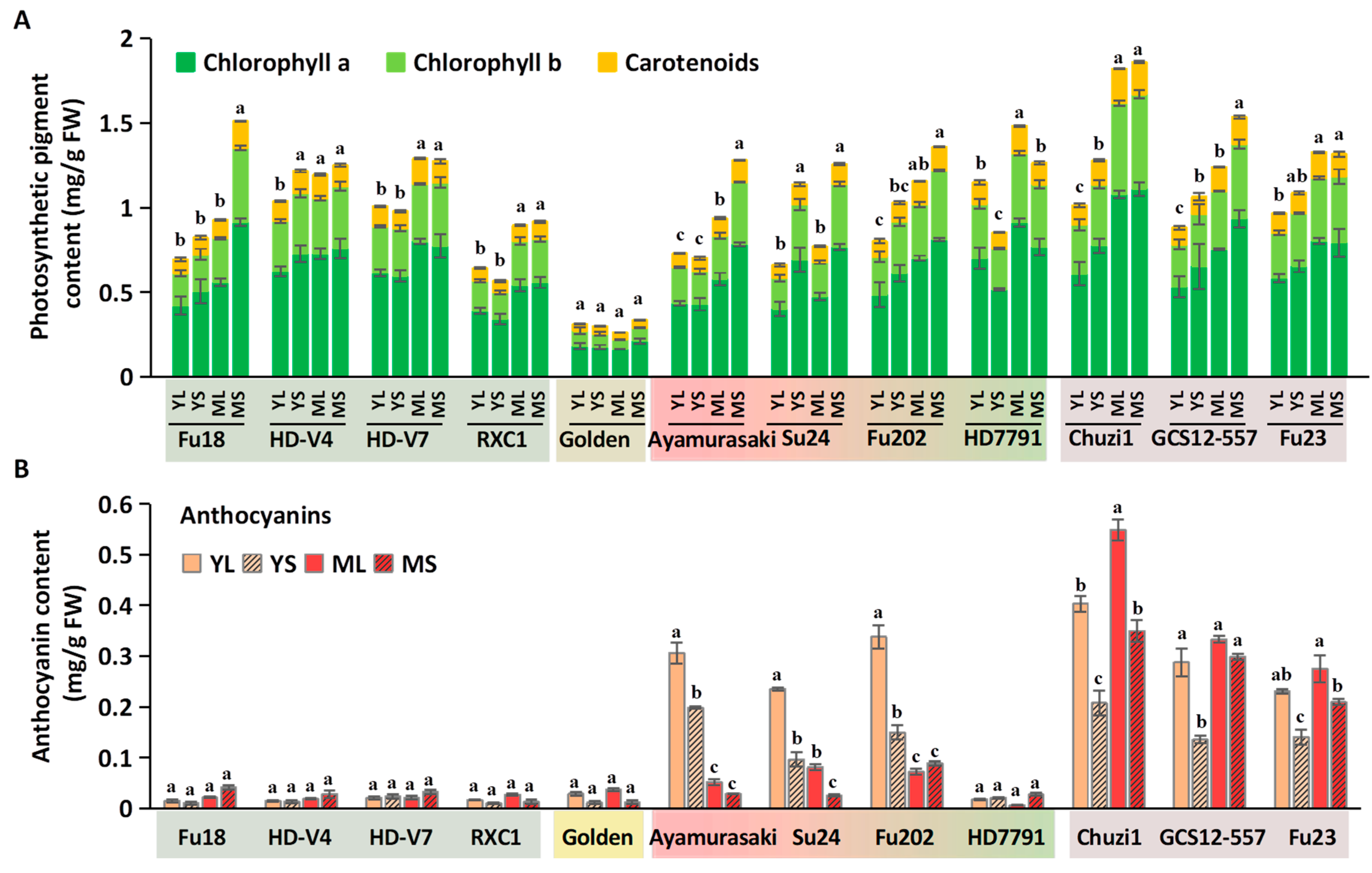
2.2.3. Effects of Shading on Leaf Pigment Contents
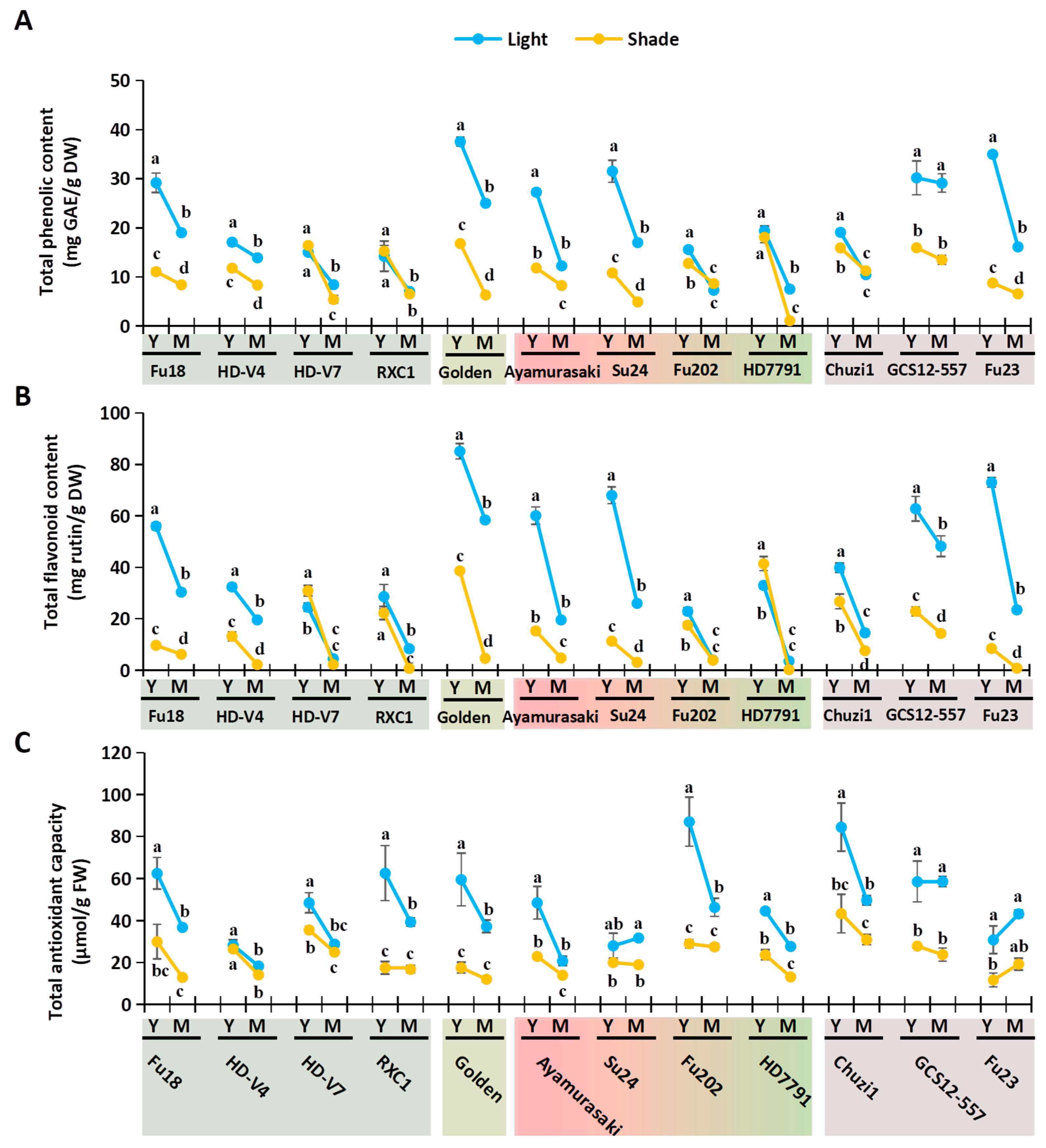
2.3. Changes in Nutritional and Antioxidant-Related Physiological Indices
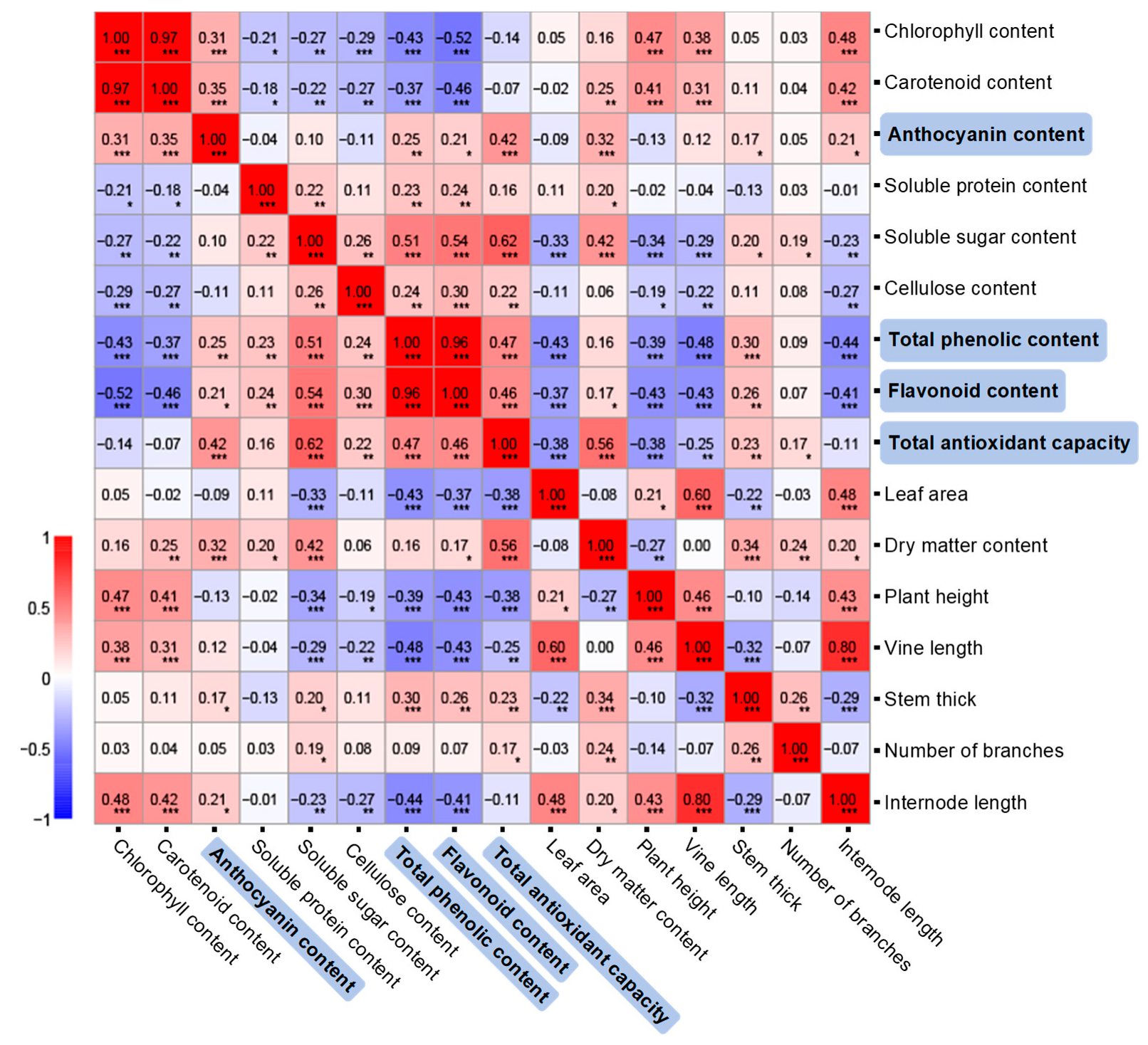
2.4. Correlation Between Agronomic, Nutritional, and Antioxidant Indices
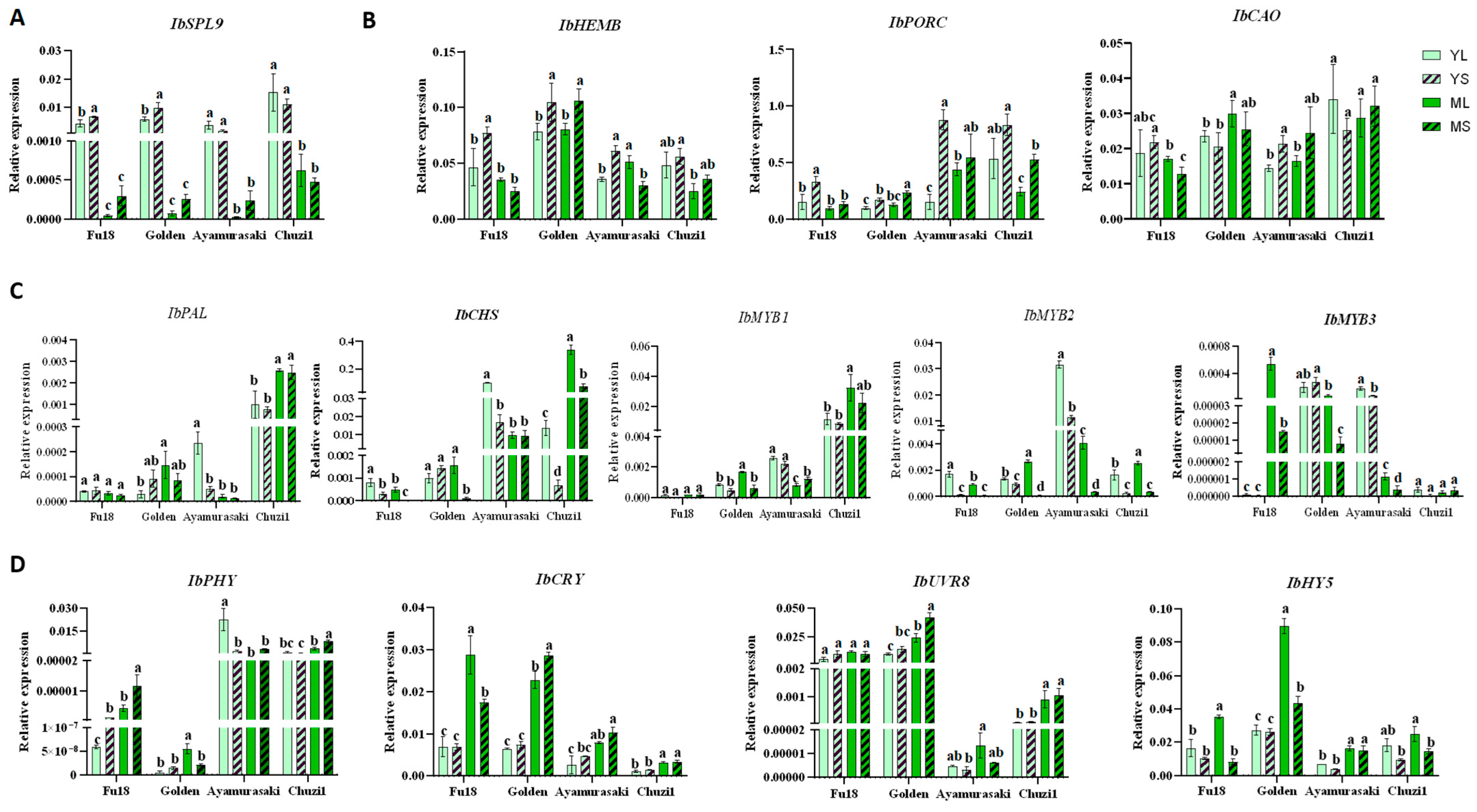
2.5. Expression of Key Leaf Color and Light-Related Genes in Sweetpotato Leaves
3. Discussion
3.1. Effects of Deep Shading on Agronomic Traits and Leaf Morphology
3.2. Integrated Regulation of Leaf Coloration, Light Signaling, and Gene Expression
3.3. Shading-Induced Changes in Nutritional and Antioxidant Properties
4. Materials and Methods
4.1. Plant Materials
4.2. Agronomic Traits
4.3. Colorimeter Measurements
4.4. Measurement of Chlorophylls, Carotenoids, and Anthocyanins
4.5. Determination of Soluble Protein, Soluble Sugar, and Cellulose Contents
4.6. Determination of TPC, TFC, and Antioxidant Capacity
4.7. RNA Extraction and qRT-PCR
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| YL | Young leaves under natural light |
| YS | Young leaves under 70% shading |
| ML | Mature leaves under natural light |
| MS | Mature Leaves under 70% Shading |
| FW | Fresh weight |
| DW | Dry weight |
| TPC | Total phenolic contents |
| TFC | Total flavonoid contents |
| SPL9 | Squamosa promoter binding protein-like 9 |
| HEMB | Heme biosynthesis gene B |
| PORC | Protochlorophyllide oxidoreductase C |
| CAO | Chlorophyll a oxygenase |
| PAL | Phenylalanine ammonia-lyase |
| CHS | Chalcone synthase |
| PHY | Phytochrome |
| CRY | Cryptochrome |
| UVR8 | UV resistance locus 8 |
| HY5 | Elongated hypocotyl 5 |
| TF | Transcription factor |
References
- Alam, M.K. A comprehensive review of sweet potato (Ipomoea batatas [L.] Lam): Revisiting the associated health benefits. Trends Food Sci. Technol. 2021, 115, 512–529. [Google Scholar] [CrossRef]
