Stem Xylem Differences in Congeneric Lianas Between Forests Are Unrelated to Hydraulic Safety but Partly Explain Efficiency
Abstract
1. Introduction
2. Results
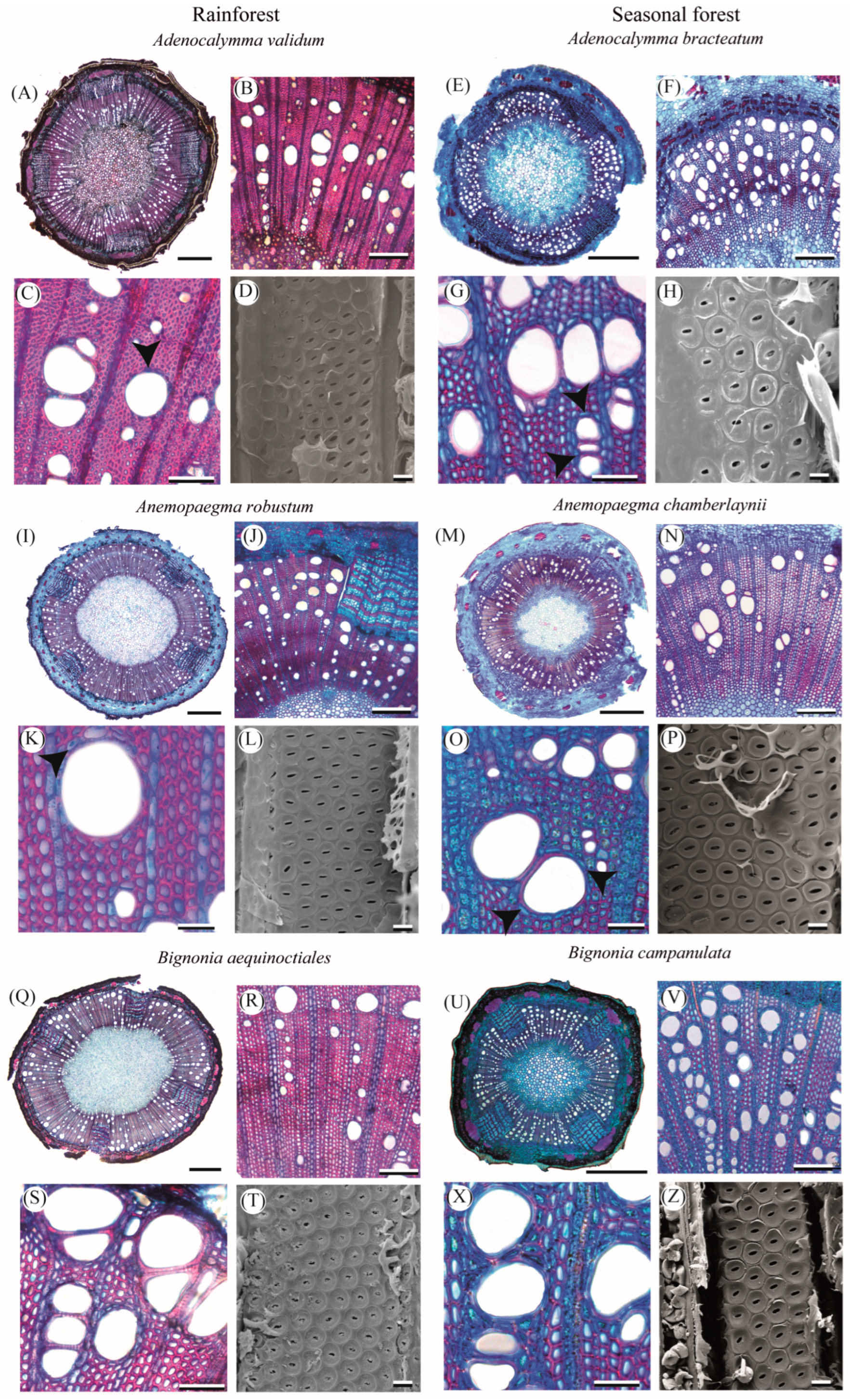
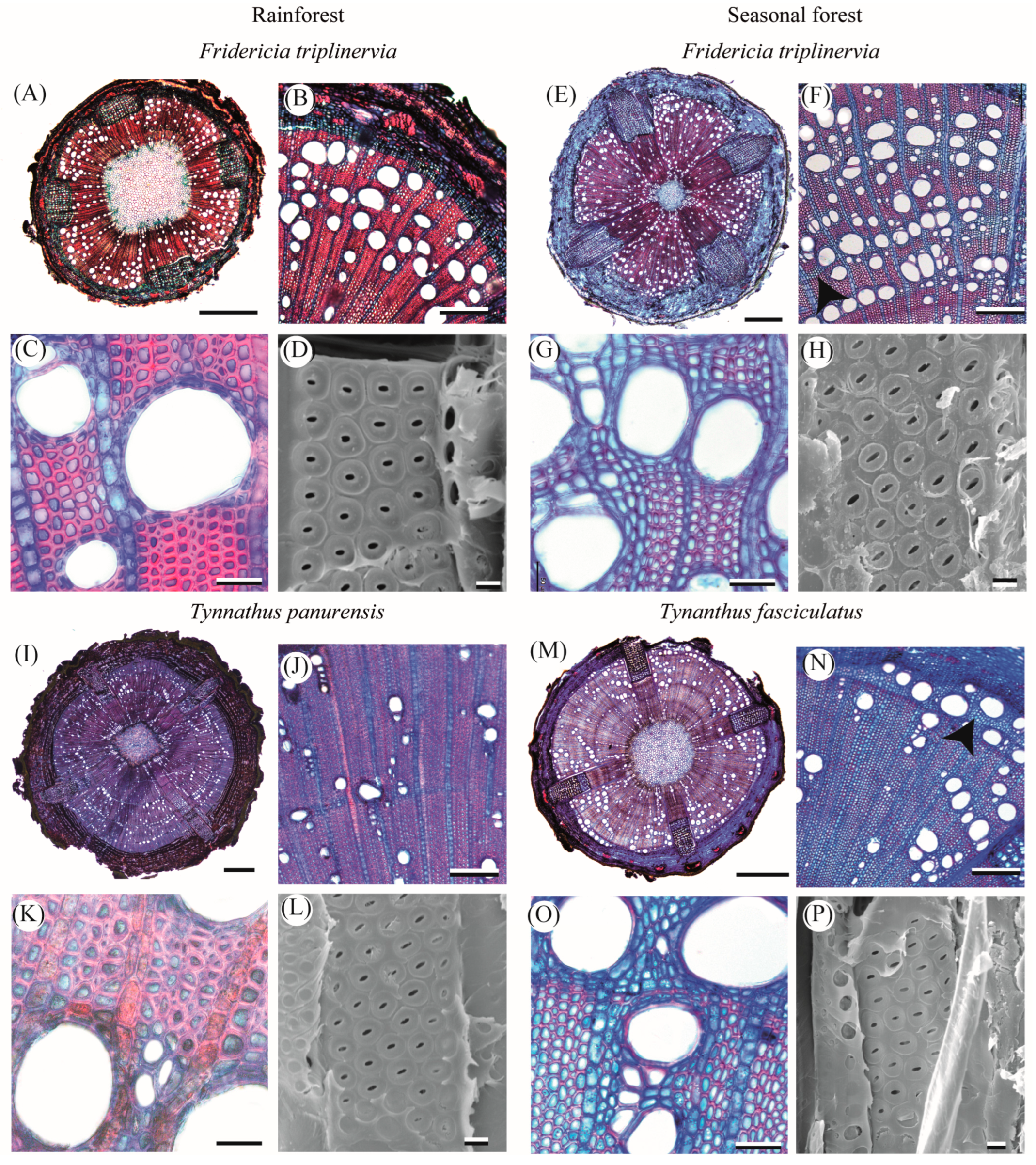
2.1. Qualitative Characterization of Lianescent Xylem
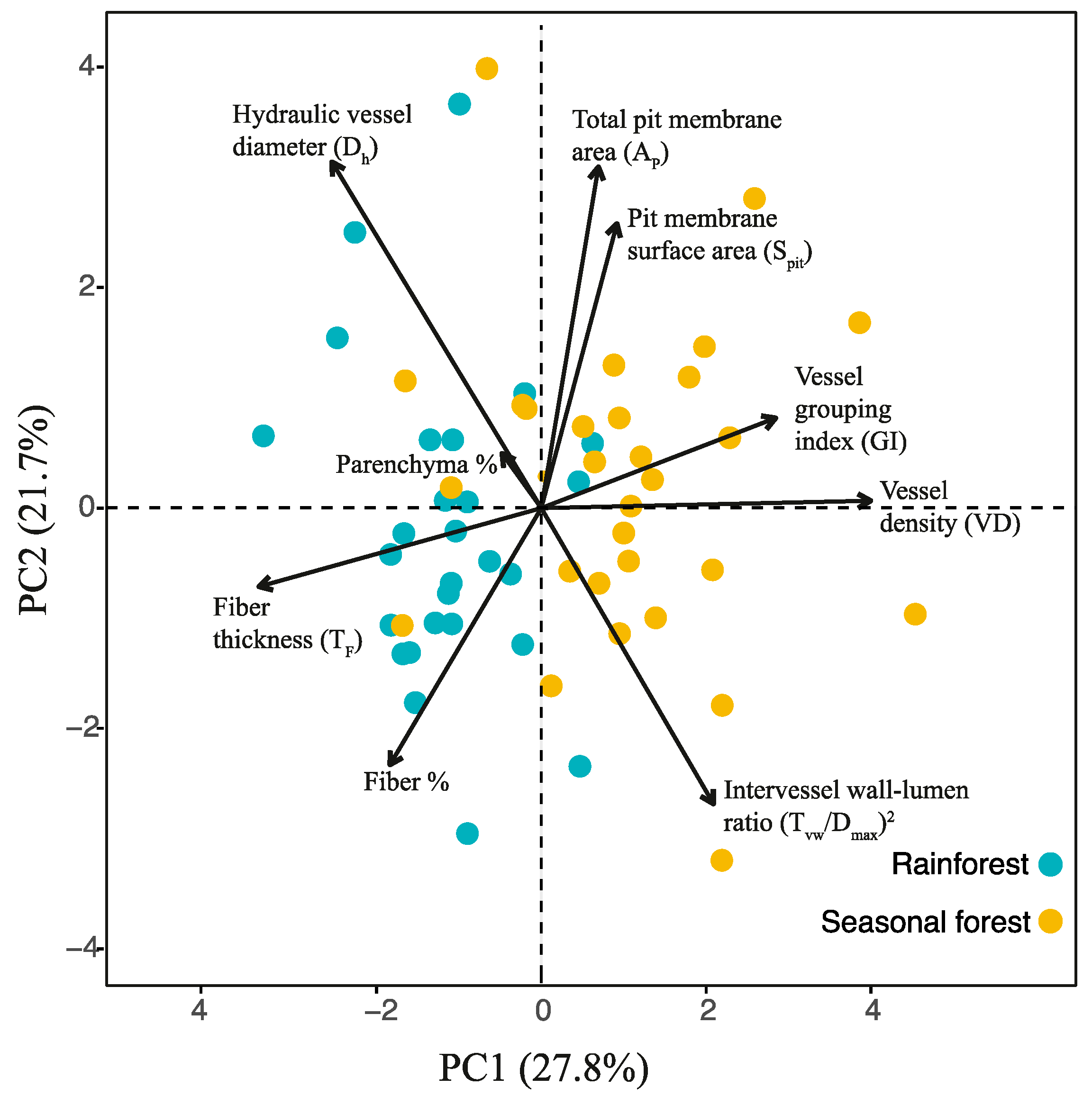
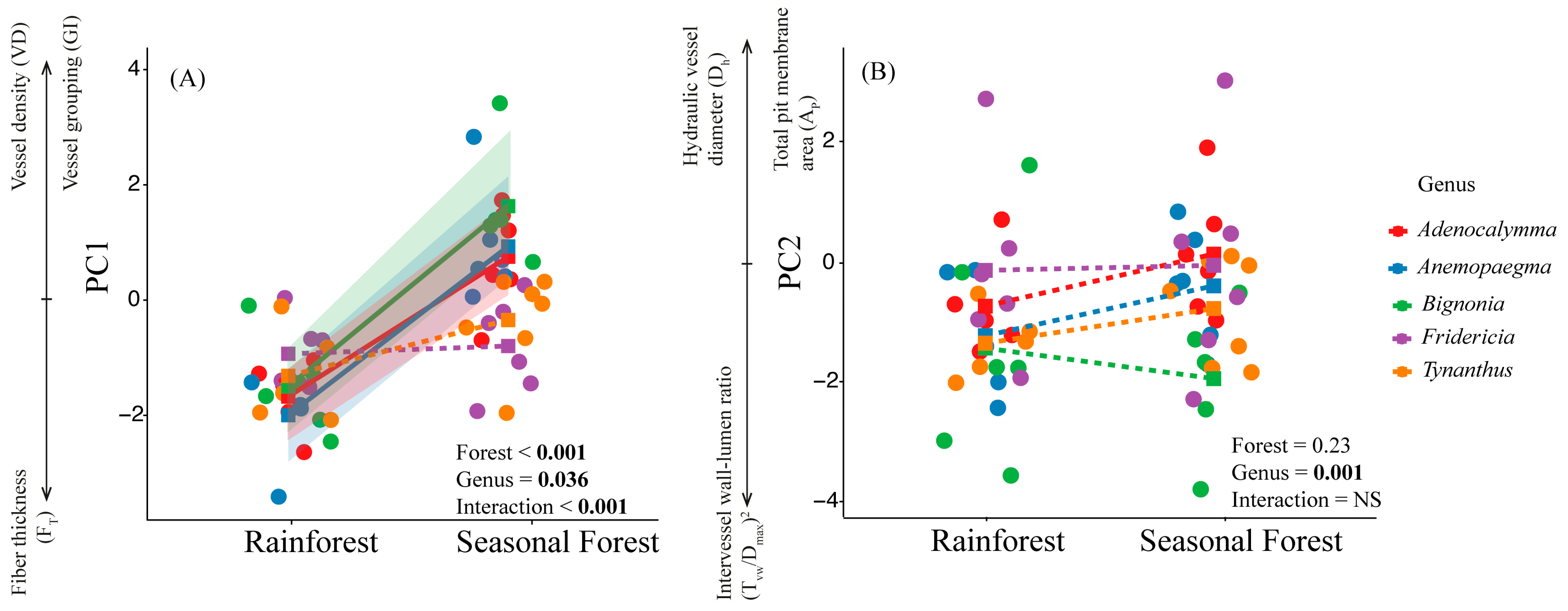
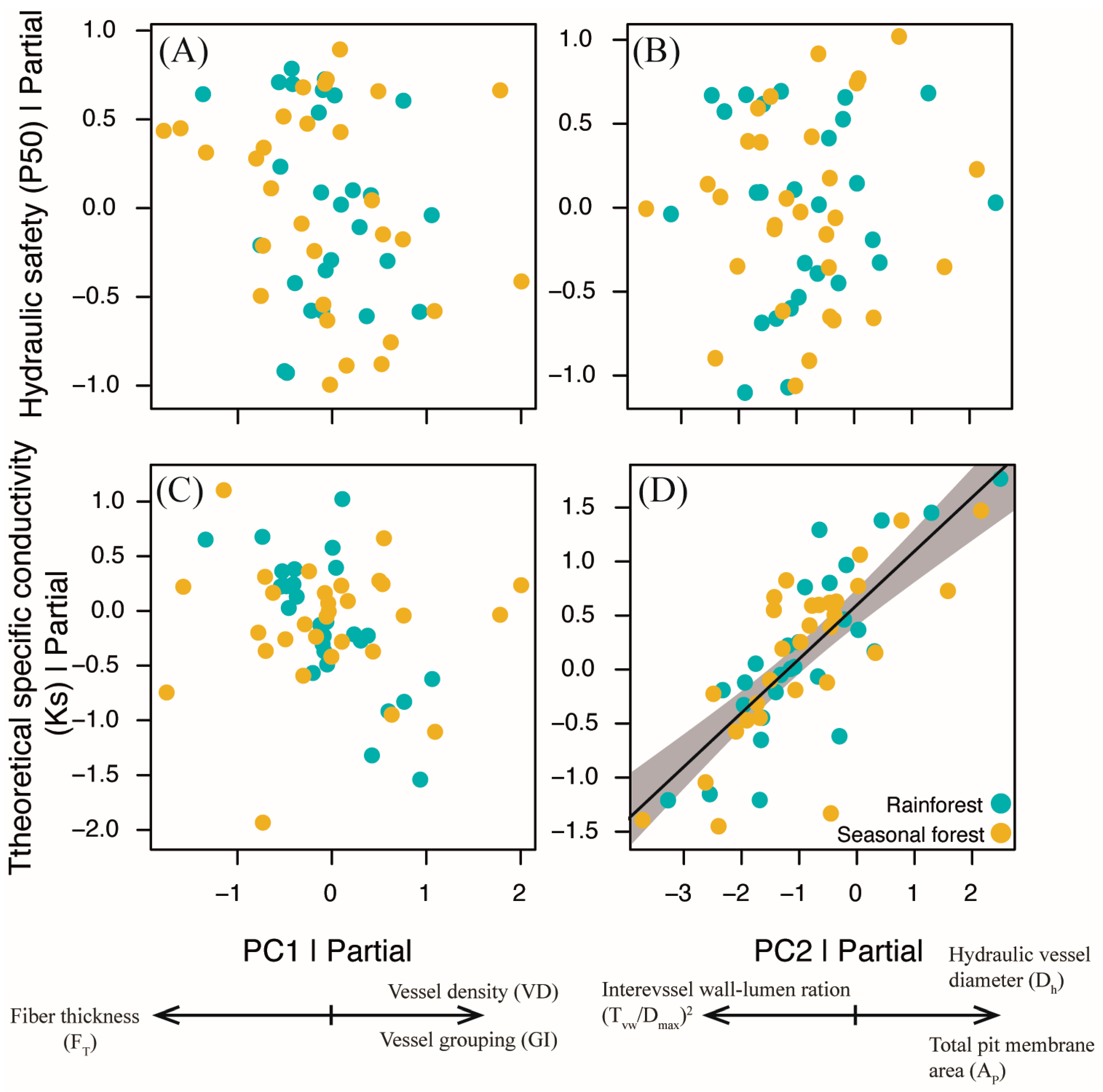
2.2. Coordination of Quantitative Xylem Features of the Lianas Studied
| Rainforest | Seasonal Dry Forest | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adenocalymma validum | Anemopaegma robustum | Bignonia aequinoctiales | Fridericia triplinervia | Tynanthus panurensis | Adenocalymma bracteatum | Anemopaegma chamberlynii | Bignonia campanulata | Fridericia triplinervia | Tynanthus fasciculatus | |
| Apex distance (m) | 1.02 ± 0.56 | 1.27 ± 0.57 | 0.93 ± 0.17 | 1.30 ± 0.66 | 0.96 ± 0.12 | 1.35 ± 0.67 | 1.42 ± 0.38 | 1.46 ± 0.58 | 1.23 ± 0.42 | 1.18 ± 0.48 |
| Stem diameter (mm) | 6.33 ± 0.45 | 6.83 ± 0.79 | 6.92 ± 0.63 | 3.83 ± 0.65 | 7.81 ± 1.95 | 3.23 ± 0.58 | 4.18 ± 1.21 | 3.21 ± 0.43 | 4.94 ± 1.42 | 3.73 ± 0.60 |
| Vessel area percentage | 0.32 ± 0.05 | 0.22 ± 0.05 | 0.23 ± 0.09 | 0.34 ± 0.11 | 0.19 ± 0.07 | 0.33 ± 0.07 | 0.31 ± 0.06 | 0.37 ± 0.10 | 0.29 ± 0.14 | 0.28 ± 0.05 |
| Fiber area percentage | 0.43 ± 0.05 | 0.51 ± 0.05 | 0.49 ± 0.10 | 0.43 ± 0.16 | 0.52 ± 0.06 | 0.37 ± 0.07 | 0.44 ± 0.08 | 0.38 ± 0.10 | 0.44 ± 0.12 | 0.43 ± 0.08 |
| Parenchyma area percentage | 0.25 ± 0.03 | 0.27 ± 0.05 | 0.26 ± 0.03 | 0.23 ± 0.05 | 0.30 ± 0.02 | 0.27 ± 0.02 | 0.22 ± 0.05 | 0.24 ± 0.02 | 0.27 ± 0.05 | 0.27 ± 0.06 |
| Vessel diameter (μm) | 31.4 ± 5.31 | 36.1 ± 7.64 | 33.2 ± 12.1 | 36.9 ± 8.01 | 30.2 ± 5.44 | 30.3 ± 5.46 | 29.8 ± 4.90 | 32.6 ± 7.15 | 40.0 ± 9.14 | 33.8 ± 4.65 |
| Maximum vessel diameter (μm) | 117 ± 29.3 | 109 ± 22.5 | 101 ± 36.5 | 107 ± 43.6 | 90.5 ± 9.10 | 79.7 ± 5.15 | 87.4 ± 12.5 | 69.3 ± 13.9 | 109 ± 33.3 | 91.3 ± 15.3 |
| Minimum vessel diameter (μm) | 9.92 ± 1.93 | 10.2 ± 1.71 | 11.2 ± 2.89 | 9.01 ± 1.18 | 10.6 ± 0.90 | 9.70 ± 1.32 | 10.9 ± 1.38 | 9.48 ± 1.59 | 12.3 ± 2.35 | 10.3 ± 1.63 |
| Vessel density (n mm−2) | 88.3 ± 17.3 | 85.0 ± 22.3 | 69.4 ± 26.0 | 123 ± 23.3 | 89.5 ± 17.0 | 160 ± 63.0 | 164 ± 53.3 | 197 ± 60.3 | 101 ± 20.7 | 100 ± 23.3 |
| Hydraulic vessel diameter (µm) | 61.8 ± 13.0 | 57.4 ± 10.7 | 55.4 ± 19.6 | 56.4 ± 15.5 | 48.6 ± 83.4 | 44.9 ± 24.6 | 50.5 ± 68.9 | 42.3 ± 10.2 | 59.4 ± 13.1 | 51.0 ± 66.8 |
| Vessel grouping index | 1.90 ± 0.51 | 1.79 ± 0.37 | 1.88 ± 0.34 | 1.89 ± 0.40 | 2.11 ± 0.78 | 2.14 ± 0.58 | 3.29 ± 0.73 | 2.13 ± 0.52 | 1.87 ± 0.22 | 2.16 ± 0.71 |
| Intervessel wall–lumen ratio | 0.003 ± 0.002 | 0.003 ± 0.001 | 0.007 ± 0.005 | 0.003 ± 0.001 | 0.006 ± 0.002 | 0.006 ± 0.001 | 0.004 ± 0.001 | 0.012 ± 0.003 | 0.006 ± 0.003 | 0.004 ± 0.002 |
| Vessel wall thickness (μm) | 6.59 ± 0.93 | 6.38 ± 1.26 | 7.14 ± 1.86 | 6.14 ± 1.95 | 6.97 ± 1.67 | 6.32 ± 1.03 | 6.06 ± 1.07 | 7.48 ± 0.99 | 8.01 ± 0.83 | 5.66 ± 0.56 |
| Fiber wall thickness (μm) | 4.83 ± 0.28 | 5.21 ± 0.79 | 4.47 ± 0.42 | 4.36 ± 0.49 | 4.78 ± 0.89 | 3.02 ± 0.30 | 2.89 ± 0.36 | 2.93 ± 0.18 | 3.72 ± 0.51 | 2.63 ± 0.43 |
| Ray width (μm) | 11.5 ± 1.68 | 16.6 ± 2.79 | 15.1 ± 3.34 | 9.54 ± 1.70 | 13.7 ± 4.36 | 10.4 ± 1.71 | 12.8 ± 1.36 | 11.6 ± 1.81 | 13.1 ± 3.11 | 8.67 ± 0.80 |