- Sun, H.N.; Mu, T.H.; Xi, L.S.; Zhang, M.; Chen, J.W. Sweet potato (Ipomoea batatas L.) leaves as nutritional and functional foods. Food Chem. 2014, 156, 380–389. [Google Scholar] [CrossRef]
- Cao, Q.H.; Ji, Z.X.; Li, Q.; Wang, X.; Tang, J.; Zhao, D.L.; Zhou, Z.L.; Zhang, A.; Dai, X.B. Breeding of a new vegetable sweetpotato cultivar ‘Shulv No.1’. China Veg. 2017, 3, 70–72. [Google Scholar] [CrossRef]
- Yuan, R.; Cao, Q.H.; Zhou, Z.L. Analysis on the registration status of vegetable sweetpotato varieties in China (2018-2020). China Seed Ind. 2021, 10, 19–22. [Google Scholar] [CrossRef]
- Islam, M.S.; Yoshimoto, M.; Yahara, S.; Okuno, S.; Ishiguro, K.; Yamakawa, O. Identification and characterization of foliar polyphenolic composition in sweetpotato (Ipomoea batatas L.) genotypes. J. Agric. Food Chem. 2002, 50, 3718–3722. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Chen, C.C.; Lin, K.H.; Chao, P.Y.; Lin, H.H.; Huang, M.Y. Bioactive compounds, antioxidants, and health benefits of sweet potato leaves. Molecules 2021, 26, 1820. [Google Scholar] [CrossRef]
- Shi, J.; Wu, Q.; Deng, J.; Balfour, K.; Chen, Z.; Liu, Y.; Kumar, S.; Chen, Y.; Zhu, Z.; Zhu, G. Metabolic profiling and antioxidant analysis for the juvenile red fading leaves of sweetpotato. Plants 2022, 11, 3014. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Yahara, S.; Okuno, S.; Islam, M.S.; Ishiguro, K.; Yamakawa, O. Antimutagenicity of mono-, di-, and tricaffeoylquinic acid derivatives isolated from sweetpotato (Ipomoea batatas L.) leaf. Biosci. Biotechnol. Biochem. 2002, 66, 2336–2341. [Google Scholar] [CrossRef]
- Wang, W.; Li, J.; Wang, Z.; Gao, H.; Su, L.; Xie, J.; Chen, X.; Liang, H.; Wang, C.; Han, Y. Oral Hepatoprotective ability evaluation of purple sweet potato anthocyanins on acute and chronic chemical liver injuries. Cell Biochem. Biophys. 2014, 69, 539–548. [Google Scholar] [CrossRef]
- Islam, S. Sweetpotatoes [Ipomoea batatas (L.) lam]: The super food of the Next Century? An intensive review on their potential as a sustainable and versatile food source for future generations. Cyta-J. Food 2024, 22, 2397553. [Google Scholar] [CrossRef]
- Wu, W.; Chen, L.; Liang, R.; Huang, S.; Li, X.; Huang, B.; Luo, H.; Zhang, M.; Wang, X.; Zhu, H. The role of light in regulating plant growth, development and sugar metabolism: A review. Front. Plant Sci. 2025, 15, 1507628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, K.; Tang, Q.; Zeng, L.; Wu, Z. Light intensity regulates low-temperature adaptability of tea plant through ROS stress and developmental programs. Int. J. Mol. Sci. 2023, 24, 9852. [Google Scholar] [CrossRef]
- Miao, C.; Yang, S.; Xu, J.; Wang, H.; Zhang, Y.; Cui, J.; Zhang, H.; Jin, H.; Lu, P.; He, L.; et al. Effects of light intensity on growth and quality of lettuce and spinach cultivars in a plant factory. Plants 2023, 12, 3337. [Google Scholar] [CrossRef]
- He, Y.; Zhu, D.; Sun, Y.; Wang, Q.; Zhu, L.; Zeng, H. Metabonomic Profiling Analyses Reveal ANS Upregulation to enhance the flavonoid pathway of purple-fleshed sweet potato storage root in response to deep shading. Agronomy 2021, 11, 737. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, Y.; Si, C.; Kumar, S.; Nie, L.; Khan, M.N. Effects of shading intensities on the yield and contents of anthocyanin and soluble sugar in tubers of purple sweet potato. Crop Sci. 2023, 63, 3013–3024. [Google Scholar] [CrossRef]
- Jing, X.; Chen, P.; Jin, X.; Lei, J.; Wang, L.; Chai, S.; Yang, X. Physiological, photosynthetic, and transcriptomics insights into the influence of shading on leafy sweet potato. Genes 2023, 14, 2112. [Google Scholar] [CrossRef]
- Deng, J.; Wu, D.; Shi, J.; Balfour, K.; Wang, H.; Zhu, G.; Liu, Y.; Wang, J.; Zhu, Z. Multiple MYB activators and repressors collaboratively regulate the juvenile red fading in leaves of sweetpotato. Front. Plant Sci. 2020, 11, 941. [Google Scholar] [CrossRef]