| Ray height (μm) | 423.3 ± 183.8 | 719.9 ± 274.2 | 817.9 ± 194.7 | 429.1 ± 142.2 | 518.3 ± 192.8 | 423.3 ± 183.8 | 776.1 ± 228.0 | 513.7 ± 220.2 | 484.8 ± 111.5 | 377.4 ± 126.8 |
| Pit membrane surface area (μm2) | 24.0 ± 4.42 | 18.8 ± 3.93 | 24.7 ± 8.94 | 26.6 ± 8.75 | 16.6 ± 1.87 | 35.2 ± 9.87 | 28.6 ± 3.16 | 22.8 ± 4.85 | 30.6 ± 7.17 | 19.7 ± 2.54 |
| Pit aperture (μm2) | 1.34 ± 0.18 | 1.28 ± 0.29 | 1.32 ± 0.41 | 1.16 ± 0.54 | 0.83 ± 0.10 | 1.82 ± 0.42 | 1.70 ± 0.21 | 1.32 ± 0.57 | 1.40 ± 0.41 | 0.96 ± 0.25 |
| Intervessel contact fraction | 0.08 ± 0.01 | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.09 ± 0.01 | 0.12 ± 0.02 | 0.12 ± 0.04 | 0.08 ± 0.01 | 0.10 ± 0.01 | 0.11 ± 0.02 | 0.10 ± 0.02 |
| Pitfield fraction | 0.70 ± 0.03 | 0.70 ± 0.05 | 0.71 ± 0.07 | 0.68 ± 0.03 | 0.64 ± 0.02 | 0.69 ± 0.04 | 0.70 ± 0.03 | 0.67 ± 0.04 | 0.64 ± 0.05 | 0.58 ± 0.05 |
| Pit fraction | 0.06 ± 0.01 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.06 ± 0.00 | 0.07 ± 0.01 | 0.08 ± 0.03 | 0.06 ± 0.00 | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.06 ± 0.01 |
| Pit density (n° 200 μm2) | 3.33 ± 0.51 | 4.5 ± 1.04 | 4 ± 1.55 | 2.71 ± 0.48 | 5.16 ± 0.98 | 1.41 ± 0.49 | 2 ± 0 | 3.08 ± 1.02 | 1.75 ± 1.17 | 2.68 ± 0.70 |
| Total pit membrane area (10−6 m2) | 1.18 ± 0.45 | 1.23 ± 0.61 | 0.90 ± 0.72 | 1.87 ± 0.53 | 1.64 ± 0.60 | 2.13 ± 1.29 | 1.36 ± 0.35 | 1.41 ± 0.72 | 2.00 ± 0.92 | 1.46 ± 0.49 |
| Abbreviation | Feature and Description | Unit |
|---|---|---|
| Vessel tissue fraction | Percentage of vessel lumen area | % |
| Fiber tissue fraction | Percentage of fiber area | % |
| Parenchyma tissue fraction | Percentage of parenchyma area (including ray and axial parenchyma) | % |
| Dv | Vessel diameter (maximum and minimum) | µm |
| Dh | Hydraulic diameter of vessel [=(] | µm |
| VD | Vessel density | N mm−2 |
| GI | Vessel grouping index: total vessels divided by the total of vessel groups with true intervessel walls; a solitary vessel counts as one vessel group | - |
| (Tvw/Dmax)2 | Intervessel wall–lumen ratio: double intervessel wall thickness (Tvw) divided by the maximum diameter of the vessel (Dmax) squared | - |
| LV | Mean vessel length | m |
| Spit | Intervessel pit membrane surface area | µm2 |
| Dpit | The number of intervessel pits per 200 µm2 | N 200 µm2 |
| FC | Intervessel contact fraction | % |
| FPF | Pitfield fraction, i.e., the ratio of the pit membrane area to the intervessel wall area | % |
| Fpit | Intervessel pit fraction of an intervessel wall (=FC·FPF) | % |
| AP | Total intervessel pit membrane area for a vessel with average diameter and length (=π·DV·LV·Fpit) | m2 |
| TF | Fiber wall thickness | µm |
| P50 | Water potential at which 50% of the maximum amount of gas is discharged | MPa |
| Ks | Theoretical specific hydraulic conductivity | Kg m−1 MPa−1 s−1 |
2.3. Testing of a Relationship Between Hydraulic and Xylem Anatomy
3. Discussion
3.1. Lianas from Tropical Forests with Different Water Regimes Have Divergent Xylem Features
3.2. Linking Anatomical Features with Hydraulic Safety and Efficiency
4. Conclusions
5. Materials and Methods
5.1. Study Site and Climate
5.2. Species Collection
5.3. Anatomical Measurements and Analyses
5.4. Hydraulic Safety
5.5. Hydraulic Efficiency
5.6. Data Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A
References
- Cosme, L.H.M.; Schietti, J.; Costa, F.R.C.; Oliveira, R.S. The importance of hydraulic architecture to the distribution patterns of trees in a central Amazonian forest. New Phytol. 2017, 215, 113–125. [Google Scholar] [CrossRef]