- Gou, J.Y.; Felippes, F.F.; Liu, C.J.; Weigel, D.; Wang, J.W. Negative regulation of anthocyanin biosynthesis in Arabidopsis by a miR156-targeted SPL transcription factor. Plant Cell 2011, 23, 1512–1522. [Google Scholar] [CrossRef]
- Banas, A.K.; Labuz, J.; Sztatelman, O.; Gabrys, H.; Fiedor, L. Expression of enzymes involved in chlorophyll catabolism in Arabidopsis is light controlled. Plant Physiol. 2011, 157, 1497–1504. [Google Scholar] [CrossRef]
- Zhang, D.; Tan, Y.; Dong, F.; Zhang, Y.; Huang, Y.; Zhou, Y.; Zhao, Z.; Yin, Q.; Xie, X.; Gao, X.; et al. The expression of IbMYB1 is essential to maintain the purple color of leaf and storage root in sweet potato [Ipomoea batatas (L.) Lam]. Front. Plant Sci. 2021, 12, 688707. [Google Scholar] [CrossRef]
- Kang, C.-Y.; Lian, H.-L.; Wang, F.-F.; Huang, J.-R.; Yang, H.-Q. Cryptochromes, phytochromes, and COP1 regulate light-controlled stomatal development in Arabidopsis. Plant Cell 2009, 21, 2624–2641. [Google Scholar] [CrossRef] [PubMed]
- Binkert, M.; Kozma-Bognar, L.; Terecskei, K.; De Veylder, L.; Nagy, F.; Ulm, R. UV-B-responsive association of the Arabidopsis bZIP transcription factor ELONGATED HYPOCOTYL5 with target genes, including its own promoter. Plant Cell 2014, 26, 4200–4213. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Z.; Lu, Y.; Zhang, X.; Chen, Y.; Li, X.; Liu, Y.; Liu, Y.; Kumar, S.; Zhu, Z.; et al. Beyond IbMYB1: Identification and characterization of two additional anthocyanin MYB activators, IbMYB2 and IbMYB3, in sweetpotato. Plants 2025, 14, 2896. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, X.; Li, C.; Chu, Q.; Cheng, S.; Su, L.; Shao, D.; Guo, X.; He, Z.; Zhou, X. Effect of light intensity on celery growth and flavonoid synthesis. Front. Plant Sci. 2024, 14, 1326218. [Google Scholar] [CrossRef]