- Oliveira, R.S.; Costa, F.R.C.; van Baalen, E.; de Jonge, A.; Bittencourt, P.R.; Almanza, Y.; Barros, F.V.; Cordoba, E.C.; Fagundes, M.V.; Garcia, S.; et al. Embolism resistance drives the distribution of Amazonian rainforest tree species along hydro-topographic gradients. New Phytol. 2019, 221, 1457–1465. [Google Scholar] [CrossRef]
- Apgaua, D.M.G.; Tng, D.Y.P.; Laurance, S.G.W. Tropical wet and dry forest tree species exhibit contrasting hydraulic architecture. Flora 2022, 291, 152072. [Google Scholar] [CrossRef]
- Baas, P.; Ewers, F.W.; Davis, S.D.; Wheeler, E.A. Evolution of xylem physiology. In Plant Diversity and Evolution: Genotypic and Phenotypic Variation in Higher Plants; Bakker, F.T., Chatrou, L.W., Gravendeel, B., Pelser, P.B., Eds.; CABI Publishing: Wallingford, UK, 2004; pp. 273–295. [Google Scholar]
- Putz, F.E.; Mooney, H.A. The Biology of Vines; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Gerolamo, C.S.; Angyalossy, V. Wood anatomy and conductivity in lianas, shrubs and trees of Bignoniaceae. IAWA J. 2017, 38, 412–432. [Google Scholar] [CrossRef]
- Tang, Y.; Yin, S.; Pace, M.R.; Gerolamo, C.S.; Nogueira, A.; Zuntini, A.R.; Lohman, L.G.; Plath, M.; Liesche, J. Diameters of phloem sieve elements can predict stem growth rates of woody plants. Tree Physiol. 2022, 42, 1560–1569. [Google Scholar] [CrossRef]
- Smith-Martin, C.M.; Jansen, S.; Brodribb, T.J.; Medina-Vega, J.A.; Lucani, C.; Huppenberger, A.; Powers, J.S. Lianas and trees from a seasonally dry and a wet tropical forest did not differ in embolism resistance but did differ in xylem anatomical traits in the dry forest. Front. For. Glob. Change 2022, 5, 34. [Google Scholar] [CrossRef]
- Pratt, R.B.; Jacobsen, A.L. Conflicting demands on angiosperm xylem: Tradeoffs among storage, transport and biomechanics. Plant Cell Environ. 2017, 40, 897–913. [Google Scholar] [CrossRef]
- Gerolamo, C.S.; Pereira, L.; Costa, F.R.C.; Jansen, S.; Angyalossy, V.; Nogueira, A. Lianas in tropical dry seasonal forests have a high hydraulic efficiency but not always a higher embolism resistance than lianas in rainforests. Ann. Bot. 2024, 134, 337–350. [Google Scholar] [CrossRef]
- Gerolamo, C.S.; Nogueira, A.; Pace, M.R.; Angyalossy, V. Interspecific anatomical differences result in similar highly flexible stems in Bignoniaceae lianas. Am. J. Bot. 2020, 107, 1622–1634. [Google Scholar] [CrossRef]
- Medina-Vega, J.A.; Bongers, F.; Poorter, L.; Schnitzer, S.A.; Sterck, F.J. Lianas have more acquisitive traits than trees in a dry but not in a wet forest. J. Ecol. 2021, 109, 2367–2384. [Google Scholar] [CrossRef]
- Hacke, U.G.; Spicer, R.; Schreiber, S.G.; Plavcová, L. An ecophysiological and developmental perspective on variation in vessel diameter. Plant Cell Environ. 2017, 40, 831–845. [Google Scholar] [CrossRef]
- Carlquist, S. Adaptive wood anatomy of chaparral shrubs. Calif. Chaparral Paradig. Reexamined Sci. Ser. 1989, 34, 25–35. [Google Scholar]
- Olson, M.E.; Anfodillo, T.; Rosell, J.A.; Petit, G.; Crivellaro, A.; Isnard, S.; Castorena, M. Universal hydraulics of the flowering plants: Vessel diameter scales with stem length across angiosperm lineages, habits and climates. Ecol. Lett. 2014, 17, 988–997. [Google Scholar] [CrossRef]
- Tyree, M.T.; Sperry, J.S. Vulnerability of xylem to cavitation and embolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 19–36. [Google Scholar] [CrossRef]