- Schenkels, L.; Saeys, W.; Lauwers, A.; De Proft, M.P. Green light induces shade avoidance to alter plant morphology and increases biomass production in Ocimum basilicum L. Scientia Hortic. 2020, 261, 6. [Google Scholar] [CrossRef]
- Qiu, X.; Sun, G.; Liu, F.; Hu, W. Functions of plant phytochrome signaling pathways in adaptation to diverse stresses. Int. J. Mol. Sci. 2023, 24, 13201. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, B.; Gardestrom, P.; Keech, O. In response to partial plant shading, the lack of phytochrome A does not directly induce leaf senescence but alters the fine-tuning of chlorophyll biosynthesis. J. Exp. Bot. 2014, 65, 4037–4049. [Google Scholar] [CrossRef]
- Ramamoorthy, P.; Bheemanahalli, R.; Meyers, S.L.; Shankle, M.W.; Reddy, K.R. Drought, low nitrogen stress, and ultraviolet-b radiation effects on growth, development, and physiology of sweetpotato cultivars during early season. Genes 2022, 13, 156. [Google Scholar] [CrossRef]
- Krause, G.; Virgo, A.; Winter, K. High susceptibility to photoinhibition of young leaves of tropical forest trees. Planta 1995, 197, 583–591. [Google Scholar] [CrossRef]
- Thi Luyen, C.; Shawon, R.A.; Cho, N.; Lee, T.H.; Ko, B.; Kim, H.C.; Bae, J.H.; Ku, Y.G. Effects of shade treatment on plant growth characteristics, phenolic contents, and antioxidant activities of all-male cultivars of asparagus (Asparagus officinalis L.). Hortic. Sci. Technol. 2022, 40, 273–285. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Lu, Y.; Wu, Q.; Liu, Y.; Liu, Y.; Kumar, S.; Zhu, G.; Zhu, Z. Pre-treatment, extraction solvent, and color stability of anthocyanins from purple sweetpotato. Foods 2024, 13, 833. [Google Scholar] [CrossRef]
- Lopes, F.L.; Formosa-Jordan, P.; Malivert, A.; Margalha, L.; Confraria, A.; Feil, R.; Lunn, J.E.; Jonsson, H.; Landrein, B.; Baena-Gonzalez, E. Sugar signaling modulates SHOOT MERISTEMLESS expression and meristem function in Arabidopsis. Proc. Natl. Acad. Sci. USA 2024, 121, e2408699121. [Google Scholar] [CrossRef] [PubMed]