- Wheeler, J.K.; Sperry, J.S.; Hacke, U.G.; Hoang, N. Inter-vessel pitting and cavitation in woody Rosaceae and other vesselled plants: A basis for a safety versus efficiency trade-off in xylem transport. Plant Cell Environ. 2005, 28, 800–812. [Google Scholar] [CrossRef]
- Hacke, U.G.; Sperry, J.S.; Wheeler, J.K.; Castro, L. Scaling of angiosperm xylem structure with safety and efficiency. Tree Physiol. 2006, 26, 689–701. [Google Scholar] [CrossRef]
- Zimmermann, M.H. Transport in the xylem. In Trees: Structure and Function; Zimmermann, M.H., Ed.; Springer: Berlin, Germany, 1971; pp. 169–220. [Google Scholar]
- Tyree, M.T.; Davis, S.D.; Cochard, H. Biophysical perspectives of xylem evolution: Is there a trade-off of hydraulic efficiency for vulnerability to dysfunction? IAWA J. 1994, 15, 335–360. [Google Scholar] [CrossRef]
- Zhang, K.-Y.; Yang, D.; Zhang, Y.-B.; Liu, Q.; Wang, Y.-S.; Ke, Y.; Xiao, Y.; Wang, Q.; Dossa, G.G.O.; Schnitzer, S.A.; et al. Vessel dimorphism and wood traits in lianas and trees among three contrasting environments. Am. J. Bot. 2023, 110, e16154. [Google Scholar] [CrossRef]
- Isasa, E.; Link, R.M.; Jansen, S.; Tezeh, F.R.; Kaack, L.; Sarmento Cabral, J.; Schuldt, B. Addressing controversies in the xylem embolism resistance–vessel diameter relationship. New Phytol. 2023, 238, 283–296. [Google Scholar] [CrossRef]
- Trueba, S.; Delzon, S.; Isnard, S.; Lens, F. Similar hydraulic efficiency and safety across vesselless angiosperms and vessel-bearing species with scalariform perforation plates. J. Exp. Bot. 2019, 70, 3227–3240. [Google Scholar] [CrossRef]
- Lens, F.; Gleason, S.M.; Bortolami, G.; Brodersen, C.; Delzon, S.; Jansen, S. Functional xylem characteristics associated with drought-induced embolism in angiosperms. New Phytol. 2022, 236, 2019–2036. [Google Scholar] [CrossRef]
- Kaack, L.; Weber, M.; Isasa, E.; Karimi, Z.; Li, S.; Pereira, L.; Trabi, C.L.; Zhang, Y.; Schenk, H.J.; Schuldt, B.; et al. Pore constrictions in intervessel pit membranes provide a mechanistic explanation for xylem embolism resistance in angiosperms. New Phytol. 2021, 230, 1829–1843. [Google Scholar] [CrossRef]
- Pereira, L.; Kaack, L.; Guan, X.; Silva, L.D.M.; Miranda, M.T.; Pires, G.S.; Ribeiro, R.V.; Schenk, H.J.; Jansen, S. Angiosperms follow a convex trade-off to optimize hydraulic safety and efficiency. New Phytol. 2023, 240, 1788–1801. [Google Scholar] [CrossRef]
- Carlquist, S. Observations on functional wood histology of vines and lianas: Vessel dimorphism, tracheids, vasicentric tracheids, narrow vessels, and parenchyma. Aliso 1985, 11, 139–157. [Google Scholar] [CrossRef]
- Carlquist, S. Non-random vessel distribution in woods: Patterns, modes, diversity, correlations. Aliso 2009, 27, 39–58. [Google Scholar] [CrossRef]
- Tyree, M.T.; Zimmermann, M.H. Xylem Structure and the Ascent of Sap; Springer: Berlin, Germany, 2002. [Google Scholar]
- Brodersen, C.R.; Choat, B.; Chatelet, D.S.; Shackel, K.A.; Matthews, M.A.; McElrone, A.J. Xylem vessel relays contribute to radial connectivity in grapevine stems (Vitis vinifera and V. arizonica; Vitaceae). Am. J. Bot. 2013, 100, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Lens, F.; Sperry, J.S.; Christman, M.A.; Choat, B.; Rabaey, D.; Jansen, S. Testing hypotheses that link wood anatomy to cavitation resistance and hydraulic conductivity in the genus Acer. New Phytol. 2011, 190, 709–723. [Google Scholar] [CrossRef]
- Levionnois, S.; Jansen, S.; Wandji, R.T.; Beauchêne, J.; Ziegler, C.; Coste, S.; Stahl, C.; Delzon, S.; Authier, L.; Heuret, P. Linking drought-induced xylem embolism resistance to wood anatomical traits in Neotropical trees. New Phytol. 2021, 229, 1453–1466. [Google Scholar] [CrossRef]
- Ewers, F.W.; Ewers, J.M.; Jacobsen, A.L.; López-Portillo, J. Vessel redundancy: Modeling safety in numbers. IAWA J. 2007, 28, 373–388. [Google Scholar] [CrossRef]
- Jacobsen, A.L.; Ewers, F.W.; Pratt, R.B.; Paddock, W.A.; Davis, S.D. Do xylem fibers affect vessel cavitation resistance? Plant Physiol. 2005, 139, 546–556. [Google Scholar] [CrossRef]
- Morris, H.; Gillingham, M.A.F.; Plavcová, L.; Gleason, S.M.; Olson, M.E.; Coomes, D.A.; Fichtler, E.; Klepsch, M.M.; Martínez-Cabrera, H.I.; McGlinn, D.J.; et al. Vessel diameter is related to amount and spatial arrangement of axial parenchyma in woody angiosperms. Plant Cell Environ. 2018, 41, 245–260. [Google Scholar] [CrossRef]
- Janssen, T.A.J.; Hölttä, T.; Fleischer, K.; Naudts, K.; Dolman, H. Wood allocation trade-offs between fiber wall, fiber lumen, and axial parenchyma drive drought resistance in Neotropical trees. Plant Cell Environ. 2020, 43, 965–980. [Google Scholar] [CrossRef]
- Silva, L.M.; Bujnowski, B.; Pereira, L.; Miranda, M.T.; Schenk, H.J.; Jansen, S. Gas diffusion kinetics in relation to embolism formation and propagation in angiosperm xylem: A mini-review. Acta Hortic. 2025, 1419, 123–134. [Google Scholar] [CrossRef]
- Dias, A.S.; Oliveira, R.S.; Martins, F.R.; Bongers, F.; Anten, N.P.; Sterck, F. How do lianas and trees change their vascular strategy in seasonal versus rainforest? Perspect. Plant Ecol. Evol. Syst. 2019, 40, 125465. [Google Scholar] [CrossRef]
- Felsenstein, J. Phylogenies and the comparative method. Am. Nat. 1985, 125, 1–15. [Google Scholar] [CrossRef]
- Alves, E.S.; Angyalossy-Alfonso, V. Ecological trends in the wood anatomy of some Brazilian species. 1. Growth rings and vessels. IAWA J. 2000, 21, 3–30. [Google Scholar] [CrossRef]
- Blagitz, M.; Nogueira, A.; Marcati, C.R. Differences of the stem vascular system across populations of two tropical species under contrasting water conditions. IAWA J. 2021, 42, 20–41. [Google Scholar] [CrossRef]
- Alves, E.S.; Angyalossy-Alfonso, V. Ecological trends in the wood anatomy of some Brazilian species. 2. Axial parenchyma, rays and fibres. IAWA J. 2002, 23, 391–418. [Google Scholar] [CrossRef]
- Martínez-Cabrera, H.I.; Jones, C.S.; Espino, S.; Schenk, H.J. Wood anatomy and wood density in shrubs: Responses to varying aridity along transcontinental transects. Am. J. Bot. 2009, 96, 1388–1398. [Google Scholar] [CrossRef]
- Fisher, F.W.; Ewers, J.B. Structural responses to stem injury in vines. In The Biology of Vines; Putz, F.E., Mooney, H.A., Eds.; Cambridge University Press: Cambridge, UK, 1991; pp. 99–126. [Google Scholar]
- Rowe, N.P.; Speck, T. Biomechanical characteristics of the ontogeny and growth habit of the tropical liana Condylocarpon guianense (Apocynaceae). Int. J. Plant Sci. 1996, 157, 406–417. [Google Scholar] [CrossRef]
- Czaninski, Y. Étude du parenchyme ligneux du Robinier (parenchyme à réserves et cellules associées aux vaisseaux) au cours du cycle annuel. J. Microsc. 1968, 7, 145–164. [Google Scholar]