- Abidi, N.; Hequet, E.; Cabrales, L. Changes in sugar composition and cellulose content during the secondary cell wall biogenesis in cotton fibers. Cellulose 2010, 17, 153–160. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Zhou, Y.; Liu, T.; Wang, M.; Liu, Y.; Liu, Y.; Li, Y.; Zhu, G. Genome reannotation of the sweetpotato (Ipomoea batatas (L.) Lam.) using extensive Nanopore and Illumina-based RNA-seq datasets. Trop. Plants 2024, 3, e008. [Google Scholar] [CrossRef]

| Variety | Usage | Treatment | Leaf Area (cm2) | Dry Matter (%) | Plant Height (cm) | Vine Length (cm) | Stem Thickness (mm) | Branch Number | Internode Length (cm) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Value | Change | Value | Change | Value | Change | Value | Change | Value | Change | Value | Change | Value | Change | |||
| Fu18 | V | L | 35.33 ± 0.66 | 156% ** | 11.50 ± 0.12 | 83.6% ** | 22.03 ± 0.79 | 116% ** | 27.38 ± 1.42 | 143% ** | 6.97 ± 0.44 | 82% | 5.33 ± 0.33 | 50% ** | 1.59 ± 0.11 | 130% ** |
| S | 55.07 ± 2.70 | 9.61 ± 0.39 | 25.48 ± 0.58 | 39.13 ± 3.46 | 5.73 ± 0.41 | 2.67 ± 0.33 | 2.07 ± 0.10 | |||||||||
| HD-V4 | V | L | 31.39 ± 3.62 | 119% | 11.07 ± 0.10 | 81.1% ** | 24.61 ± 0.54 | 113% ** | 26.04 ± 1.14 | 142% ** | 6.80 ± 0.26 | 69% ** | 4.33 ± 0.67 | 77% | 2.04 ± 0.13 | 142% ** |
| S | 37.32 ± 2.06 | 8.98 ± 0.23 | 27.74 ± 0.53 | 36.93 ± 2.22 | 4.70 ± 0.28 | 3.33 ± 0.33 | 2.89 ± 0.20 | |||||||||
| HD-V7 | V&R | L | 34.09 ± 1.60 | 123% ** | 11.60 ± 0.06 | 82.2% ** | 24.16 ± 0.62 | 102% | 39.41 ± 2.34 | 129% ** | 4.03 ± 0.20 | 91% | 3.33 ± 0.33 | 100% | 2.78 ± 0.22 | 174% ** |
| S | 41.84 ± 0.44 | 9.53 ± 0.19 | 24.53 ± 0.42 | 50.99 ± 1.52 | 3.68 ± 0.26 | 3.33 ± 0.33 | 4.83 ± 0.16 | |||||||||