- Braun, H.J. The significance of the accessory tissues of the hydrosystem for osmotic water shifting as the second principle of water ascent, with some thoughts concerning the evolution of trees. IAWA Bull. 1984, 5, 275–294. [Google Scholar] [CrossRef]
- Salleo, S.; Lo Gullo, M.A.; Trifilò, P.; Nardini, A. New evidence for a role of vessel-associated cells and phloem in the rapid xylem refilling of cavitated stems of Laurus nobilis L. Plant Cell Environ. 2004, 27, 1065–1076. [Google Scholar] [CrossRef]
- Li, S.; Lens, F.; Espino, S.; Karimi, Z.; Klepsch, M.; Schenk, H.J.; Schmitt, M.; Schuldt, B.; Jansen, S. Intervessel pit membrane thickness as a key determinant of embolism resistance in angiosperm xylem. IAWA J. 2016, 37, 152–171. [Google Scholar] [CrossRef]
- Jansen, S.; Choat, B.; Pletsers, A. Morphological variation of intervessel pit membranes and implications to xylem function in angiosperms. Am. J. Bot. 2009, 96, 409–419. [Google Scholar] [CrossRef]
- Sperry, J.S.; Donnelly, J.R.; Tyree, M.T. A method for measuring hydraulic conductivity and embolism in xylem. Plant Cell Environ. 1988, 11, 35–40. [Google Scholar] [CrossRef]
- Tyree, M.T.; Ewers, F.W. The hydraulic architecture of trees and other woody plants. New Phytol. 1991, 119, 345–360. [Google Scholar] [CrossRef]
- Meunier, F.; Krishna Moorthy, S.M.; De Deurwaerder, H.P.T.; Kreus, R.; Van den Bulcke, J.; Lehnebach, R.; Verbeeck, H. Within-site variability of liana wood anatomical traits: A case study in Laussat, French Guiana. Forests 2020, 11, 523. [Google Scholar] [CrossRef]
- Gasson, P.; Dobbins, D.R. Wood anatomy of the Bignoniaceae, with a comparison of trees and lianas. IAWA J. 1991, 12, 389–415. [Google Scholar] [CrossRef]
- Angyalossy, V.; Pace, M.R.; Lima, A.C. Liana anatomy: A broad perspective on structural evolution of the vascular system. In Ecology of Lianas; Schnitzer, S.A., Bongers, F., Burnham, R.J., Putz, F.E., Eds.; Wiley-Blackwell: Chichester, UK, 2015; pp. 251–287. [Google Scholar]
- Ewers, F.W.; Fisher, J.B.; Fichtner, K. Water flux and xylem structure in vines. In The Biology of Vines; Putz, F.E., Mooney, H.A., Eds.; Cambridge University Press: Cambridge, UK, 1991; pp. 127–160. [Google Scholar]
- Sano, Y.; Morris, H.; Shimada, H.; Ronse De Craene, L.P.; Jansen, S. Anatomical features associated with water transport in imperforate tracheary elements of vessel-bearing angiosperms. Ann. Bot. 2011, 107, 953–964. [Google Scholar] [CrossRef]
- Zimmermann, M.H. The Hydraulic Architecture of Plants. In Xylem Structure and the Ascent of Sap; Zimmermann, M.H., Ed.; Springer: Berlin, Germany, 1983; pp. 66–82. [Google Scholar]
- Sperry, J.S.; Meinzer, F.C.; McCulloh, K.A. Safety and efficiency conflicts in hydraulic architecture: Scaling from tissues to trees. Plant Cell Environ. 2008, 31, 632–645. [Google Scholar] [CrossRef]
- Pittermann, J.; Sperry, J.S.; Hacke, U.G.; Wheeler, J.K.; Sikkema, E.H. Inter-tracheid pitting and the hydraulic efficiency of conifer wood: The role of tracheid allometry and cavitation protection. Am. J. Bot. 2006, 93, 1265–1279. [Google Scholar] [CrossRef]
- Beltrán-Rodríguez, L.; Romero-Manzanares, A.; Borja-de la Rosa, M.A.; Valdez-Hernández, J.I.; Luna-Cavazos, M.; Blancas-Vázquez, J. Adaptive advantages of wood anatomical–hydraulic features linked to sex in a tropical dioecious species. Trees 2022, 36, 39–52. [Google Scholar] [CrossRef]
- Ribeiro, J.E.L.S.; Hopkins, M.J.G.; Vicentini, A.; Sothers, C.A.; Costa, M.A.S.; Brito, J.M.; Procópio, L.C. Flora da Reserva Ducke; Instituto Nacional de Pesquisas da Amazônia (INPA) & Department for International Development (DFID): Manaus, Brazil, 1999. [Google Scholar]
- Morellato, P.C.; Leitão-Filho, H.F. Reproductive phenology of climbers in a Southeastern Brazilian forest. Biotropica 1996, 28, 180–191. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen–Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Marques Filho, A.O.; Ribeiro, M.N.G.; Santos, H.M.; Santos, J.M. Estudos climatológicos da Reserva Florestal Ducke, Manaus-AM. IV. Precipitação. Acta Amaz. 1981, 11, 759–768. [Google Scholar] [CrossRef]
- Esteban, E.J.L.; Castilho, C.V.; Melgaço, K.L.; Costa, F.R.C. The other side of droughts: Wet extremes and topography as buffers of drought negative effects in an Amazonian forest. New Phytol. 2020, 228, 1965–1978. [Google Scholar] [CrossRef]
- Guillaumet, J.L.; Kahn, F. Structure et dynamisme de la forêt. Acta Amaz. 1982, 12, 61–77. [Google Scholar] [CrossRef]
- Rocha, E.X.; Nogueira, A.; Costa, F.R.C.; Burnham, R.; Gerolamo, C.; Honorato, C.; Schietti, J. Liana functional assembly along the hydrological gradient in Central Amazonia. Oecologia 2022, 200, 183–197. [Google Scholar] [CrossRef]
- Chauvel, A.; Lucas, Y.; Boulet, R. On the genesis of the soil mantle of the region of Manaus. Experientia 1987, 43, 234–241. [Google Scholar] [CrossRef]
- Mertens, J. The Characterization of Selected Physical and Chemical Soil Properties of the Surface Soil Layer in the “Reserva Ducke”, Manaus, Brazil, with Emphasis on Their Spatial Distribution. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2004. [Google Scholar]
- Mendes, D.R.; Caroline, J.; Cunha, M.; Lammoglia, R.; Rodriguez, F.D.; Longo, R.M. Caracterização físico-química do solo em área de relevante interesse ecológico (A.R.I.E.)—Mata de Santa Genebra—Campinas/SP. In Anais do IV Congresso Brasileiro de Gestão Ambiental; Instituto Brasileiro de Estudos Ambientais: Belém, Brazil, 2013; pp. 1–13. [Google Scholar]
- Schnitzer, S.A.; DeWalt, S.J.; Chave, J. Censusing and measuring lianas: A quantitative comparison of the common methods. Biotropica 2006, 38, 581–591. [Google Scholar] [CrossRef]
- Gasson, P.; Baas, P. New Perspectives in Wood Anatomy; Springer: Dordrecht, The Netherlands, 1983. [Google Scholar]
- Rosell, J.A.; Olson, M.E. Do lianas really have wide vessels? Vessel diameter–stem length scaling in non-self-supporting plants. Perspect. Plant Ecol. Evol. Syst. 2014, 16, 288–295. [Google Scholar] [CrossRef]
- Johansen, D.A. Plant Microtechnique; McGraw-Hill: New York, NY, USA, 1940. [Google Scholar]
- Rupp, P. Polyglykol als Einbettungsmedium zum Schneiden botanischer Präparate. Mikrokosmos 1964, 53, 123–128. [Google Scholar]
- Barbosa, A.C.; Pace, M.R.; Witovisk, L.; Angyalossy, V. A new method to obtain good anatomical slides of heterogeneous plant parts. IAWA J. 2010, 31, 373–383. [Google Scholar] [CrossRef]
- Bukatsch, F. Remarks on the double staining Astra blue-safranin. Microkosmos 1972, 61, 255–258. [Google Scholar]
- Kraus, J.E.; Arduin, M. Manual Básico de Métodos em Morfologia Vegetal; EDUR: Rio de Janeiro, Brazil, 1997. [Google Scholar]
- IAWA Committee. IAWA list of microscopic features for hardwood identification. IAWA Bull. N.S. 1989, 10, 219–332. [Google Scholar]
- Franklin, G.L. Preparation of thin sections of synthetic resins and wood–resin composites, and a new macerating method for wood. Nature 1945, 155, 51–52. [Google Scholar] [CrossRef]