| RXC1 | V | L | 53.27 ± 1.46 | 120% * | 10.77 ± 0.24 | 86.3% * | 21.19 ± 0.20 | 131% ** | 33.48 ± 2.10 | 130% ** | 5.58 ± 0.25 | 79% * | 3.67 ± 0.67 | 73% | 2.33 ± 0.10 | 119% |
| S | 64.11 ± 2.06 | 9.29 ± 0.26 | 27.70 ± 0.80 | 43.62 ± 1.95 | 4.43 ± 0.34 | 2.67 ± 0.33 | 2.77 ± 0.30 | |||||||||
| Golden | O | L | 47.74 ± 0.74 | 159% ** | 11.78 ± 0.19 | 80.0% ** | 13.72 ± 0.22 | 125% ** | 25.89 ± 1.14 | 133% * | 4.83 ± 0.17 | 99% | 3.67 ± 0.67 | 91% | 1.61 ± 0.06 | 128% ** |
| S | 75.66 ± 2.77 | 9.42 ± 0.10 | 17.18 ± 0.86 | 34.40 ± 2.54 | 4.78 ± 0.20 | 3.33 ± 0.67 | 2.06 ± 0.11 | |||||||||
| Ayamurasaki | R | L | 44.20 ± 1.61 | 149% ** | 10.08 ± 0.28 | 84.7% ** | 16.87 ± 0.70 | 134% ** | 37.69 ± 3.32 | 140% ** | 5.67 ± 0.27 | 86% * | 4.33 ± 0.67 | 100% | 1.81 ± 0.09 | 155% ** |
| S | 65.93 ± 3.02 | 8.54 ± 0.01 | 22.62 ± 1.60 | 52.63 ± 2.39 | 4.85 ± 0.17 | 4.33 ± 0.33 | 2.81 ± 0.11 | |||||||||
| Su24 | R | L | 40.73 ± 1.38 | 168% ** | 10.60 ± 0.15 | 100.2% | 22.68 ± 0.78 | 116% * | 28.25 ± 2.03 | 188% ** | 5.65 ± 0.11 | 87% | 2.00 ± 0.00 | 184% * | 2.28 ± 0.06 | 169% ** |
| S | 68.55 ± 1.63 | 10.62 ± 0.18 | 26.35 ± 0.96 | 53.11 ± 1.02 | 4.93 ± 0.31 | 3.67 ± 0.67 | 3.86 ± 0.18 | |||||||||
| Fu202 | R | L | 44.50 ± 2.86 | 134% * | 14.15 ± 0.08 | 73.9% ** | 18.12 ± 0.75 | 113% | 33.99 ± 1.50 | 130% * | 5.07 ± 0.29 | 82% * | 4.00 ± 0.58 | 75% | 2.84 ± 0.07 | 134% ** |
| S | 59.62 ± 1.84 | 10.46 ± 0.08 | 20.55 ± 0.80 | 44.31 ± 4.91 | 4.17 ± 0.25 | 3.00 ± 0.58 | 3.81 ± 0.09 | |||||||||
| HD7791 | V&O | L | 68.57 ± 1.25 | 118% * | 14.09 ± 0.34 | 74.1% ** | 22.01 ± 0.56 | 131% ** | 38.69 ± 1.81 | 162% ** | 5.95 ± 0.18 | 82% ** | 5.00 ± 0.58 | 73% | 3.04 ± 0.15 | 135% ** |
| S | 80.72 ± 2.96 | 10.44 ± 0.03 | 28.73 ± 0.94 | 62.57 ± 6.18 | 4.88 ± 0.22 | 3.67 ± 0.67 | 4.09 ± 0.23 | |||||||||
| Chuzi1 | R | L | 50.55 ± 0.78 | 143.5% ** | 14.35 ± 0.13 | 83.7% ** | 21.32 ± 0.40 | 129.1% ** | 54.12 ± 3.30 | 111% | 6.00 ± 0.22 | 91% | 4.00 ± 0.00 | 83% | 4.59 ± 0.28 | 122% * |