- Scholz, A.; Klepsch, M.; Karimi, Z.; Jansen, S. How to quantify conduits in wood? Front. Plant Sci. 2013, 4, 56. [Google Scholar] [CrossRef]
- Jacobsen, A.L.; Pratt, R.B.; Tobin, M.F.; Hacke, U.G.; Ewers, F.W. A global analysis of xylem vessel length in woody plants. Am. J. Bot. 2012, 99, 1583–1591. [Google Scholar] [CrossRef]
- Pereira, L.; Bittencourt, P.R.L.; Oliveira, R.S.; Junior, M.B.M.; Barros, F.V.; Ribeiro, R.V.; Mazzafera, P. Plant pneumatics: Stem air flow is related to embolism—New perspectives on methods in plant hydraulics. New Phytol. 2016, 211, 357–373. [Google Scholar] [CrossRef]
- Bittencourt, P.; Pereira, L.; Oliveira, R. Pneumatic method to measure plant xylem embolism. Bio-Protocol 2018, 8, e2752. [Google Scholar] [CrossRef]
- Zhang, Y.; Lamarque, L.J.; Torres-Ruiz, J.M.; Schuldt, B.; Karimi, Z.; Li, S.; Qin, D.W.; Bittencourt, P.; Burlett, R.; Cao, K.F.; et al. Testing the plant pneumatic method to estimate xylem embolism resistance in stems of temperate trees. Tree Physiol. 2018, 38, 1016–1025. [Google Scholar] [CrossRef]
- Scholander, P.F.; Hammel, H.T.; Bradstreet, E.D.; Hemmingsen, E.A. Sap pressure in vascular plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Pammenter, N.W.; Vander Willigen, C. A mathematical and statistical analysis of the curves illustrating vulnerability of xylem to cavitation. Tree Physiol. 1998, 18, 589–593. [Google Scholar] [CrossRef]
- Poorter, L.; McDonald, I.; Alarcón, A.; Fichtler, E.; Licona, J.C.; Peña-Claros, M.; Sterck, F.; Villegas, Z.; Sass-Klaassen, U. The importance of wood traits and hydraulic conductance for the performance and life history strategies of 42 rainforest tree species. New Phytol. 2010, 185, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Björklund, M. Be careful with your principal components. Evolution 2019, 73, 2151–2160. [Google Scholar] [CrossRef] [PubMed]
- Bates, D. lme4: Mixed-Effects Modeling with R. J. Stat. Softw. 2010, 37, 1–48. [Google Scholar]
- Thiers, B. Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. New York Botanical Garden’s Virtual Herbarium. Available online: http://sweetgum.nybg.org/science/ih/ (accessed on 22 August 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerolamo, C.S.; Nogueira, A.; Pereira, L.; Jansen, S.; Rocha, E.X.; Angyalossy, V. Stem Xylem Differences in Congeneric Lianas Between Forests Are Unrelated to Hydraulic Safety but Partly Explain Efficiency. Plants 2025, 14, 2951. https://doi.org/10.3390/plants14192951
Gerolamo CS, Nogueira A, Pereira L, Jansen S, Rocha EX, Angyalossy V. Stem Xylem Differences in Congeneric Lianas Between Forests Are Unrelated to Hydraulic Safety but Partly Explain Efficiency. Plants. 2025; 14(19):2951. https://doi.org/10.3390/plants14192951
Chicago/Turabian StyleGerolamo, Caian S., Anselmo Nogueira, Luciano Pereira, Steven Jansen, Elisangela X. Rocha, and Veronica Angyalossy. 2025. "Stem Xylem Differences in Congeneric Lianas Between Forests Are Unrelated to Hydraulic Safety but Partly Explain Efficiency" Plants 14, no. 19: 2951. https://doi.org/10.3390/plants14192951
APA StyleGerolamo, C. S., Nogueira, A., Pereira, L., Jansen, S., Rocha, E. X., & Angyalossy, V. (2025). Stem Xylem Differences in Congeneric Lianas Between Forests Are Unrelated to Hydraulic Safety but Partly Explain Efficiency. Plants, 14(19), 2951. https://doi.org/10.3390/plants14192951