| S | 72.53 ± 1.32 | 12.01 ± 0.10 | 27.53 ± 1.04 | 60.12 ± 4.63 | 5.48 ± 0.30 | 3.33 ± 0.33 | 5.60 ± 0.27 | |||||||||
| GCS12-557 | V | L | 29.14 ± 0.37 | 178% ** | 10.26 ± 0.24 | 82% ** | 20.15 ± 0.84 | 124% ** | 21.17 ± 1.05 | 152% ** | 5.78 ± 0.24 | 86% | 4.33 ± 0.88 | 85% | 1.33 ± 0.05 | 147% ** |
| S | 52.00 ± 1.70 | 8.45 ± 0.10 | 25.06 ± 0.94 | 32.19 ± 2.47 | 4.97 ± 0.31 | 3.67 ± 0.67 | 1.96 ± 0.09 | |||||||||
| Fu23 | V | L | 44.21 ± 1.75 | 168% ** | 12.16 ± 0.21 | 86% ** | 23.03 ± 0.49 | 103% | 36.75 ± 4.00 | 124% | 5.52 ± 0.15 | 87% ** | 3.33 ± 0.33 | 140% | 2.33 ± 0.24 | 189% ** |
| S | 74.30 ± 2.24 | 10.50 ± 0.25 | 23.82 ± 0.20 | 45.42 ± 1.10 | 4.82 ± 0.15 | 4.67 ± 0.67 | 4.41 ± 0.17 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Wang, J.; Chen, Y.; Li, J.; Li, Z.; Kumar, S.; Zhu, Z.; Liu, Y.-H.; Zhu, G. Effects of Deep Shading on Agronomic Traits, Coloration, and Antioxidant Properties in Sweetpotato Leaves. Plants 2025, 14, 2969. https://doi.org/10.3390/plants14192969
Lu Y, Wang J, Chen Y, Li J, Li Z, Kumar S, Zhu Z, Liu Y-H, Zhu G. Effects of Deep Shading on Agronomic Traits, Coloration, and Antioxidant Properties in Sweetpotato Leaves. Plants. 2025; 14(19):2969. https://doi.org/10.3390/plants14192969
Chicago/Turabian StyleLu, Yang, Jian Wang, Yizhao Chen, Jingjing Li, Zengrui Li, Sunjeet Kumar, Zhixin Zhu, Yong-Hua Liu, and Guopeng Zhu. 2025. "Effects of Deep Shading on Agronomic Traits, Coloration, and Antioxidant Properties in Sweetpotato Leaves" Plants 14, no. 19: 2969. https://doi.org/10.3390/plants14192969
APA StyleLu, Y., Wang, J., Chen, Y., Li, J., Li, Z., Kumar, S., Zhu, Z., Liu, Y.-H., & Zhu, G. (2025). Effects of Deep Shading on Agronomic Traits, Coloration, and Antioxidant Properties in Sweetpotato Leaves. Plants, 14(19), 2969. https://doi.org/10.3390/plants14192969








